Calcium pyrophosphate deposition (CPPD) disease is arthritis caused by calcium pyrophosphate (CPP) crystals (Fig. 1). Until recently, CPPD disease has been referred to as pseudogout. This term stems from an early description of this disease in patients with an acute goutlike arthritis whose synovial-fluid crystals were resistant to digestion by uricase and who thus did not have gout.1 Almost simultaneously, a case series was published of 27 patients in Hungary who had chronic episodic inflammatory oligoarthritis affecting primarily the knee.2 These patients shared a radiographic finding that was characterized by a “dense narrow band following the contour of the epiphysis” in the articular cartilage, a finding that was termed chondrocalcinosis articularis (Fig. 2A and 2B). These two early descriptions foreshadowed the broad range of clinical presentations that currently constitute CPPD disease.
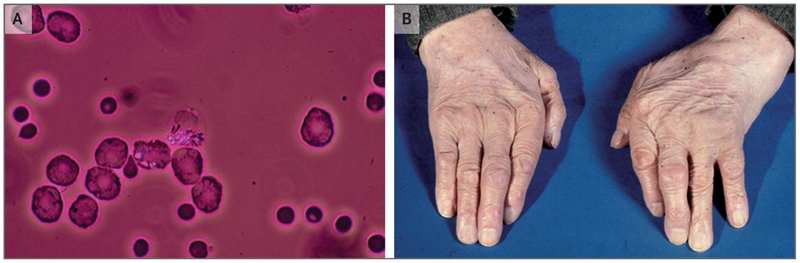
Figure 1. Calcium Pyrophosphate Deposition (CPPD).
Rhomboid, birefringent calcium pyrophosphate (CPP) crystals are seen under polarizing light microscopy in this sample of synovial fluid that was obtained from a patient with acute CPP crystal arthritis of the wrist (Panel A). The hands of an elderly patient with CPPD disease show swelling in the left wrist and the third proximal interphalangeal joint of the left hand (Panel B).
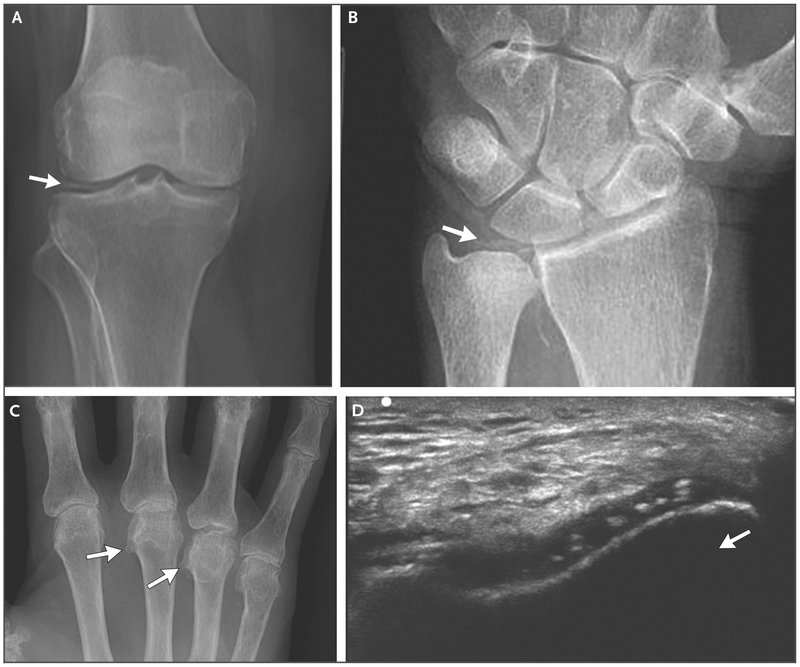
Figure 2. Imaging of Chondrocalcinosis in Patients with CPPD Disease.
Panel A shows a radiograph of a knee with meniscal chondrocalcinosis (arrow). Panel B shows a radiograph of a wrist with chondrocalcinosis of the triangular cartilage (arrow). Panel C shows a radiograph of a hand with hooklike osteophytes (arrows). Panel D shows an ultrasonographic image of a right knee, which was obtained with the transducer in the anatomical axial plane, with the knee flexed 90 degrees. The probe was pointed at the femoral cartilage on the “V” of the patellar groove. Chondrocalcinosis is seen in the substance of the cartilage; the arrow indicates the direction of the probe.
Nomenclature issues have plagued CPPD disease since its original description. Various cumbersome terms such as “calcium pyrophosphate dihydrate deposition disease” achieved common use. In 2011, a group from the European League against Rheumatism recommended that calcium pyrophosphate crystals be referred to as CPP crystals, that the term “acute CPP crystal arthritis” refer to the acute inflammatory arthritis that was formerly known as pseudogout, and that the term “chronic CPP crystal arthritis” be used to denote other types of arthritis associated with CPP crystals.3 The term “chondrocalcinosis” refers to the common radiographic correlate of CPPD disease and does not imply clinical arthritis. We use the term “CPP deposition” (CPPD) to refer to the presence of CPP crystals and the term “CPPD disease” to include all the related clinical presentations.
CLINICAL PRESENATION
Acute CPP crystal arthritis (or pseudogout) is the most widely recognized form of CPPD disease. Patients typically present with the acute onset of monoarticular or oligoarticular arthritis. The vigorous inflammatory response to CPP crystals manifests as warmth, erythema, and swelling in and around the affected joint, and the clinical picture is often indistinguishable from acute gouty arthritis or septic arthritis. Along with other findings, the distribution of joint involvement may provide a helpful clue with regard to the presence of acute CPP crystal arthritis. The knee is the most commonly involved joint, followed by the wrist; acute podagra in the first metatarsophalangeal joint is rare. Systemic symptoms including fevers, chills, and constitutional symptoms often occur with acute CPP crystal arthritis. In contrast to the brief attacks of acute gouty arthritis that typically last for several days to 1 week, acute attacks of CPPD disease may last for weeks to months.4
Chronic CPP crystal arthritis comprises several clinical phenotypes. Most affected patients have a polyarticular form of arthritis that resembles osteoarthritis. This osteoarthritis-like arthritis is usually distinguishable from typical osteoarthritis by flares of inflammatory signs and symptoms and by unusually severe articular damage. The involvement of joints such as the glenohumeral joint, the wrist, and the metacarpophalangeal joints, which are not often affected by typical osteoarthritis, should lead one to suspect the presence of CPPD disease (Fig. 1B). A rarer form of polyarticular CPPD disease resembles rheumatoid arthritis. Patients with this condition have persistent inflammatory arthritis that affects large and small joints. Flares in this phenotypic variant of CPPD disease often involve joints sequentially, and involvement is less symmetric than that seen with rheumatoid arthritis. McCarty estimated that the chronic degenerative polyarticular form of CPPD disease accounts for roughly 50% of the cases of CPPD disease, whereas acute CPP crystal arthritis represents approximately 25% of the cases.5
Other less common clinical presentations of CPPD disease have been described. CPP crystals are commonly seen in spinal tissues, including inter-vertebral disks and spinal ligaments.6,7 The crowned dens syndrome is caused by the deposition of CPP crystals around the C2 vertebra and manifests as acute severe neck pain, fever, and high levels of inflammatory markers.8 This syndrome is often confused with meningitis or sepsis. CPP crystals have also been associated with a severely destructive arthritis that is similar to neurotrophic (Char-cot’s) arthropathy.9 Rarely, tumoral deposits of CPP crystals occur in soft tissues, where they can cause considerable tissue damage and may be mistaken for cancers.10 In an unknown percentage of patients, chondrocalcinosis is present without clinical arthritis. We believe that this condition represents a presymptomatic phase of clinical arthritis, similar to that of hyperuricemia in gout, but the proof of this will require further study.
PATHOGENESIS
Although the pathogenesis of CPPD disease is not fully understood, the formation of CPP crystals in the pericellular matrix of cartilage is the essential first step in the disease process (Fig. 3). CPP crystals rarely form in noncartilaginous tissues.11 Thus, the cells and highly specialized extra-cellular matrix of cartilage are clearly conducive to CPPD. For example, chondrocytes constitutively generate pericellular exosome-sized vesicles, termed “articular cartilage vesicles,” which serve as important sites of crystal formation in cartilage (Fig. 3).12 Chondrocytes also produce high levels of extracellular inorganic pyrophosphate, which is critical to the formation of CPP crystals.13
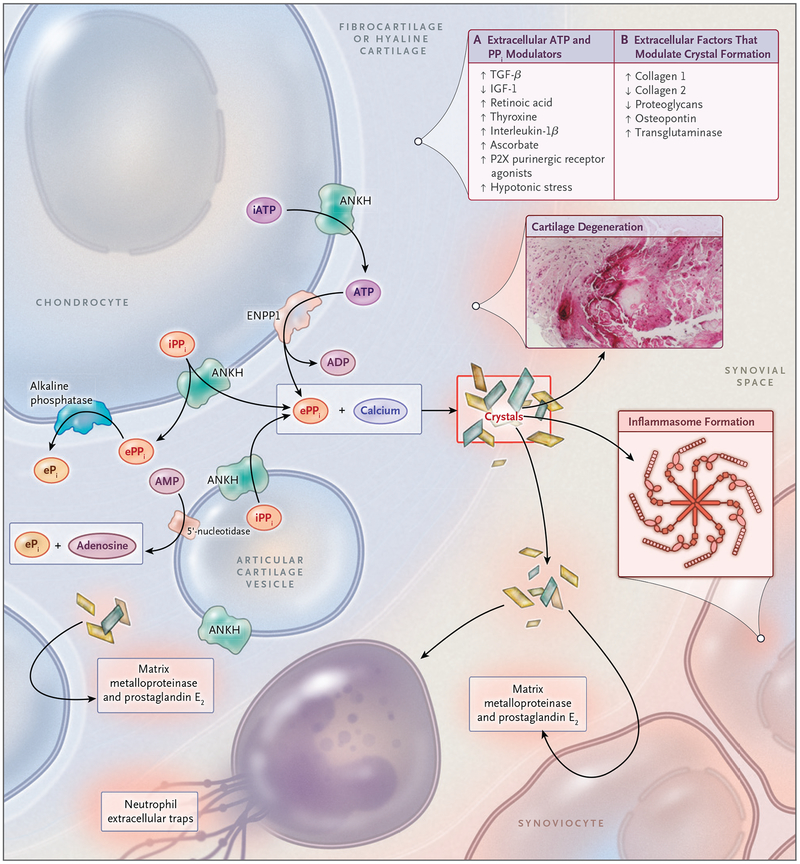
Figure 3. Pathophysiological Features of CPPD Disease.
The formation of CPP crystals occurs in the articular cartilage pericellular matrix and is facilitated by extracellular vesicles known as articular cartilage vesicles. Pyrophosphate (PPi) is generated from extracellular ATP and forms complexes with calcium to create CPP crystals. Panel A of the box, upper right, lists the factors that are known to modulate levels of extracellular ATP and PPi, and Panel B the extracellular matrix factors that regulate the formation of CPP crystals. P2X indicates one class of purinergic receptors. CPP crystals induce inflammation in the synovial space but also have adverse biomechanical consequences and direct catabolic effects on joint tissues owing to the production of prostaglandin E2 and matrix metalloproteinases. These factors ultimately produce cartilage degeneration, as shown by the CPP crystal deposit in cartilage in situ (inset). ANKH denotes human homologue of the protein product of the progressive ankylosis gene, ENPP1 ectonucleotide pyrophosphatase 1, ePi extracellular phosphate ion, ePPi extracellular PPi, IGF-1 insulin-like growth factor 1, iPPi intracellular PPi, and TGF-β transforming growth factor β.
Inorganic pyrophosphate plays a central role in CPPD that is analogous to that of urate in gout and may be a key therapeutic target. In cartilage, most inorganic pyrophosphate is generated from extracellular ATP.14 ATP efflux and thus the levels of inorganic pyrophosphate are critically regulated by the multipass membrane protein known as ANKH (the human homologue of protein product of the murine progressive ankylosis gene).15 ANKH may represent a therapeutic target in CPPD, and existing drugs, such as probenecid, act as potent antagonists of ANKH action in vitro.15 Extra-cellular ATP is metabolized to inorganic pyrophosphate by enzymes with nucleoside triphosphate pyrophosphohydrolase activity, such as ectonucleotide pyrophosphatase 1, whereas alkaline phosphatase and other pyrophosphatases degrade inorganic pyrophosphate. In addition, growth factors, cytokines, and some pharmacologic agents modulate the levels of inorganic pyrophosphate in cartilage (Fig. 3).14
Once CPP crystals are generated, they mediate tissue damage by means of multiple mechanisms. They initiate inflammation by activating components of the NLRP3 inflammasome16 and by creating neutrophil extracellular traps.17 Apart from inducing inflammation, CPP crystals have important direct catabolic effects on chondrocytes18 and synoviocytes,19 eliciting the production of destructive matrix metalloproteinases and prostaglandins. CPP crystal deposits in articular cartilage also alter the mechanical properties of cartilage, which may cause or accelerate joint damage (Fig. 3, inset).20
PREVALENCE
Estimates vary, but CPPD disease appears to affect 4 to 7% of the adult population in Europe and the United States.21,22 Unfortunately, our current understanding of the prevalence of CPPD disease is based largely on radiographically detected chon drocalcinosis rather than on clinically important CPP crystal arthritis. The presence of chondrocalcinosis probably identifies only approximately 40% of the patients with clinically significant CPPD disease,23 and chondrocalcinosis is particularly difficult to visualize on radiography in patients with severe cartilage loss. Conversely, chondrocalcinosis, particularly in the fibrocartilage of the knee, may occur in patients without arthritis and can be composed of a non-CPP mineral, primarily dicalcium phosphate dihydrate.24
PISK FACTORS AND ASSOCIATED CONDITIONS
CPPD disease is clearly a disease of aging and is rare in patients younger than 60 years of age.21 In radiographic examinations that include the knee, pelvis, and wrist, chondrocalcinosis is detected in 44% of patients older than 84 years of age; the prevalence doubles with each decade over 60 years of age.25 Previous trauma to the joint is also a strong risk factor for CPPD. This association is best shown in the meniscus of the knee. One study showed that decades after meniscectomy, chondrocalcinosis developed in 20% of the knees treated with surgery, as compared with only 4% of the contralateral knees not treated with surgery.26
CPPD is often found in the context of osteoarthritis. There is some overlap in the clinical presentations of CPPD disease and osteoarthritis, so that diagnostic mimicry may explain some of the association. Osteoarthritis and CPPD disease are both relatively common with advanced age, and thus co-occurrence by chance might explain the association. However, because of the strong evidence supporting a detrimental effect of CPP crystals on articular tissues, it is certain that CPP crystals worsen cartilage damage and likely that they initiate such damage. The latter theory is bolstered by studies of familial CPPD disease in which crystal formation predates joint degeneration and by the co-occurrence of radiographic and clinical features of CPPD disease and osteoarthritis in joints that are usually spared in osteoarthritis, such as the metacarpophalangeal, radiocarpal, or glenohumeral joints (Fig. 2C).
A handful of metabolic conditions are well-established risk factors for CPPD disease.27 CPPD disease results from a high ratio of inorganic pyro-phosphate to phosphate ions in patients with hypophosphatasia, a congenital syndrome that is caused by low functional levels of alkaline phosphatase. Hyperparathyroidism is also clearly associated with CPPD disease. Hyperparathyroidism alters calcium metabolism, but the persistence of CPPD disease years after the correction of hyper-calcemia suggests a complex link between these diseases.28 Hemochromatosis is also strongly associated with CPPD and may be caused by the inhibitory action of iron on pyrophosphatases29 or by high levels of parathyroid hormone in cartilage.30 Hypomagnesemia is also an important risk factor for CPPD disease,31,32 and CPPD disease with the Gitelman’s variant of Bartter’s syndrome is believed to stem from hypomagnesemia.33 Magnesium increases the solubility of CPP crystals and acts as a cofactor for pyrophosphatases.34 As many as 5% of patients with gout have CPP crystals in their synovial fluid,35 which supports the hypothesis that these diseases share common local and systemic risk factors. In patients younger than 60 years of age who present with CPPD disease, testing and examination for all these associated metabolic diseases is indicated, because arthritis may be the presenting symptom. We recommend iron studies, including measurement of iron, transferrin, and ferritin levels, as well as measurement of levels of serum calcium, alkaline phosphatase, and parathyroid hormone.
Acute attacks of CPPD disease often occur in the context of acute illness or joint trauma or in the postoperative period, particularly after parathyroidectomy36 or hip-fracture repair.37 There are no known dietary associations of CPPD disease. Several medications may precipitate acute CPP crystal arthritis. Although this association is somewhat controversial, the administration of intraarticular hyaluronan preparations may induce acute CPP crystal arthritis.38 Other possible associations include the use of loop diuretics,39 granulocyte–macrophage colony-stimulating factor,40 and pamidronate.41
Patients with CPPD disease may have other subtle manifestations of dysfunctional tissue mineralization, because inorganic pyrophosphate is a potent regulator of normal and pathologic mineralization. Lymphocytes and skin fibroblasts from patients with familial CPPD disease show high levels of inorganic pyrophosphate,42 which suggests a systemic disorder. Recently, Abhishek et al. found that patients with nonfamilial chondrocalcinosis had lower cortical bone mineral density and higher rates of vascular and soft-tissue calcification than those without chondrocalcinosis.43
FAMILIAL CPPD
Although most CPPD disease is sporadic, multiple kindreds with premature or extensive CPPD have been described worldwide. CPPD disease that occurs in patients younger than 60 years of age should prompt inquiry about similarly affected family members. Interestingly, the first descriptions of CPPD disease in Hungary included five patients with affected relatives.2 Chondrocalcinosis develops in most affected patients before the onset of clinical degenerative arthritis, a finding that supports a causative role for CPP crystals in joint damage.
Two genetic loci are associated with familial CPPD. Mutations in the CCAL2 locus on chromo-some 5p produce an autosomal dominant pattern of inheritance (probably resulting from a gain of function of the ANKH protein)44 and provide additional support for a key role of ANKH in the pathogenesis of CPPD disease. The CCAL1 locus on chromosome 8 has not yet been fully characterized. Recently, a gain-of-function mutation in the TNFRSF11B (osteoprotegerin) gene was described in a family with early-onset osteoarthritis and chondrocalcinosis.45
DIAGNOSIS
CPPD disease is underdiagnosed. It has been shown that 20% of unselected patients who are examined at the time of total joint replacement for osteoarthritis of the knee have CPP crystals in their synovial fluid.46 CPPD occurs at identical rates in synovial-fluid samples and in tissue samples that are obtained during knee or hip replacement due to osteoarthritis.23,47
CPPD disease is most accurately diagnosed by the finding of positively birefringent, rhomboid-shaped crystals in synovial fluid from the affected joint (Fig. 1A). Birefringence is a property of highly ordered material such as crystals in which the double refraction of light results in characteristic color changes with the movement of the crystal in relation to the light source. Under compensated polarizing light microscopy, which is typically performed with a red filter, CPP crystals appear blue when they are parallel to the axis of the polarizer and yellow when they are perpendicular. In contrast, monosodium urate crystals appear yellow when they are parallel to the axis of the polarizer and blue when they are perpendicular. The identification of CPP crystals can be difficult, because these crystals are small and often show weak birefringence. Intracellular and extracellular CPP crystals in synovial fluid have equal significance. Cell counts in synovial fluid can vary widely.
A single set of diagnostic criteria for CPPD disease, which was proposed by Ryan and McCarty, has been published.34 Until validated diagnostic and classification criteria for CPPD disease are available, it seems prudent to define definite CPPD disease as the presence of CPP crystals in synovial fluid or tissues with appropriate clinical findings.
Conventional radiography provides important support for the diagnosis of CPPD disease and may assist in distinguishing CPPD disease from other types of arthritis. Although chondrocalcinosis is the radiographic finding that is most closely aligned with CPPD disease, it should not be used as a sole diagnostic criterion in the absence of clinical arthritis. Other useful radiographic clues that assist in differentiating primary osteoarthritis from CPPD disease include the following: hooklike osteophytes; axial skeletal involvement, such as annulus fibrosis calcification, severe disk degeneration with the vacuum phenomenon and subchondral erosions, and the vacuum phenomenon of sacroiliac joints; radiocarpal- or patella-femoral-predominant narrowing of the joint space; subchondral cyst formation; severe articular destruction, such as subchondral collapse, bony fragments, and microfractures; and tendon or fascial calcifications, such as at the Achilles tendon, plantar fascia, gastrocnemius, quadriceps, rotator cuff, or triceps at the elbow or shoulder.
The increasing use of musculoskeletal ultrasonography in the clinic may aid in the diagnosis of CPPD disease. Chondrocalcinosis on ultrasonography appears as linear densities in the hyaline cartilage or fibrocartilage (Fig. 2D). Although some early studies touted the higher sensitivity of ultrasonography as compared with conventional radiography, it may be challenging to differentiate between gout and CPPD disease with ultrasonography.48
Advanced imaging techniques may be useful to detect CPPD disease in some contexts.49 Computed tomographic (CT) scanning accurately detects calcifications and is particularly useful in detecting axial CPPD. Although magnetic resonance imaging (MRI) is the preferred advanced imaging method for the assessment of painful joints, in its present iteration it is insensitive to tissue calcification. New imaging technologies, including advanced MRI techniques, diffraction-enhanced synchrotron imaging, and dual-energy CT, hold promise for improved diagnostic accuracy.49
MANAGEMENT
Acute CPP crystal arthritis is managed with strategies that are aimed at reducing inflammation and that are borrowed from therapies used for acute gouty arthritis (Fig. 4). Intraarticular glucocorticoids work well for patients with acute CPP crystal arthritis, and this treatment is typically recommended as first-line therapy for joints that are amenable to injection.4 Oral colchicine at a daily dose of 0.6 to 1.2 mg is used in patients without clinically significant renal or hepatic impairment, and an optional loading regimen of 1.2 mg may be administered once. Some evidence supports the efficacy of daily colchicine, given prophylactically, to decrease the frequency of acute attacks.50 Nonsteroidal antiinflammatory drugs (NSAIDs), given at antiinflammatory daily doses and preferably used along with gastroprotection, may be effective for treating acute flares. Systemic glucocorticoids are also frequently used in elderly persons, who are susceptible to CPPD, and most experts agree that these agents are moderately effective in reducing pain and inflammation in patients with acute CPP crystal arthritis.51 Case reports also support the effectiveness of systemic interleukin-1β inhibitors in patients with acute CPP crystal arthritis.52
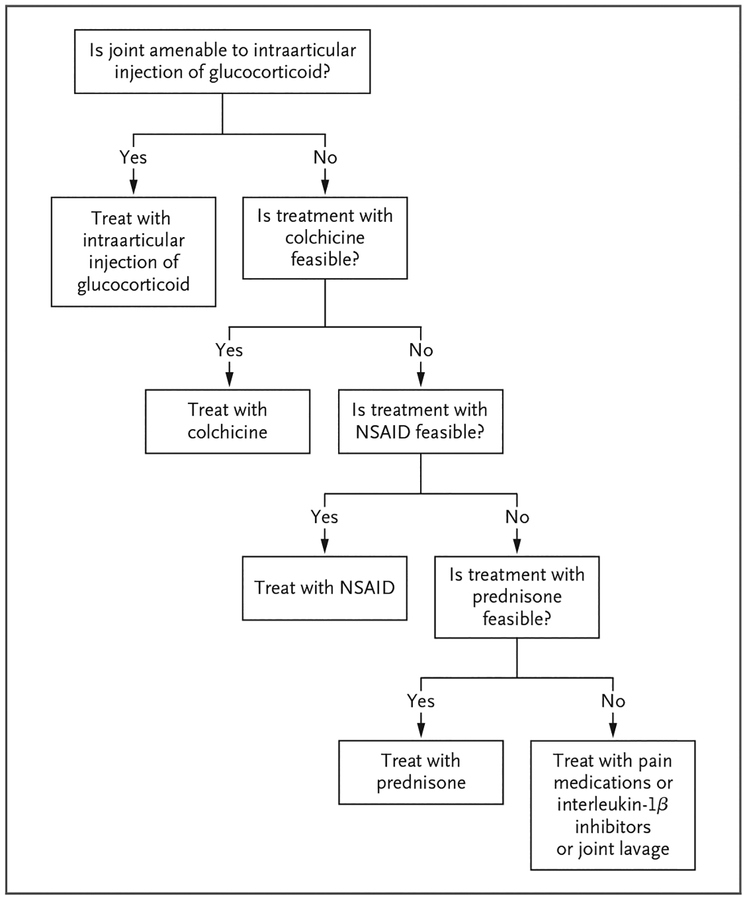
Figure 4. Management Strategy for Acute CPP Crystal Arthritis.
A treatment is considered to be feasible if it is not associated with unacceptable side effects. NSAID denotes nonsteroidal antiinflammatory drug.
For a patient with acute CPP crystal arthritis, we use intraarticular glucocorticoids as a first-line agent. If this intervention is not feasible, we use new dosing recommendations for oral colchicine53 or low-to-moderate doses of prednisone, depending on coexisting conditions.
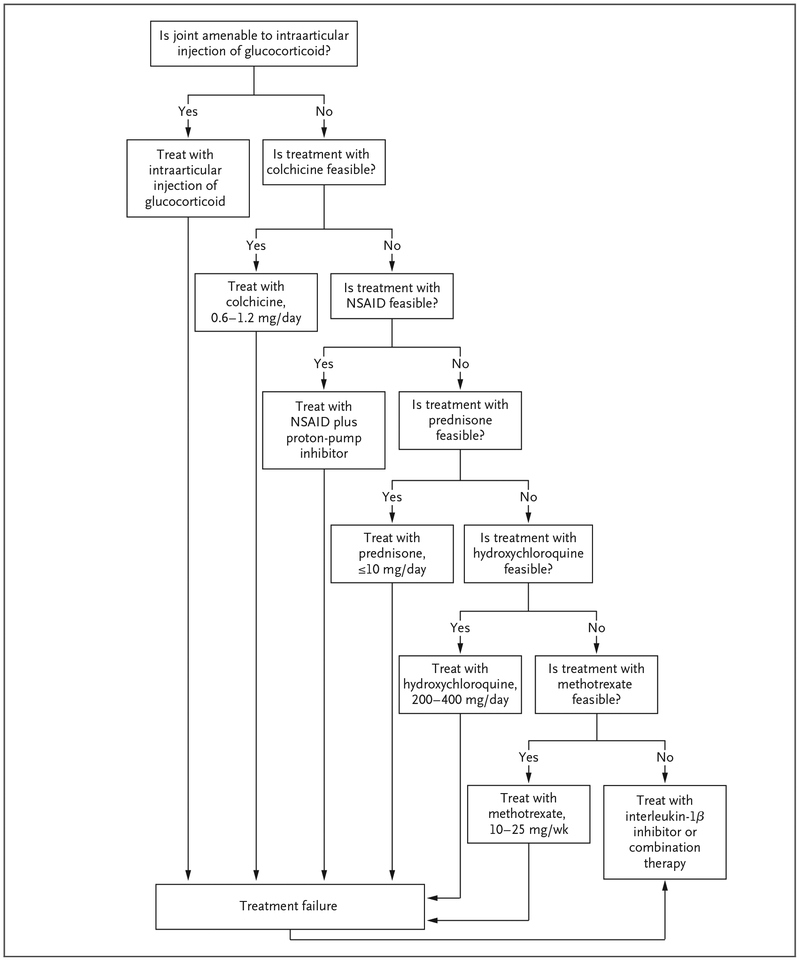
Chronic CPP crystal arthritis is much more difficult to manage than acute CPP crystal arthritis (Fig. 5). Few controlled trials of any therapies exist,54,55 and all the current therapeutic strategies are aimed at reducing inflammation. Unlike the case with gout, in which long-term therapies reduce the urate burden, no current disease-modulating treatments are available for CPPD disease. For patients with monoarticular or oligoarticular large-joint involvement, repeated intraarticular injections of glucocorticoids may control symptoms. The daily use of oral colchicine at a low dose (0.6 to 1.2 mg) may be useful in reducing the frequency of acute attacks.50 Alternatively, NSAIDs may produce similar beneficial effects if that they do not cause bothersome side effects. Low-dose systemic glucocorticoids may be necessary to control pain and inflammation in patients in whom colchicine or NSAIDs are ineffective or are associated with unacceptable side effects. Some data support the use of hydroxychloroquine in patients with CPPD disease.54
Figure 5. Management Strategy for Chronic CPP Crystal Arthritis.
Combination therapy may include various combinations of colchicine, prednisone, methotrexate, and hydroxychloroquine.
In an uncontrolled trial, methotrexate at doses of 5 to 10 mg per week showed a clinically significant benefit in patients with chronic CPP crystal arthritis.56 However, a recent randomized, controlled trial involving similar patients who were assigned to methotrexate, administered subcutaneously at a dose of 15 mg per week, or placebo showed no difference between the drug and placebo.55 Methotrexate was associated with few side effects in these patients and remains an option for selected patients in whom other therapies have failed. Anecdotal evidence supports the use of interleukin-1β inhibitors in patients with chronic CPP crystal arthritis, and further long-term studies of these agents are warranted.
We use a trial-and-error approach for the treatment of patients with chronic CPP crystal arthritis, and we often combine low doses of several different medications. In patients in whom intraarticular glucocorticoid therapy does not control symptoms or in whom oral colchicine or NSAIDs do not provide adequate relief, we use low-dose (5 to 10 mg daily) oral prednisone. If there are no contraindications and low-dose prednisone alone is not adequate to control symptoms, we may try various combinations of colchicine, hydroxychloroquine, and weekly methotrexate. Interleukin-1β inhibitors can also be used in patients with refractory disease.
It is important to remember that CPPD disease can be a presenting sign of hyperparathyroidism, hypophosphatasia, hemochromatosis, or hypomagnesemia. Screening is indicated for these conditions, particularly in patients younger than 60 years of age who present with CPPD disease.
Ultimately, joint replacement may be necessary in patients with CPPD disease. Current evidence shows similar outcomes of knee and hip replacement in patients with osteoarthritis and in those with CPPD disease.57
FUTURE DIRECTIONS
Approximately 55 years after the initial description of CPPD disease, this common form of arthritis has garnered little attention in the medical community. Diagnostic challenges result in under-diagnosis, but most important, there is a paucity of specific and effective therapies for affected patients. Although no proven disease-modulating agents are available, we can improve outcomes in patients by the careful diagnosis of CPPD disease with the use of a thorough analysis of synovial fluid and the initiation of appropriate treatment strategies.
Acknowledgments
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Daniel Malone, M.D., and Guillermo Carrera, M.D., for providing images, and Claudia Gohr, B.S., and Gordon Gohr, B.A., for assistance with earlier versions of the figures.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.McCarty D Jr, Kohn NN, Faires JS. The significance of calcium pyrophosphate crystals in the synovial fluid of arthritic patients: the “pseudogut syndrome.” 1. Clinical aspects. Ann Intern Med 1962; 56: 711–37. [DOI] [PubMed] [Google Scholar]
- 2.Žitňan D, Sit’Aj Š. Chondrocalcinosis articularis. I. Clinical and radiological study. Ann Rheum Dis 1963; 22: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. I. terminology and diagnosis. Ann Rheum Dis 2011; 70: 563–70. [DOI] [PubMed] [Google Scholar]
- 4.Masuda I, Ishikawa K. Clinical features of pseudogout attack: a survey of 50 cases. Clin Orthop Relat Res 1988; 229: 173–81. [PubMed] [Google Scholar]
- 5.McCarty D Jr. Diagnostic mimicry in arthritis: patterns of joint involvement associated with calcium pyrophosphate dihydrate crystal deposits. Bull Rheum Dis 1975; 25: 804–9. [Google Scholar]
- 6.Lee RS, Kayser MV, Ali SY. Calcium phosphate microcrystal deposition in the human intervertebral disc. J Anat 2006; 208: 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yayama T, Kobayashi S, Sato R, et al. Calcium pyrophosphate crystal deposition in the ligamentum flavum of degenerated lumbar spine: histopathological and immunohistological findings. Clin Rheumatol 2008; 27: 597–604. [DOI] [PubMed] [Google Scholar]
- 8.Godfrin-Valnet M, Godfrin G, Godard J, et al. Eighteen cases of crowned dens syndrome: presentation and diagnosis. Neurochirurgie 2013; 59: 115–20. [DOI] [PubMed] [Google Scholar]
- 9.McCarty D Jr. Patterns of joint involvement associated with calcium pyro- phosphate dihydrate crystal deposition. Bull Rheum Dis 1975; 25: 804–9. [Google Scholar]
- 10.Yamakawa K, Iwasaki H, Ohjimi Y, et al. Tumoral calcium pyrophosphate dihydrate crystal deposition disease: a clinicopathologic analysis of five cases. Pathol Res Pract 2001; 197: 499–506. [DOI] [PubMed] [Google Scholar]
- 11.Ryan L, McCarty D. Calcium pyro-phosphate crystal deposition disease: pseudogout: articular chondrocalcinosis In: McCarty D, ed. Arthritis and allied conditions: a textbook of rheumatology. 11th ed Philadelphia: Lea & Febiger, 1989: 1711–36. [Google Scholar]
- 12.Derfus BA, Rachow JW, Mandel NS, et al. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum 1992; 35: 231–40. [DOI] [PubMed] [Google Scholar]
- 13.Ryan LM, Rosenthal AK. Metabolism of extracellular pyrophosphate. Curr Opin Rheumatol 2003; 15: 311–4. [DOI] [PubMed] [Google Scholar]
- 14.Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res 2011; 52: 139–46. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res Ther 2013; 15: R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflamma-some. Nature 2006; 440: 237–41. [DOI] [PubMed] [Google Scholar]
- 17.Pang L, Hayes CP, Buac K, Yoo DG, Rada B. Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J Immunol 2013; 190: 6488–500. [DOI] [PubMed] [Google Scholar]
- 18.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal- induced nitric oxide generation. J Immunol 2005; 174: 5016–23. [DOI] [PubMed] [Google Scholar]
- 19.Reuben PM, Wenger L, Cruz M, Cheung HS. Induction of matrix metalloproteinase-8 in human fibroblasts by basic calcium phosphate and calcium pyro-phosphate dihydrate crystals: effect of phosphocitrate. Connect Tissue Res 2001; 42: 1–12. [DOI] [PubMed] [Google Scholar]
- 20.Muehleman C, Li J, Aigner T, et al. Association between crystals and cartilage degeneration in the ankle. J Rheumatol 2008; 35: 1108–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Neame RL, Carr AJ, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis 2003; 62: 513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum 1987; 30: 914–8. [DOI] [PubMed] [Google Scholar]
- 23.Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 2009; 60: 2694–703. [DOI] [PubMed] [Google Scholar]
- 24.McCarty DJ Jr, Hogan JM, Gatter RA, Grossman M. Studies on pathological calcifications in human cartilage. I. Prevalence and types of crystal deposits in the menisci of two hundred fifteen cadavera. J Bone Joint Surg Am 1966; 48: 309–25. [PubMed] [Google Scholar]
- 25.Wilkins E, Dieppe P, Maddison P, Evison G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis 1983; 42: 280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty M, Watt I, Dieppe PA. Localised chondrocalcinosis in post-meniscectomy knees. Lancet 1982; 1: 1207–10. [DOI] [PubMed] [Google Scholar]
- 27.Jones AC, Chuck AJ, Arie EA, Green DJ, Doherty M. Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum 1992; 22: 188–202. [DOI] [PubMed] [Google Scholar]
- 28.Glass JS, Grahame R. Chondrocalcinosis after parathyroidectomy. Ann Rheum Dis 1976; 35: 521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarty DJ, Pepe PF. Erythrocyte neutral inorganic pyrophosphatase in pseudogout. J Lab Clin Med 1972; 79: 277–84. [PubMed] [Google Scholar]
- 30.Pawlotsky Y, Le Dantec P, Moirand R, et al. Elevated parathyroid hormone 44–68 and osteoarticular changes in patients with genetic hemochromatosis. Arthritis Rheum 1999; 42: 799–806. [DOI] [PubMed] [Google Scholar]
- 31.Richette P, Ayoub G, Bardin T, Bouvet S, Orcel P, Badran AM. Hypomagnesemia and chondrocalcinosis in short bowel syndrome. J Rheumatol 2005; 32: 2434–6. [PubMed] [Google Scholar]
- 32.Punzi L, Calò L, Schiavon F, Pianon M, Rosada M, Todesco S. Chondrocalcinosis is a feature of Gitelman’s variant of Bartter’s syndrome: a new look at the hypomagnesemia associated with calcium pyrophosphate dihydrate crystal deposition disease. Rev Rhum Engl Ed 1998; 65: 571–4. [PubMed] [Google Scholar]
- 33.Ea HK, Blanchard A, Dougados M, Roux C. Chondrocalcinosis secondary to hypomagnesemia in Gitelman’s syndrome. J Rheumatol 2005; 32: 1840–2. [PubMed] [Google Scholar]
- 34.Ryan L, McCarty D. Calcium pyro-phosphate crystal deposition disease; pseudogout; articular chondrocalcinosis. In: McCarty D, ed. Arthritis and allied conditions: a textbook of rheumatology 10th ed Philadelphia: Lea & Febiger, 1985: 1515–46. [Google Scholar]
- 35.Jaccard YB, Gerster JC, Calame L. Mixed monosodium urate and calcium pyrophosphate crystal-induced arthropathy: a review of seventeen cases. Rev Rhum Engl Ed 1996; 63: 331–5. [PubMed] [Google Scholar]
- 36.Bilezikian JP, Connor TB, Aptekar R, et al. Pseudogout after parathyroidectomy. Lancet 1973; 1: 445–6. [DOI] [PubMed] [Google Scholar]
- 37.Harato K, Yoshida H. Pseudogout at the knee joint will frequently occur after hip fracture and lead to the knee pain in the early postoperative period. J Orthop Surg Res 2015; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammesfahr JF, Knopf AB, Stitik T. Safety of intra-articular hyaluronates for pain associated with osteoarthritis of the knee. Am J Orthop (Belle Mead NJ) 2003; 32: 277–83. [PubMed] [Google Scholar]
- 39.Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudo-gout in the general population. Rheumatology (Oxford) 2012; 51: 2070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ames PR, Rainey MG. Consecutive pseudogout attacks after repetitive granulocyte colony-stimulating factor administration for neutropenia. Mod Rheumatol 2007; 17: 445–6. [DOI] [PubMed] [Google Scholar]
- 41.Malnick SD, Ariel-Ronen S, Evron E, Sthoeger ZM. Acute pseudogout as a complication of pamidronate. Ann Pharmacother 1997; 31: 499–500. [DOI] [PubMed] [Google Scholar]
- 42.Lust G, Faure G, Netter P, Gaucher A, Seegmiller JE. Evidence of a generalized metabolic defect in patients with hereditary chondrocalcinosis: increased inorganic pyrophosphate in cultured fibro-blasts and lymphoblasts. Arthritis Rheum 1981; 24: 1517–21. [DOI] [PubMed] [Google Scholar]
- 43.Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Association between low cortical bone mineral density, soft-tissue calcification, vascular calcification and chondrocalcinosis: a case-control study. Ann Rheum Dis 2014; 73: 1997–2002. [DOI] [PubMed] [Google Scholar]
- 44.Williams CJ, Pendleton A, Bonavita G, et al. Mutations in the amino terminus of ANKH in two US families with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum 2003; 48: 2627–31. [DOI] [PubMed] [Google Scholar]
- 45.Ramos YF, Bos SD, van der Breggen R, et al. A gain of function mutation in TNFRSF11B encoding osteoprotegerin causes osteoarthritis with chondrocalcinosis. Ann Rheum Dis 2015; 74: 1756–62. [DOI] [PubMed] [Google Scholar]
- 46.Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol 2002; 29: 570–4. [PubMed] [Google Scholar]
- 47.Fuerst M, Niggemeyer O, Lammers L, Schäfer F, Lohmann C, Rüther W. Articu lar cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet Disord 2009; 10: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Löffler C, Sattler H, Peters L, Löffler U, Uppenkamp M, Bergner R. Distinguishing gouty arthritis from calcium pyrophosphate disease and other arthritides. J Rheumatol 2015; 42: 513–20. [DOI] [PubMed] [Google Scholar]
- 49.Miksanek J, Rosenthal AK. Imaging of calcium pyrophosphate deposition disease. Curr Rheumatol Rep 2015; 17(3): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarellos A, Spilberg I. Colchicine prophylaxis in pseudogout. J Rheumatol 1986; 13: 804–5. [PubMed] [Google Scholar]
- 51.Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. II. management. Ann Rheum Dis 2011; 70: 571–5. [DOI] [PubMed] [Google Scholar]
- 52.Ottaviani S, Brunier L, Sibilia J, et al. Efficacy of anakinra in calcium pyrophosphate crystal-induced arthritis: a report of 16 cases and review of the literature. Joint Bone Spine 2013; 80: 178–82. [DOI] [PubMed] [Google Scholar]
- 53.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum 2010; 62: 1060–8. [DOI] [PubMed] [Google Scholar]
- 54.Rothschild B, Yakubov LE. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Compr Ther 1997; 23: 327–31. [PubMed] [Google Scholar]
- 55.Finckh A, Mc Carthy GM, Madigan A, et al. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther 2014; 16: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chollet-Janin A, Finckh A, Dudler J, Guerne PA. Methotrexate as an alternative therapy for chronic calcium pyrophosphate deposition disease: an exploratory analysis. Arthritis Rheum 2007; 56: 688–92. [DOI] [PubMed] [Google Scholar]
- 57.Kumar V, Pandit HG, Liddle AD, et al. Comparison of outcomes after UKA in patients with and without chondrocalcinosis: a matched cohort study. Knee Surg Sports Traumatol Arthrosc 2015. March 19 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]