Abstract
Objective.
Monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD) crystals have been observed in synovial joints both before and after the onset of osteoarthritis (OA). The relationship between crystals and OA, however, remains controversial. We compared histologic and immunohisto-chemical patterns in articular cartilage of ankle joints with and without crystals.
Methods.
A sample of 7855 human cadaveric tali was examined for the presence of surface and beneath-the-surface crystals. A random subsample of tali with and without crystals underwent crystal analysis by Fourier transform infrared spectroscopy (FTIR), histologic examination, and immunohisto-chemistry for S100 protein, superficial zone protein, collagen X, and cSRC.
Results.
The prevalence of grossly visible crystals in the pool of donors over 18 years of age was 4.7% and correlated with advanced age, male sex, and obesity. Crystals were strongly associated with cartilage lesions and these lesions appeared to be biomechanically induced, being located where opposing articular surfaces might not be in congruence with each other. Thirty-four percent of the random subsamples of crystals upon which FTIR was performed contained CPPD, and the remainder were MSU crystals. Both crystal types were associated with higher levels of superficial zone protein and collagen X.
Conclusion.
We show that the presence of surface crystals of either MSU or CPPD is strongly correlated with cartilage lesions in the talus. The histologic similarities in cartilage from joints with CPPD crystals compared to those with MSU crystals, together with what is known about the dramatically different etiologic factors producing these crystals, strongly suggest that these lesions are biomechanically induced. (First Release April 15 2008; J Rheumatol 2008;35:1108–17)
Keywords: ANKLE OSTEOARTHRITIS, GOUT, CALCIUM PYROPHOSPHATE DIHYDRATE, MONOSODIUM URATE CRYSTALS, TALUS, CARTILAGE
Osteoarthritis (OA) is defined by pathological changes in cartilage, bone, and associated soft tissues of the synovial joint. The causes of OA are multiple, and how contributing factors work together to initiate and advance joint degeneration in OA is unclear and often difficult to approach experimentally. For example, the contribution of articular crystals, such as monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD), to disease inception and progression remains controversial1–3. Do crystals preferentially form in degenerated cartilage? Or do crystals initiate changes in articular tissues that are indistinguishable from OA?
Gout, a metabolic disease associated with hyperuricemia, has been noted to occur both before and after the onset of clinical and radiographic OA4. It is possible that the articular damage from gout is the result of repeated episodes of inflammation in the joint. But gout is also thought to preferentially attack previously damaged joints. For example, it often occurs in longstanding Heberden’s nodes in postmenopausal women5. Recently, Roddy, et al6 reported that acute attacks of gout at individual joint sites were associated with the presence of clinically assessed “OA” at that joint. Whether this association represents secondary OA from crystal-induced damage to the joint, or whether MSU crystals preferentially deposit in damaged joints remains unclear.
CPPD disease, on the other hand, is a primary disorder of articular cartilage associated with overproduction of inorganic pyrophosphate by chondrocytes7. It is often radiographically defined by the presence of chondrocalcinosis. The relationship between CPPD disease and the development and progression of OA has been the subject of much controversy. Although several cross-sectional studies have reported an association between chondrocalcinosis/CPPD and OA8 and between chondrocalcinosis/CPPD and osteophytes9–11, a few longitudinal studies have found that cartilage loss/joint space narrowing were not associated with chondrocalcinosis2,10.
Noninvasive means of identifying these crystals likely miss a significant portion of affected patients. The most accurate way in which to identify crystal deposits is through examination of tissues, an opportunity that is rarely available except from endstage patients undergoing surgery12. We had the unique opportunity to examine the prevalence of crystals in a large sample of cadaveric human tali (examined within 24 h of death) ranging from healthy to endstage cartilage degradation, and associate their presence with cartilage lesions. The relative rarity of primary OA in the ankle joint13 adds further interest to our study.
MATERIALS AND METHODS
Donors and specimens.
Adult human tali (n = 7855) from 4007 donors were harvested within 24 hours of death, through the Gift of Hope Organ and Tissue Donor Network from April 1998 to October 2006. The tali were harvested as an ongoing tissue procurement program for departmental projects on the study of the biochemistry of OA. The tibial articulating surfaces were not available to us. These donors were age 18 years or older and were selected from the total larger group of 4033 donors that included those younger than 18. Information on the age, sex, race, general body type (obese, normal, light, as assessed through visual inspection), and cause of death of the donor was available. No details of the donor’s medical or surgical history were known.
Specimen grading.
Immediately upon harvesting the tali from the donor, the cartilage on the trochlear articular surface was examined for the presence of macroscopically visible crystal deposits. Surface crystals from 34 representative samples (from 34 tali of 24 donors) were subjected to polarized light microscopy and Fourier transform infrared analyses as described below. Three independent observers (CM, JL, AKR) also examined the specimens for possible macroscopic determination of crystal type, either due to crystal color or morphological characteristics or location without knowledge of crystal type. Each trochlear surface was then graded for gross morphological appearance according to the following scale: Grade 0 = normal, healthy looking cartilage; Grade 1 = early fibrillation, flaking, shallow pits or grooves and/or small blisters affecting the cartilage; Grade 2 = deep fibrillation and fissuring, flaking, pitting and/or blistering; Grade 3 = 30% or less of the articular surface area eroded down to the subchondral bone; Grade 4 = greater than 30% of the entire articular surface eroded down to the subchondral bone14. Subsequent to grading, the tali were stored at −20°C for further study.
Fourier transform infrared spectroscopy (FTIR) for identification of crystal deposits.
Thirty-four 5-μm thick frozen sections of unstained cartilage were placed on Kevley reflective slides and dehydrated by incubation at 37°C. Using a microscope focused on the crystal area of the specimen, synchrotron FTIR was performed at the Synchrotron Radiation Center (Madison, WI, USA). The spectra obtained were compared with standard spectra for MSU and CPPD crystals15.
Polarized light microscopy of crystals.
Crystal material was scraped from the cartilage surface with a scalpel and placed on a microscope slide. A drop of 0.9% saline was added, and the material was examined by an expert examiner (AKR) with compensated polarizing light microscopy. In all cases, the identity of the crystals under light microscopy was confirmed with FTIR analysis.
Histology.
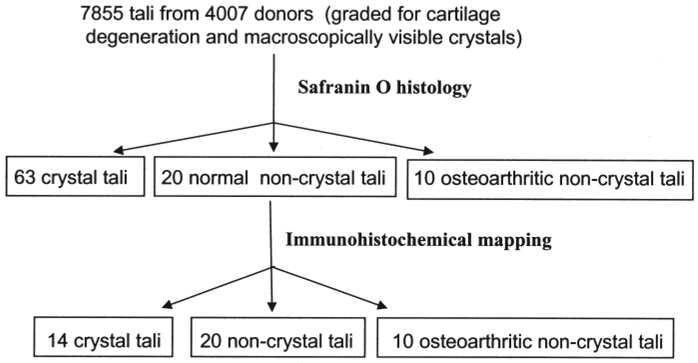
Selection of groups of tali for histological study is shown in Figure 1. The articular cartilage on each trochlea was subdivided into 8 regions with a sharp scalpel. Each of these was divided into 2 groups: 1 group was preserved in formalin (as CPPD crystals are soluble in alcohol, but not in formalin) and the other in 95% ethyl alcohol (as MSU crystals are soluble in formalin, but not in alcohol). Cartilage was then separated from the subchondral bone for histological mapping, and prepared for paraffin histology. Six-micron sections from each block were stained with Safranin O/Fast green16 and by one of the immunohistochemical methods listed below.
Figure 1.
The process of selecting tali used for histology and immunohistochemistry.
Immunohistochemistry.
Immunostaining for S100 protein and collagen type X were performed as described by Aigner, et al17 using a streptavidin-biotin complex method with alkaline phosphatase as detection enzyme.
Steroid receptor coactivator (cSRC).
Deparaffinized tissue sections were pretreated with 2 mg/ml bovine testicular hyaluronidase for 30 min at 37°C. The primary antibody was rabbit polyclonal steroid receptor coactivator 2 (SRC-2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1/100. The secondary antibody was horseradish peroxidase F’2-conjugated goat anti-rabbit IGG (Accurate Chemical and Scientific Corp., Westbury, NY, USA) applied at a 1/50 dilution in 1% bovine serum albumin (BSA) in phosphate buffered saline in a moist chamber for 2 h at room temperature.
Superficial zone protein (SZP).
An ImmunoPore® ABC alkaline mouse IgG phosphatase staining kit (Pierve 32044) was used. Tissue sections were incubated for 40 min with the primary antibody, mouse anti-SZP (Glaxo-SmithKline GW), at a 20 mg/ml concentration. They were then incubated for 120 min with the biotinylated secondary antibody, horse anti-mouse IgG in 1/50 dilution in TBS/2.5 mM CaCl2/1% BSA/0.5% Tween 20.
Immunohistochemical grading.
Visual inspection of sections for intensity and prevalence of a particular immunostain were described and then graded as follows: – = negative, + = weak/isolated, ++ = strong and widespread.
Statistical evaluation.
Statistical analyses were performed using StatView. Analysis of variance (ANOVA) was used to determine significant differences between groups. Significance was taken at p ≤ 0.05.
RESULTS
Gross appearance and demographics.
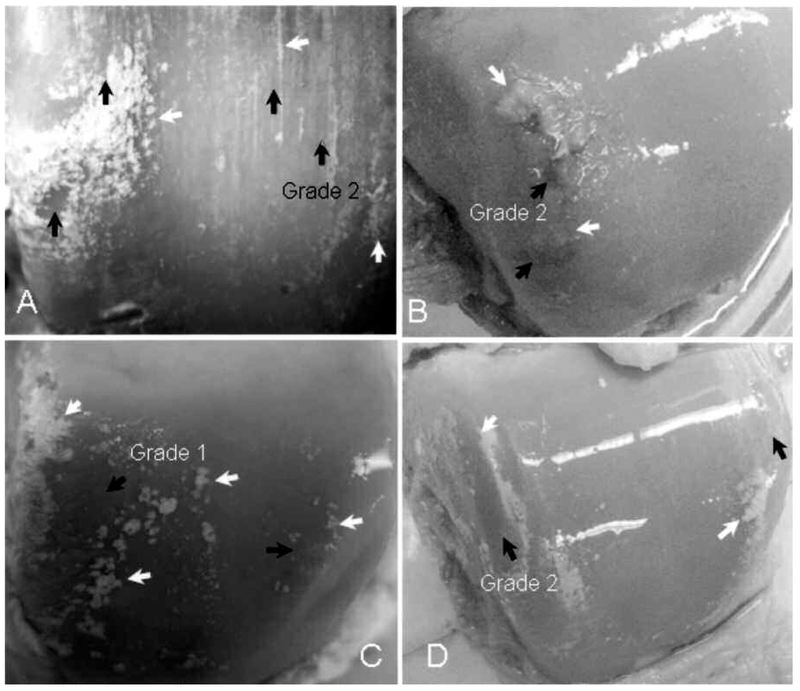
Figure 2 shows the dramatic appearance of surface crystal deposits on these talar cartilages. The normally smooth cartilage surfaces of the involved tissues were studded or streaked with white to yellow deposits that appeared to be firmly attached to or just beneath the cartilage surface. Some of this material occurred in linear streaks across the cartilage surface in the anterior/posterior direction, while other deposits were round or confluent. Often, similar material appeared to be present in the attached surrounding synovial tissue and occasionally in the ligaments. It was not possible to determine whether the surface crystals were MSU or CPPD by visual inspection as there were no discriminating characteristics, i.e., color, morphological appearance, amount, or location on the joint surface. Even after having the results of the FTIR testing, and thus knowing the crystal type, no discrimination could be made.
Figure 2.
Crystals (white arrows) associated with cartilage lesions (black arrows). The crystals surround the lesion in more severe cases. Lesions are located in regions that could be subject to biomechanically-induced damage from the opposing articular surface, particularly in joint instability. Note that in regions with a smooth normal appearance, without lesions, no crystals are present.
There were a few specimens (the number from the total pool is not known because full descriptive accounts of the location of the crystals were not made until the final year of sample collection) in which crystals could be seen in isolated regions beneath the surface of a normal-appearing, Grade 0 articular cartilage. These are presumed to be CPPD crystals based on location.
Basic descriptive data on the samples are given in Table 1. Of the pool of tali from 4007 donors 18 years of age and older, 187 (4.7%) demonstrated surface crystal deposits. Of those 40 years of age and older the prevalence was 5.3%, and of those 60 years and older 6.8%. ANOVA showed that donors with crystals were a decade older than those without crystals (p < 0.05), and were less likely to be female. In addition, they were more likely to be obese (p < 0.05). While 84% of the crystal-positive donors had evidence of bilateral deposits, the remainder had grossly visible crystals on only one side.
Table 1.
Demographics of donor tali.
| Total Pool of Tali, n (%) | Non-Crystal Group, n (%) | Crystals Group, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donors | Sex | Obese | Donors | Sex | Obese | Donors | Sex | Obese | ||||
| Age, yrs | Male | Female | Male’ | Female | Male | Female | ||||||
| 18–29 | 120 | 98 (82) | 22 (18) | 11 (9) | 120 | 98 (82) | 22 (18) | 11 (9) | 0 | 0 | 0 | 0 |
| 30–39 | 138 | 97 (70) | 41 (30) | 23 (17) | 138 | 97 (70) | 41 (30) | 23 (17) | 0 | 0 | 0 | 0 |
| 40–49 | 388 | 279 (72) | 109 (28) | 115 (30) | 383 | 274 (72) | 109 (28) | 113 (30) | 5 | 5 (100) | 0 | 2 (40) |
| 50–59 | 750 | 565 (75) | 185 (25) | 215 (29) | 735 | 552 (75) | 183 (25) | 207 (28) | 15 | 13 (87) | 2(13) | 8 (53) |
| 60–69 | 986 | 761 (77) | 225 (23) | 274 (28) | 954 | 732 (77) | 222 (23) | 261 (27) | 32 | 29 (91) | 3(9) | 13 (41) |
| 70–79 | 1123 | 748 (67) | 375 (33) | 287 (26) | 1041 | 684 (66) | 357 (34) | 257 (25) | 82 | 64 (78) | 18 (22) | 30 (37) |
| > 80 | 502 | 284 (57) | 218 (43) | 89 (18) | 449 | 252 (56) | 197 (44) | 68 (15) | 53 | 32 (60) | 21 (40) | 21 (40) |
| Total | 4007 | 2837 (71) | 1170 (29) | 1014 (25) | 3820 | 2689 (70) | 1131 (30) | 940 (25) | 187 | 143 (76) | 44 (24) | 74 (40) |
| No. tali | Total pool = 7855 | Non-crystal group = 7511 | Crystal group = 344 | |||||||||
All figures are n (%).
Characterization of surface crystal deposits.
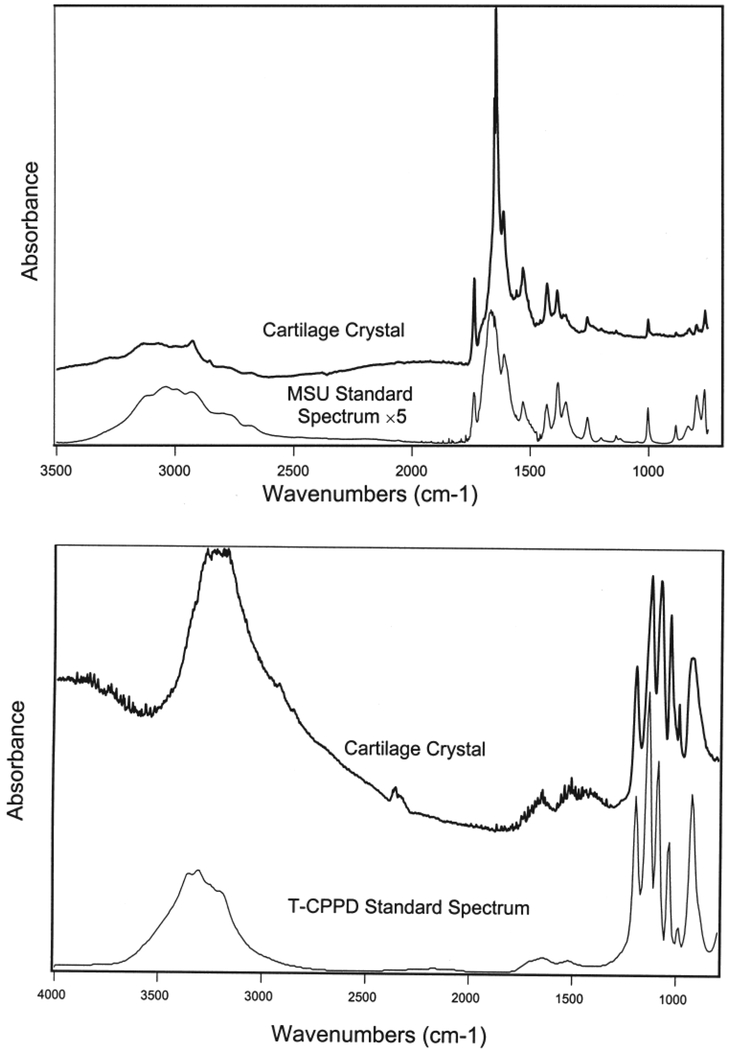
We examined surface crystal deposits with polarized light microscopy and FTIR spectroscopy on thin unfixed/unprocessed slices and scraped crystal samples from 34 random samples from 24 donors. Areas of interest appeared as streaks of white powdery deposits and could be easily identified grossly on the slides. Crystal material was consistent with pure MSU in 23 tali of 16 donors (67% of sampled donors with surface crystals; Figure 3, top panel) and consistent with pure CPPD in 11 tali of 8 donors (33% of sampled donors with surface crystals; Figure 3, bottom panel). None of the samples examined contained both CPPD and MSU crystals.
Figure 3.
FTIR curves for the tested material (MSU, top; CPPD, bottom) on histological sections of tali compared to the sodium urate standard.
Cartilage degeneration in crystal-negative specimens.
For specimens in which there was cartilage degeneration but the absence of crystals, typical characteristics of OA cartilage were present: loss of cartilage integrity, cell loss, cell cloning and hypertrophy adjacent to lesions, and reduced, or loss of, Safranin O staining.
Cartilage degeneration in crystal-positive specimens.
The crystal deposits were nearly always grossly associated with a cartilage lesion, either being found within, or adjacent to, a large lesion and on the surface of adjacent fibrillated lesions (Figures 2 and 4). Further, the lesions were located either at the margin of the trochlea where it rubs against the opposing articular surface of the fibula or tibia or they were “tram-track” lesions indicative of erosion caused by apposition with exostosis (osteophytes) from the anterior margin of the tibia during joint motion18. Table 2 shows the grades of gross cartilage degeneration for the entire pool of tali as well as for those displaying crystals. Only 7.8% of the tali with crystals had no gross signs of cartilage degeneration. Cartilage degeneration of grade 2 or higher occurred in 54.7% of tali with crystals, and 26.0% of tali without crystals (p < 0.05). The mean grade of gross cartilage degeneration was also higher in tali with crystals. Average grades were 1.57 (out of a maximum of 4) in crystal-containing specimens and 0.83 in non-crystal-containing specimens (p < 0.05). In donors with unilateral crystals (16% of those displaying crystals), 37% had more cartilage degeneration on the side containing visible crystals. Thirteen percent had a lower grade of cartilage degeneration on the affected side, and 50% had the same level of cartilage degeneration on both sides. Most tali without gross deposits of crystals, however, were not further examined for crystals, but of those that were examined, none showed crystals microscopically.
Figure 4.
Representative macroscopic (top row) and microscopic views of normal, MSU crystal, and CPPD crystal tali (left, middle, and right columns, respectively). Note the middle zone CPPD crystal cysts that are often in continuity with the articular surface.
Table 2.
Cartilage degeneration scores for the total pool of tali and for the subset displaying crystals.
| Total Sample, n (%) | Non-crystal tali, n (%) | Crystal tali, n (%) | |
|---|---|---|---|
| No. donors | 4007 | 3820 (95.3) | 187 (4.7) |
| No. individual tali | 7855 | 7511 (95.6) | 344 (4.4) |
| Grade 0 | 2948 (38) | 2921 (39) | 27 (7.8) |
| Grade 1 | 2856 (36) | 2727 (36.3) | 129 (37.5) |
| Grade 2 | 1614 (20.5) | 1455 (19.4) | 159 (46.3) |
| Grade 3 | 413 (5.2) | 384 (5) | 29 (8.4) |
| Grade 4 | 24 (0.3) | 24 (0.3) | 0(0) |
| Mean grade | 0.83 | 0.74 | 1.57 |
Microscopic patterns of deposition.
For specimens in which MSU crystals were positively identified through FTIR, crystals were found on the surface of superficial zone cartilage as well as within fibrillations, fissures, and crevices extending down into the lower zones of degenerated areas (Figure 4, middle column).
For specimens in which CPPD crystals were positively identified through FTIR, crystals were found on the cartilage surface and within fibrillations and fissures extending from the surface. A defining characteristic for CPPD, however, which distinguished these crystals from MSU, was the presence of crystal cysts in the middle and deep zones (Figure 4, right column). These cysts were frequently in continuity with the cartilage surface, either directly or by way of a narrow tunnel to the surface as determined by examination of serial sections.
There were multiple (8 of 14; 57%) tali in which MSU or CPPD crystals could be found covering the articular surface but in which small regions of the superficial zone were intact and without cell loss or cloning.
Immunohistochemistry.
Table 3 is a summary of the immunohistochemical staining. In normal cartilage, Col X was found only in deep regions of the cartilage adjacent to the tidemark. In degenerated cartilage, Col X was found adjacent to the tide-mark and in regions of cartilage degeneration. In degenerated cartilage with CPPD crystals, Col X was found in particularly high levels around the crystal cysts as well as in the lower half of the middle zone and in the portions of calcified cartilage removed with the uncalcified cartilage during cartilage isolation (Figure 4). However, from examination of all mapped regions on an articular surface, Col X could be found in regions not directly affected by CPPD crystal deposits, as long as crystals were found somewhere within the talus. No difference could be found between levels of Col X in MSU versus CPPD tali, possibly because cartilage degeneration was present in both sets of tali. Interestingly, hypertrophic chondrocytes were found adjacent to cartilage lesions characteristic of OA, but not necessarily adjacent to CPPD crystal cysts, even if there was cartilage loss at that point and Col X was present in the region. Thus, the presence of Col X was not necessarily indicative of the presence of hypertrophic chondrocytes.
Table 3.
Summary of means of immunohistochemical staining levels in the uncalcified cartilage of the trochlea of the talus.
| N | Collagen X | S-100 | cSRC | SZP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | D | S | M | D | S | M | D | S | M | D | ||
| Control | 20 | − | − | − | ++ | ++ | ++ | + | + | + | + | − | − |
| Degenerated | 10 | − | + | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | + | − |
| MSU crystals | 7 | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | ++ | ++ |
| CPPD crystals | 7 | − | ++ | ++ | ++ | +++ | ++ | ++ | ++ | + | +++ | ++ | ++ |
0 = no staining, 1 = weak and/or isolated staining, 2 = moderate staining, 3 = strong and widespread staining. SZP: superficial zone protein; MSU: mono-sodium urate; CPPD: calcium pyrophosphate dihydrate. S: superficial, M: medium; D: deep.
Overall, the level of S-100 immunostaining was similar in the chondrocytes of normal, degenerated, and crystal-containing cartilages since all cells demonstrated fairly strong and homogeneously distributed S-100 staining (Figure 4). An exception was observed in some degenerated cartilages with CPPD crystals, which demonstrated a high level of S-100 immunostaining in cell clones located adjacent to large CPPD crystal accumulations beneath the articular surface.
There was a significant positive correlation between the visible level of SZP with the levels of cartilage degeneration (r = 0.63; p < 0.05) and of crystal deposition (r = 0.66; p <0.05), although no difference could be discerned between MSU and CPPD specimens. SZP was found in particularly high levels adjacent to crystals both at the surface and beneath the articular surface, whereas, in normal control sections, SZP was found only within and on the superficial zone (Figure 4).
There was no significant correlation between crystal deposition and cSRC immunostaining. However, 18% of the mapped sections from the degenerated and crystal/degenerated specimens showed more intense staining than any of the control specimens, but only adjacent to the cartilage lesions (Figure 4). The remaining 82% of the sections showed the same intensity of cSRC staining as the controls.
DISCUSSION
In our study, a large sample of human organ bank tali was examined for the simultaneous presence and location of crystals and cartilage degradation. This is of significance in that the sensitivity of magnetic resonance imaging (MRI) for chondrocalcinosis is extremely low19 and as low as 39% for plain radiographs20. The MSU crystals of gout are not detectable through either MRI or radiography and are generally only detected through clinical evaluation of serum samples during acute episodes.
In a sample of 7855 tali from 4007 donors, 4.7% of those 18 years of age and older had visible crystal deposits. Crystals were more common with advanced age. These data are consistent with those of others showing that both gout and chondrocalcinosis have an increased prevalence with age1,11,21–23. Crystal-affected tali were more likely to be from male donors and from obese rather than lean individuals. It is known that gout is most prevalent in adult men, particularly between the ages of 40 and 50, although there is no sex distinction for CPPD disease10. It is also known that there is a strong association of obesity with hyperuricemia and gout and that weight reduction associated with a change in proportional macronutrient intake reduces serum urate levels in gout24. Surprisingly, both MSU and CPPD crystals were present in this group of specimens, and were impossible to differentiate on gross appearance.
For the specimens whose crystals were definitively identified through FTIR, we found that MSU crystals were always associated with the articular surface and CPPD crystals were found in the middle zone, although this middle zone was often located at the surface once degeneration reached a point at which the superficial zone was lost. This is in agreement with Grassi, et al25, who observed, through ultrasonography, hyperechoic enhancement of the superficial cartilage margin due to MSU crystal adhesion to the surface and a hyperechoic band within the cartilage middle layer due to CPPD crystals.
Of our crystal-associated specimens, 92.2% displayed concurrent visible cartilage degeneration. Although 7.8% of crystal-associated tali in our entire data bank displayed no signs of cartilage degeneration, it may be difficult to macroscopically detect early fibrillation beneath a covering of crystals. Thus, it appears that crystal deposits of either MSU or CPPD are strongly associated with cartilage degeneration. The location and pattern of histologic change in the MSU and CPPD affected tali were identical, and were strongly suggestive of a bio-mechanical effect.
Few studies have systematically examined the co-occurrence of OA and articular crystals. In a study of prognostic factors of cartilage loss in the knee in the general population, there was no statistically significant relationship with uric acid concentration (a surrogate for gout) and OA26. Some early reports focus on gout as a precursor or cause of erosive OA27,28. Recently, Roddy, et al6 noted a strong association between acute attacks of gout and the presence of OA in the 1st metatarsophalangeal joint, mid-foot, knee, and distal interphalangeal joints. This work, however, as well as our own, does not shed light on the important question of causality. Roddy, et al6 interpreted the close correlation between gouty arthritis and OA in individual joints to support the hypothesis that the presence of OA at an individual joint site predisposes to the formation of urate crystals at that site. In 1975, Wilcox and Khalaf29 set out to explain the observation that joint injury initiates gout. They hypothesized that a mechanical or metabolic upset resulted in a lowering of local pH, thereby favoring MSU crystal nucleation. Similarly, it has been shown that proteoglycans increase urate solubility, the hypothesis being that when cartilage damage occurs, proteoglycans are decreased or degraded, thus favoring the precipitation of urate crystals30. Additionally, it has been suspected that not only is chronic saturation of tissue by urate necessary for MSU crystal deposition, but so too is altered connective tissue turnover and composition, thus explaining why some individuals with chronic hyperuricemia succumb to gout while others do not4,31.
As for the relationship between CPPD and OA, there is a strong and well established association between these 2 conditions32–34, but whether CPPD deposition or OA is the inciting factor is not known. Radiographic studies have shown that chondrocalcinosis is associated with osteophytosis11,12, providing some support for the hypothesis that chondrocalcinosis, at least in the form of CPPD disease, may be a marker for hypertrophic and, thus, reparative tissue response to injury11,35. However, we were able to identify several specimens in which crystals, located beneath the cartilage surface, were present prior to the onset of any morphological changes — the articular surface being smooth and normal-appearing. Thus, it can be stated that CPPD crystals can accumulate in the absence of OA in a given joint.
Traditionally, cartilage damage in crystal-induced arthritis was thought to be mediated by recurrent episodes of inflammation. The important findings of Martinez-Sanchez and Pascual36 and Pascual, et al37, however, suggest that both MSU and CPPD crystals may be present in uninflamed joints and between attacks of inflammatory arthritis, thus having the opportunity to contribute to cartilage damage without initiating an inflammatory response36. Crystals have also been shown to have negative consequences on cartilage wear38.
In our study, both MSU and CPPD crystals were clearly associated with cartilage lesions. All of these cartilage lesions appeared to be biomechanically induced by virtue of their being: (1) located at margins where apposition with even a slightly noncongruent articulating surface would create abnormal stress/friction; or (2) the result of articulation with an anterior tibial osteophyte39–41.
These findings, however, do not tell us whether surface crystals appeared prior to, or after, the inception of cartilage lesions. At least for middle zone crystals (CPPD) we demonstrated that they can accumulate in the absence of cartilage lesions, at least in some instances. However, we also showed that middle zone crystals often continue superficially and make contact with the articular surface and, in this scenario, are intimately associated with cartilage lesions. It may be speculated that surface cartilage is eventually eroded just above the middle zone crystals, allowing crystals to escape to the surface. Concerning surface crystals, it is possible that cartilage degradation occurs first, followed by surface crystal deposition. Once crystals are attached to the cartilage surface, further biochemical/frictional cartilage damage may ensue. Alternatively, crystals may be the initiating factor in the degradation of cartilage in particularly stressed locations. In either scenario, it is apparent that surface crystals are intimately associated with each of the cartilage lesions on a given articular surface. We favor a combination of both hypotheses, whereby the cartilage is already metabolically altered, stressed, and perhaps fibrillated or fissured, thus opening up minute channels for the nucleation of crystals, which then perpetuate or worsen mechanically induced damage.
We also examined crystal-containing cartilage immunohistochemically to determine whether we could further identify patterns of cartilage degeneration unique to cartilage-containing crystals. SZP was found in particularly high levels in association with both types of crystals and their associated cartilage lesions. This finding may represent an attempt at increasing surface lubrication in the face of crystal-induced friction between articular surfaces. Collagen X was found in abundance in the lower half of the cartilage affected by both types of crystals. Interestingly, collagen X appeared to be increased even at sites distant to the crystal deposition and cartilage lesions as long as crystals were found somewhere on the articular surface. The presence of collagen X was not necessarily indicative of the presence of hypertrophic chondrocytes. This leads us to speculate that perhaps chondrocytes in slightly distant regions lead to the presence of collagen X beyond their immediate vicinity. The significance of these findings will require further investigation.
S100 protein did not correlate significantly with the presence of crystals in degenerated cartilage. However, it was found in greater levels in enlarged cell clones adjacent to CPPD crystal cysts. S100 plays a role in local chondrocyte hypertrophy, and S100A8 and S100A8/A9 are essential to neutrophil migration induced by MSU crystals42. The level of cSRC staining did not correlate with the presence of MSU crystals. However, some degenerated/crystal-containing tissues displayed increased intensity of staining never found in controls. These results may provide some evidence of the role of cSRC in MSU-associated kinase caspase signaling.
A caveat of our study is that only 18% of the tali displaying crystals were examined through FTIR and polarized light microscopy for definitive identification as either MSU or CPPD. Definitive identification of crystals in the earlier samples was impossible as the tali themselves were no longer available. It was also unfortunate that the tibial surfaces of the ankle joints were not available for study. This might have provided greater insight into the relationship of crystals between articulating surfaces. It would be interesting to determine differences in location and amount of crystals between opposing surfaces. This will be examined in future studies of other joints.
The presence of either MSU or CPPD crystals is strongly correlated with cartilage lesions in the ankle joint. The histo-logic similarities in cartilage from joints with CPPD crystals compared to those with MSU crystals, together with what is known about the dramatically different etiologic factors producing these crystals, strongly suggest that these lesions are at least partially biomechanically induced.
ACKNOWLEDGMENT
We thank the families of the donors to the Gift of Hope Organ and Tissue Donor Network of Illinois. We also thank Dr. Arkady Margulis for procurement of the human tali.
Supported by National Institutes of Health grant NIAMS RO1 AR 40292–05.
Contributor Information
THOMAS AIGNER, University of Leipzig, Leipzig, Germany.
ANN K. ROSENTHAL, Medical College of Wisconsin, Milwaukee, Wisconsin, USA..
REFERENCES
- 1.Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage 2007;15:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neogi T, Nevitt M, Niu J, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis. Arthritis Rheum 2006;54:1822–8. [DOI] [PubMed] [Google Scholar]
- 3.Doherty M, Belcher C, Regan M, Jones A, Ledingham J. Association between synovial fluid levels of inorganic pyrophosphate and short term radiographic outcome of knee osteoarthritis. Ann Rheum Dis 1996;55:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lally EV, Ho G Jr, Kaplan SR. The clinical spectrum of gouty arthritis in women. Arch Intern Med 1986;146:2221–5. [PubMed] [Google Scholar]
- 5.Simkin PA, Campbell PM, Larson EB. Gout in Heberden’s nodes. Arthritis Rheum 1985;26:461–8. [DOI] [PubMed] [Google Scholar]
- 6.Roddy E, Zhang W, Doherty M. Are joints affected by gout also affected by osteoarthritis? Ann Rheum Dis 2007;66:1374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silcox DC, McCarty DJ. Elevated inorganic pyrophosphate concentration in synovial fluid in osteoarthritis and pseudogout. J Lab Clin Med 1974;83:518–31. [PubMed] [Google Scholar]
- 8.Doherty M, Watt I, Dieppe PA. Localized chondrocalcinosis in post-meniscectomy knee. Lancet 1982;1:1207–10. [DOI] [PubMed] [Google Scholar]
- 9.Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR. Synovial fluid features and their relations to osteoarthritis severity: new finding from sequential studies. Osteoarthritis Cartilage 2003;11:50–4. [DOI] [PubMed] [Google Scholar]
- 10.Neame RL, Carr AJ, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis 2003;62:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaosa Y, Lanyon P, Doherty M. Characterisation of size and direction of osteophyte in knee osteoarthritis: a radiographic study. Ann Rheum Dis 2002;61:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieppe P, Watt I. Crystal deposition in osteoarthritis: an opportunistic event? Clin Rheum Dis 1985;11:367–93. [PubMed] [Google Scholar]
- 13.Peyron JG. The epidemiology of osteoarthritis In: Moskowitz RW, Howell DS, Goldberg VM, et al. , editors. Osteoarthritis: Diagnosis and treatment. Philadelphia: Saunders; 1984:9–27. [Google Scholar]
- 14.Muehleman C, Bareither D, Huch K, Cole A, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage 1997;5:23–37. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal AK, Mandel N. Identification of crystals in synovial fluids and joint tissues. Curr Rheumatol Rep 2001;3:11–6. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg L Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am 1971;53:69–82. [PubMed] [Google Scholar]
- 17.Aigner T, Loos S, Muller S, Sandell LJ, Unni KK, Kirchner T. Cell differentiation and matrix gene expression in mesenchymal chondrosarcomas. Am J Pathol 2000;156:1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massada JL. Ankle overuse injuries in soccer players. Morphological adaptation of the talus in the anterior impingement. J Sports Med Phys Fitness 1991;31:447–51. [PubMed] [Google Scholar]
- 19.Abreu M, Johnson K, Chung CB, et al. Calcification in calcium pyrophosphate dihydrate (CPPD) crystal deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol 2004;33:392–8. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal AK. Update in calcium deposition diseases. Curr Opin Rheumatol 2007:19;158–62. [DOI] [PubMed] [Google Scholar]
- 21.Mikuls TE, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005;64:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HY, Pan WH, Yeh WT, Tsai KS. Hyperuricaemia and gout in Taiwan: results from the nutritional and health survey in Taiwan (1993–96). J Rheumatol 2001;28:1640–6. [PubMed] [Google Scholar]
- 23.Agudelo C, Wise CM. Crystal-associated arthritis. Clin Geriatr Med 1998;14:495–513. [PubMed] [Google Scholar]
- 24.Dessein PH, Shipton FA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie-carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear” — Sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum 2006;36:197–202. [DOI] [PubMed] [Google Scholar]
- 26.Schouten JS, van den Ouweland FA, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis 1992;51:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parhami N, Greenstein N, Juozevivius JL. Erosive osteoarthritis and gout: gout in 36 joints. J Rheumatol 1986;13:469–71. [PubMed] [Google Scholar]
- 28.Erlich GE. Inflammatory arthritis: The clinical syndrome. J Chron Dis 1972:25:317–28. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox WR, Khalaf AA. Nucleation of monosodium urate crystals. Ann Rheum Dis 1975;34:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz WA, Schubert M. The interaction of monosodium urate with connective tissue components. J Clin Invest 1970:49:1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terkeltaub R Gout in 2006: the perfect storm. Bull Hosp Jt Dis 2006;64:82–6 [PubMed] [Google Scholar]
- 32.Sokoloff L, Varma A. Chondrocalcinosis in surgically resected joints. Arthritis Rheum 1988;31:750–6. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher H, Gordon G, Paul H, et al. Osteoarthritis, crystal deposition and inflammation. Semin Arthritis Rheum 1981; 11:116–9. [Google Scholar]
- 34.Dieppe P, Crocker P, Corke C, Doyle DV, Huskisson EC, Willoughby DA. Synovial fluid crystals. Q J Med 1979;48:533–55. [PubMed] [Google Scholar]
- 35.Doherty M, Dieppe P. Crystal deposition disease in the elderly. Clin Rheum Dis 1986;2:97–116. [PubMed] [Google Scholar]
- 36.Martinez-Sanchez A, Pascual E. Intracellular and extracellular CPPD crystals are a regular feature in synovial fluid from uninflamed joints of patients with CPPD related arthropathy. Ann Rheum Dis 2005;64:1769–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual E, Batlle-Gualda E, Martinez A, Rosas J, Vela P. Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med 1999;131:756–9. [DOI] [PubMed] [Google Scholar]
- 38.Hayes A, Harris B, Dieppe P, Clift S. Wear of articular cartilage: The effect of crystals. Proc Inst Mech Eng 1993:207:41–58. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Jadin K, Masuda K, Sah R, Muehleman C. Characterization of lesions of the talus and description of tram track lesions. Foot Ankle Int 2006;27:344–55. [DOI] [PubMed] [Google Scholar]
- 40.Berberian WS, Hecht PJ, Wapner KL, DiVerniero R. Morphology of tibiotalar osteophytes in anterior ankle impingement. Foot Ankle Int 2001;22:313–7. [DOI] [PubMed] [Google Scholar]
- 41.Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int 1997;18:418–23. [DOI] [PubMed] [Google Scholar]
- 42.Ryckman C, McColl SR, Vandal K, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum 2003;48:2310–20. [DOI] [PubMed] [Google Scholar]