Abstract
Adding rapamycin or acarbose to diet at 9-10 months of age has been shown to significantly increase life span in both male and female UM-HET3 mice. The current study examined cochleae of male and female UM-HET3 mice at 22 months of age to determine if either treatment also influenced age-related loss of cochlear hair cells. A large loss of cochlear outer hair cells was observed at 22 months of age in untreated mice in both apical and basal halves of the cochlear spiral. Addition of acarbose to diet had no significant effect on the amount of outer hair cell loss at 22 months of age or in its pattern, with large loss in both apical and basal halves. The addition of rapamycin to diet, however, significantly reduced outer hair cell loss in the basal half of the cochlea at 22 months of age when compared to untreated mice. There was no significant difference between male and female mice in any of the conditions. Age-related outer hair cell loss in the apical cochlea precedes outer hair cell loss in the base in many mouse strains. The results of the present study suggest that rapamycin but not acarbose treatment can delay age-related loss of outer hair cells at doses at which each drug increases life span.
Keywords: rapamycin, acarbose, aging, cochlea, age-related hearing loss, auditory
1. Introduction
Age-related hearing loss (ARHL) is the most prevalent form of hearing loss in humans, affecting close to a third of people over the age of 65, with 63% percent of male and 48% of female adults between 70 and 79 years of age affected (Gates and Mills, 2005). A decreased ability to communicate reduces quality of life, increases the risks from other age-related changes and illnesses and contributes to the incidence of depression and dementia (e.g. Davis and Smith, 2013; Gates et al., 2010).
The present study took advantage of the availability of cochleae of 22 month old mice from the Paul F. Glenn Center for Biology of Aging Research at the University of Michigan. The National Institute on Aging (NIA) Intervention Testing Program (ITP) identifies and tests drugs with the potential to increase life span and delay aging-induced disorders using the UM-HET3 mouse model (e.g. Nadon et al., 2017) and the UM Glenn Center follows the same protocol used by the ITP. Drugs are added to diet at 4 months of age. Rapamycin and acarbose are two of the most successful interventions that have been identified. Rapamycin extended life span by 26% in male mice and 23% in females (Miller et al., 2014) and acarbose extended life span by 19% in male mice and 5% in females (Harrison et al., 2014; Strong et al., 2016). Rapamycin acts on mammalian-target-of-rapamycin (mTOR) pathways. These pathways are multifaceted and act on other functional signaling pathways including those associated with metabolism, proliferation, immune response and cell survival (Wataya-Kaneda, 2015; Perl, 2015 for reviews). Acarbose inhibits digestion of polysaccharides and reduces uptake of sugar in the gut (Joshi et al., 2015; Derosa & Maffioli, 2012 for reviews). Acarbose may mimic some influences of caloric restriction (CR) but may also have different influences, since acarbose effects on body weight are more dramatic in females while the effects on lifespan are much larger in males and differences in effects of CR versus acarbose on gene expression have been noted (Strong et al., 2016). CR has been shown to reduce/delay age-related hearing loss with variable effects across different animal models and mouse strains (Willott et al. 1995 for review).
Loss of cochlear outer hair cells (OHCs) is a component of age-related hearing loss in people and across multiple animal models (Nelson and Hinojosa, 2006; Gates and Mills, 2005; Ohlemiller, 2009; Frisina, 2009 for reviews). In most mouse strains OHC loss appears earliest in the apical portion of the cochlear spiral (low frequencies) and then appears in the more basal portion (high frequencies) and finally progresses to mid cochlea, while in people OHC loss occurs first in basal cochlea, then apical cochlea and finally mid-cochlear portions (Frisina, 2009; Nelson and Hinojosa, 2006; Ohlemiller 2009; Gates and Mills, 2005 for reviews). The present study compared cochlear OHC loss in 22 month old untreated UM-HET3 mice to that in age-matched littermate controls with rapamycin or acarbose added to diet at 4 months of age as well as to untreated 4 month old young controls.
The UM-HET3 mice used in the NIA ITP studies are a four-way cross that provides genetic heterogeneity in the progeny and reduces the potential for strain-specific influences on results. However, three of the UM-HET3 grandparent strains are homozygous for the Cdh23753A variant at the ahl (age-related hearing loss) locus that results in a genetically recessive susceptibility to early onset, progressive deafness and substantial hair cell loss in young adult mice (Noben-Trauth et al., 2003; Johnson et al., 1997, 2006; Willott and Erway, 1998). This study therefore tested the UM-HET3 mice for this variant and analyzed cochleae only from progeny that carried the variant in heterozygous form and were thus resistant to early onset hearing loss.
2. Methods
2.1. Approvals
This study was reviewed and approved by the Institutional Animal Care & Use Committee at the University of Michigan, which is fully accredited by AAALAC International. All procedures conformed to the National Research Council’s Guidelines for the Care and Use of Laboratory Animals: Eighth Edition.
2.2. Mice
UMHET3 mice were produced as the progeny of (BALB/cByJ × C57BL/6J) F1 dams and (C3H/HeJ × DBA/2) F1 sires. They were weaned at 19 – 21 days of age, and housed in groups of 3 males or 4 females, with free access to Purina 5LG6 food and water, with other husbandry details as in the ITP longevity protocols (e.g. Harrison et al., 2009; Miller et al., 2014; Strong et al., 2016). At four months of age, randomly selected cages were switched to a diet containing either encapsulated rapamycin at a dose of 14.7 parts per million (ppm = mg drug per kg chow) or acarbose at 1000 ppm. Other cages remained on the control diet (5LG6) until the mice were euthanized at 22 months.
2.3. Euthanization
Mice were euthanized by decapitation at 22 months of age. Left cochleae were removed and received gentle intrascalar perfusion of fixative (4% paraformaldehyde in phosphate buffer) followed by immersion in fixative overnight at room temperature. A piece of the tail was also removed and frozen.
2.4. Genetic Assessment
Genomic DNA was prepared from tail biopsies of UM-HET3 mice using a Puregene Tissue Kit (Qiagen). Genotypes at the ahl locus were determined by PCR amplification of genomic DNA using primers derived from sequences flanking exon 7 of Cdh23 (5’-AAAAGCCTGCAGCATTAGGA-3’; 5’-ATATGCGTGGGTGTTCACAA-3’). The 606 bp products were purified using a QIAquick PCR Purification Kit (Qiagen), digested with excess MspI restriction enzyme, and fragments were separated by agarose gel electrophoresis. The ahl resistant allele (c.753G) introduces an MspI recognition site that produces 405 and 200 bp fragments upon digestion, while the ahl sensitive allele (c.753A) lacks any MspI site.
2.5. Hair Cell Counts
Cochleae from the left side were further processed for hair cell assessment. After the removal of the otic capsule, tectorial membrane and lateral wall, the remaining tissue was permeabilized with 0.3% Triton X in phosphate buffered saline (PBS) for 30 minutes followed by three rinses in PBS. Cochlear tissues were then labeled with Phalloidin Alexafluor 568 (Life Technologies) diluted 1:100 in PBS followed by three rinses in PBS in the dark. The cochlear epithelium was further trimmed and then microdissected into three segments, apex, base and hook, and each segment was mounted separately as a surface preparation on a glass slide with Fluoromount (EMS Inc.) and covered with a coverslip. Slides were then stored at 4°C until examination.
Phalloidin labeling of hair cells was used to identify presence or absence of hair cells (Figure 1). Hair cells were counted under epifluorescence optics on a Leica fluorescent microscope using a 50x objective and a 0.19 mm reticule in the microscope eyepiece, under double blind conditions. The number of inner hair cells (IHC) and outer hair cells (OHC) that were present or absent for each 0.19 mm reticule length was entered into a cytocochleogram program (developed in house e.g. Lemke et al., 2009, Piu et al., 2011) starting at the apex and moving basally until the entire length of the cochlear spiral had been assessed. The program compares hair cell numbers to a normal data base. The program can generate a graph of hair cell loss by position along the cochlear spiral for each cochlea (cytocochleogram) (e.g. Figure 2) and can also provide the analysis in absolute numbers or as the total percent of hair cells lost (e.g. Figures 3 and 4) in each animal assessed. The loss of OHCs in the basal half of the cochleae was compared for significant differences between 22 month old groups of mice with no treatment versus treatment with rapamycin or acarbose using the non-parametric Mann-Whitney test with Bonferroni adjustment.
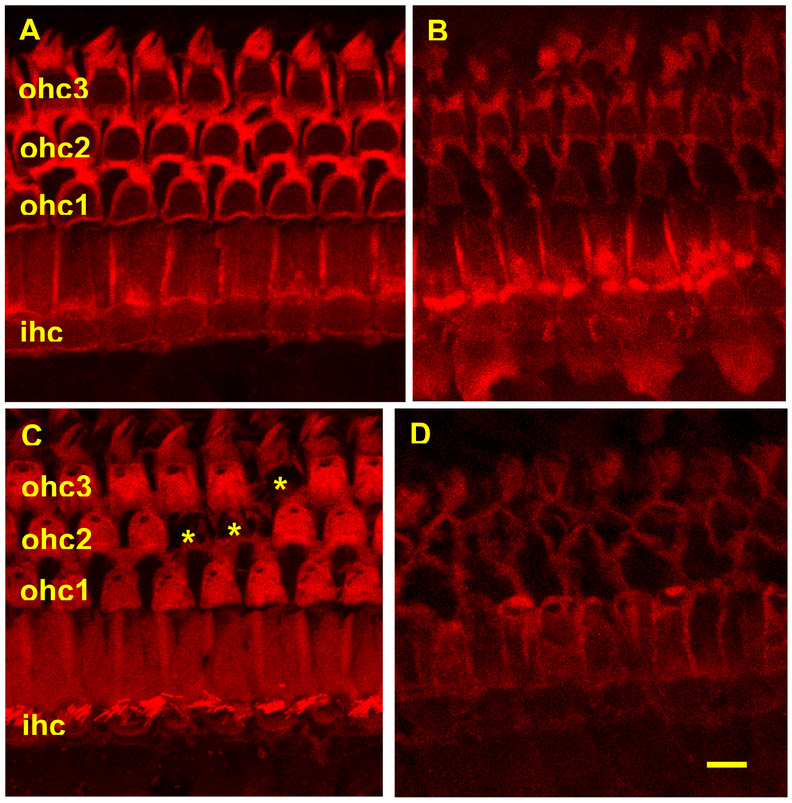
Figure 1:
Photomicrographs showing images of basal cochlea representative of four conditions: (A) At four months of age (no treatment) there are three rows (ohc 1, 2, 3) of phalloidin labeled (red chromophore) outer hair cells (OHC) without any OHC loss and one row of labeled inner hair cells (ihc) with no loss; (B) At twenty two months of age without any treatments, there is large loss of inner and out hair cells; (C) At twenty two months of age with rapamycin added to diet at 4 months of age there is small loss of outer hair cells (asterisks show loss of one OHC in row 3 and loss of two OHCs in row 2); (1D) At twenty two months of age with acarbose added to diet at 4 months of age there is large loss of inner and out hair cells. Scale bar = 10 microns
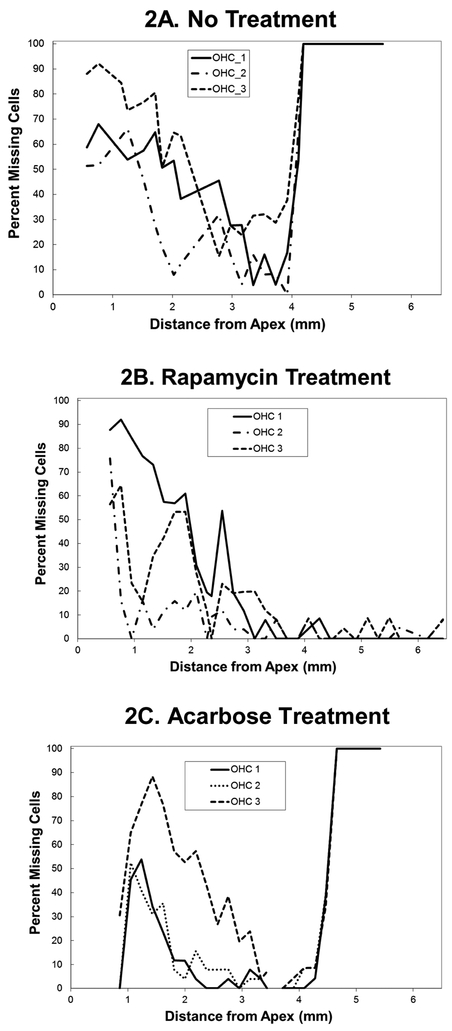
Figure 2:
Cytochleograms comparing age-related outer hair cell loss in representative 22 month old mice that were either (2A) untreated; (2B) treated with rapamycin added to diet at 4 months of age; or (2C) treated with rapamycin added to diet at 4 months of age. Apical cochlea is to the left and base to the right, the transition from apical turn to basal turn is approximately 1.75 mm from the apex and the transition from basal turn to the hook is at approximately 3.9 mm from apex. There is large loss of outer hair cells in the basal half of the cochlea (including hook) in the untreated and acarbose treated rats at twenty two months of age. In the rat with rapamycin added to diet at four months of age there is only minor outer hair cell loss in the basal half of the cochlea. There is large outer hair cell loss at twenty two months of age in the apical cochlea in all three conditions
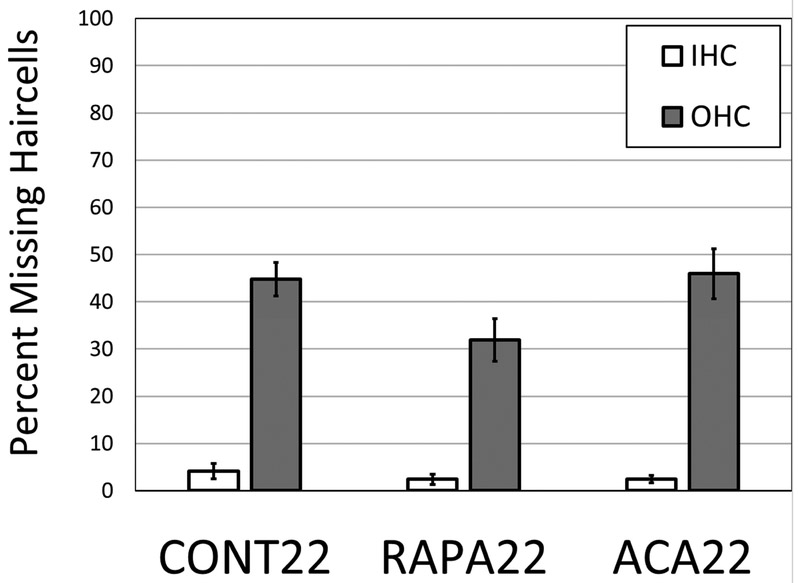
Figure 3:
A comparison of the mean percent loss of outer hair cells (OHC) and inner hair cells (IHC) in all turns (apex, base and hook) of the cochlear spiral in the group of mice at twenty two months of age without any treatments (CONT22); in the group of mice at twenty two months of age with rapamycin added to diet at 4 months of age (RAPA22) and in the group of mice at twenty two months of age with acarbose added to diet at 4 months of age (ACA22). There is a significant difference in mean OHC loss between the control (untreated) and rapamycin treated groups (p <0.01).
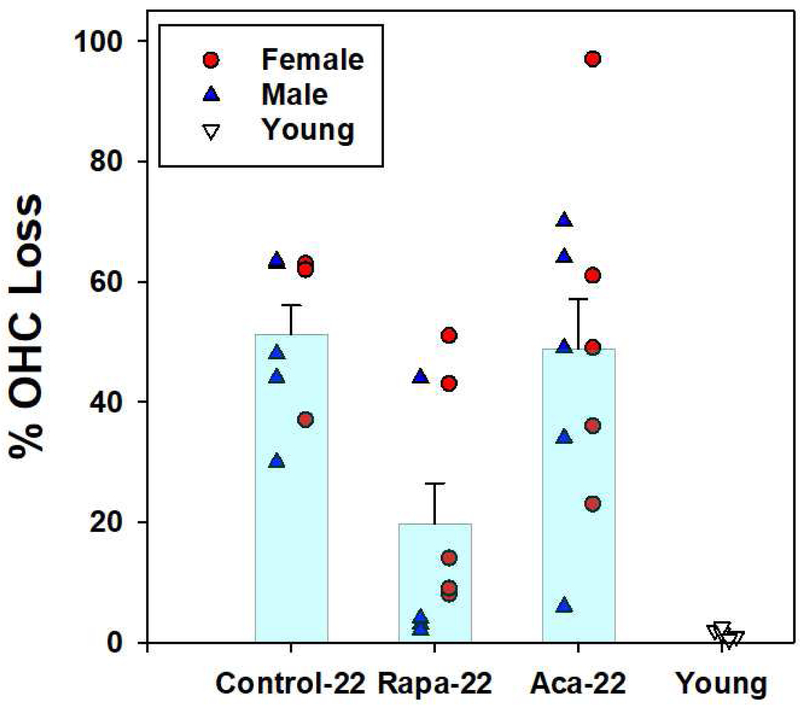
Figure 4:
Scatter plot showing the mean percent loss of outer hair cells in the basal half of the cochlear spiral in the group of mice at twenty two months of age without any treatments (Control-22); in the group of mice at twenty two months of age with rapamycin added to diet at 4 months of age (Rapa-22); in the group of mice at twenty two months of age with acarbose added to diet at 4 months of age (Aca-22) and in the group of mice at 4 months of age (Young). Mice at 22 months of age are divided into males (blue triangles) and females (red circles). Young (4 month old) mice are not divided and shown as unfilled triangles. Error bars show standard error of the mean. There is a significant difference between control (untreated) and rapamycin treated groups (p <0.01).
3. RESULTS
3.1. Genetic Sorting based on ahl genotyping
Three of the four UM-HET3 grandparental strains (BALB/cByJ, C57BL/6J, and DBA/2J) are homozygous for the ahl-susceptible Cdh23753A variant, while the fourth strain (C3H/HeJ) is homozygous for the ahl-resistant Cdh23753G variant (Noben-Trauth et al., 2003). UM-HET3 progeny are therefore expected to be either homozygous (Cdh23753A/A) and at increased risk of early onset, progressive hair cell loss and deafness, or heterozygous (Cdh23753G/A) and relatively resistant to the early onset deficits. Consistent with this expectation, all evaluated homozygous Cdh23753A/A mice exhibited over 90% OHC loss across all turns by 22 months of age regardless of treatment or sex (data not shown). We therefore included in the following evaluations of treated and control UM-HET3 mice only those that carried the resistant genotype (Cdh23753G/A).
3.2. Hair Cell Loss - Untreated
There was little or no hair cell loss found in cochleae from untreated mice at four months of age (Figures 1A, 2-4). Cochleae from eight untreated 22 mos. old mice were assessed (5 male and 3 female). There were large OHC losses in both base and apex in all eight mice (Figures 1B and 2A show representative examples). There was a similar pattern of hair cell loss in males and females (Figure 4). There was a mean OHC loss of 44.75 +/−10.09 (standard deviation) across all turns of the cochlear spiral. There was a mean OHC loss of 51.3 +/−13.4% (standard deviation) in the basal half (including hook) of the cochlea, ranging from 30.4 to 63.4% (Figure 3). There was small IHC loss across turns, averaging 4.11 +/− 4.68%.
3.3. Hair Cell Loss - Rapamycin treatment
Cochleae were assessed from nine 22 mos. old rapamycin treated mice (5 males and 4 females). There was a mean OHC loss of 31.91 +/− 14.3 across all turns of the cochlear spiral (Figure 3). The rapamycin treated mice all showed large OHC loss in the apical half of the cochleae, comparable to that in untreated mice (representative example in Figure 1C). In the basal half of the cochleae, however, six (3 male and 3 female) of the nine rapamycin treated mice had only minimal OHC loss (6.6 +/− 3.9%) ranging from 2.4 to 13.9% (Figures 2C and 4). This was only slightly elevated from the less than 1% loss in normal (untreated) 4 month old mice. The other three rapamycin treated mice (2 male and 1 female) had basal OHC loss between 43.4 and 50.6% (mean of 46.1 +/− 3.9%), close to that seen in untreated 22 month old mice. When all nine rapamycin treated mice were combined the mean OHC loss in the basal half of the cochleae was 19.7 +/− 20.1%, significantly (p <0.01) reduced from mean of 51.3% in the untreated 22 mos. old mice (Figure 4). There was a similar pattern of hair cell loss in males and females (Figure 3). There was no significant difference in the pattern or amount of IHC loss compared to untreated mice.
3.4. Hair Cell Loss - Acarbose treatment
Cochleae were assessed from ten acarbose treated mice (5 males and 5 females). There was a mean OHC loss of 45.93 +/−16.73 across all turns of the cochlear spiral, not significantly different from the loss in untreated 22 mos. old mice Figure 3). There was wide variation in basal OHC loss in the acarbose group (Figure 4) and no significant treatment effect in nine of these ten mice (Figures 1D and 2C for a representative examples), with large OHC loss in both base and apex. When all ten acarbose treated mice were combined, there was a basal half OHC loss of 48.8 +/− 25.9%, similar to the 51.3% loss in untreated 22 month old mice (Figure 4). There was a similar pattern of hair cell loss in males and females (Figure 3). There was no significant difference in the pattern or amount of IHC loss compared to untreated mice.
4. DISCUSSION
The results of the study show that rapamycin significantly decreased age-related OHC loss in the basal half of the cochleae of 22 month old UM-HET3 mice, comparing treated and untreated mice that carried the resistant ahl genotype (Cdh23753G/A). There was large OHC loss in the apical half of the cochleae in both treated and untreated mice. Since age-related apical OHC loss precedes that in base in most mouse strains (e.g. Altschuler et al., 2015; Frisina, 2009; Frisina & Zhu, 2010; Ohlemiller, 2009; Sha et al., 2008; Sergeyenko et al., 2013) this suggests that rapamycin treatment is delaying basal OHC loss. If so, loss in basal portion of the cochlea could then appear at late ages and eventually catch up to the loss in untreated mice. It is possible that rapamycin delays OHC loss in the apical cochlea, which could be observed if younger ages are assessed; future studies would be needed to address this. The effect in base versus apex could also reflect differences in age-related changes in base and apex or differences in the sensitivity of basal versus apical OHC to age-related changes and stresses. This would have implications to treating people where ARHL occurs first in the basal cochlea. It would be useful in future studies to add the functional assessments that were not possible under the present study conditions in which specimens from each mouse were shared among multiple groups of investigators. It would also be valuable to examine the effects of rapamycin treatment on other components of ARHL, such as loss of inner hair cell – auditory nerve synaptic connections and spiral ganglion neurons, changes in gap detection and temporal processing, and to examine other central auditory system components of ARHL.
It is interesting that while both rapamycin and acarbose treatments have been shown to increase lifespan in UM-HET3 mice (Miller et al., 2014; Harrison et al., 2014; Strong et al., 2016) that rapamycin but not acarbose delayed / reduced age-related outer hair cell loss. This suggests differences in underlying mechanisms and that not all lifespan extending treatments will necessarily have the same benefit on hearing. The treatment effect of rapamycin is likely to involve inhibition of mTOR, but it is not possible at present to determine if preservation of function in basal OHC reflects direct effects on the OHC themselves, or alterations in other supporting cell types of the cochlea, or potentially system wide effects on metabolic or inflammatory pathways. It would also be interesting to compare rapamycin induced changes in molecular signaling pathways between mice showing treatment effect compared to those in which a treatment effect on OHC in basal cochlea is not seen.
Highlights.
Adding rapamycin to diet at 4 months decreased hair cell loss in 22 month old mice
Decreased age-related hair cell loss with rapamycin was in basal half of the cochlea
Adding acarbose to diet did not decrease hair cell loss in 22 month old mice
Acknowledgements
These studies were supported by the Paul F. Glenn Center for Biology of Aging Research at the University of Michigan; NIH NIA grants AG022303 and AG024824 (RAM); VA Merit grant (RAA) and NIH NIDCD grants P30 DC005188 and T32 DC000011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altschuler RA, Dolan DF, Halsey K, Kanicki A, Deng N, Martin C, Eberle J, Kohrman D, Miller RA, Schacht J (2015) Age-related Changes in Auditory Nerve – Inner Hair Cell Connections, Hair Cell Numbers, Auditory Brain Stem Response and Gap Detection in UM-HET4 Mice, Neuroscience 292:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, Smith P (2013) Adult hearing screening: health policy issues--what happens next? Am J Audiol. 22(1):167–70. PMID: [DOI] [PubMed] [Google Scholar]
- Frisina RD (2009) Age-related hearing loss: ear and brain mechanisms. Ann N Y Acad Sci. 1170:708–17 [DOI] [PubMed] [Google Scholar]
- Frisina RD, Zhu X. (2010) Auditory sensitivity and the outer hair cell system in the CBA mouse model of age-related hearing loss. Open Access Anim Physiol. 2:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA. (2006) The effect of noise on cochlear aging. Ear Hear. 27(1):91. [DOI] [PubMed] [Google Scholar]
- Gates GA, Gibbons LE, McCurry SM, Crane PK, Feeney MP, Larson EB. (2010) Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cogn Behav Neurol. 23(4):218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. , Miller RA. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K. (2006) Strain background effects and genetic modifiers of hearing in mice. Brain Res. 1091(1):79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. (1997) A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 114(1-2):83–92. [DOI] [PubMed] [Google Scholar]
- Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. (2015) Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 16(13):1959–81. [DOI] [PubMed] [Google Scholar]
- Lemke LE, McGee DH, Prieskorn DM, Wall GM, Dolan DF, Altschuler RA, Miller JM. (2009) Safety of ciprofloxacin and dexamethasone in the guinea pig middle ear. Arch Otolaryngol Head Neck Surg. 135(6):575–80. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. (2014) Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 13(3):468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL, Strong R, Miller RA, Harrison DE. (2017) NIA Interventions Testing Program: Investigating Putative Aging Intervention Agents in a Genetically Heterogeneous Mouse Model. EBioMedicine. 21:3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R (2006) Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 116:1–12 [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. (2003) Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 35(1):21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK (2009) Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 1277:70–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A. (2105) mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 1346(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Oinag Y, Sweet J, Tapp R, Dolan DF, Altschuler RA, Lichter J and LeBel C, (2011) OTO-104: A sustained release dexamethasone hydrogel for the treatment of otic disorder, Otology and Neurotology, 32:171–9 [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 33(34):13686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. (2008) Age-related auditory pathology in the CBA/J mouse, Hearing Res, 243:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA et al. , (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 15(5):872–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataya-Kaneda M. (2015) Mammalian target of rapamycin and tuberous sclerosis complex. J Dermatol Sci. 79(2):93–100. [DOI] [PubMed] [Google Scholar]
- Willott JF, Erway LC, Archer JR, Harrison DE. (1995) Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res. 88(1-2): 143–55. [DOI] [PubMed] [Google Scholar]
- Willott JF, Erway LC. (1998) Genetics of age-related hearing loss in mice. IV. Cochlear pathology and hearing loss in 25 BXD recombinant inbred mouse strains. Hear Res. 119(1-2):27–36. [DOI] [PubMed] [Google Scholar]