Abstract
Haematopoietic stem cell transplantation (HSCT) is central to the management of many haematological disorders. A frequent complication of HSCT is acute graft-versus-host disease (GVHD), a condition in which immune cells from the donor attack healthy recipient tissues. The gastrointestinal system is among the most common sites affected by acute GVHD, and severe manifestations of acute GVHD of the gut portends a poor prognosis in patients after HSCT. Acute GVHD of the gastrointestinal tract presents both diagnostic and therapeutic challenges. Although the clinical manifestations are nonspecific and overlap with those of infection and drug toxicity, diagnosis is ultimately based on clinical criteria. As reliable serum biomarkers have not yet been validated outside of clinical trials, endoscopic and histopathological evaluation continue to be utilized in diagnosis. Once a diagnosis of gastrointestinal acute GVHD is established, therapy with systemic corticosteroids is typically initiated, and non-responders can be treated with a wide range of second-line therapies. In addition to treating the underlying disease, the management of complications including profuse diarrhoea, severe malnutrition and gastrointestinal bleeding is paramount. In this Review, we discuss strategies for the diagnosis and management of acute GVHD of the gastrointestinal tract as they pertain to the practising gastroenterologist.
Haematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for patients with haematological malignancies (such as leukaemia, lymphoma, myeloma and myelodysplasia) and non-malignant diseases (such as haemoglobinopathies, aplastic anaemia and immune deficiency syndromes)1. In the USA, >8,000 patients received allogeneic HSCT in 2013 (REF. 1). Before HSCT, patients commonly undergo conditioning therapy, which comprises treatment with preparative chemotherapy to eradicate diseased haematological cells, provide an immuno-suppressed environment for donor cell engraftment and prevent the rejection of haematopoietic progenitor cells from the donor, which are subsequently infused1. In autologous HSCT, donor cells are obtained from the patient, frozen and infused after conditioning chemo-therapy2. In allogeneic HSCT, donor cells are derived from a healthy donor with varying degrees of HLA matching with respect to the recipient2. Haematopoietic stem cells are most commonly obtained from the bone marrow, peripheral blood or umbilical cord blood1. The period between conditioning therapy and donor cell engraftment is typically 10–12 days and is marked by profound neutropenia, particularly in the setting of myeloablative conditioning3.
Graft-versus-host disease (GVHD) occurs when alloreactive T cells from the donor attack healthy tissues in the recipient and arises almost exclusively in patients who have undergone allogeneic HSCT3. Following allogeneic HSCT, functional reconstitution of the immune system occurs gradually over several months, during which time immunosuppressive prophylaxis of GVHD is gradually tapered if GVHD does not develop4. Patient outcomes and survival after HSCT depend upon the underlying disease as well as individual patient factors4. The two most common underlying causes of death after allogeneic HSCT are disease relapse and GVHD5.
The NIH criteria subcategorize GVHD into classic acute GVHD, which has defining features and occurs within 100 days after transplantation; persistent, recurrent or late acute GVHD, which presents with the features of acute disease but occurs more than 100 days after transplantation; classic chronic GVHD, which can occur at any time after transplantation, has defining characteristics and lacks the features of acute disease; and an overlap syndrome, which includes features of both chronic and acute GVHD6,7. The incidence of acute GVHD varies with respect to several clinical variables, with cumulative incidence rates ranging from 40–80% of HSCT recipients8. Risk factors for acute GVHD include differences in histocompatibility between donor and recipient, patient age, source of donor cells, conditioning and prophylaxis regimens used and graft manipulation techniques9. The most common tissues affected by acute GVHD are the skin, liver and gastrointestinal tract. Gastrointestinal acute GVHD is the most difficult of these conditions to treat and is the greatest cause of GVHD-related mortality9. Although reports vary, the cumulative incidence of gastrointestinal acute GVHD might be as high as 60% (REF. 8). Although hepatic manifestations are relatively less common than skin and gastrointestinal conditions, gastroenterologists might be tasked with their management, for which we refer the reader to a review of the hepatobiliary manifestations of GVHD10.
In this Review, we focus our discussion on the gastro intestinal manifestations of acute GVHD, as this condition is often the most severe and therefore poses substantial diagnostic and therapeutic challenges. For an overview of chronic GVHD and its management, we refer the reader to two review articles9,11. We discuss the aspects of gastrointestinal acute GVHD that are most pertinent to the practising gastroenterologist, including pathophysiology, endoscopic characteristics, diagnosis, risk stratification, treatment, management of complications and supportive care. Importantly, we pay special attention to the latest paradigm-shifting developments in the study of gastrointestinal acute GVHD, including the role of gut dysbiosis in pathogenesis, data regarding endoscopic diagnosis, the search for novel biomarkers and changes in staging and risk stratification.
Pathophysiology
The pathophysiology of acute GVHD is incompletely understood. Donor T cell responses and inflammatory cytokines are the most studied immune components that mediate tissue damage. Downregulation or inhibition of the recipient regulatory T (Treg) cell response and upregulation of the recipient effector T cell response, which is possibly exacerbated by treatment with immunosuppressive regimens, contributes to the lack of tolerance of allogeneic donor cells to recipient tissues12. Dysfunction in recipient antigen-presenting cells also influences immune activation in acute GVHD12. In the gut, emerging evidence suggests that the immune dysregulation observed in acute GVHD leads to further perturbation of the intestinal epithelium, specifically intestinal stem cells, Paneth cells and goblet cells13.
Imbalances in the gut microbiota are also likely to have an important role in the development of acute GVHD of the gastrointestinal tract; conditioning regimens, broad-spectrum antibiotics, immunosuppressants and the introduction of foreign lymphocytes from the donor profoundly alter the composition of the gut microbiota14–16. The degree of microbial imbalance has been shown to correlate with long-term outcomes and transplant-related mortality in gastrointestinal acute GVHD15. Moreover, Clostridium difficile has been implicated as a potential trigger of immune dysregulation that might contribute to the development of acute GVHD via local immune activation17. As the complex interplay between the immune system and the gut micro-biota is further elucidated, the intestinal bacteria might become novel therapeutic targets in the management of gastrointestinal acute GVHD. Gut dysbiosis has been observed in both acute GVHD and IBD18,19. Moreover, acute GVHD and IBD share clinical, histopathological and genetic features20,21. For example, polymorphisms in NOD2, which encodes the intracellular bacterial sensor nucleotide-binding oligomerization domain-containing protein 2 (NOD2), are associated with IBD and might also be linked with acute GVHD development in candidate gene studies20,21; however, the latter observation remains to be fully validated.
A striking consequence of the immune dysregulation observed in acute GVHD is a multifactorial disturbance of the gut mucosal barrier, which manifests with severe secretory diarrhoea22. The immune-mediated destruction of the intestinal mucosa leads to a failure of fluid resorption, particularly in the ileum, and is largely responsible for voluminous diarrhoea in patients with acute GVHD22,23. In addition, the destruction of mucosal cells at the brush border and consequent reduction in luminal levels of disaccharidase enzymes creates an osmotic effect due to the passage of unabsorbed carbohydrates22,23. Dysbiosis depletes the gut of bacterial populations, which might otherwise mitigate this effect via metabolism of these luminal polysaccharides23. Furthermore, compromise of brush border tight junctions (also known as zonulae occludentes) in the setting of a vigorous inflammatory response can lead to substantial mucosal protein loss, therefore drawing fluid into the intestinal lumen via oncotic pressure24. Moreover, inflammatory-cell-mediated destruction of apical bile salt transporters in the ileal brush border leads to bile acid malabsorption and might lead to bile salt diarrhoea25. Finally, a number of the agents that are used to prevent and treat acute GVHD and its complications are themselves cathartic agents, most notably the motilin agonist tacrolimus, mycophenolate mofetil (MMF) and magnesium salts25. In some instances, these agents might contribute to the voluminous diarrhoea that is frequently observed in acute GVHD.
Diagnosis
As discussed in this section, serological markers, radiology and endoscopy all have roles in the evaluation of patients with suspected acute GVHD of the gastrointestinal tract. Although these tools help to confirm the diagnosis or screen for alternative or coexisting pathologies, the diagnosis of acute GVHD ultimately depends on classic clinical features and the exclusion of alternative diagnoses26.
Signs and symptoms.
Acute GVHD can affect any segment of the gastrointestinal tract and manifest with nonspecific symptoms. In the oropharynx, mucositis — which is characterized by oral pain, odynophagia, anorexia, blistering, aphthae and gingivitis — might be a manifestation of either acute GVHD or the result of myeloablative conditioning therapy27,28. Conditioning regimens are the predominant cause of mucositis in the initial 2–3 weeks after HSCT, with acute GVHD becoming a common culprit thereafter28. Severe oral and oesophageal involvement might lead to bleeding, super-infection and, rarely, airway obstruction29. Although isolated acute GVHD of the oesophagus is rare, it might present with odynophagia, dysphagia or chest pain30,31. The earliest manifestations of gastroduodenal acute GVHD can be insidious and include loss of appetite, early satiety, dyspepsia and weight loss32–34. Recognizing these symptoms might offer the best chance for early intervention, which could potentially alter the clinical course of the disease. Symptoms might progress to severe nausea, incessant vomiting, epigastric pain and upper gastrointestinal bleeding32–34. Importantly, before engraftment, chemotherapy-induced toxicity and opportunistic infections can cause gastrointestinal manifesta tions similar to those of acute GVHD. In addition, it is not uncommon for drug toxicity, acute GVHD and infections to coexist35.
Acute GVHD of the lower gastrointestinal tract, which affects both the small and large intestine, manifests with abdominal pain and diarrhoea36. The occurrence of diarrhoea 2 weeks after HSCT is the most common presenting symptom of lower gastrointestinal acute GVHD; however, notably, diarrhoea can develop at any time after engraftment36. The diarrhoea is secretory, occurs independently of oral intake and can be profound and incessant, with up to several litres of output per day36. Although initially watery, the stool might turn mucoid owing to loss of transmucosal proteins and might be bloody in the setting of mucosal denudation37. Moreover, small intestinal mucosal injury and protein loss due to acute GVHD can lead to malabsorption and malnutrition38. Paradoxically, severe disease can lead to paralytic ileus, either spontaneously or as a result of analgesic and anti-motility agents such as morphine, loperamide or diphenoxylate36.
The incidence of severe gastrointestinal bleeding after HSCT has markedly declined owing to improvements in fungal, antiviral and acute GVHD prophylaxis. The cumulative incidence of gastrointestinal bleeding of any severity has been reported as 9%, and as 2% for severe gastrointestinal bleeding37,39. Although patients who have received HSCT can certainly present with causes of gastrointestinal bleeding that are not related to the transplantation (such as oesophagitis, peptic ulcer disease, angiodysplasia, diverticulosis and haemorrhoids), acute GVHD remains the most common aetiological factor40. Predisposing factors to gastrointestinal bleeding in acute GVHD include prolonged thrombocytopenia, an advanced stage of acute GVHD, the presence of thrombotic microangiopathy and secondary infection39. Importantly, severe gastrointestinal bleeding in acute GVHD is an independent predictor of mortality, with mortality approaching 40% (REF. 37).
Imaging findings.
Imaging findings in acute GVHD are nonspecific and of limited clinical utility. Nonetheless, CT scan with intravenous and oral contrast reveals abnormal mucosal enhancement of thickened bowel segments in the majority of patients with acute GVHD41,42. The distribution of bowel wall thickening in acute GVHD can be patchy or diffuse. Of note, small intestinal involvement is present in ~75% of cases, which aids in the exclusion of pathologies such as C. difficile colitis41,42. Focal thickening of the ileum and right colon wall might suggest typhlitis (also known as neutropenic enterocolitis)42. The degree of bowel wall thickening in acute GVHD is typically moderate, while severe thickening is more commonly observed in patients with a coexisting infection or typhlitis41. In rare circumstances, CT scan might also reveal pneumatosis intestinalis (intramural bowel gas) with multiple thin-walled, air-filled pockets in the colonic submucosa or subserosa43. Magnetic resonance enterography (MRE) represents an alternative method for assessing gastrointestinal GVHD, and findings are similar to those obtained using CT scan44. In addition, contrast-enhanced ultrasonography (CEUS) and PET–CT are non-invasive modalities that are currently being investigated for the assessment of gastrointestinal tract involvement in GVHD45,46.
Biomarkers.
The search is underway for effective bio-markers that anticipate a patient’s risk of developing acute GVHD, diagnose the disease at its earliest stages, estimate the prognosis and predict response to treatment. Several promising biomarkers have been identified, although none have yet been validated to guide diagnosis or treatment. Of the inflammatory bio markers that are elevated in GVHD, those with the greatest relevance to GVHD of the gastrointestinal tract are suppression of tumorigenicity 2 (ST2; also known as IL-1RL1, T1 and IL-33R), regenerating islet-derived protein 3α (REG3α), TNF receptor 1 (TNFR1) and T cell immunoglobulin and mucin domain-containing protein 3 (TIM3; also known as HAVcr-2).
The two most informative biomarkers for acute GVHD are ST2 and REG3α. ST2 is shed from activated T cells and might function as a decoy receptor for IL-33, therefore inhibiting the IL-33-dependent induction of a pro-inflammatory phenotype in T cells47. REG3α is secreted primarily by Paneth cells and functions as an antimicrobial peptide and regulator of Gram-positive bacteria in the gut47. A large study (n = 1,287) that utilized a two-biomarker signature measuring ST2 and REG3α concentrations in the blood 1 week after HSCT identified patients who were at high risk of developing lethal GVHD (~20%) and reported a cumulative incidence of 6-month non-relapse mortality (NRM) of 28% in the high-risk group compared with 7% in the low-risk group (P < 0.001)48. Compared with the low-risk group, high-risk patients had a significantly greater GVHD-related mortality (4% versus 18%, P <0.001) and risk of severe gastrointestinal acute GVHD (8% versus 17%, P <0.001). The Ann Arbor (AA) scoring system, which originally combined the plasma concentrations of three biomarkers (TNFR1, REG3α and ST2) to define three distinct risk groups at the symptomatic onset of acute GVHD, can also now be defined successfully by only two biomarkers, REG3α and ST2 (REF. 49). The AA scores identify patients who will later develop acute GVHD of the lower gastrointestinal tract but who present with only a rash. Patients who present with only skin involvement at the time of diagnosis are classified as AA3 (high risk) by their bio-marker profiles and are twice as likely to develop gastrointestinal acute GVHD compared with patients who are classified as AA1 (low risk)49. The two-biomarker AA algorithm is superior to the three-biomarker algorithm, as it can accurately identify more patients who are at low risk, which is perhaps a result of the adoption of a commercial enzyme-linked immunosorbent assay (ELISA) that has increased sensitivity for detection of ST2, the best single biomarker, compared with that used when the initial three-biomarker AA algorithm was developed48. Although promising, this algorithm is currently best used in the context of a clinical trial, as it has not yet been demonstrated that a therapeutic intervention based on the biomarker risk can change long-term outcomes. Further refinements, including an increase in the positive predictive value (perhaps through serial monitoring) will be required before the widespread adoption of biomarkers to guide clinical practice.
Of the two other serum biomarkers for gastrointestinal GVHD, levels of TNFR1 (a membrane receptor for TNF that is cleaved into a soluble form after ligand binding) also strongly correlates with the overall severity of acute GVHD, response to treatment, NRM and survival50,51. In smaller studies, high serum concentrations of TIM3, an activation marker on CD4+ T cells that regu lates macrophage function, have been shown to predict the development of severe GVHD, and high levels of ST2 and TIM3 have been correlated with 2-year NRM and overall survival in patients with GVHD52,53.
In addition, numerous inflammatory markers (such as erythrocyte sedimentation rate and C-reactive protein (CRP) levels), angiogenic markers (such as follistatin and angiopoietin 2) and microbiota-derived metabolites (such as butyrate) are under active investigation as biomarkers; however, these markers are non-specific to gastrointestinal acute GVHD54–56. In small studies, faecal calprotectin levels were demonstrated to have potential as a diagnostic tool57,58. Citrulline levels have also been shown to correlate with enterocyte damage59. Finally, serum albumin levels might be a potential prognostic biomarker, possibly as a surrogate for the degree of protein-losing enteropathy or for nutritional status22,24,60.
Endoscopic findings.
Endoscopic findings in acute GVHD vary broadly, which reflects the phases of progressive mucosal inflammation, including normal mucosa, mucosal oedema, mild erythema, mucosal erosions, superficial ulcerations, severe mucosal sloughing and mucosal denudation61,62. (FIG. 1) Studies using the magnification endoscopy with water immersion technique have demonstrated the presence of short, blunted villi in the mucosa of the duodenum and ileum and mucosal oedema, erosion, and ‘tortoiseshell-like’ mucosa in the colon63. The most commonly used system for the classification of endoscopic findings in acute GVHD is the Freiburg Criteria64 (TABLE 1). However, the diagnostic reliability of endoscopic findings is unclear; studies have reported diagnostic sensitivities and specificities of 34–89% and 65–79%, respectively, when compared with histological diagnosis64–66. In addition, some studies have shown that the concordance between the macroscopic and histological findings in acute GVHD are as low as 38.9% and that a difference of two grades or more is observed in 28% of cases64–67. Accordingly, mucosal biopsy is necessary for a reliable diagnosis, and both endoscopically normal and abnormal mucosa should be sampled68,69. It is worth reiterating that although the diagnosis of acute GVHD should be confirmed with biopsy of the affected organ or tissue when possible, the diagnosis is ultimately made on the basis of clinical findings. In addition, biopsies should not delay the management of the disease in patients who have classical clinical features of acute GVHD26,70.
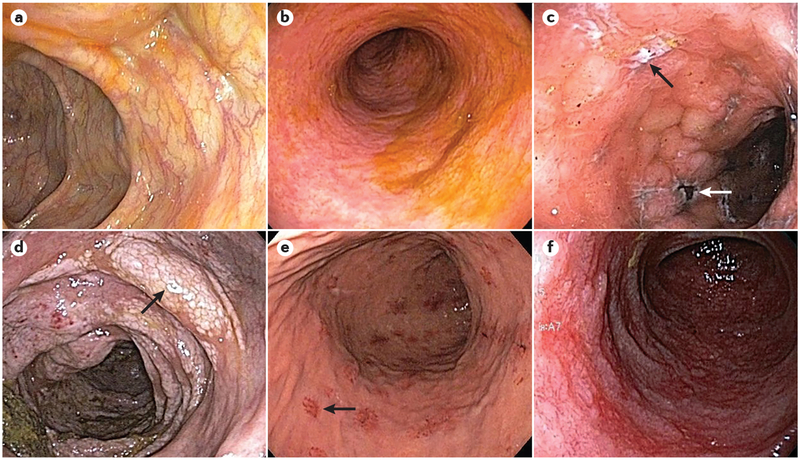
Figure 1 |. Endoscopic findings in acute GVHD of the gastrointestinal tract.
Endoscopic images depict various endoscopic findings in acute graft-versus-host disease (GVHD) of the gastrointestinal tract. a | Colonic mucosa with a normal appearance following haematopoietic stem cell transplantation (HSCT) in a patient with diarrhoea. Non-targeted biopsies revealed mild acute GVHD. b | Sigmoid colonic mucosa with mucosal oedema, loss of normal haustra and complete loss of vascularity following HSCT in a patient with diarrhoea. Biopsies revealed mild acute GVHD. c | Colonic mucosa with oedema, exudates, deep ulcers (black arrow) and areas of necrotic tissue (white arrow) in a patient following HSCT with biopsies confirming severe acute GVHD. d | Colonic mucosa with oedema, friability, loss of vascularity and white plaques (arrow) in a patient following HSCT. Biopsies revealed moderate acute GVHD and pneumatosis intestinalis. e | Upper endoscopy in a patient with nausea and epigastric pain following HSCT, showing patchy, raised erythematous lesions (arrow) as well as localized superficial mucosal erosions. Biopsies of the lesions revealed mild acute GVHD. f | Duodenal mucosa in a patient with severe epigastric pain following HSCT, revealing loss of normal plicae circulares, the presence of friable and oedematous mucosa and a complete loss of normal vascularity. Biopsies revealed severe acute GVHD.
Table 1 |.
Grading endoscopic severity in gastrointestinal acute GVHD
| Grade | Freiburg Classification for endoscopic findings64 |
|---|---|
| 1 | Normal mucosa or the absence of higher-grade findings |
| 2 | Spotted erythema or initial aphthous lesion |
| 3 | Aphthous lesions or focal erosions |
| 4 | Confluent defects, ulcerations and/or complete denudation of the mucosa |
Table from REF. 64, Macmillan Publishers Limited.
Oesophagogastroduodenoscopy (EGD) and colonos-copy are both appropriate diagnostic approaches and can be chosen based on the patient’s most prominent symptoms. One study reported diagnostic accuracies of 67–80% for EGD, 58–80% for sigmoidoscopy, 83–87% for colonoscopy, 87–100% for ileocolonoscopy and 92–93% for EGD with sigmoidoscopy64. However, several studies have reported that biopsies from the distal colon yield the highest sensitivities for detecting acute GVHD, ranging from 82% to 95% (REFS 67,71). It has also been demonstrated that very few diagnoses of isolated upper gastrointestinal acute GVHD would be missed by performing lower endoscopy alone72. Thus, owing to its diagnostic yield, safety and the non-requirement for sedation, flexible sigmoidoscopy with biopsy might be considered the initial endoscopic test of choice73,74. Notably, such an approach might not detect superimposed cytomegalo-virus infection, which might be scattered throughout the bowel or localized to the ascending colon75. In our clinical practice, we generally do not use purgatives in patients who have received HSCT and who are undergoing lower endoscopy; the volume of diarrhoea in this patient population usually allows for adequate inspection of the mucosa without the need for additional bowel cleansers. Importantly, newer data suggest that upper and lower endoscopy have similar diagnostic yields, even in patients who present with diarrhoea76,77. If EGD is performed in such patients, duodenal biopsies have a diagnostic yield higher than other sites of the upper gastrointestinal tract78. However, the endoscopist must be aware of the risk of biopsy-induced duodenal haematoma79.
Owing to the often patchy or diffuse distribution of endoscopic findings in acute GVHD in the gastrointestinal tract, a common pitfall in diagnosis is the failure to obtain biopsy samples from multiple sites. The American Society of Gastrointestinal Endoscopy (ASGE) suggests two potential approaches in patients with suspected GVHD80. The first is to obtain four biopsy samples from the rectosigmoid and the descending colon. If this method is non-diagnostic, an EGD with four biopsy samples from the gastric antrum, gastric body, duodenum and distal oesophagus is recom mended. An alternative approach is to perform ileo colonoscopy to obtain four biopsy samples from each anatomic segment of the colon80. Our recommendation (BOX 1; FIG. 2) in patients with diarrhoea and suspected acute GVHD is to perform lower endoscopy (advancing the scope as far proximally as can be safely performed), taking biopsy samples from each colonic segment and any discrete lesions. We perform EGD with biopsies from each anatomic segment in patients with a negative finding on colonoscopy and symptoms consistent with upper gastrointestinal involvement (such as dyspepsia, nausea, vomiting, epigastric pain or upper gastro intestinal bleeding). We recommend that biopsy specimens from different locations be placed in separate containers with 10% buffered formalin to enable disease localization. As with routine endoscopic biopsies, specimen orientation by the gastroenterologist is not required80.
Box 1 |. Proposed endoscopic approach in suspected acute GVHD
Numerous variables must be taken into account when considering an endoscopic evaluation in a patient following haematopoietic stem cell transplantation (HSCT). This box summarizes our recommendations for the pre-procedural and post-procedural considerations. It also provides our proposed endoscopic approach in patients after HCST who are being evaluated for gastrointestinal acute graft-versus-host disease (GVHD).
Pre-endoscopy
Optimize haemodynamics and pulmonary status
Ensure platelet count is >50 × 109/L and international normalized ratio (INR) is <1.5
No bowel preparation is necessary for lower endoscopy
No antibiotic prophylaxis is necessary
Endoscopic approach
- For diarrhoea-predominant disease:
- Lower endoscopy, advancing the scope to the furthest proximal extent as can be accomplished safely
- Biopsies of any discrete lesions and random biopsies of mucosa in each colonic segment
- If the above are non-diagnostic, consider oesophagogastroduodenoscopy (EGD) with biopsies from each anatomic segment
- If upper gastrointestinal symptoms are most prominent:
- EGD with biopsies from each anatomic segment
- If the above is non-diagnostic, consider lower endoscopy, advancing the scope to the furthest proximal extent as can be accomplished safely and obtaining biopsies accordingly
Post-endoscopy
- Clear communication between the gastroenterologist and pathologist regarding the differential diagnosis
- Assess for GVHD, superimposed cytomegalovirus infection, and so on
Monitor response to treatment and consider repeat endoscopy if there is no clinical improvement
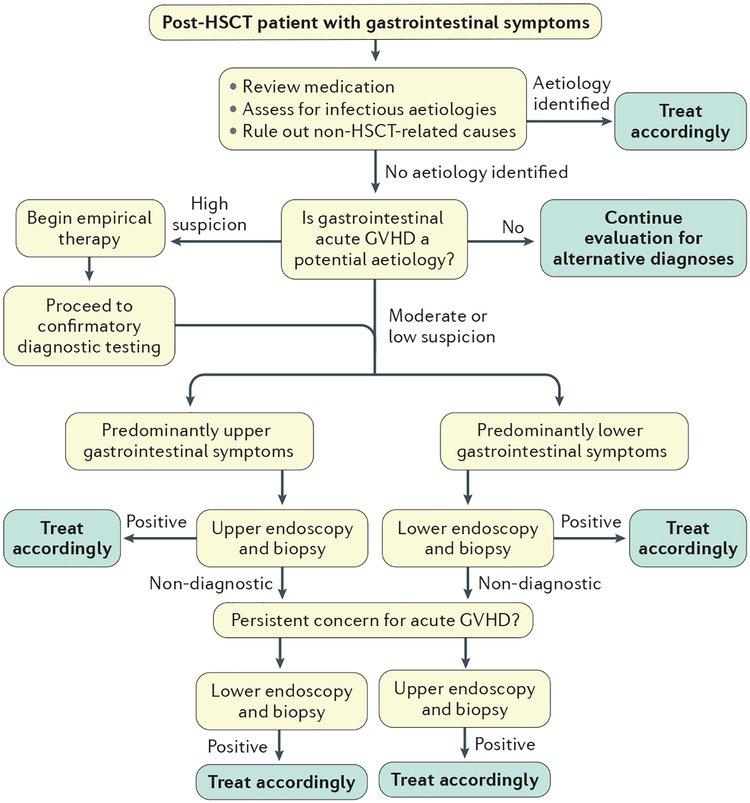
Figure 2 |. Recommended diagnostic algorithm for patients with suspected gastrointestinal acute GVHD after HSCT.
Post-haematopoietic stem cell transplantation (HSCT) patients who develop gastrointestinal symptoms must be carefully evaluated. The initial step is to review medications that might cause gastrointestinal adverse effects, assess for acute infection and rule out non-transplant-related gastrointestinal illness. If these aetiologies are excluded, graft-versus-host disease (GVHD) must be considered as a potential aetiology. If there is a high suspicion for gastrointestinal acute GVHD, empirical treatment can be initiated as the diagnostic work-up is undertaken. If there is a moderate or low suspicion for gastrointestinal acute GVHD, an endoscopic evaluation should be undertaken in accordance with the patient’s predominant symptoms (upper or lower gastrointestinal). Once a diagnosis is established, treatment should be promptly initiated. If the initial study is non-diagnostic but gastrointestinal acute GVHD is still a concern, further endoscopic evaluation should be undertaken. Once a diagnosis is established, appropriate therapy must be initiated. First-line therapy for GVHD typically includes corticosteroids.
The precise site of endoscopic biopsy is also important. In general, we recommend that biopsy of areas of sloughed mucosa should be avoided, as they are less likely to be diagnostic. In addition, biopsy of frankly ulcerated areas might be difficult for the pathologist to interpret, as they contain necrotic cells that lack specific histological features. In the stomach, there is evidence that the use of PPIs might artificially increase the number of apoptotic bodies in the antrum, but not in the fundus, which might obscure the histopathological diagnosis of acute GVHD. Thus, the acquisition of fundic biopsies should be considered in patients receiving treatment with PPIs81.
If discrete ulcers are identified during endoscopy and superimposed cytomegalovirus infection is suspected, biopsies of the ulcer base are essential owing to the cytopathic effect of cytomegalovirus in endothelial cells, ganglion cells and other mesenchymal cells82. Adequate tissue sampling is again vital, as studies have demonstrated that the sensitivity of performing three, six and ten biopsies is 80%, 90% and 99%, respectively82. Endoscopic features of herpes simplex virus (HSV) infection in the oesophagus include vesiculation and ulceration83. These ulcers tend to be multiple, well circumscribed, uniform and smaller than those observed during cytomegalovirus infection. Although HSV colitis is rare, it can mimic the endoscopic features of acute GVHD, cytomegalovirus or other forms of colitis. Endoscopic findings in HSV colitis might include mucosal oedema, erythema and well-demarcated ulcers of variable size and depth, with or without purulent exudate, that are often separated by normal-appearing mucosa84. HSV infection is more likely to be detected by sampling the edge of an ulcer, as HSV infects squamous epithelial cells83. Biopsy material for viral culture can be stored in containers with viral transport medium, and PCR can be used to test for the presence of viral pathogens85.
In patients with acute GVHD who fail to respond to first-line treatment, repeat endoscopy might be needed to re-evaluate the stage of disease, assess response to therapy and exclude alternative or superimposed diagnoses. In one study, approximately one-quarter of patients who were unresponsive to first-line acute GVHD treatment were found to have cytomegalovirus infection following repeat endoscopy86. A second study reported that 71% of patients with ongoing symptoms had histological findings on repeat endoscopy that differed from those of the initial endoscopic procedure, which led to changes in therapy in 77% of such patients with ongoing symptoms87.
Endoscopy with mucosal biopsy is generally safe in patients with acute GVHD. However, bleeding from biopsy sites and intra-mucosal haematomas in the setting of thrombocytopenia have been reported79. In a systematic review of the safety of endoscopic procedures in patients with thrombocytopenia (not exclusively with GVHD), the overall bleeding rate was found to be 1.9–2.9% (REF. 88). According to the ASGE guidelines, a platelet count >50 × 109/L is recommended before endoscopy with biopsy, and a count >20 × 109/L is recommended for diagnostic endoscopy89,90. Although data are lacking, frank perforation due to endoscopic biopsy is rare91. Finally, although patients with neutropenia after HSCT are at a higher risk of clinically relevant bacteraemia, there are insufficient data in the literature to support or oppose the use of prophylactic antibiotics before endoscopy with biopsies in patients with absolute neutrophil counts <500 cells/μl (equivalent to >0.5 × 109/L)92.
Small bowel capsule endoscopy has also been evaluated as a diagnostic tool in acute GVHD. The disease distribution identified by capsule endoscopy ranges from scattered lesions to contiguous enteritis93. Findings include normal mucosa, diffuse erythema, mucosal oedema, erosions and ulceration93. In one small trial that involved a cohort of 13 patients, capsule endoscopy showed high sensitivity (100%) and negative predictive value for diagnosis of acute GVHD94. Another study suggested that capsule endoscopy can serve as a diagnostic modality that spares the need for EGD in patients who have a high likelihood of small bowel acute GVHD and a high risk of complications from endo scopy95. Nevertheless, we recommend against using capsule endoscopy owing to the lack of specificity for acute GVHD compared with conventional endoscopy, the inability to obtain tissue biopsy samples and the potential risk of capsule retention in patients with diffuse enteritis. Another emerging modality is confocal laser endomicroscopy (CLE), which enables in vivo histo-logical assessment to be performed during endoscopy, therefore obviating the need for biopsy. Several small studies have shown that CLE has excellent sensitivity and specificity for the diagnosis of acute GVHD96,97.
Histopathological findings.
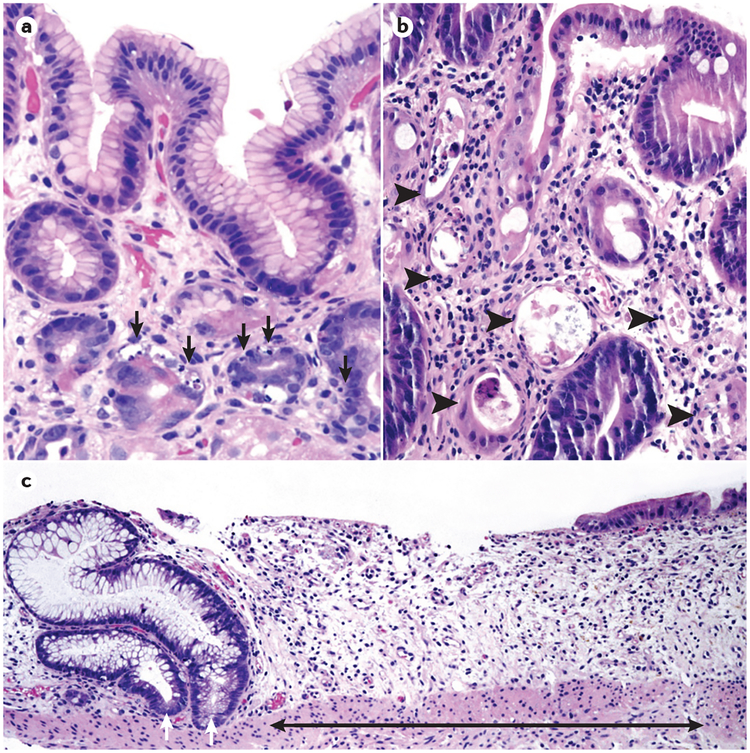
The most common histo-logical finding in acute GVHD is epithelial cell apoptosis; as intestinal stem cells are targets of the disease, the most prominent histological manifestation is apoptotic bodies in the regenerative compartment of the crypt98,99. Crypt dropout (effacement of the crypt epithelium) is more widespread in high-grade disease and involves dropout of individual crypt cells in low-grade specimens (grade 2) and entire crypts or multiple contiguous crypts in high-grade specimens (grade 3)100 (FIG. 3). The highest-grade histopathology (grade 4) demonstrates mucosal sloughing and loss of the epithelium101. In addition, the number of Paneth cells — which are primarily located next to stem cells in small intestinal crypts — inversely correlates with the risk of mortality in acute GVHD102. Accordingly, the quantification of Paneth cells on duodenal biopsy might aid in establishing the diagnosis and in prognosticating disease severity. Although high-grade histopathology, particularly grade 4, is specific for acute GVHD, it might be difficult to distinguish low-grade acute GVHD from alternative diagnoses such as medication toxicity103.
Figure 3 |. Histopathological findings in GVHD of the gastrointestinal tract.
Haematoxylin and eosin (H&E) staining of acute graft-versus-host disease (GVHD) of the gastrointestinal tract, illustrating common histopathological findings. a | Oxyntic mucosa of the stomach showing apoptotic epithelial cells in multiple crypts (black arrows, ×200). The nuclei of the apoptotic cells shrink in size, the chromatin condenses and nuclear fragmentation occurs. b | Colonic mucosa depicting multiple withered and necrotic crypts (arrow heads, ×200). c | Colonic biopsy showing areas of extensive crypt loss (black arrow). The remaining two crypts (white arrows) show reactive epithelial changes (×100).
The most commonly used histological classification for acute GVHD is a four-tiered grading system104 (TABLE 2). Grade 1 histology is identified in 90% of patients with acute GVHD, grade 2 histology in 2%, and grades 3 or 4 in 30% combined104. Importantly, a single pathological specimen might contain focal areas of varying grade, making definitive grading difficult and probably introducing some element of subjectivity to the final pathological grade assignment in these instances. However, despite well-defined grading schema, histological grade has not been shown to correlate with clinical manifestations of disease105. In addition, with the exception of grade 4 histology, which portends a poor prognosis, histological grade is not predictive of treatment response, morbidity or mortality36,105. More so, because of the patchy, diffuse distribution of GVHD, the absence of histological findings should not preclude treatment in the appropriate clinical setting.
Table 2 |.
Grading histological severity in gastrointestinal acute GVHD
| Grade | Histological classification |
|---|---|
| 1 | Isolated apoptotic epithelial cells without crypt loss |
| 2 | Crypt necrosis, withering and individual crypt loss |
| 3 | Contiguous areas of multiple crypt loss |
| 4 | Extensive crypt dropout with denudation of the epithelium |
Staging and prognosis
Several clinical staging systems have been proposed to classify the severity of acute GVHD. Historically, these have included the Glucksberg and the International Bone Marrow Transplant Registry (IBMTR) grading systems106. Both systems were updated by Przepiorka et al.107 to include skin, liver and both upper and lower gastrointestinal involvement (BOX 2). Grading is based solely on clinical features, and endoscopic, histological and radiological findings are not taken into account. Note that patients might be classified with high-grade acute GVHD if skin or liver stage is advanced, despite having either absent or minimal-stage gastrointestinal involvement.
Box 2 |. Clinical staging and grading of acute GVHD
The severity of acute graft-versus-host disease (GVHD) is classified according to the stage of individual target organ involvement and the overall grade, which takes into account the stages of each organ system. The clinical stage and grade of acute GVHD correlate with treatment response and mortality.
Clinical stage of acute GVHD
| Stage | Target organ | |||
|---|---|---|---|---|
| Skin (active erythema only) | Liver (serum total bilirubin) | Upper gastrointestinal | Lower gastrointestinal (stool output) | |
| 0 | No active (erythematous) rash | <2 mg/dL (<34.21 μmol/L) | No or intermittent nausea, vomiting or anorexia |
|
| 1 | Maculopapular rash, <25% BSA | 2–3 mg/dL (34.21–51.31 μmol/L) | Persistent nausea, vomiting or anorexia |
|
| 2 | Maculopapular rash, 25–50% BSA | 3.1–6 mg/dL (53.02–102.62 μmol/L) | – |
|
| 3 | Maculopapular rash, >50% BSA | 6.1–15 mg/dL (104.33–256.56 μmol/L) | – |
|
| 4 | Generalized erythroderma (>50% BSA), plus bullous formation and desquamation (>5% BSA) | >15 mg/dL (>256.56 μmol/L) | – | Severe abdominal pain with or without ileus or grossly bloody stool (regardless of volume) |
Overall clinical grade of acute GVHD (based upon the most severe target organ involvement)
| Grade | Criteria |
|---|---|
| 0 | No stage 1–4 of any organ involvement |
| I | Stage 1–2 skin involvement, without liver, upper gastrointestinal or lower gastrointestinal involvement |
| II | Stage 3 skin involvement and/or stage 1 liver involvement and/or stage 1 upper gastrointestinal involvement and/or stage 1 lower gastrointestinal involvement |
| III | Stage 2–3 liver involvement and/or stage 2–3 lower gastrointestinal involvement with stage 0–3 skin involvement and/or stage 0–1 upper gastrointestinal involvement |
| IV | Stage 4 skin, liver or lower gastrointestinal involvement, with stage 0–1 upper gastrointestinal involvement |
BSA, body surface area. Tables from REF. 107, Macmillan Publishers Limited.
Overall, the clinical stage and grade of acute GVHD correlates with treatment response and mortality, with high scores being associated with poor patient outcomes36,108. In a large retrospective cohort study in patients who received an allogeneic HSCT for chronic myeloid leukaemia, the transplant-related mortality for grades 0–IV acute GVHD was 28%, 27%, 43%, 68% and 92%, respectively109. Another study demonstrated a relative risk of acute GVHD-related mortality of 2.28 for grades III–IV compared with grade II disease110. In 2015, MacMillan and colleagues111 proposed a novel risk score for acute GVHD that used multiple regression analysis to stratify patients into standard-risk and high-risk groups. By use of this model, high-risk patients were shown to be much less likely to respond to initial corticosteroid therapy and had at least a twofold increased risk of mortality. The authors proposed that patients who were deemed high risk should be considered for more intensive first-line therapy than corticosteroids alone.
There are several important limitations to the current grading schemas for acute GVHD. First, the systems are not based on data from validated clinical trials. Second, they do not take into account the dynamic nature of the disease and usually reflect only maximal disease severity. Finally, they are calculated retrospectively and thus cannot be used as a prognostic tool. Some investigators have proposed that a scoring system based on an area under a receiver operating characteristic (ROC) curve model of disease activity might better reflect disease activity and enable more accurate prognostication of NRM112.
In addition to standardized staging, clinical features have been used to predict outcomes in acute GVHD. Severe gastrointestinal bleeding, hypoalbuminaemia and serum biomarker levels (including ST2, REG3α, TNFR1 and TIM3) are associated with increased mortality in acute GVHD37,60. A study demonstrated that a composite of clinical features — including jaundice, age >18 years, lack of response to corticosteroids and gastrointestinal bleeding — correlated with poor outcomes in patients with acute GVHD113. Another composite score that included timing of onset, disease severity at diagnosis and visceral organ involvement was reported to be associated with poor survival110. By use of radiography, the presence of diffuse small bowel thickening and of pneumatosis intestinalis portended a poor prognosis in patients with acute GVHD43,114.
Differential diagnosis
Given the nonspecific presentation of acute GVHD, a broad differential diagnosis should be considered when evaluating patients with gastrointestinal complaints following HSCT (TABLE 3). The timing of symptom onset is a key consideration; conditioning therapy is the most common cause of gastrointestinal symptoms in the period immediately following HSCT and is the most likely cause of diarrhoea during the first 3 weeks. Thereafter, acute GVHD becomes more common36. After engraftment, it is not unusual for both acute GVHD and conditioning therapy-induced gastrointestinal symptoms to coexist or for one to transition into the other. Diarrhoea secondary to conditioning regimens is typically self-limited and less severe than in acute GVHD91.
Table 3 |.
Differential diagnosis for acute GVHD of the gastrointestinal tract
| Aetiology | Differential diagnosis |
|---|---|
| Anorexia, dysphagia, nausea and vomiting | |
| Iatrogenic |
|
| Infectious |
|
| Inflammatory |
|
| Anatomical |
|
| Abdominal pain | |
| Iatrogenic |
|
| Infectious |
|
| Inflammatory |
|
| Hepatic, biliary, pancreatic |
|
| Vascular |
|
| Genitourinary |
|
| Diarrhoea | |
| Iatrogenic |
|
| Infectious |
|
| Other |
|
EBV, Epstein–Barr virus; HSV, herpes simplex virus; MAC, Mycobacterium avium complex; MMF, mycophenolate mofetil; VZV, varicella zoster virus.
In addition to myeloablative conditioning regimens, ongoing immunosuppressive prophylaxis can cause adverse gastrointestinal effects. Mycophenolate acid (MPA), an inhibitor of purine synthesis in lymphocytes, can cause a colitis that is difficult to distinguish from acute GVHD115. MPA is available in two formulations: the prodrug MMF and the sodium salt, enteric-coated mycophenolate sodium (EC-MPS)116. Gastrointestinal symptoms occur in nearly half of patients who receive MMF therapy115. The most frequent complaint with MMF is watery, voluminous diarrhoea that starts 2–4 weeks follow ing initiation of therapy115. Findings on endoscopy range from normal mucosa to ulceration and mucosal sloughing. Notably, rectal sparing is observed almost universally in MMF-induced colitis115. Moreover, the histology of MMF-induced colitis closely resembles that of acute GVHD; however, eosinophilia and the absence of apoptotic micro-abscesses are more common in MMF-induced colitis117. Initial treatment of MMF-induced colitis consists of dose reduction or discontinuation of the medication. Alternatively, some centres utilize EC-MPS, which might improve gastrointestinal tolerability compared with the standard formulation MMF116.
Infections account for 10–15% of cases of diarrhoea in the period following HSCT36,118, and enteric viral infections are more common than bacterial infections in this setting119. Gastrointestinal cytomegalovirus infection should be considered in all patients who receive HSCT (FIG. 4). Cytomegalovirus infection can present as diarrhoea, abdominal pain, odynophagia or fever occurring weeks to months after HSCT86. Diarrhoea is bloody in half of such cases120. Similar to acute GVHD, lesions might be found endoscopically anywhere along the gastrointestinal tract with prominent mucositis, mucosal ulceration and sloughing86. As patients with active gastrointestinal cytomegalovirus infection might demonstrate negative serum PCR results, intestinal biopsy is required for definitive diagnosis36. Owing to the fact that histological findings might mimic those of acute GVHD and that characteristic cytomegalovirus inclusions might be difficult to identify with haematoxylin and eosin (H&E) staining, immunohistochemical staining (typically antibodies targeting the cytomegalovirus tegument component pp65 or other early or immediate early cytomegalovirus antigens) or viral culture (with subsequent confirmatory PCR testing) should be performed121. Timely diagnosis and therapy are crucial, as cytomegalovirus infection is an independent predictor of mortality in patients who receive HSCT86,122. In addition, varicella zoster virus (VZV) is another possible viral pathogen that might confound the diagnosis of acute GVHD, whereby disseminated VZV infection presents with abdominal pain, diarrhoea and transaminitis that might precede dermatological manifestations121.
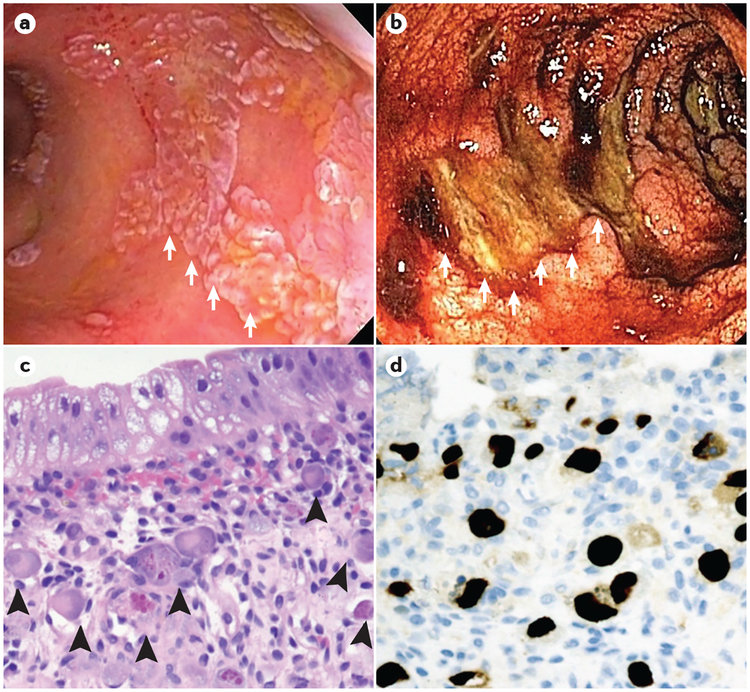
Figure 4 |. Gastrointestinal cytomegalovirus infection following HSCT.
Endoscopic and histopathological images from patients with acute graft-versus-host disease (GVHD) of the gastrointestinal tract, depicting cytomegalovirus infection in the post-haematopoietic stem cell transplantation (HSCT) setting. a | Colonoscopy in a patient following HSCT with established severe acute GVHD who is unresponsive to corticosteroids. Numerous raised white plaques (white arrows) are present throughout the colon. Biopsy samples of the lesions revealed extensive cytomegalovirus-infected cells and positive immunostaining for cytomegalovirus proteins. b | Colonoscopy in a patient following HSCT with severe acute GVHD and profuse haematochezia. Large, deep ulcers in the transverse colon (white arrows, which trace the rim of an ulcer), areas of active bleeding (asterisk) and diffusely oedematous colonic mucosa are seen. Biopsies of the mucosa revealed acute GVHD, whereas biopsies of the ulcer base stained positive for cytomegalovirus proteins by use of immunohistochemistry. c | Haematoxylin and eosin (H&E)-stained slide showing an area of a colon with crypt loss and multiple cytomegalovirus-infected endothelial cells (arrowheads, ×200). The cytomegalovirus-infected cells have large, smudgy, eccentric nuclei with prominent intranuclear and intracytoplasmic inclusions. d | Immunohistochemical staining of cytomegalovirus proteins (typically cytomegalovirus tegument component pp65) highlights cytomegalovirus-infected endothelial cells (dark brown immunostaining, ×200).
A number of non-culturable viruses can cause severe diarrhoea in post-HSCT patients123,124. These viruses, including adenovirus, astrovirus, rotavirus and noro-virus, predominate during the winter, are often health care-associated and might cause severe and prolonged illness following HSCT123,124. Upon infection with these viruses, severe diarrhoea might develop with or without upper gastrointestinal symptoms. Viral gastroenteritis and acute GVHD can sometimes coexist, and viral gastroenteritis might be predisposing to or worsen the symptoms of acute GVHD124. A growing number of reports suggest that norovirus infection can result in severe, and occasionally life-threatening, complications in patients who are immunocompromised125. Norovirus is highly transmissible, and outbreaks can spread rapidly within health care centres. PCR-based testing of stool samples is the standard confirmatory test in suspected cases of norovirus gastroenteritis. Endoscopic findings are variable and might include normal mucosa or severe erythema. Histological assessment might demonstrate villous blunting and prominent cytotoxic lymphocytosis125.
Among bacterial pathogens, C. difficile is perhaps the most common and most worrisome. The incidence of C. difficile infection among patients who receive allogeneic HSCT ranges from 12 to 27% and is associated with increased mortality17,126,127. Predisposing factors to C. difficile infection include age >60 years, allogeneic HSCT, chemotherapy before conditioning therapy, severe acute GVHD, use of broad-spectrum antimicrobials and prior vancomycin-resistant enterococci colonization126. Clinically, C. difficile infection can be indistinguishable from acute GVHD36,128. Endoscopically, pseudo-membranes are typically absent in C. difficile-infected immuno suppressed patients, making C. difficile infection more difficult to endoscopically distinguish from acute GVHD129. Various treatment guidelines recommend antibiotic therapy with oral metronidazole for mild to moderate cases of C. difficile infection, and oral vancomycin for severe cases130. Notably, these guidelines rely on severity scores that utilize white blood cell count, serum creatinine concentrations and serum albumin levels, which might be problematic in the post-HSCT population17.
Typhlitis due to Clostridium septicum infection is characterized by inflammation of the caecum and presents with fever and right lower quadrant abdominal pain 2–4 weeks following cytotoxic chemotherapy or conditioning chemotherapy, at which point neutrophil counts are at their nadir131. In contrast to acute GVHD, typhlitis most often presents before bone marrow engraftment. If typhlitis is suspected, imaging must be performed and might reveal bowel wall thickening and mucosal enhancement, predominantly in the ileocaecal region132. If detected, empirical therapy with broad-spectrum antibiotics should be initiated and surgical consultation should be considered132. Initial antibiotic coverage must be active against C. septicum, Pseudomonas aeruginosa and other colonic microbiota that might translocate through a bowel wall that has been compromised by clostridial toxins133. Typical empirical regimens include piperacillin–tazobactam monotherapy, cefepime in combination with metronidazole, or ceftazidime with metronidazole.
Cord colitis syndrome (CCS) is a proposed clinical entity that presents as a persistent diarrhoeal illness in ~10% of patients who receive cord blood transplantation and causes a granulomatous inflammation of the upper and lower gastrointestinal tract134. CCS is considered a diagnosis of exclusion once acute GVHD and demonstrable infectious aetiologies have been ruled out134. Although the syndrome is responsive to antibiotics, a causative pathogen has not been definitively identified135. CCS most often presents several months following cord blood transplantation with watery diarrhoea and fever. Colonoscopy reveals erythematous mucosa with or without ulceration, and biopsy demonstrates chronic active colitis, frequently with associated granulomas136. This clinical entity remains controversial, as studies have suggested that CCS is not a histopathologically distinct disease from acute GVHD137,138.
Management
Once alternative causes of post-HSCT gastro intestinal symptoms have been thoroughly considered and excluded and a diagnosis of acute GVHD has been established, prompt treatment should be initiated. Following HSCT, patients should receive GVHD prophylaxis, and those who later develop GVHD should be assessed for the adequacy of their prophylactic regimen, with adjustment as required. First-line treatment regimens consist of systemic and/or oral non-absorbable corticosteroids. Unfortunately, non-response to first-line treatment is common, and data regarding the effectiveness of various second-line treatment modalities is limited. Supportive care throughout the duration of illness is of critical importance and focuses on symptomatic management139.
Prophylaxis of acute GVHD.
Prophylaxis of acute GVHD is based upon suppression of the engrafted cytotoxic T cell-mediated immune response. Factors considered in selecting a prophylactic regimen include the underlying malignancy, the chemotherapy regimen used for conditioning, the extent of HLA discordance between the donor and recipient, the susceptibility of the patient to infection and the desire of the physician to balance graft immunosuppression with the desired graft-versus-tumour effect140. A common prophylactic regimen consists of a course of methotrexate combined with a prolonged tapering dose of a calcineurin inhibitor141,142. Among patients who receive less aggressive conditioning regimens, methotrexate might be replaced with MMF or EC-MPS (to avoid methotrexate-related toxicity)143,144. Patients who receive an unrelated donor HSCT or an HLA‑mismatched transplantation are at greater risk of developing acute GVHD, and such patients can undergo T cell depletion or post-transplantation treatment with cyclophosphamide, which might reduce the incidence of acute GVHD145,146.
Treatment of acute GVHD.
The initial step in the management of acute GVHD is the evaluation of the patient’s prophylactic regimen147. Among patients who discontinue prophylaxis, the prophylactic regimen can be restarted; in patients still receiving prophylaxis, drug levels can be optimized via dosage increase if serum drug levels are low.
The mainstay first-line treatment strategy for gastrointestinal acute GVHD comprises corticosteroids147. A common starting regimen with systemic corticosteroids is prednisone or methylprednisolone at doses of 1–2 mg/kg per day in divided doses (typically every 12 hours). For patients with less severe disease (grade II), initial treatment with a lower dose of prednisone (1 mg/kg/day) has also been shown to be effective148. Conversely, corticosteroid doses >2 mg/kg/day do not provide additional therapeutic benefit across grades of severity149. At any grade of severity, concomitant use of oral non-absorbable corticosteroids, such as budesonide or beclomethasone, might improve response rates and also helps to moderate the dose of systemic cortico steroid therapy150,151. For patients with mild to moderate disease severity, monotherapy with oral beclomethasone dipropionate is a reasonable initial therapeutic approach152. In patients with mild to moderate colonic acute GVHD, we have also prescribed budesonide MMX (Cosmo Pharmaceuticals NV), an oral formulation that uses a multi-matrix system to extend budesonide release throughout the colon, which has been studied in patients with ulcerative colitis153. Corticosteroids are generally continued for several weeks and slowly tapered to prevent relapse154. Patients who demonstrate disease progression at day 5 of treatment, or no improvement by day 7, are deemed steroid-refractory and are likely to require second-line agents147,154.
A substantial proportion of patients with acute GVHD fail to respond to first-line corticosteroids. In a study from 2015, 31% of standard-risk patients and 57% of high-risk patients were steroid-refractory.111 There are also data showing that patients with lower gastrointestinal involvement are at particularly high risk of non-response155. Unfortunately, there is an insufficient amount of quality data to guide the management of patients who fail to respond to corticosteroids, and their outcomes are generally poor139. Several second-line therapies are currently available to treat patients who are steroid-refractory, including lymphocyte-depleting agents, cell cycle inhibitors, antimetabolites, TNF antagonists, inhibitors of leukocyte trafficking, antibodies directed against various interleukins, extra corporeal photopheresis and others139. A detailed discussion of second-line therapies is beyond the scope of this article, and we direct the reader to a relevant review139.
Supportive care and management of gastrointestinal complications in acute GVHD.
In addition to immunosuppressive therapies, treatment of acute GVHD includes symptomatic management of nausea, vomiting, mucositis, dysphagia, diarrhoea, abdominal pain, bleeding and malnutrition. Patients with severe disease might be placed on bowel rest (‘nil per os’ (NPO)) to prevent exacerbation of symptoms. However, oral intake should be resumed as soon as the patient can tolerate it to prevent gut atrophy. Nausea secondary to upper gastro intestinal acute GVHD might be initially managed with 5-hydroxytryptamine 3 (5-HT3) receptor antagonists156. Low-dose corticosteroids are also effectively used as prophylactics157. Other agents for symptomatic management include phenothiazines, metoclopramide, lorazepam, haloperidol and dronabinol. Aprepitant, a neurokinin 1 receptor antagonist, is effective for symptomatic management; however, it should be noted that aprepitant interacts with various immunosuppressants, as it is a substrate and a moderate inhibitor of human cytochrome P450 3A4, which has a crucial role in the hepatic metabolism of calcineurin inhibitors158.
Oropharyngeal mucositis, dysphagia and odynophagia can lead to anorexia and malnutrition; supportive measures include the use of topical anaesthetics. Viscous lidocaine is included in formulations known as ‘magic mouthwashes’, which contain components such as antibiotics, antifungals, antihistamines, steroids and coating agents. Saline solution has been shown to be as effective as a ‘magic mouthwash’ for analgesia and healing of mucositis159. In addition, antiseptic mouth rinses such as chlorhexidine gluconate aid in the prophylaxis of oral superinfection but might also exacerbate symptoms of mucositis. For symptoms of oesophagitis, the mainstay of therapy is PPIs158. Although coating agents such as sucralfate are often used in conjunction with PPIs, there is little evidence to support their efficacy, and there is concern that they might decrease the absorption of other medications; thus, they are not recommended by expert guidelines160.
Chronic abdominal pain related to acute GVHD is difficult to treat. The pain is attributed to inflammation, oedema, increased mucosal friability and ileus161. The use of NSAIDs and paracetamol might be limited by bleeding and hepatotoxicity risks, respectively. Therefore, opioid analgesics (morphine, hydro morphone and so on) are often necessary. Opioids should be used with caution in patients with acute GVHD, as they can further impair neuromuscular activity, hinder gut propulsion and inadvertently exacerbate pain162. In an effort to combat these adverse effects, the μ-opioid receptor antagonist methylnaltrexone might be used pre-emptively163. Alternatively, buprenorphine, a μ-opioid receptor partial agonist and a κ-opioid receptor and δ-opioid receptor antagonist, might offer analgesia with less frequent and less severe adverse gastrointestinal effects164. Finally, non-narcotic analgesics such as GABA analogues, serotonin–noradrenaline reuptake inhibitors and tri-cyclic anti depressants might have a role as adjuncts for neuropathic gastrointestinal pain162.
The management of diarrhoea is one of the greatest challenges in acute GVHD (TABLE 4). Anti-diarrhoeal agents should be initiated after infection is excluded, as decreasing stool output might delay clearance of pathogenic bacteria and their associated toxins, thus lengthening the disease course165. Loperamide is the initial anti-diarrhoeal agent of choice, but if ineffective, octreotide or tincture of opium might be used and titrated to elicit the desired response166. The combination of atropine and diphenoxylate is a commonly used anti-motility agent; however, it must be used with caution, as it can provoke ileus (pseudo-obstruction)167. A trial of pancreatic enzyme supplementation might also be considered, as there is evidence that acute GVHD can cause pancreatic insufficiency168,169. The addition of a bile-acid-binding resin (known as a bile acid sequestrant) might also be beneficial168,169; however, such agents should be used judiciously, as they can interfere with the absorption of other medications. Stool output must be measured diligently, and anti-motility agents should be tapered as soon as diarrhoea begins to subside to prevent the development of ileus59.
Table 4 |.
Management of diarrhoea in gastrointestinal acute GVHD
| Medication | Dosing |
|---|---|
| Loperamide |
|
| Diphenoxylate–atropine |
|
| Octreotide |
|
| Tincture of opium |
|
| Pancrelipase |
|
| Cholestyramine |
|
Patients who receive HSCT commonly develop macronutrient and micronutrient deficiencies secondary to profound gastrointestinal symptoms, systemic inflammation, protein-losing enteropathy and medication toxicity59. Malnutrition and hypoalbuminaemia are well-established factors associated with poor prognosis and thus must be addressed60. Patients should receive formal nutritional evaluations to determine their need for supplemental enteral and/or parenteral nutrition. Oral feeding is preferable in order to maintain both intestinal function and the mucosal barrier and concomitantly avoids the potential complications of total parenteral nutrition (TPN)170. However, TPN might be the only available option for patients with severe diarrhoea and an inability to tolerate sufficient oral intake. If TPN is initiated, an early re-introduction of oral intake leads to improved nutritional outcomes171.
Gastrointestinal bleeding secondary to acute GVHD typically results from diffuse mucosal oozing of blood and is rarely amenable to endoscopic intervention158. Nonetheless, endoscopic evaluation is warranted to exclude lesions that might be treatable. The mainstay of therapy for acute GVHD-related haemorrhage include supportive therapy to correct thrombocytopenia and coagulopathy, acid suppression and treatment of GVHD158. Continuous infusion of octreotide has been studied for the treatment of GVHD-related gastrointestinal bleeding, but its efficacy is lost when the drug is discontinued172. In the setting of ongoing bleeding that cannot be managed with conservative approaches, cases of successful arterial embolization have been reported173. Another technique is intra-arterial platelet transfusion174. Surgical management is typically not feasible, given the diffuse nature of the disease and the high surgical risk of these patients.
Conclusions
Although the pathophysiology of acute GVHD remains to be fully elucidated, acute GVHD seems to be largely a result of dysregulated cell-mediated immunity, which prompts an aggressive cytotoxic response by donor lymphocytes. Emerging evidence suggests that imbalances in the gut microbiota probably also have an important role in the development of acute GVHD of the gastrointestinal tract. Although serological markers, radiology and endoscopy all have important roles in the evaluation of patients with gastrointestinal acute GVHD, the diagnosis ultimately depends on identification of classical clinical features and the exclusion of alternative diagnoses. Given the nonspecific presentation of acute GVHD, a broad differential diagnosis should be considered and should include the effects of myeloablative conditioning regimens, ongoing immunosuppressive prophylaxis, viral infection and bacterial infection (particularly with C. difficile). Endoscopic findings might vary broadly and reflect the phases of progressive mucosal inflammation. Although the diagnosis of acute GVHD should be confirmed using tissue biopsy when possible, the diagnosis is ultimately made on the basis of clinical findings, and biopsies should not delay the management of the disease in patients who present with classic clinical features. Biopsy samples from the distal colon yield the highest sensitivities for detecting acute GVHD and should be obtained from multiple sites given the typically patchy, diffuse nature of the disease. The histological grade of biopsy specimens has not been shown to correlate with clinical manifestations of disease and is not predictive of treatment response, morbidity or mortality. Several novel biomarkers have been identified that might help anticipate the risk of developing acute GVHD, diagnose the disease at earlier stages, estimate the prognosis and predict response to treatment; however, to date, none have been integrated into routine clinical practice. First-line treatment regimens for acute GVHD consist of corticosteroids; higher dose corticosteroids are not superior to more moderate dosages, and the use of oral, non-absorbable corticosteroids can help to further reduce systemic dose requirements. Failure to respond to first-line treatment is common and should prompt the re-evaluation of the patient for possible alternative or concomitant diagnoses. Although a number of second-line treatments are available, outcomes among corticosteroid non-responders remains dismal.
Accordingly, acute GVHD of the gastrointestinal tract is a major challenge in the clinical management of patients with haematological malignancies who receive HSCT. In spite of ongoing advances, GVHD remains a common occurrence at centres that perform HSCT. Severe gastrointestinal acute GVHD has a poor prognosis and can be catastrophic if it is not expediently diagnosed and treated. Although the diagnosis and treatment of acute GVHD generally falls within the realm of the haematologist and oncologist, the involvement of the gut necessitates the consultation of a gastroenterologist. Gastroenterologists have a central role in both diagnosis and risk stratification of acute GVHD of the gut via endoscopy and tissue biopsy. They also manage gastro intestinal complications, including gastro intestinal bleeds, and can guide aspects of supportive care such as treatment of diarrhoea and malnutrition. A collaborative, multidisciplinary approach involving haematologists, gastroenterologists and pathologists is most likely to lead to improved outcomes in the vulnerable population of patients with acute GVHD of the gut.
Allogeneic HSCT
Transplantation in which a patient receives haematopoietic stem cells from a genetically similar, but not identical, donor.
Autologous HSCT
Transplantation in which a patient’s haematopoietic stem cells are harvested, stored for the duration of the patient’s conditioning regimen, and later returned to that same patient for re-engraftment.
Myeloablative conditioning
The complete or near complete depletion of native bone marrow cells via the administration of cytotoxic chemotherapy such as cyclophosphamide, often along with radiation therapy.
Oesophagogastroduodenoscopy
A diagnostic endoscopic procedure used to visualize, and sometimes intervene upon, regions of the upper gastrointestinal tract, down to the level of the duodenum.
Small bowel capsule endoscopy
A procedure in which a capsule containing a wireless camera is ingested by a patient and used to visualize areas of the small bowel that are difficult to access using conventional endoscopy.
Unrelated donor HSCT
A type of allogeneic transplant in which the donor is not related to the patient.
HLA-mismatched transplantation
A type of allogeneic transplantation in which the HLA typing of the donor and recipient are not identical.
Key points.
Acute graft-versus-host disease (GVHD) of the gastrointestinal tract is a common complication in patients after haematopoietic stem cell transplantation (HSCT) that results in considerable morbidity and mortality
As the clinical, serological and radiographical findings in gastrointestinal acute GVHD are nonspecific, a broad differential diagnosis should be considered, particularly potential infectious causes and chemotherapeutic or immunosuppressant toxicity
Expedient endoscopy and histopathology are helpful in excluding possible conditions that mimic gastrointestinal acute GVHD; nevertheless, the diagnosis is ultimately based on clinical criteria
Several novel diagnostic, prognostic, risk and predictive biomarkers have been identified for gastrointestinal acute GVHD; however, none have yet been integrated into routine clinical practice
Upon diagnosis of gastrointestinal acute GVHD, timely first-line therapy with systemic corticosteroids (such as prednisone or methylprednisolone) and/or oral non-absorbable corticosteroids (such as beclomethasone or budesonide) is crucial
Acute GVHD leads to substantial gastrointestinal symptom burden, including profuse diarrhoea, abdominal pain, severe malnutrition and gastrointestinal bleeding; providing supportive and palliative care is a critical role of the gastroenterologist
Footnotes
Competing interests statement
J.F. and J.L. declare that they have a patent application for a biomarker array discussed in this Review (Title: Method of predicting graft versus host disease. Provisional Application No: 62/411,230; Filing Date: 10/21/16). The other authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gratwohl A et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 303, 1617–1624 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Forman SJ, Negrin RS & Antin JH (eds) Thomas’ Hematopoietic Cell Transplantation 5th Edn Ch. 83 (Wiley-Blackwell, 2016). [Google Scholar]
- 3.Seggewiss R & Einsele H Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 115, 3861–3868 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Barrett AJ & Battiwalla M Relapse after allogeneic stem cell transplantation. Expert Rev. Hematol 3, 429–441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 110, 3784–3792 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipovich AH et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant 11, 945–956 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Jagasia MH et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant 21, 389–401.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AC et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant 22, 4–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socie G & Ritz J Current issues in chronic graft-versus-host disease. Blood 124, 374–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald GB Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology 51, 1450–1460 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke KR et al. The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant 23, 211–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeiser R et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108, 390–399 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teshima T, Reddy P & Zeiser R Acute graft-versus-host disease: novel biological insights. Biol. Blood Marrow Transplant 22, 11–16 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Shono Y, Docampo MD, Peled JU, Perobelli SM & Jenq RR Intestinal microbiota-related effects on graft-versus-host disease. Int. J. Hematol 101, 428–437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taur Y et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shono Y et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl Med 8, 339ra71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso CD & Marr KA Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr. Opin. Infect. Dis 26, 326–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi J & Chang EB The gut microbiota and inflammatory bowel diseases. Transl Res. 179, 38–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peled JU, Hanash AM & Jenq RR Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood 128, 2395–2402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holler E, Landfried K, Meier J, Hausmann M & Rogler G The role of bacteria and pattern recognition receptors in GVHD. Int. J. Inflam 2010, 814326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting C, Alterovitz G, Merlob A & Abdi R Genomic studies of GVHD-lessons learned thus far. Bone Marrow Transplant. 48, 4–9 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Weisdorf SA, Salati LM, Longsdorf JA, Ramsay NK & Sharp HL Graft-versus-host disease of the intestine: a protein losing enteropathy characterized by fecal alpha 1-antitrypsin. Gastroenterology 85, 1076–1081 (1983). [PubMed] [Google Scholar]
- 23.Zeiser R, Socie G & Blazar BR Pathogenesis of acute graft-versus-host disease: from intestinal microbiota alterations to donor T cell activation. Br. J. Haematol 175, 191–207 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Rezvani AR et al. Decreased serum albumin as a biomarker for severe acute graft-versus-host disease after reduced-intensity allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant 17, 1594–1601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruutu T et al. Improved survival with ursodeoxycholic acid prophylaxis in allogeneic stem cell transplantation: long-term follow-up of a randomized study. Biol. Blood Marrow Transplant 20, 135–138 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Dignan FL et al. Diagnosis and management of acute graft-versus-host disease. Br. J. Haematol 158, 30–45 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Schubert MM & Sullivan KM Recognition, incidence, and management of oral graft-versus-host disease. NCI Monogr 135–143 (1990). [PubMed] [Google Scholar]
- 28.Chaudhry HM et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol. Blood Marrow Transplant 22, 605–616 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Vera-Llonch M, Oster G, Ford CM, Lu J & Sonis S Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support. Care Cancer 15, 491–496 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Trabulo D et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-versus-host disease. World J. Gastroenterol 21, 9217–9222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald GB, Sullivan KM, Schuffler MD, Shulman HM & Thomas ED Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology 80, 914–921 (1981). [PubMed] [Google Scholar]
- 32.Yeh SP et al. Gastric bleeding due to graft-versus-host disease: discrepancy between endoscopic and histologic assessment. Am. J. Clin. Pathol 122, 919–925 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Weisdorf DJ et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood 76, 624–629 (1990). [PubMed] [Google Scholar]
- 34.Wu D et al. Persistent nausea and anorexia after marrow transplantation: a prospective study of 78 patients. Transplantation 66, 1319–1324 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Wakui M et al. Prospective evaluation for upper gastrointestinal tract acute graft-versus-host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 23, 573–578 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Cox GJ et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology 107, 1398–1407 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Schwartz JM et al. Severe gastrointestinal bleeding after hematopoietic cell transplantation, 1987–1997: incidence, causes, and outcome. Am. J. Gastroenterol 96, 385–393 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Joshi NM et al. Bile acid malabsorption in patients with graft-versus-host disease of the gastrointestinal tract. Br. J. Haematol 157, 403–407 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Labrador J et al. Incidence and risk factors for life-threatening bleeding after allogeneic stem cell transplant. Br. J. Haematol 169, 719–725 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Spencer GD, Shulman HM, Myerson D, Thomas ED & McDonald GB Diffuse intestinal ulceration after marrow transplantation: a clinicopathologic study of 13 patients. Hum. Pathol 17, 621–633 (1986). [DOI] [PubMed] [Google Scholar]
- 41.Mahgerefteh SY et al. Radiologic imaging and intervention for gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. Radiology 258, 660–671 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick ID & Greenberg HM Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology 226, 668–674 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Hepgur M et al. Medical management of pneumatosis intestinalis in patients undergoing allogeneic blood and marrow transplantation. Bone Marrow Transplant. 46, 876–879 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Budjan J et al. Assessment of acute intestinal graft versus host disease by abdominal magnetic resonance imaging at 3 Tesla. Eur. Radiol 24, 1835–1844 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Benedetti E et al. Prospective qualitative and quantitative non-invasive evaluation of intestinal acute GVHD by contrast-enhanced ultrasound sonography. Bone Marrow Transplant. 48, 1421–1428 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Bodet-Milin C et al. 18F-FDG PET/CT for the assessment of gastrointestinal GVHD: results of a pilot study. Bone Marrow Transplant. 49, 131–137 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Ferrara JL et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 118, 6702–6708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartwell MJ et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2, e89798 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine JE et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2, e21–e29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YB & Cutler CS Biomarkers for acute GVHD: can we predict the unpredictable? Bone Marrow Transplant. 48, 755–760 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Paczesny S Discovery and validation of graft-versus-host disease biomarkers. Blood 121, 585–594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald GB et al. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood 126, 113–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu Zaid M et al. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood 129, 162–170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apostolova P & Zeiser R The role of purine metabolites as DAMPs in acute graft-versus-host disease. Front. Immunol 7, 439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holtan SG et al. Circulating angiogenic factors associated with response and survival in patients with acute graft-versus-host disease: results from Blood and Marrow Transplant Clinical Trials Network 0302 and 0802. Biol. Blood Marrow Transplant 21, 1029–1036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathewson ND et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol 17, 505–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenz F et al. Fecal calprotectin as a biomarker of intestinal graft versus host disease after allogeneic hematopoietic stem cell transplantation. Sci. Rep 5, 7920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adam B et al. Endoscopic and histological findings are predicted by fecal calprotectin in acute intestinal graft-versus-host-disease. Dig. Dis. Sci 61, 2019–2026 (2016). [DOI] [PubMed] [Google Scholar]
- 59.van der Meij BS et al. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant. 48, 474–482 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Ayuk F et al. Serum albumin level predicts survival of patients with gastrointestinal acute graft-versus-host disease after allogeneic stem cell transplantation. Ann. Hematol 93, 855–861 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Ponec RJ, Hackman RC & McDonald GB Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest. Endosc 49, 612–621 (1999). [DOI] [PubMed] [Google Scholar]
- 62.ASGE Standards of Practice Committee et al. The role of endoscopy in the management of patients with diarrhea. Gastrointest. Endosc 71, 887–892 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Hiejima E et al. Diagnostic accuracy of endoscopic features of pediatric acute gastrointestinal graft-versus-host disease. Dig. Endosc 28, 548–555 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Kreisel W et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 47, 430–438 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velasco-Guardado A et al. Endoscopic evaluation and histological findings in graft-versus-host disease. Rev. Esp. Enferm. Dig 104, 310–314 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Cruz-Correa M et al. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy 34, 808–813 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Thompson B, Salzman D, Steinhauer J, Lazenby AJ & Wilcox CM Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 38, 371–376 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Terdiman JP et al. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy 28, 680–685 (1996). [DOI] [PubMed] [Google Scholar]
- 69.Khan K et al. Diagnostic endoscopy in children after hematopoietic stem cell transplantation. Gastrointest. Endosc 64, 379–385 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Martin PJ et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant 18, 1150–1163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross WA et al. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am. J. Gastroenterol 103, 982–989 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Ma C, Maluf HM & Liu TC Acute graft-versus-host disease is more prevalent and severe in the lower than the upper gastrointestinal tract. Hum. Pathol 46, 1480–1487 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Crowell KR et al. Endoscopy in the diagnosis of intestinal graft-versus-host disease: is lower endoscopy with biopsy as effective in diagnosis as upper endoscopy combined with lower endoscopy? Pediatr. Blood Cancer 60, 1798–1800 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Lee KJ et al. Stepwise endoscopy based on sigmoidoscopy in evaluating pediatric graft-versus-host disease. Pediatr. Gastroenterol. Hepatol. Nutr 19, 29–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kakugawa Y, Fukuda T & Saito Y Cautionary note on using rectosigmoid biopsies to diagnose graft-versus-host disease: necessity of ruling out cytomegalovirus colitis. Am. J. Gastroenterol 103, 2959–2960 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Cloutier J, Wall DA, Paulsen K & Bernstein CN Upper versus lower endoscopy in the diagnosis of graft-versus-host disease. J. Clin. Gastroenterol 51, 701–706 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Wild D et al. The diagnostic yield of site and symptom-based biopsies for acute gastrointestinal graft-versus-host disease: a 5-year retrospective review. Dig. Dis. Sci 61, 806–813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ip S, Marquez V, Schaeffer DF & Donnellan F Sensitivities of biopsy sites in the endoscopic evaluation of graft-versus-host disease: retrospective review from a tertiary center. Dig. Dis. Sci 61, 2351–2356 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Sierra A et al. Biopsy-induced duodenal hematoma is not an infrequent complication favored by bone marrow transplantation. J. Pediatr. Gastroenterol. Nutr 63, 627–632 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Committee, A. S.o. P. et al. Endoscopic mucosal tissue sampling. Gastrointest. Endosc 78, 216–224 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Welch DC et al. Gastric graft-versus-host disease revisited: does proton pump inhibitor therapy affect endoscopic gastric biopsy interpretation? Am. J. Surg. Pathol 30, 444–449 (2006). [DOI] [PubMed] [Google Scholar]
- 82.DiMarino AJ & Benjamin SB Gastrointestinal disease: an endoscopic approach (Slack, 2002). [Google Scholar]
- 83.Bhaijee F, Subramony C, Tang SJ & Pepper DJ Human immunodeficiency virus-associated gastrointestinal disease: common endoscopic biopsy diagnoses. Patholog. Res. Int 2011, 247923 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phadke VK, Friedman-Moraco RJ, Quigley BC, Farris AB & Norvell JP Concomitant herpes simplex virus colitis and hepatitis in a man with ulcerative colitis: case report and review of the literature. Medicine (Baltimore) 95, e5082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arai Y et al. Usefulness of quantitative PCR in biopsy specimens for early therapeutic intervention in gastrointestinal cytomegalovirus infections after allogeneic stem cell transplantation. Rinsho Ketsueki 55, 2400–2407 (in Japanese) (2014). [DOI] [PubMed] [Google Scholar]
- 86.Bhutani D et al. Incidence, risk factors, and outcome of cytomegalovirus viremia and gastroenteritis in patients with gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant 21, 159–164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez C et al. Serial intestinal endoscopic examinations of patients with persistent diarrhea after allo-SCT. Bone Marrow Transplant. 47, 694–699 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Tong MC, Tadros M & Vaziri H Endoscopy in neutropenic and/or thrombocytopenic patients. World J. Gastroenterol 21, 13166–13176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu CF, Zhu LX, Xu XM, Chen WC & Wu DP Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J. Gastroenterol 14, 2262–2267 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.ASGE Standards of Practice Committee et al. Adverse events of upper GI endoscopy. Gastrointest. Endosc 76, 707–718 (2012). [DOI] [PubMed] [Google Scholar]
- 91.Feldman M, Friedman LS & Brandt LJ Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management 10th Edn Ch. 41 (Saunders, 2016). [Google Scholar]
- 92.ASGE Standards of Practice Committee et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc 81, 81–89 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Malard F & Mohty M New insight for the diagnosis of gastrointestinal acute graft-versus-host disease. Mediators Inflamm. 2014, 701013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neumann S et al. Wireless capsule endoscopy for diagnosis of acute intestinal graft-versus-host disease. Gastrointest. Endosc 65, 403–409 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Yakoub-Agha I et al. Impact of small bowel exploration using video-capsule endoscopy in the-management of acute gastrointestinal graft-versus-host disease. Transplantation 78, 1697–1701 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Bojarski C et al. In vivo diagnosis of acute intestinal graft-versus-host disease by confocal endomicroscopy. Endoscopy 41, 433–438 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Coron E et al. Early detection of acute graft-versus-host disease by wireless capsule endoscopy and probe-based confocal laser endomicroscopy: results of a pilot study. United European Gastroenterol. J 2, 206–215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sale GE, Shulman HM, McDonald GB & Thomas ED Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am. J. Surg. Pathol 3, 291–299 (1979). [DOI] [PubMed] [Google Scholar]
- 99.Washington K & Jagasia M Pathology of graft-versus-host disease in the gastrointestinal tract. Hum. Pathol 40, 909–917 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Shidham VB et al. Colon biopsies for evaluation of acute graft-versus-host disease (A-GVHD) in allogeneic bone marrow transplant patients. BMC Gastroenterol. 3, 5 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fenoglio-Preiser CM Gastrointestinal pathology: an atlas and text (Wolters Kluwer/Lippincott Williams & Wilkins, 2008). [Google Scholar]
- 102.Levine JE et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood 122, 1505–1509 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin J, Fan R, Zhao Z, Cummings OW & Chen S Is the presence of 6 or fewer crypt apoptotic bodies sufficient for diagnosis of graft versus host disease? A decade of experience at a single institution. Am. J. Surg. Pathol 37, 539–547 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Lerner KG et al. Histopathology of graft-versus-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant. Proc 6, 367–371 (1974). [PubMed] [Google Scholar]
- 105.Massi D et al. The impact of histopathologic examination of graft-versus-host disease in the era of reduced-intensity conditioning regimen: a study from the Gruppo Italiano Trapianto di Midollo Osseo. Hum. Pathol 42, 254–268 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Rowlings PA et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br. J. Haematol 97, 855–864 (1997). [DOI] [PubMed] [Google Scholar]
- 107.Przepiorka D et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 15, 825–828 (1995). [PubMed] [Google Scholar]
- 108.Goyal RK, Goyal M & Sankaranarayan K Grading acute graft-versus-host disease: time to reconsider. Pediatr. Transplant 19, 252–254 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Gratwohl A et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantion. Blood 86, 813–818 (1995). [PubMed] [Google Scholar]
- 110.Lee SE et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 48, 587–592 (2013). [DOI] [PubMed] [Google Scholar]
- 111.MacMillan ML et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol. Blood Marrow Transplant 21, 761–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leisenring WM et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood 108, 749–755 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castilla-Llorente C et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 49, 966–971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimoni A et al. CT in the clinical and prognostic evaluation of acute graft-versus-host disease of the gastrointestinal tract. Br. J. Radiol 85, e416–e423 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J & Bhamidimarri KR Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann. Gastroenterol 28, 366–373 (2015). [PMC free article] [PubMed] [Google Scholar]
- 116.Ortega F et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation 92, 426–432 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Star KV, Ho VT, Wang HH & Odze RD Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am. J. Surg. Pathol 37, 1319–1328 (2013). [DOI] [PubMed] [Google Scholar]
- 118.Kamboj M, Mihu CN, Sepkowitz K, Kernan NA & Papanicolaou GA Work-up for infectious diarrhea after allogeneic hematopoietic stem cell transplantation: single specimen testing results in cost savings without compromising diagnostic yield. Transpl. Infect. Dis 9, 265–269 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Bowden RA, Ljungman P & Snydman DR Transplant infections (Wolters Kluwer Health/Lippincott Williams & Wilkins, 2010). [Google Scholar]
- 120.Harano Y, Kotajima L & Arioka H Case of cytomegalovirus colitis in an immunocompetent patient: a rare cause of abdominal pain and diarrhea in the elderly. Int. J. Gen. Med 8, 97–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yagi T et al. Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: importance of empiric therapy with acyclovir. Bone Marrow Transplant. 25, 1003–1005 (2000). [DOI] [PubMed] [Google Scholar]
- 122.Cho BS et al. Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy. Ann. Hematol 92, 497–504 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Ghosh N, Malik FA, Daver RG, Vanichanan J & Okhuysen PC Viral associated diarrhea in immunocompromised and cancer patients at a large comprehensive cancer center: a 10-year retrospective study. Infect. Dis. (Lond.) 49, 113–119 (2016). [DOI] [PubMed] [Google Scholar]
- 124.van Montfrans J et al. Viral PCR positivity in stool before allogeneic hematopoietic cell transplantation is strongly associated with acute intestinal graft-versus-host disease. Biol. Blood Marrow Transplant 21, 772–774 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Schwartz S et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117, 5850–5856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trifilio SM, Pi J & Mehta J Changing epidemiology of Clostridium difficile-associated disease during stem cell transplantation. Biol. Blood Marrow Transplant 19, 405–409 (2013). [DOI] [PubMed] [Google Scholar]
- 127.Chakrabarti S, Lees A, Jones SG & Milligan DW Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transplant. 26, 871–876 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Collini PJ, Bauer M, Kuijper E & Dockrell DH Clostridium difficile infection in HIV-seropositive individuals and transplant recipients. J. Infect 64, 131–147 (2012). [DOI] [PubMed] [Google Scholar]
- 129.Nomura K et al. Absence of pseudomembranes in Clostridium difficile-associated diarrhea in patients using immunosuppression agents. Scand. J. Gastroenterol 44, 74–78 (2009). [DOI] [PubMed] [Google Scholar]
- 130.Surawicz CM et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol 108, 478–498 (2013). [DOI] [PubMed] [Google Scholar]
- 131.King A, Rampling A, Wight DG & Warren RE Neutropenic enterocolitis due to Clostridium septicum infection. J. Clin. Pathol 37, 335–343 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Machado NO Neutropenic enterocolitis: a continuing medical and surgical challenge. N. Am. J. Med. Sci 2, 293–300 (2010). [PMC free article] [PubMed] [Google Scholar]
- 133.Nesher L & Rolston KV Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin. Infect. Dis 56, 711–717 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Gupta NK & Masia R Cord colitis syndrome: a cause of granulomatous inflammation in the upper and lower gastrointestinal tract. Am. J. Surg. Pathol 37, 1109–1113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhatt AS et al. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N. Engl. J. Med 369, 517–528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herrera AF et al. Cord colitis syndrome in cord-blood stem-cell transplantation. N. Engl. J. Med 365, 815–824 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Shimoji S et al. Evaluating the association between histological manifestations of cord colitis syndrome with GVHD. Bone Marrow Transplant. 48, 1249–1252 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Milano F et al. Late-onset colitis after cord blood transplantation is consistent with graft-versus-host disease: results of a blinded histopathological review. Biol. Blood Marrow Transplant 20, 1008–1013 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Renteria AS, Levine JE & Ferrara JLM Therapeutic targets and emerging treatment options in gastrointestinal acute graft-versus-host disease. Expert Opin. Orphan Drugs 4, 469–484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruutu T et al. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 47, 1459–1464 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Inamoto Y et al. A retrospective comparison of tacrolimus versus cyclosporine with methotrexate for immunosuppression after allogeneic hematopoietic cell transplantation with mobilized blood cells. Biol. Blood Marrow Transplant 17, 1088–1092 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ringden O et al. Decreased incidence of graft-versus-host disease and improved survival with methotrexate combined with cyclosporin compared with monotherapy in recipients of bone marrow from donors other than HLA identical siblings. Bone Marrow Transplant. 9, 19–25 (1992). [PubMed] [Google Scholar]
- 143.Perkins J et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol. Blood Marrow Transplant 16, 937–947 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Weber T, Niestadtkotter J, Wienke A, Muller-Tidow C & Muller LP Enteric-coated mycophenolate sodium containing GvHD prophylaxis reduces GvHD rate after allogeneic HSCT. Eur. J. Haematol 97, 232–238 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Theurich S et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst. Rev 9, CD009159 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Klein OR et al. Alternative-donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for nonmalignant disorders. Biol. Blood Marrow Transplant 22, 895–901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruutu T et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 49, 168–173 (2014). [DOI] [PubMed] [Google Scholar]
- 148.Mielcarek M et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica 100, 842–848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Lint MT et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood 92, 2288–2293 (1998). [PubMed] [Google Scholar]
- 150.McDonald GB et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology 115, 28–35 (1998). [DOI] [PubMed] [Google Scholar]
- 151.Hockenbery DM et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood 109, 4557–4563 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Castilla C et al. Oral beclomethasone dipropionate for the treatment of gastrointestinal acute graft-versus-host disease (GVHD). Biol. Blood Marrow Transplant. 12, 936–941 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Sandborn WJ et al. Once-daily budesonide MMX(R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 143, 1218–1226.e2 (2012). [DOI] [PubMed] [Google Scholar]
- 154.Deeg HJ How I treat refractory acute GVHD. Blood 109, 4119–4126 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.MacMillan ML et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol. Blood Marrow Transplant 8, 387–394 (2002). [DOI] [PubMed] [Google Scholar]
- 156.Trigg ME & Inverso DM Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: a review of antiemetic regimens. Bone Marrow Transplant. 42, 501–506 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Navari RM & Aapro M Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N. Engl. J. Med 374, 1356–1367 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Tuncer HH, Rana N, Milani C, Darko A & Al-Homsi SA Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J. Gastroenterol 18, 1851–1860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dodd MJ et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol Endod 90, 39–47 (2000). [DOI] [PubMed] [Google Scholar]
- 160.Lalla RV et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120, 1453–1461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McDonald GB How I treat acute graft-versus-host disease of the gastrointestinal tract and the liver. Blood 127, 1544–1550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lauro A, De Giorgio R & Pinna AD Advancement in the clinical management of intestinal pseudo-obstruction. Expert Rev. Gastroenterol. Hepatol 9, 197–208 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Thomas J et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N. Engl. J. Med 358, 2332–2343 (2008). [DOI] [PubMed] [Google Scholar]
- 164.Khanna IK & Pillarisetti S Buprenorphinean attractive opioid with underutilized potential in treatment of chronic pain. J. Pain Res 8, 859–870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DuPont HL & Hornick RB Adverse effect of lomotil therapy in shigellosis. JAMA 226, 1525–1528 (1973). [PubMed] [Google Scholar]
- 166.Ippoliti C et al. Use of octreotide in the symptomatic management of diarrhea induced by graft-versus-host disease in patients with hematologic malignancies. J. Clin. Oncol 15, 3350–3354 (1997). [DOI] [PubMed] [Google Scholar]
- 167.Beatson N Atropine and paralytic ileus. Postgrad. Med. J 58, 451–453 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Akpek G, Valladares JL, Lee L, Margolis J & Vogelsang GB Pancreatic insufficiency in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 27, 163–166 (2001). [DOI] [PubMed] [Google Scholar]
- 169.Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ & Booth IW Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch. Dis. Child 75, 208–213 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guieze R et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin. Nutr 33, 533–538 (2014). [DOI] [PubMed] [Google Scholar]
- 171.Imataki O et al. Nutritional support for patients suffering from intestinal graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Am. J. Hematol 81, 747–752 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Ohashi K et al. Use of octreotide in the management of severe duodenal bleeding after unrelated-donor bone marrow transplantation. Int. J. Hematol 78, 176–177 (2003). [DOI] [PubMed] [Google Scholar]
- 173.Chen RZ, Zhao G, Jin N, Chen BA & Ding JH Superselective arterial embolization of the superior mesenteric artery for the treatment of gastrointestinal hemorrhage following allogeneic hematopoietic stem cell transplantation. Patient Prefer. Adherence 8, 1581–1585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kably IM, Ziga ED & Andreansky M Intra-arterial platelet infusion for intractable hemorrhage and refractory thrombocytopenia in children with gastrointestinal graft-versus-host disease. Pediatr. Blood Cancer 62, 2226–2228 (2015). [DOI] [PubMed] [Google Scholar]