Abstract
Purpose:
To conduct a pilot randomized trial testing an exercise program specifically adapted for post-bariatric patients.
Methods:
A total of 51 post-bariatric patients, 6-24 months post-surgery, were randomly assigned to usual care control (n = 25) or the exercise intervention (n = 26). The intervention included twice weekly 60 minute group exercise classes with functional strength, flexibility, and aerobic activities; at least three days per week of self-directed exercise; daily pedometer; recording of steps and activities; and weekly telephone counseling. There was also a six month maintenance period.
Results:
Patients were 49 ± 12 years old, 84% female, 59% non-Hispanic white, with a BMI of 32.9 ± 5.7 kg/m2 and percent excess BMI loss since surgery of 56% ± 35%. Patients were 14 ± 5 months post-surgery. A total of 44 patients (86%) completed both phases of the program and all assessments. The following measures improved significantly for intervention participants with no significant change in control participants: yards walked in six minutes, seconds for 8-foot up-and-go, number of arm curls, and distance in inches for chair sit-and-reach. Intervention changes remained after six months of maintenance.
Conclusions:
When compared to patients in usual care, a specially adapted exercise program for post-bariatric patients resulted in significant improvements in objectively monitored health outcomes. This program was delivered in a clinical setting and could be implemented in a variety of settings to improve health outcomes for post-bariatric patients.
Introduction
Bariatric surgery has now emerged as the most successful treatment for severe obesity.(1) Despite these findings, there is a wide range in weight loss and regain after surgery even within the same procedure, leading many researchers to investigate the predictors of weight loss success.(2-4) Observational studies have shown that one of the most important predictors of weight loss post-surgery is increases in self-reported physical activity.(5, 6)
In spite of the clear benefits that physical activity may have for bariatric patients, one of the referral criteria for bariatric surgery is that they have made multiple attempts at weight maintenance and have not been successful.(7) Thus there is skepticism that these patients will be able to adopt and maintain an active lifestyle after surgery. There are some published studies testing various exercise interventions for bariatric patients. Some have focused on the pre-surgical period(8, 9) and others on the post-surgical period, primarily in the first year after surgery.(10-13) Some have shown a positive impact on body composition and weight loss, however, only one of the intervention studies followed U.S. federal guidelines for regular moderate-to-vigorous physical activity (MVPA),(13) and only one included different types of exercise such as strength and flexibility.(14)
By 2020, it is estimated that there will be over one million people who have had bariatric surgery.(7) Developing exercise programs for post-bariatric patients in clinical settings that meet public health recommendations for regular physical activity is important to protect the healthcare system‟s investment in bariatric surgery as a viable treatment for severe obesity. However, there is still relatively little known about what types of exercise programs are tolerated well by post-bariatric patients and what exercises are best for improvements in daily functioning.(15) We adapted an effective program for adults with arthritis(16) for use with post-bariatric patients and tested it using a randomized controlled trial. This pilot was called Fitness and Exercise for Post-Bariatric Patients (FEPP). We hypothesized that 1) post-bariatric patients participating in FEPP would have greater changes in physical fitness measures and self-reported physical activity as compared to a usual care control group of post-bariatric patients during a six month exercise program, and 2) that these changes would be maintained after a six month maintenance phase where participants were transitioned to more self-directed exercise.
Methods
Setting
The study was conducted with Kaiser Permanente Southern California (KPSC) patients in the San Diego county service region of the healthcare system. Weight loss surgeries are performed in 10 different hospital facilities by 13 surgeons. The details of weight loss surgery at KPSC have been published elsewhere.(17)
Participants
Patients were eligible for our study if they had the following characteristics: 1) having had an initial bariatric procedure 6 – 24 months prior to the date we began recruitment, 2) having no revisions of this procedure during this time, 3) having no conditions which would prevent them from doing moderate weight-bearing exercise, 4) living in San Diego county, and 5) planning to stay in the county for at least one year. Confirmation of their ability to participate in a regular exercise program was obtained from each participant‟s physician before scheduling the baseline data collection appointment.
Recruitment was done by generating an eligible list of participants from a clinical bariatric surgery registry(17) and mailing an introductory letter and “opt out/in” card to these eligible patients. These mailings were followed-up by phone calls. If patients were contacted they were informed about the study. If they indicated they were interested in participating, they were scheduled for a 90 minute in person group baseline measurement session. Participants received a $25 gift certificate for each measurement session they attended (baseline, end of program [6 months], and end of maintenance [12 months]). They also received an additional $25 gift certificate at each time point after they returned their pedometers. At no time were patients reimbursed for participating in the exercise program. Incentives were only used to encourage participation in the outcome measurements.
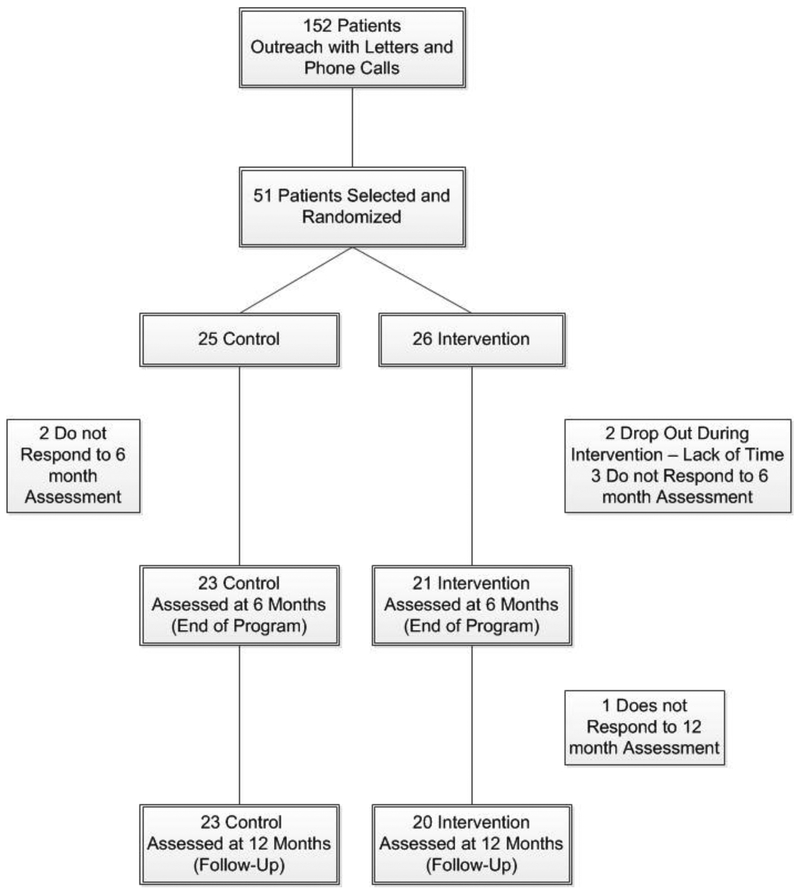
Participants were randomized with a 1:1 ratio to control or intervention conditions after the collection of baseline data. Randomization codes were generated based on block randomization with varying block sizes stratified by gender and bariatric procedure (gastric sleeve, bypass, adjustable gastric band). All procedures were approved by the Institutional Review Boards for Human Subjects at both Kaiser Permanente Southern California and San Diego State University. The study design is shown in Figure 1.
Figure 1.
Design figure for Fitness and Exercise for Post-Bariatric Patients (FEPP) with sample sizes for each group and assessment period.
Measurements
Objective physical measures.
The following measures were used for all time points to assess changes in physical activity and fitness: Four consecutive day pedometer (New Lifestyles NL-800, Lees Summit, MO) activity counts (two weekday and both weekend days) and the six minute walk for assessment of aerobic fitness which have both been used with other bariatric populations to measure physical activity,(18) weight and height for calculation of body mass index (BMI; kg/m2) and percent excess weight loss (%EWL) (calculated using a BMI of 25 mg/k2 as the ideal target for 100% EWL), the 8-foot up-and-go(19) and 30 second chair rise(20) for balance, mobility and coordination, the chair sit-and-reach(21) for flexibility, and the arm curl(22) for strength and muscular endurance.
Self-Report.
A single questionnaire was administered to all participants at baseline, after the program ended (6 months), and after the maintenance phase (12 months). This questionnaire contained measures of sedentary activity(23) and the aerobic exercise, flexibility, and muscle strength questions from the Behavioral Risk Factor Surveillance Survey (BRFSS).(24)
Exercise Protocol
Although there are a variety of exercise programs available for people with severe obesity and a few for post-bariatric patients tested in the literature,(8–14) we felt there were none that addressed all the challenges faced by post-bariatric patients when trying to exercise. These included arthritis that is not substantially improved after weight loss,(25) hypoglycemic episodes specific to the metabolic changes following bariatric surgery, peripheral neuropathy in patients with diabetes as a result of poor glycemic control before surgery which interferes with balance and coordination, loss of muscle and bone density seen in rapid weight loss, nutrient supplementation and protein intake recommendations specific to bariatric patients, and poor adherence with consistent exercise routines.
We began by adapting an exercise program designed specifically for people with poor functional fitness and/or arthritis to increase all aspects of fitness (aerobic endurance, strength, flexibility, and balance, with emphasis on dynamic balance and mobility).(16) Mobility issues are often cited as barriers to exercise in these bariatric patients.(26) We modified this curriculum to include a mastery learning approach(27, 28) to achieving the final goals of MVPA at least 150 minutes/week and to include the most enduring strategies for behavior change including self-monitoring, feedback, and goal setting, social support, modeling, and tailoring prescriptions and messages.(28, 29) Finally, we added a maintenance phase with booster exercise sessions and social support. These are all fundamental strategies for successful behavior change that are the foundation of all good exercise programs.
Finally, there were a number of modifications we made to tailor the program specifically to post-bariatric patients in various phases of the weight loss trajectory (6 – 24 months after surgery). These included the concept of “functional resistance exercise”(30) where patients were taught to use their body weight as the resistance challenge to the neuromuscular system and to focus on proper biomechanical movement during activities of daily living instead of adding a traditional resistance component like weight lifting or the use of resistance bands. This helped patients avoid further musculoskeletal injury in addition to assisting them to adapt to the postural and balance changes that were necessary with large amounts of weight loss.
Another adaptation was teaching patients to do flexibility and strength exercises while standing or sitting in a chair instead of lying down, which was very uncomfortable for most participants because of excess skin and body fat. It was also difficult and awkward for patients to rise to a standing position from the floor. All exercises were tailored to the specific needs of each patient according to their abilities and patients were allowed to increase the frequency and intensity of activities as they mastered lower levels of exercise. All exercises had intensity/difficulty levels so that they could be done safely and consistently at home. Handouts with instructions for proper exercise technique were given to all participants for home use.
We also incorporated discussions during the exercise class about specific nutritional considerations for exercise in post-bariatric patients. These included being prepared for post-prandial hypoglycemia, the importance of using vitamin and mineral supplements, and preparing for increases in appetite and the urge to overeat because they would “burn” the extra calories off.
The final exercise prescription and program requirements were as follows: 1) attendance twice per week at a 60 minute structured exercise class, 2) once per week phone counseling sessions to review progress, set new goals, and problem solve barriers to change, 3) wearing a pedometer daily, 4) reading the program curriculum, 5) and recording all out-of-class physical activity and daily pedometer counts in the 10,000 Steps™ website. The maintenance phase was designed to “wean” participants off the structure and requirements of the program by reducing class attendance to once per week and counseling sessions to once per month. The pedometer and website recording were made optional during maintenance.
Usual Care
Regular post-operative care for bariatric patients included routine laboratory testing, weight assessment, and phone calls from nurse care managers that included guidance about dietary changes necessary throughout the post-operative period and counseling to encourage regular MVPA. Exercise counseling varied widely and did not contain any standardized recommendations. Phone calls and monitoring were done within the first two weeks of surgery, and then at two months, six months, and annually thereafter.
Analyses
Descriptive statistics were presented as means ± standard deviations for continuous variables and frequency (percentages) for categorical variables, or otherwise as indicated. Baseline characteristics were compared between control and intervention groups, and between participants retained and lost to follow-up, using independent sample t-tests for continuous and the chi-square statistic for categorical variables. Outcome measures over time were analyzed using piecewise linear mixed-effect models with random intercept and linear slopes assessing changes during the intervention period (baseline to 6 months) and the maintenance period (6 months to 12 months) separately. Differences within and between groups for these changes were evaluated and significance was set at p < .05. Data were analyzed following the intent-to-treat principle where all randomized participants were included regardless of whether or not they had outcome measures. Pedometer steps and 8-foot up-and-go scores were log-transformed to approximate a normal distribution for statistical testing. Chair sit-and-reach results were also transformed to remove negative values by adding a value of 15 to each score. SAS Enterprise Guide 4.3 (SAS Institute Inc., Cary, North Carolina) was used for all data analyses.
Results
Participants
Baseline characteristics were similar between control and intervention groups, and between patients who did and did not complete the outcome assessments (Table 1). In general, the patient population was middle aged, primarily women, non-Hispanic white, had gastric bypass surgery, were just over a year post-surgery, and lost about 34 kg after surgery but before beginning the study. All participants self-reported low levels of functional impairment and moderate rates of physical activity at baseline.
Table I.
Baseline characteristics and statistical comparisons for intervention and control groups, and for participants who did and did not complete the study.
| Group | Completed Assessments | |||||
|---|---|---|---|---|---|---|
| Control (n = 25) |
Intervention (n = 26) |
p | No (n = 8) |
Yes (n = 43) |
p | |
| Age | 46.6 ± 12.0 | 52.0 ± 10.9 | 0.14 | 47.1 ± 13.6 | 49.8 ± 11.4 | 0.31 |
| % Female | 21 (84%) | 22 (84.6%) | 1.00 | 8 (100%) | 35 (81.4%) | 0.33 |
| Ethnicity | 0.14 | 0.67 | ||||
| African American/Black | 1 (4%) | 3 (11.5%) | 0 (0%) | 4 (9.3%) | ||
| Hispanic | 8 (32%) | 5 (19.2%) | 3 (37.5%) | 10 (23.3%) | ||
| Other | 0 (0%) | 4 (15.4%) | 1 (12.5%) | 3 (7%) | ||
| White | 16 (64%) | 14 (53.8%) | 4 (50%) | 26 (60.5%) | ||
| Type of Surgery | 0.69 | 0.06 | ||||
| Lap Band | 1 (4%) | 1 (3.8%) | 1 (12.5%) | 1 (2.3%) | ||
| Gastric Sleeve | 7 (24%) | 4 (15.4%) | 3 (25%) | 8 (18.6%) | ||
| Bypass | 17 (68%) | 21 (80.8%) | 4 (50%) | 34 (79.1%) | ||
| Time from Surgery to Baseline (months) | 14.3 ± 5.9 | 13.2 ± 4.6 | 0.39 | 12.1 ± 5.2 | 14.0 ± 5.3 | 0.31 |
| Weight Lost before Baseline (kgs) | 33.1 ± 32.1 | 35.2 ± 37.4 | 0.88 | 40.5 ± 33.1 | 33.0 ± 35.1 | 0.57 |
| Objectively Monitored Outcomes | ||||||
| 6 Min Walk (meters) | 503.1 ± 77.2 | 495.7 ± 111.2 | 0.62 | 488.7 ± 109.6 | 501.4 ± 93.5 | 1.00 |
| 8 Foot Up-and-Go (seconds) | 6.2 ± 1.2 | 6.6 ± 2.0 | 0.71 | 6.9 ± 1.9 | 6.3 ± 1.6 | 0.26 |
| Arm Curls (#) | 15.0 ± 3.8 | 15.1 ± 6.1 | 0.71 | 14.6 ± 3.5 | 15.1 ± 5.3 | 0.69 |
| Sit and Reach (cm) + 15 | 14.8 ± 4.4 | 16.1 ± 4.2 | 0.22 | 15.1 ± 3.3 | 15.6 ± 4.5 | 0.81 |
| Chair Rise (#) | 11.0 ± 3.5 | 11.0 ± 3.5 | 0.97 | 11.0 ± 3.4 | 11.0 ± 3.5 | 0.60 |
| Steps per Day (Goal: 10,000) | 6640.5 ±2795.5 | 6633.3 ±3352.8 | 0.865 | 4683.7 ±2019.8 | 7000.2 ±3100.85 | 0.03 |
| Weight (kg) | 93.4 ± 19.8 | 90.8 ± 23.0 | 0.52 | 94.4 ± 22.6 | 91.6 ± 21.3 | 0.82 |
| BMI (kg/m2) at Surgery | 44.5 ± 5.5 | 45.0 ± 7.6 | 0.77 | 45.6 ± 4.4 | 44.6 ± 6.9 | 0.71 |
| BMI (kg/m2) at the Time of Enrollment in the Program | 33.1 ± 5.8 | 32.7 ± 5.8 | 0.81 | 34.3 ± 5.8 | 32.6 ± 5.8 | 0.50 |
| Self-Reported Outcomes | ||||||
| Flexibility (days/week) | 1.7 ± 2.0 | 2.4 ± 2.7 | 0.52 | 1.8 ± 2.3 | 2.1 ± 2.4 | 0.86 |
| Strength (days/week) | 1.3 ± 1.8 | 2.6 ± 2.6 | 0.09 | 2.0 ± 1.6 | 1.9 ± 2.4 | 0.565 |
| MVPA (min/week) | 252.9 ± 180.5 | 291.1 ± 192.8 | 0.37 | 190.3 ± 153.4 | 288.4 ± 189.2 | 0.185 |
| Sedentary Activity (min/day) | 179.1 ± 70.4 | 166.5 ± 87.8 | 0.49 | 174.1 ± 87.2 | 172.2 ± 78.9 | 0.93 |
Ten of the 25 intervention participants (40%) had a pre-existing condition that limited exercise participation, despite being cleared for participation by their physicians and self-reporting low levels of functional impairment at baseline. Of the remaining 15 intervention participants, 44% developed a condition during the program limiting participation. Program retention rates were 92% for controls and 81% for intervention participants. This remained the same for controls at the end of the maintenance period but decreased to 77% for intervention participants. Overall program retention was 84% for one year (see Figure 1).
Program Outcomes
Intervention phase (baseline to 6 months).
Means and standard deviations for study outcomes are shown in Table 2 for control and intervention participants. The following measures increased significantly for intervention participants with no significant change in control participants: meters walked in six minutes (change of 30.45 ± 8.8 [p = .001] for intervention vs. change of 0.91± 0.2 [p = .92] for control), log transformed seconds for 8-foot up-and-go (change of −0.075 ± 0.035 [p = .035] for intervention vs. change of 0.045 ± 0.03 [p = .17] for control), number of arm curls (change of 1.60 ± 0.69 [p = .02] for intervention vs. change of 1.03 ± 0.64 [p = .11] for control), and distance in cm + 15 for chair sit-and-reach (change of 1.57 ± 0.68 [p = .02] for intervention vs. change of 0.39 ± 0.63 [p = .54] for control). No other significant changes were seen during the intervention phase for either intervention or control participants.
Table II.
Outcome results presented for intervention and control participants in the intent-to-treat analyses. Data are presented as means ± standard deviations.
| Usual Care Control Participants | Intervention Participants | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 25) |
6 Months (n = 23) |
12 Months (n = 23) |
Baseline (n = 26) |
6 Months (n = 21) |
12 Months (n = 20) |
|
| 6 Min Walk (meters) | 503.1 ± 77.2 | 503.1 ± 79.7 | 508.0 ± 94.6 | 495.7 ± 111.2 | 526.7 ± 87.0 | 521.2 ± 78.6 |
| 8 Foot Up-and-Go (seconds) | 6.2 ± 1.2 | 6.5 ± 1.5 | 6.1 ± 2.0 | 6.6 ± 2.0 | 6.2 ± 1.6 | 5.9 ± 1.1 |
| Arm Curls (#) | 15.0 ± 3.8 | 16.2 ± 4.0 | 16.9 ± 4.3 | 15.1 ± 6.1 | 17.4 ± 5.8 | 16.5 ± 4.7 |
| Sit and Reach (cm) | −0.16 ± 4.4 | 0.43 ± 3.7 | 2.4 ± 4.1 | 1.1 ± 4.2 | 2.5 ± 3.4 | 2.1 ± 4.7 |
| Chair Rise (#) | 11.0 ± 3.5 | 11.6 ± 3.8 | 12.4 ± 3.6 | 11.0 ± 3.4 | 11.6 ± 4.2 | 12.3 ± 2.6 |
| Steps per Day (Goal: 10,000) | 6640.5 ±2795.5 | 6800.7 ±3650.3 | 7409.0 ±4254.7 | 6633.3 ±3352.8 | 7615.3 ±4944.6 | 8624.9 ±5436.8 |
| Weight (kg) | 93.4 ± 19.8 | 93.6 ± 21.8 | 94.6 ± 21.8 | 90.8 ± 23.0 | 87.0 ± 21.0 | 89.0 ± 21.0 |
| Flexibility (days/week) | 1.7 ± 1.9 | 1.6 ± 2.4 | 1.4 ± 2.1 | 2.4 ± 2.7 | 3.2 ± 1.9 | 3.4 ± 2.8 |
| Strength (days/week) | 1.3 ± 1.8 | 1.3 ± 2.0 | 1.2 ± 1.6 | 2.6 ± 2.6 | 2.3 ± 1.5 | 1.9 ± 2.4 |
| MVPA (min/week) | 252.9 ± 180.5 | 272.7 ± 253.4 | 281.9 ± 293.2 | 291.1 ± 192.8 | 353.01 ± 308.6 | 253.9 ± 202.8 |
| Sedentary Activity (min/day) | 179.1 ± 70.4 | 167.2 ± 104.1 | 171.4 ± 101.8 | 166.5 ± 87.8 | 153.3 ± 92.7 | 153.3 ± 108.3 |
Maintenance phase.
During the maintenance phase of the program, intervention participants maintained the changes they had in yards walked in six minutes, log transformed seconds for 8-foot up-and-go, number of arm curls, and distance in cm + 15 for chair sit-and-reach (i.e. no significant differences between changes in maintenance phase when compared to intervention phase). Control participants had a significant increase in chair sit-and-reach during the maintenance phase (change of 1.96 ± 0.63 during maintenance phase [p = .003] vs. change of 0.39 ± 0.63 during intervention phase [p = .54]).
Program Participation
During the intervention phase of the program, participants attended 56% of all classes offered (an average of one class per week) and exercised 3.1 ± 1.7 days a week outside of class for 139.4 ± 86.9 minutes per week. If the 60 minute classes are added to this total, then participants exercised an average of four days a week for a total of 200 minutes. Participants were able to make contact with the behavioral counselor 69% of the time and meet their goals 59% of the time (as assessed each week of the program). In addition, 87% of them used the 10,000 Steps™ website and recorded their steps on 6.3 ± 0.7 days per week.
During the maintenance phase, only 32% of intervention participants were able to attend one class per week (an average of 1.5 classes a month) and exercised 2.9 ± 1.8 days a week outside of class for 122.9 ± 87.7 minutes per week. If the 60 minute class is added to this total, then participants exercised an average of 3 days a week for a total of 146 minutes. Participants were able to make contact with the behavioral counselor 93% of the time and meet their goals 65% of the time. The website and 10,000 Steps™ website were made optional during the maintenance phase; 44% of intervention participants used the website and recorded their steps on 4.5 ± 3.1 days per week.
Discussion
Overall we found a number of significant health benefits for post-bariatric patients who participated in FEPP as compared to participants randomized to usual care. These included increases in objectively monitored aerobic fitness (six minute walk), strength (arm curl), balance, mobility, and coordination (8-foot up-and-go), and flexibility (chair sit-and-reach) after six months of a combined in class structured and home-based exercise program. These improvements remained following six months of maintenance.
It is difficult to compare these findings to other post-operative bariatric exercise studies in the literature because of the disparate exercise prescriptions and length of programs.(8-14) To our knowledge, FEPP is the longest program with a focus on all aspects of physical fitness (aerobic, strength, and flexibility) with special attention to exercise-limiting conditions such as arthritis which are very common in severely obese patients having bariatric surgery. Our program was based in sound theoretical principles of behavior change and evidence-based public health recommendations,(27-30,31-33) which is not the case for many of the programs studied to date.
Even with a strong theoretical, evidence-based strategy, our program was only able to encourage patients to exercise consistently for four days a week with intensive support and monitoring. When the monitoring and accountability was made optional during the maintenance phase, patients were only able to achieve three days a week on a regular basis. Without the structured exercise class, patients exercised an average of 140 minutes per week during the intervention and 123 minutes per week during maintenance. Patients were able to attend at least one structured exercise class per week during the intensive intervention phase, increasing their total exercise to an average of 200 minutes per week. With optional monitoring and accountability during maintenance, the exercise class attendance dropped to an average of one class every two weeks, increasing their total exercise during this phase to an average 146 minutes per week.
There were a number of limitations with our study that may have contributed to the fact that we did not find improvements in excess weight loss, BMI, sedentary behavior, and either self-reported or objectively measured MVPA. The study was designed primarily as a feasibility study to understand the functional limitations of post-bariatric patients and their physical and behavioral capacity for adopting the national recommendations for adult regular physical activity, including the incorporation of strength and flexibility activities. This was not powered for efficacy outcomes. In addition, we did not limit the study to patients who were completely sedentary. Patients who were already physically active were eligible for the study which may have limited the impact of our intervention on changes in pedometer and self-reported physical activity. When compared to other populations in the literature, however, baseline pedometer counts for all participants were typical for those seen in sedentary adults(34) but lower than other published studies with bariatric surgery patients.(5)
Another limitation of the study was that patients could be 6 – 18 months post-surgery which is a very wide range in weight loss experience. We chose to use this wide range to have our findings as generalizable as possible to the post-bariatric population. However, a narrower post-operative time frame could have reduced variability in responding to the intervention and thus we may have had more power to detect effects in weight and self-reported and objectively monitored physical activity. In addition, most of our patients were in the BMI range of 30 – 35 kg/m2 when they began the program. Our findings might not be generalizable to patients at much higher BMI ranges who might have additional health challenges compromising their ability to fully participate in all exercises.
Conclusions
Based upon our findings, it may be unreasonable to expect that most post-bariatric patients can maintain the public health recommendations of 150 minutes per week of MVPA or 10,000 steps per day even after a six month gradual, mastery-based approach to exercise adoption. It may be more reasonable to prescribe 3 – 4 days a week for 30 – 60 minutes with a social support and monitoring component to improve adherence. We found large variability in patients‟ responses to the exercise program. Future protocols should test strategies that include 1) use of individualized exercise targets, 2) broadening of aerobic prescriptions from the traditional focus on walking, and 3) including circuit-based formats with supervised, individualized exercise modification and an emphasis on strength, flexibility, mobility, and improved functional status.
Acknowledgments
This program was funded with a grant from the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) #R21 DK089269-01A1. None of the authors have any conflicts of interest to disclose.
Footnotes
Conflict of Interest Disclosure Statement
None of the authors have any conflicts of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014. March;24(3):437–55. PubMed PMID: . Pubmed Central PMCID: PMC3916703. Epub 2014/01/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinzl JF, Schrattenecker M, Traweger C, Mattesich M, Fiala M, Biebl W. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16(12):1609–14. [DOI] [PubMed] [Google Scholar]
- 3.Pontiroli AE, Fossati A, Vedani P, Fiorilli M, Folli F, Paganelli M, et al. Post-surgery adherence to scheduled visits and compliance, more than personality disorders, predict outcome of bariatric restrictive surgery in morbidly obese patients. Obes Surg. 2007;17(11):1492–7. [DOI] [PubMed] [Google Scholar]
- 4.Toussi R, Fujioka K, Coleman KJ. Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obesity (Silver Spring, Md). 2009;17(5):996–1002. [DOI] [PubMed] [Google Scholar]
- 5.King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2). Surg Obes Relat Dis. 2012. Sep-Oct;8(5):522–32. PubMed PMID: . Pubmed Central PMCID: PMC3248952. Epub 2011/09/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Exercise following bariatric surgery: systematic review. Obes Surg. 2010. May;20(5):657–65. PubMed PMID: . Pubmed Central PMCID: 2850994. Epub 2010/02/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Spitz AF, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring, Md). 2009. April;17 Suppl 1:S1–70, v. PubMed PMID: . Epub 2009/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 8.Baillot A, Mampuya WM, Comeau E, Meziat-Burdin A, Langlois MF. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg. 2013. July;23(7):882–91. PubMed PMID: . Epub 2013/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 9.Parikh M, Dasari M, McMacken M, Ren C, Fielding G, Ogedegbe G. Does a preoperative medically supervised weight loss program improve bariatric surgery outcomes? A pilot randomized study. Surg Endosc. 2012. March;26(3):853–61. PubMed PMID: . Epub 2011/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 10.Castello V, Simoes RP, Bassi D, Catai AM, Arena R, Borghi-Silva A. Impact of aerobic exercise training on heart rate variability and functional capacity in obese women after gastric bypass surgery. Obes Surg. 2011. November;21(11):1739–49. PubMed PMID: . Epub 2010/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 11.Nijamkin MP, Campa A, Sosa J, Baum M, Himburg S, Johnson P. Comprehensive nutrition and lifestyle education improves weight loss and physical activity in Hispanic Americans following gastric bypass surgery: a randomized controlled trial. Journal of the Academy of Nutrition and Dietetics. 2012. March;112(3):382–90. PubMed PMID: . Epub 2012/06/22. eng. [DOI] [PubMed] [Google Scholar]
- 12.Shah M, Snell PG, Rao S, Adams-Huet B, Quittner C, Livingston EH, et al. High-volume exercise program in obese bariatric surgery patients: a randomized, controlled trial. Obesity (Silver Spring, Md). 2011. September;19(9):1826–34. PubMed PMID: . Epub 2011/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 13.Zagarins SE, N.A. A, Skinner SS, Kepmer AJ, Welch G. Improved Exercise Behaviors Associated with a Comprehensive Structured Exercise Program Following Bariatric Surgery. Bariatr Nurs Surg Patient Care. 2011;6(2):85–90. [Google Scholar]
- 14.Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obes Surg. 2011. January;21(1):61–70. PubMed PMID: . Epub 2009/12/10. eng. [DOI] [PubMed] [Google Scholar]
- 15.Herring LY, Stevinson C, Davies MJ, Biddle SJ, Sutton C, Bowrey D, Carter P. Changes in physical activity behaviour and physical function after bariatric surgery: A systematic review and meta-analysis. Obes Rev. 2016; January 18, doi: 10.1111/obr.12361. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Levy SS, Macera CA, Hootman JM, Coleman KJ, Lopez R, Nichols JF, et al. Evaluation of a multi-component group exercise program for adults with arthritis: Fitness and Exercise for People with Arthritis (FEPA). Disabil Health J. 2012. October;5(4):305–11. PubMed PMID: . Epub 2012/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 17.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014; 10:396–403. PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Josbeno DA, Jakicic JM, Hergenroeder A, Eid GM. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surg Obes Relat Dis. 2010. Jul-Aug;6(4):361–6. PubMed PMID: . Epub 2008/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 19.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. [DOI] [PubMed] [Google Scholar]
- 20.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999. June;70(2):113–9. PubMed PMID: . Epub 1999/06/25. eng. [DOI] [PubMed] [Google Scholar]
- 21.Jones CJ, Rikli RE, Max J, Noffal G. The reliability and validity of a chair sit-and-reach test as a measure of hamstring flexibility in older adults. Res Q Exerc Sport. 1998. December;69(4):338–43. PubMed PMID: . Epub 1998/12/29. eng. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence RH, Jette AM. Disentangling the disablement process. J Gerontol B Psychol Sci Soc Sci. 1996;51(4):S173–S82. [DOI] [PubMed] [Google Scholar]
- 23.Laska MN, Murray DM, Lytle LA, Harnack LJ. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity (Silver Spring, Md). 2012. January;20(1):118–25. PubMed PMID: . Pubmed Central PMCID: PMC3402912. Epub 2011/06/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl HW III, et al. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;39(8):1267–74. [DOI] [PubMed] [Google Scholar]
- 25.Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014; 10:335–339. [DOI] [PubMed] [Google Scholar]
- 26.Zabatiero J, Hill K, Gucciardi DF, Hamdorf JM, Taylor SF, Hagger MS, Smith A. Beliefs, barriers and facilitators to physical activity in bariatric surgery candidates. Obes Surg. 2015; September 1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Coleman KJ, Raynor HR, Mueller DM, Cerny FJ, Dorn JM, Epstein LH. Providing sedentary adults with choices for meeting their walking goals. Prev Med. 1999;28(5):510–9. [DOI] [PubMed] [Google Scholar]
- 28.Kulik C, Kulik J, Bangert-Drowns R. Effectiveness of mastery learning programs: A meta-ayalsis. Rev Educ Res. 1990;60(2):265–306. [Google Scholar]
- 29.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009. November;28(6):690–701. PubMed PMID: . Epub 2009/11/18. eng. [DOI] [PubMed] [Google Scholar]
- 30.Neves LM, Fortaleza AC, Rossi FE, Diniz TA, Codogno JS, Gobbo LA, Gobbi S, Freitas Júnior IF. Functional training reduces body fat and improves functional fitness and cholesterol levels in postmenopausal women: a randomized clinical trial. J Sports Med Phys Fitness. 2015. December 18. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Muller-Riemenschneider F, Reinhold T, Nocon M, Willich SN. Long-term effectiveness of interventions promoting physical activity: a systematic review. Prev Med. 2008. October;47(4):354–68. PubMed PMID: . Epub 2008/08/05. eng. [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Papandonatos G, Fava JL, Gorin AA, Phelan S, McCaffery J, et al. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol. 2008. December;76(6):1015–21. PubMed PMID: . Pubmed Central PMCID: 2677901. Epub 2008/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. [DOI] [PubMed] [Google Scholar]
- 34.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? For adults. The international journal of behavioral nutrition and physical activity. 2011;8:79 PubMed PMID: . Pubmed Central PMCID: PMC3197470. Epub 2011/07/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]