Abstract
Purpose of review
Articular cartilage vesicles (ACVs) are small extracellular vesicles that serve as foci of pathologic calcium crystal deposition in articular cartilage matrix. In this review, I have summarized the role of ACVs in calcium crystal formation and discuss recent findings that impact our understanding of the content, behavior, and origin of ACVs in healthy and diseased joints. The burgeoning interest in extracellular vesicles in other fields renders this a timely and relevant topic.
Recent findings
I have highlighted recent studies demonstrating that some ACVs originate in the autophagic pathway in healthy articular chondrocytes. I have reviewed accumulating evidence that nonmineralizing functions of ACVs contribute to osteoarthritis. I have also discussed new work supporting a role for extracellular vesicles in interleukin-1 β-induced mineralization and in mediating the catabolic effects of synovial inflammation in osteoarthritis.
Summary
We are making slow and steady progress in understanding the origin and function of ACVs and other relevant extracellular vesicles in arthritis. Further work in this interesting area is warranted.
Keywords: articular cartilage vesicles, autophagy, basic calcium phosphate crystals, calcium pyrophosphate, chondrocalcinosis, chondrocytes, exosomes, matrix vesicles, pseudogout
INTRODUCTION
Articular cartilage vesicles (ACVs) are 50–250 nm diameter extracellular vesicles found in the pericellular matrix of healthy articular cartilage. They were initially described in the context of pathologic articular cartilage calcification [1], mirroring similar work performed 20 years earlier identifying matrix vesicles in growth plate cartilage [2]. ACVs concentrate enzymes and substrates necessary for the formation of arthritogenic calcium crystals; and isolated ACVs serve as an important in-vitro model of pathologic articular crystal formation [1]. Although it is highly unlikely that these structures possess only pathologic functions, remarkably little is known about the role of ACVs in healthy cartilage.
Recent studies of ACVs have been informed by the explosion of interest in cell-derived extracellular vesicles. It is now clear that multiple types of extracellular vesicles are released by all cell types in a highly regulated fashion. Two major categories of extracellular vesicles are loosely defined by size. Exosomes are 50–200 nm in diameter, whereas microparticles are 500–1000 nm in diameter. Studies of extracellular vesicles at other sites of pathologic mineralization, such as atherosclerotic plaques [3], heart valves [4], and in the kidney during stone formation [5], may also illuminate processes involved in the formation and function of ACVs.
In this review, I have summarized current knowledge of ACVs in the context of articular calcium-containing crystal formation and osteoarthritis, and highlight new advances relevant to processes of pathologic calcification in the joint and to our understanding of ACVs in healthy and diseased cartilage.
ARTHRITOGENIC CALCIUM-CONTAINING CRYSTALS
Two varieties of calcium-containing crystals are associated with arthritis. Calcium pyrophosphate (CPP) crystals are fairly unique to articular cartilage, whereas basic calcium phosphate (BCP) crystals are broadly distributed and found in many types of calcifications. CPP crystals cause an acute arthritis known as pseudogout, but more commonly produce painful degenerative arthritis with or without inflammatory features. Approximately 20% of unselected patients with osteoarthritis have CPP crystals in their joint fluids [6] or cartilage [7]. BCP crystals contain a trio of carbonated substitute apatite, octacalcium phosphate, and tricalcium phosphate. They are extremely common in end-stage osteoarthritis [6,7]. They also cause a heterogeneous group of musculoskeletal pathologies, including calcific tendonitis, Milwaukee shoulder syndrome, and periarthritis syndromes such as pseudopodagra [8]. Both types of calcium-containing crystals predict severe cartilage damage in osteoarthritis [9].
MECHANISMS OF ARTICULAR CARTILAGE VESICLE MINERALIZATION
Although considerable attention has been paid to understanding the mechanisms of damage induced by calcium crystals [10,11], major knowledge gaps exist in our understanding of calcium crystal formation. Much of the work on pathologic crystal formation builds on our understanding of processes of normal mineralization in bones and teeth. The advent of electron microscopy revealed lipid-rich deposits in the pericellular matrix of mineralizing growth plate chondrocytes. These were ultimately shown to contain membrane-bound extracellular vesicles called ‘matrix vesicles’ [12]. In the ensuing years, matrix vesicles were enzymatically isolated from growth plate cartilage and shown to generate hydroxyapatite-like mineral [13].
Twenty years after the isolation and characterization of matrix vesicles from growth plate cartilage, similar-sized extracellular vesicles were noted in normal articular cartilage [14]. Enzymatic isolation from the surrounding extracellular matrix and differential centrifugation yields a heterogeneous population of membrane-delineated vesicles (Fig. 1). Isolated ACVs generate arthritogenic calcium crystals in vitro identical to those seen in human synovial fluid [15]. ACVs are easily isolated from normal cartilage as well as osteoarthritic cartilage [16]. They are also generated and released into conditioned media by chondrocytes in monolayer cultures [17].
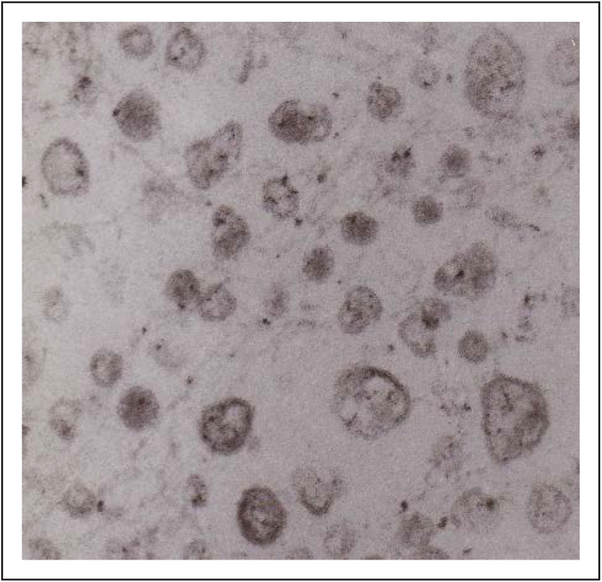
FIGURE 1.
Articular cartilage vesicles. Electron micrographs of articular cartilage vesicles isolated from adult porcine articular cartilage showing a heterogeneous population of membrane-delineated vesicles 50–250 nm in diameter.
FACTORS REGULATING ARTICULAR CARTILAGE VESICLE MINERALIZATION
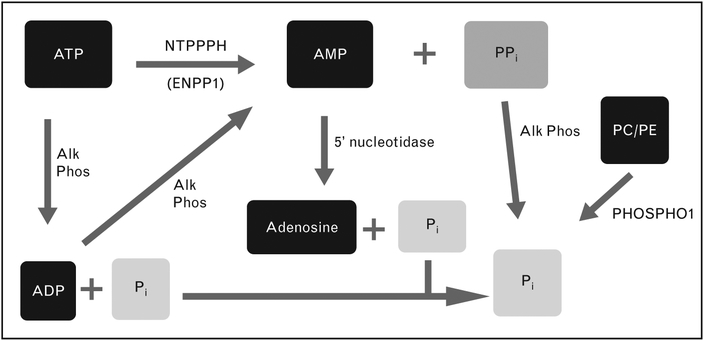
The type and quantity of mineral generated by ACVs are regulated by the ratio of pyrophosphate (PPi) to phosphate (Pi). This critical ratio also controls mineralization in other tissues. ACVs generate CPP crystals in the presence of ATP. BCP crystal formation predominates when B-glycerophosphate is the primary phosphate source [15,18]. Specific activities of ATP metabolizing ecto-enzymes on ACVs drive mineralization by affecting levels of Pi and PPi (Fig. 2). These ecto-enzymes include alkaline phosphatase, nucleotide triphosphate pyrophos-phohydrolases, such as ectonucleoside pyrophosphohydrolase/phosphodiesterase1 (ENPP1), and 5′ nucleotidase. Although the PPi/Pi ratio is primarily controlled by the ratio of NTPPPH to alkaline phosphatase enzyme activities, recent work suggests that this equation is considerably more complex. Zhang et al. [19] demonstrated that alkaline phosphatase in matrix vesicles possesses phosphodiesterase activity which can generate PPi from ATP. Elegant studies implicate the PHOSPHO1 enzyme as an additional participant in matrix vesicle mineralization. PHOSPHO1 is a phosphoethanolamine/phosphocholine phosphatase that functions to pull phosphate into the interior of the vesicle, where mineralization begins [20]. PHOSPHO1 has not been studied in ACVs, but this enzyme and others that regulate the critical PPi/Pi ratio warrant further study.
FIGURE 2.
Ecto-enzymes regulating ratio of pyrophosphate and phosphate levels. Specific enzymes with NTPPPH activity include ectonucleoside pyrophosphohydrolase/phosphodiesterase1. Alk Phos, alkaline phosphatase; NTPPPH, nucleoside triphosphate pyrophosphohydrolase; PPi, ratio of pyrophosphate; Pi, ratio of phosphate.
Studies of calcification in nonarticular cells and vesicles may reveal additional factors regulating ACV-induced mineralization. For example, bone marrow-derived mesenchymal stem cells undergo massive mineralization in response to interleukin-1β (Il-1β). Interesting work exploring the mechanism of this effect demonstrated that mesenchymal stem cells do not undergo osteoblastic differentiation in response to IL-1β [21]. Instead, mineralization correlated with a decrease in cellular levels of ENPP1. Interestingly, analysis of the extracellular vesicles produced by IL-1β treated mesenchymal stem cells showed a clearly altered ratio of ENPP1 to alkaline phosphatase and less PPi in the vesicle fraction. This work supports the hypothesis that inflammatory cytokines can induce mineralization by altering the enzymes regulating the PPi/Pi ratio on extracellular vesicles [21].
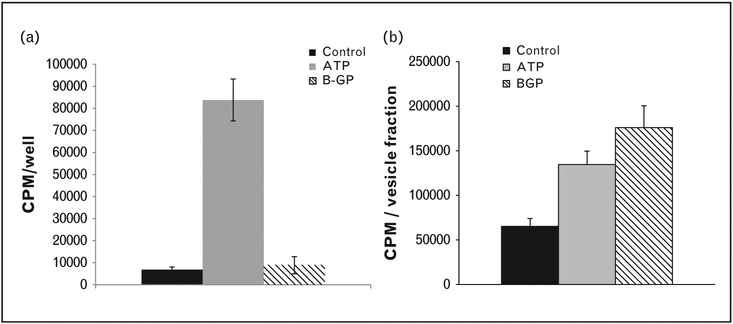
The composition of the extracellular matrix surrounding ACVs clearly also plays an important role in regulating mineralization. The unique qualities of articular cartilage extracellular matrix may explain why CPP crystals are located almost exclusively in cartilage and at sites of cartilage metaplasia. This is supported by several interesting indirect observations. As shown in Fig. 3, in response to various phosphate sources, the mineralization pattern of articular chondrocytes differs from that of isolated ACVs. Quantities of extracellular matrix are significantly higher in chondrocyte cultures than in ACV fractions. Secondly, while CPP crystals are not known to be present in any animal smaller than a dog [22] and have never been identified in growth plate cartilage, isolated matrix vesicles derived from chicken growth plate cartilage produce CPP crystals when provided with high concentrations of ATP [23]. In more direct studies, mineralization was assessed when ACVs were embedded in agarose gels containing various matrix components. Normal cartilage matrix, modeled by adding proteoglycans and type II collagen to the gel, suppressed ACV-induced mineralization [15]. Mimicking osteoarthritic extracellular matrix by adding type I collagen increased ACV-induced mineralization [15]. Thus, the compositional changes that occur in osteoarthritic matrix promote ACV mineralization.
FIGURE 3.
Mineralization patterns differ in chondrocyte monolayers and ACVs in response to ATP and β-glycerophosphate. (a) Adult porcine chondrocyte monolayers were incubated with 1mM ATP or 1mM β-glycerophosphate (BGP) in media trace labeled with 45Ca2+. After 48 h, media were removed and 45Ca2+ in the cell layer was quantified with liquid scintigraphy. (b) ACVs isolated from adult porcine cartilage were incubated in a solution trace labeled with 45Ca2+ containing 1mM ATP or 1mM β-glycerophosphate for 72 h. ACVs were washed and 45Ca2+ in the ACV fraction was quantified using liquid scintigraphy. ACV, articular cartilage vesicle.
ARTICULAR CARTILAGE VESICLE CONTENT
We have much to learn about the content of ACVs. Proteomics methodologies are well suited to studying the interior contents of ACVs, and vesicles typically contain a manageable-sized proteome [24]. Our proteomic work highlighted differences and similarities between ACVs isolated from normal and osteoarthritic cartilage [24]. We showed that the majority of the proteome was shared by both types of ACVs. The small group of proteins that were different primarily represented extracellular proteins in osteoarthritic cartilage matrix. A recent publication describes an open web-based database with proteomics data from matrix vesicles derived from bone, cartilage, and teeth of six different species [25■]. This type of tool will aid further investigations of vesicle content.
ARTICULAR CARTILAGE VESICLE ORIGIN
In general, processes involved in extracellular vesicle generation and release remain poorly understood. Matrix vesicles from growth plate chondrocytes are believed to arise from microvilli through a microtubule-dependent process involving zeoitic blebbing [26■]. In smooth muscle cells forced to adopt an osteoblast-like phenotype, exosome-like vesicles are derived from multivesicular bodies in a process responsive to inflammatory cytokines[27]. We recently demonstrated that ACVs from primary articular chondrocytes are generated through the autophagic pathway [28■■]. Autophagy is the process through which internal cell contents are recycled in intracellular acidic vesicles. Autophagy is a constitutive process in healthy chondrocytes, whose normal environs are nutrient-poor and hypoxic [29]. Using a dynamic model, we showed that agents that induce autophagy increase ACVs numbers in the conditioned media of primary articular chondrocyte cultures. ACVs carry markers of autophagosomes and are externalized in a caspase-3-dependent process which is independent of apoptosis [28■■]. A role for membrane blebbing was illustrated by the ability of Rho associated protein kinase pathway inhibitors to reduce ACV externalization [28■■]. This pathway is the first identified pathway for ACV formation and release. This work has implications for understanding the role of ACVs as well as the potential to provide new tools to manipulate ACV production.
NONMINERALIZING CONTRIBUTION TO OSTEOARTHRITIS
ACVs may contribute to osteoarthritis by affecting processes other than mineralization. Extracellular vesicles safely carry proteins and RNA from one cell to another. This might be particularly important in tissues with relatively geographically isolated cells such as articular cartilage. Several years ago, Mitton et al. [30] demonstrated that ACVs contain measurable quantities of RNA. ACV RNA is highly resistant to RNases, and is predominantly comprising small RNAs similar in size to microRNAs. Radiolabeled ACV RNA could be transferred to chondrocytes. Importantly, chondrocytes exposed to ACVs displayed markers of an osteoarthritic phenotype, including high levels of alkaline phosphatase and osteopontin.
Increasing recognition of a critical role for synovial inflammation in osteoarthritis spurred work on synovial cell-derived extracellular vesicles in osteoarthritis. Microparticles and exosomes from many tissues modulate inflammation though a variety of mechanisms [31]. Kato et al. [32■■] analyzed exosomes derived from human synovial fibroblasts from normal joints. Using a gel-based binding method of concentrating exosomes from conditioned media, they characterized synovial exosomes using antibodies to exosome markers. IL-1β, a critical factor in osteoarthritis [33], increased exosome formation. Exosomes derived from IL-1β-treated synovial cultures produced high levels of the catabolic mediator, matrix metalloproteinase 13, when added to chondrocyte cultures [32■■]. Exosomes from IL-1β-derived synovial cells also stimulated proteoglycan release from mouse femoral head cartilage and increased markers of angiogenesis in human umbilical vein endothelial cells. This interesting work suggests that synovial exosomes formed in an inflammatory environment may directly contribute to articular damage in osteoarthritis.
Exosomes also participate in nonclassical protein secretion and may contribute to osteoarthritis by releasing these proteins into the extracellular space. IL-1β plays a critical role in osteoarthritis and may be exported via vesicular transport [34]. Type II transglutaminase is present in high concentrations in ACVs [35], has critical functions in extracellular matrix and in osteoarthritis [36], and lacks a leader sequence essential for classical protein export. Further efforts to understand the mechanisms through which individual proteins are selected and processed into vesicles for transport are necessary.
FUTURE DIRECTIONS
ACVs remain poorly understood. Additional information about content, including lipid analysis and better methods to identify membrane proteins are necessary. Because of the difficulties inherent in studying ACVs in situ in articular cartilage, most functional studies require that an ACV fraction be concentrated and isolated and this requires relatively large quantities of articular cartilage or chondrocytes. Interestingly, ACVs from conditioned media, cell layers, and whole cartilage may not be equivalent in terms of composition and behavior, and a better understanding of how isolation methods affect the form and function of ACV will be needed to move this field forward. Although protein secretion and matrix repair have been proposed as possible functions in healthy cartilage, the role of ACVs in situ in normal cartilage remains essentially unknown.
CONCLUSION
Slow progress has been made in understanding the mechanisms though which ACVs contribute to mineralization in situ in cartilage. Work accomplished in the last several years has provided us with a potential mechanism through which ACVs are generated, and an ability to manipulate their production. Further studies of this interesting area of cartilage biology are warranted.
KEY POINTS.
ACVs play a key role in pathologic mineralization in cartilage.
ACV-induced mineralization is regulated by the PPi/Pi ratio and extracellular matrix components.
Some ACVs originate in the autophagic pathway.
Additional information about the content and behavior of these important vesicles will shed light on their functions in healthy and diseased cartilage.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the Department of Veterans Affairs, Merit Review Grants BX000812 (A.K.R.) and CX001143 (A.K.R.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Wortmann RL, Chowdhury M, Rachow JW. ATP-dependent mineralization of hyaline articular cartilage matrix vesicles In: Mikanagi K, Nishioka K, Kelley W, editors. Purine and pyrimidine metabolism in man. New York, NY: Plenum; 1989. pp. 55–62. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 1969; 41:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 2013; 113:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc 1976; 35:156–162. [PubMed] [Google Scholar]
- 5.Khan SR, Rodriguez DE, Gower LB, Monga M. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol 2012; 187:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in preoperative knees. J Rheumatol 2002; 29:570–574. [PubMed] [Google Scholar]
- 7.Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 2009; 60:2694–2703. [DOI] [PubMed] [Google Scholar]
- 8.Fam AG, Rubenstein J. Hydroxyapatite pseudopodagra. A syndrome of young women. Arthritis Rheum 1989; 32:741–747. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst M, Niggemeyer O, Lammers L, et al. Articular cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet Disord 2009; 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy GM, Westfall PR, Masuda I, et al. Basic calcium phosphate crystals activate human osteoarthritic synovial fibroblasts and induce matrix metalloproteinase-13 (collagenase-3) in adult porcine articular chondrocytes. Ann Rheum Dis 2001; 60:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazar B, Ea HK, Narayan S, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol 2011; 186:2495–2502. [DOI] [PubMed] [Google Scholar]
- 12.Anderson HC. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 1967; 35:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean FM, Keller PJ, Genge BR, et al. Disposition of preformed mineral in matrix vesicles. Internal localization and association with alkaline phosphatase. J Biol Chem 1987; 262:10481–10488. [PubMed] [Google Scholar]
- 14.Ali SY. Apatite-type crystal deposition in arthritic cartilage. Scan Electron Microsc 1985; 4:1555–1566. [PubMed] [Google Scholar]
- 15.Jubeck B, Gohr C, Fahey M, et al. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum 2008; 58:2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derfus B, Kranendonk S, Camacho N, et al. Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif Tissue Int 1998; 63:258–262. [DOI] [PubMed] [Google Scholar]
- 17.Derfus BA, Camacho NP, Olmez U, et al. Transforming growth factor beta-1 stimulates articular chondrocyte elaboration of matrix vesicles capable of greater calcium pyrophosphate precipitation. Osteoarthritis Cartilage 2001; 9:189–194. [DOI] [PubMed] [Google Scholar]
- 18.Derfus BA, Rachow JW, Mandel NS, et al. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum 1992; 35:231–240. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Balcerzak M, Radisson J, et al. Phosphodiesterase activity of alkaline phosphatase in ATP-initiated Ca(2+) and phosphate deposition in isolated chicken matrix vesicles. J Biol Chem 2005; 280:37289–37296. [DOI] [PubMed] [Google Scholar]
- 20.Simao AM, Bolean M, Hoylaerts MF, et al. Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles’ biomimetics. Calcif Tissue Int 2013; 93:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira E, Porter RM, Wehling N, et al. Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow. J Biol Chem 2013; 288:29494–29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan L, McCarty D. Calcium pyrophosphate crystal deposition disease; pseudogout; articular chondrocalcinosis In: McCarty D, editor. Arthritis and allied conditions. Philadelphia, PA: Lea & Febiger; 1985. pp. 1515–1546. [Google Scholar]
- 23.Thouverey C, Bechkoff G, Pikula S, Buchet R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthritis Cartilage 2009; 17:64–72. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum 2011; 63:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Xu Q, Luan J, et al. MVsCarta: a protein database of matrix vesicles to aid understanding of biomineralization. Biosci Trends 2015; 9:190–192. [DOI] [PubMed] [Google Scholar]; ■ The study describes a new open database available to investigators interested in matrix vesicles from mineralizing tissues.
- 26.Abdallah D, Hamade E, Merhi RA, et al. Fatty acid composition in matrix vesicles and in microvilli from femurs of chicken embryos revealed selective recruitment of fatty acids. Biochem Biophys Res Commun 2014; 446:1161–1164. [DOI] [PubMed] [Google Scholar]; ■ The interesting work supports the notion that a select population of lipids participates in matrix vesicle formation and may be important in mediating vesicle-induced mineralization.
- 27.Kapustin AN, Chatrou ML, Drozdov I, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 2015; 116:1312–1323. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, et al. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J Biol Chem 2015; 290:13028–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ The work demonstrates a role for autophagy, membrane blebbing and caspase-3 in ACV formation and release in primary articular chondrocytes.
- 29.Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol 2015; 67:1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitton E, Gohr CM, McNally MT, Rosenthal AK. Articular cartilage vesicles contain RNA. Biochem Biophys Res Commun 2009; 388:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suades R, Padro T, Badimon L. The role of blood-borne microparticles in inflammation and hemostasis. Semin Thromb Hemost 2015; 41:590–606. [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Miyaki S, Ishitobi H, et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther 2014; 16:R163. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ The studies support a role for synovial exosomes in mediating cartilage damage in osteoarthritis.
- 33.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011; 7:33–42. [DOI] [PubMed] [Google Scholar]
- 34.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 2007; 179:1913–1925. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum 1997; 40:966–970. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal AK, Gohr CM, Henry LA, Le M. Participation of transglutaminase in the activation of latent transforming growth factor beta1 in aging articular cartilage. Arthritis Rheum 2000; 43:1729–1733. [DOI] [PubMed] [Google Scholar]