Abstract
Background:
The arterial switch operation (ASO) became the procedure of choice for dextro-transposition of the great arteries (d-TGA) almost 30 years ago, but the long-term results of this operation are unknown. We aimed to compare the long-term transplant-free survival of patients with d-TGA who underwent ASO vs. atrial switch in the Pediatric Cardiac Care Consortium (PCCC).
Methods:
We performed a retrospective cohort study of d-TGA patients undergoing ASO or atrial switch in the US between 1982 and 1991. Long-term transplant-free survival was obtained by linking PCCC data with the National Death Index and the Organ Procurement and Transplant Network. Kaplan-Meier survival plots were constructed and multivariable regression was used to compare long-term transplant-free survival.
Results:
Of 554 d-TGA patients who underwent ASO (n=259) or atrial switch (n=295) the 20-year overall transplant-free survival was 82.1% for those undergoing ASO and 76.3% for those who had atrial switch procedure. Adjusted overall transplant-free survival beyond 10 years post-operation was superior for ASO compared to atrial switch (HR=0.07, 95% CI 0.01–0.52, p-value=0.009). During this time period the ASO had higher in-hospital mortality than the atrial switch (21.6% vs 12.9%, p=0.007). After excluding those with in-hospital mortality, the transplant-free survival 20 years post-repair was 97.7% for the ASO vs. 86.3% for the atrial switch.
Conclusions:
Despite initial higher in-hospital mortality for ASO during the study period, there is a significant long-term transplant-free survival advantage for ASO as compared to atrial switch for d-TGA surgery. Ongoing monitoring is required to assess late risk of cardiovascular disease.
Keywords: congenital heart disease, transposition, arterial switch, outcomes, survival analysis
Dextro-Transposition of the Great Arteries (d-TGA) is the second most common cyanotic congenital heart defect, with an estimated annual incidence of 860 cases in the United States [1]. Around 1960, the Mustard and Senning atrial switch procedures became the first corrective surgeries available for d-TGA [2,3]. Although the atrial switch had good perioperative survival, complications of the repair were common [4].
In 1975, Jatene introduced the arterial switch operation (ASO) [5], but his initial experience was notable for a high perioperative mortality (71%), much greater than the well-established atrial switch procedure [4,6]. At this time some surgeons recommended ASO only for complex d-TGA, citing the potential unknown long-term complications of the ASO [7]. Others sought to perform the ASO in most cases of d-TGA despite poor initial results, anticipating improved results with increasing experience [8]. Around 1990, with the improved perioperative ASO experience, the ASO superseded the atrial switch as the preferred corrective surgery for d-TGA [9,10].
Although the ASO has been shown to have better mid-term results, long-term results beyond the second decade of life are unknown for those undergoing the procedure in the US [10–14]. Common ASO complications include neo-pulmonary stenosis and neo-aortic dilation which may impact long-term results [9]. Additionally, reports of ASO follow-up show that coronary artery obstruction are found at a low persistent rate [11,12,15], but late deaths due to myocardial infarction and sudden cardiac death in ASO patients are rare [11,16,17]. Nevertheless, coronary lesions and other ASO complications underlie concerns about the long-term results of the operation. Our objective, therefore, was to compare the long-term transplant-free survival of patients with d-TGA between the ASO and atrial switch performed in the decade when the surgery preference transitioned.
PATIENTS AND METHODS
Study Design
We performed a retrospective cohort study using data from the Pediatric Cardiac Care Consortium (PCCC), an international registry for interventions for pediatric heart diseases established in 1982 to allow collaboration between pediatric cardiovascular centers [18,19]. Cardiovascular centers voluntarily participated in the PCCC, with 47 US centers participating from 1982–2011.
Patients were included in this study if they were US residents operated for d-TGA with an ASO or an atrial switch (Mustard or Senning) during infancy at a PCCC center in the US during the decade from 1982 to 1991, the time period during which the transition from atrial to arterial switch type of correction for d-TGA took place in the US. The Mustard and Senning procedures were considered together as the atrial switch group in the initial analyses, with supplemental analyses then performed considering these operations separately. Complex d-TGA was defined as d-TGA accompanied by any combination of: ventricular septal defect (VSD), coarctation, native pulmonary outflow tract obstruction (POTO), or native systemic outflow tract obstruction. Simple d-TGA included patients with an atrial septal defect (ASD), patent ductus arteriosus, or no accompanying defects. Available PCCC forms were reviewed for the collection of variables such as cardiopulmonary bypass and cross-clamp times, as well as coronary artery anatomy. In-hospital deaths were defined as post-operative death during the admission for d-TGA repair. Among hospital survivors, those who had adequate identifiers [first name, middle initial (if available), last name, birth day, birth month, birth year, sex, and state of birth] were submitted to the National Death Index (NDI) and the Organ Procurement and Transplant Network (OPTN) [20]. Ascertainment of vital status and cause of death with NDI and transplant status with OPTN was complete through December 31st, 2014. The study was approved by the Institutional Review Board of Emory University.
Statistical Methods
Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and statistical significance was assessed at the 0.05 level, unless otherwise noted. Normality of continuous variables was assessed with histograms, normal probability plots and the Anderson-Darling test for normality. Descriptive statistics are presented as counts and percentages for categorical variables and median (25th–75th percentile) for continuous data with skewed distributions. Continuous data were compared between surgical groups with the use of Wilcoxon rank-sum tests and comparisons between categorical variables were performed with Chi-square tests, or Fisher exact tests when the expected cell counts were <5. Unadjusted and adjusted in-hospital mortality was compared between surgical interventions using generalized linear mixed models (controlling for sex and transposition complexity and treating surgical center as a random effect). Kaplan-Meier survival plots were constructed to display long-term transplant-free survival data, with statistical comparisons performed using the log-rank test. These plots were created for overall transplant-free survival and for long-term transplant-free survival, conditional on survival to hospital discharge following d-TGA repair.
Survival without transplant after d-TGA intervention was treated as a time-dependent outcome and analyzed using survival analysis methods. Prior to modeling, the proportional hazard assumption was assessed using log-log survival curves and by formally testing the interaction between time and d-TGA intervention group using an extended Cox model. For both overall and conditional long-term survival, the proportional hazard assumption was violated. As a result, we utilized Heaviside functions in the extended Cox Model. When such a function is used, the hazard ratio (HR) formula yields constant HR for different time intervals. Our intervals were chosen such that a) an event occurred in both groups so that the hazard ratio was estimable and b) the hazard function was relatively stable within each window of time. Stability of the hazard function was assessed using kernel smoothed hazard plots with bandwidth of 2 years. The date of intervention was used as our starting point and the effects of ASO intervention on the probability of survival in the models are given as HR with 95% confidence interval (CI), unadjusted and adjusted.
RESULTS
Baseline Characteristics
There were 554 patients with d-TGA who met the study inclusion criteria. Among those, 259 patients underwent surgical correction with ASO and had a median follow-up time of 24.3 years; there were 295 patients with atrial switch and a median follow-up time of 26.5 years. The Senning procedure accounted for 63.7% (n=188) of all atrial switch procedures. There were 460 patients discharged alive after surgical correction, and 336 of the hospital survivors (73.0%) had sufficient identifiers to be submitted to NDI. The patient flow diagram is depicted in Figure 1. The number of ASO performed each year increased until 1990 when the ASO outpaced the atrial switch (Figure 2).
Figure 1.
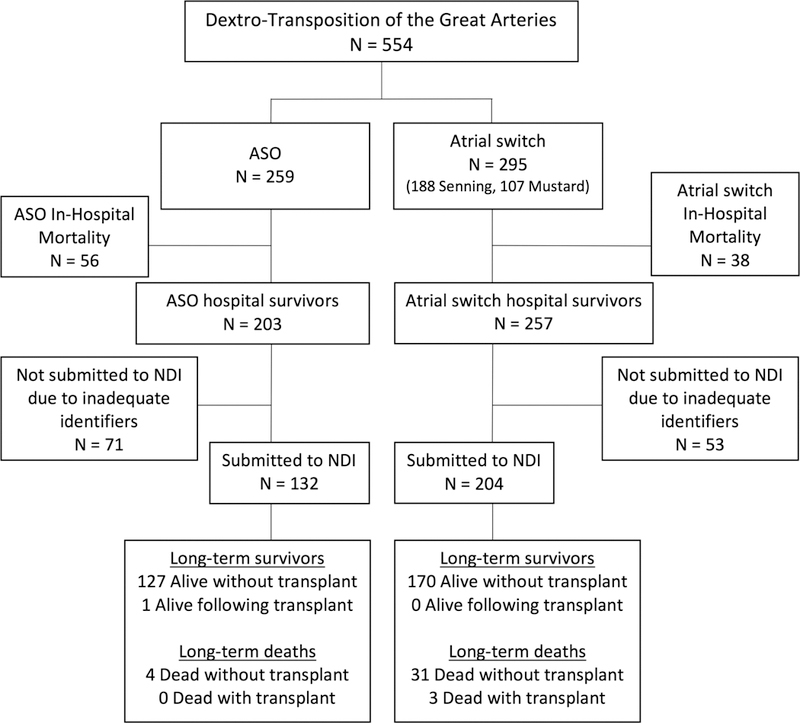
Patient inclusion diagram. NDI = National Death Index, ASO = arterial switch operation.
Figure 2.
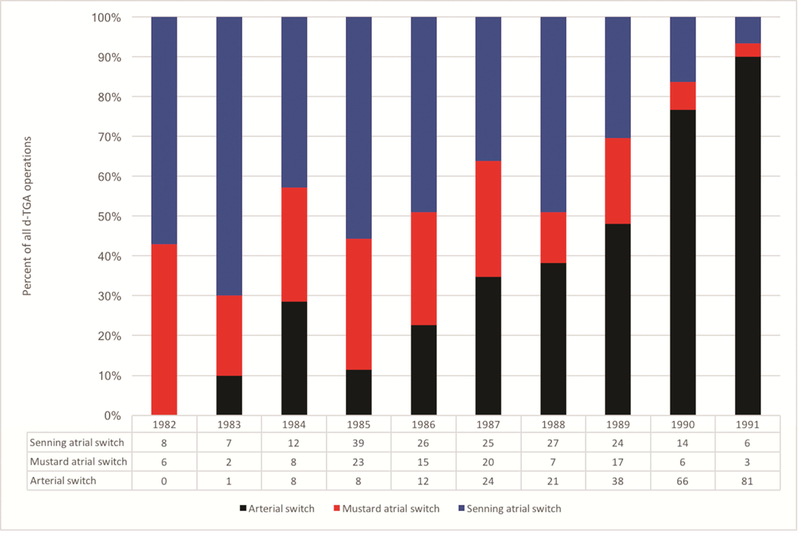
Operations for dextro-transposition of the great arteries in the Pediatric Cardiac Care Consortium, expressed as percent of total d-TGA operations in the bar graph as well as raw number of each operation. d-TGA = dextro-transposition of the great arteries.
The characteristics of all d-TGA patients are shown in Table 1. There were no differences between the two groups with regards to sex, anatomic complexity, or presence of an accompanying VSD or coarctation. Those who underwent ASO were more likely to have an ASD and less likely to have POTO. Of the 116 total patients who had documented coronary artery anatomy, atypical coronary anatomy was present in a similar percentage of ASO and atrial switch patients (18.6% vs 13.0%, p=0.43). Not surprisingly given the nature and timing of the two operations, age and weight at index operation were both lower among patients who underwent ASO compared to those undergoing atrial switch (7 days vs 167 days, p<0.001; 3.6 kg vs 6.0 kg, p<0.001); patients undergoing ASO were less likely to have had prior balloon atrial septostomy than those undergoing atrial switch (49.8% vs 73.9%, p<0.0001). Patient characteristics stratified by hospital discharge status are shown in Table 2. Hospital survivors were less likely to have an accompanying VSD (24.6% vs 40.4%, p=0.002) or an additional cardiac lesion (complex d-TGA) than those who died prior to discharge (26.3% vs 40.4%, p=0.003). Sex, coronary anatomy, cardiopulmonary bypass time, or cross-clamp time were not significantly different between hospital survivors and those who died in-hospital. Demographic information stratified by hospital discharge status for each specific surgical group is shown in Supplemental Table 1 and Supplemental Table 2.
Table 1.
Characteristics of all dextro-transposition of the great arteries patients (including in-hospital deaths), overall and stratified by operation
| N | Overall n=554 |
ASO n=259 |
Atrial Switch n=295 |
p-value | |
|---|---|---|---|---|---|
| Sex | 554 | 0.65 | |||
| Female | 157(28.3%) | 71(27.4%) | 86(29.2%) | ||
| Male | 397(71.7%) | 188 (72.6%) | 209 (70.8%) | ||
| Genetic defect | 554 | 2 (0.4%) | 0(0.0%) | 2(0.7%) | 0.50 |
| Type of transposition | 554 | 0.55 | |||
| Simple d-TGA | 394(71.1%) | 181 (69.9%) | 213 (72.2%) | ||
| Complex d-TGA | 160(28.9%) | 78(30.1%) | 82(27.8%) | ||
| Accompanying defect | 554 | ||||
| VSD | 151(27.3%) | 76(29.3%) | 75(25.4%) | 0.30 | |
| Coarctation | 16(2.9%) | 10 (3.9%) | 6(2.0%) | 0.20 | |
| ASD | 115(20.8%) | 65(25.1%) | 50(16.9%) | 0.02 | |
| POTO | 13(2.3%) | 2(0.8%) | 11 (3.7%) | 0.02 | |
| Coronary anatomy | 116 | 0.43 | |||
| Usual | 97 (83.6%) | 57(81.4%) | 40(87.0%) | ||
| Abnormal | 19 (16.4%) | 13(18.6%) | 6 (13.0%) | ||
| Prior balloon atrial septostomy | 554 | 347(62.6%) | 129 (49.8%) | 218 (73.9%) | <0.001 |
| Birth weight (kg) | 531 | 3.4 (3.0 – 3.8) | 3.4 (3.1 – 3.7) | 3.3 (2.9 – 3.8) | 0.009 |
| Age at index operation (days) | 554 | 72 (7 – 177) | 7 (4 – 12) | 167 (97 – 215) | <0.001 |
| Weight at TGA operation (kg) | 489 | 4.2 (3.5 – 6.1) | 3.6 (3.2 – 3.9) | 6.0 (4.9 – 7.1) | <0.001 |
| Cardiopulmonary bypass time (min) | 90 | 117 (85 – 152) | 123 (80 – 150) | 106 (88 – 180) | 0.35 |
| Cross clamp time (min) | 84 | 66 (56 – 87) | 67 (56 – 86) | 62 (52 – 94) | 0.97 |
| Median length of follow-up (years) | 394 | 25.2 (23.3 – 27.5) | 24.3 (23.3 – 25.6) | 26.5 (23.4 – 28.7) | <0.001 |
ASO = arterial switch operation, VSD = ventricular septal defect, ASD = atrial septal defect, POTO = native pulmonary outflow tract obstruction
Values expressed as N (%) or median (IQR 25th–75th)
Continuous variables are compared using Wilcoxon rank sum tests and categorical variables using Chi-square tests or Fisher’s exact test if expected cell count <5
Table 2.
Characteristics of all dextro-transposition of the great arteries patients stratified by hospital discharge status
| N | Hospital Survivors n=460 |
In-hospital Deaths n=94 |
p-value | |
|---|---|---|---|---|
| Index operation | 554 | 0.006 | ||
| ASO | 203(44.1%) | 56(59.6%) | ||
| Atrial switch operation | 247(55.9%) | 38(40.4%) | ||
| Sex | 554 | 0.34 | ||
| Female | 127(27.6%) | 30(31.9%) | ||
| Male | 333(72.4%) | 64(68.1%) | ||
| Genetic defect | 554 | 1 (0.2%) | 1(1.1%) | 0.31 |
| Type of transposition | 554 | 0.003 | ||
| Simple d-TGA | 339(73.7%) | 55(58.5%) | ||
| Complex d-TGA | 121(26.3%) | 39(41.5%) | ||
| Accompanying defect | 554 | |||
| VSD | 113(24.6%) | 38(40.4%) | 0.002 | |
| Coarctation | 13(2.8%) | 3(3.2%) | 0.74 | |
| ASD | 90 (19.6%) | 25(26.6%) | 0.13 | |
| POTO | 13(2.8%) | 0(0.0%) | 0.14 | |
| Coronary anatomy | 116 | 0.36 | ||
| Usual | 74 (81.3%) | 23(92.0%) | ||
| Abnormal | 17 (18.7%) | 2(8.0%) | ||
| Prior balloon atrial septostomy | 554 | 297(64.6%) | 50(53.2%) | 0.04 |
| Birth weight (kg) | 531 | 3.4 (3.0 – 3.8) | 3.3 (3.0 – 3.7) | 0.92 |
| Age at index operation (days) | 554 | 83 (7 – 182) | 21 (5 – 134) | 0.02 |
| Weight at TGA operation (kg) | 489 | 4.5 (3.6 – 6.3) | 3.8 (3.2 – 5.2) | 0.001 |
| Cardiopulmonary bypass time (min) | 90 | 123 (86 – 152) | 86 (78 – 146) | 0.28 |
| Cross clamp time (min) | 84 | 65 (57 – 88) | 67 (43 – 86) | 0.42 |
ASO = arterial switch operation, VSD = ventricular septal defect, ASD = atrial septal defect, POTO = native pulmonary outflow tract obstruction
Values expressed as N (%) or median (25th–75th)
Continuous variables are compared using Wilcoxon rank sum tests and categorical variables using Chi-square tests or Fisher’s exact test if expected cell count <5
Overall Survival
As shown in Figure 3, the 20-year overall transplant-free survival for children who had surgery for d-TGA was 82.1% for those undergoing ASO and 76.3% for those who had atrial switch procedure (log-rank p-value=0.14). Given that the hazard of transplant/mortality changed over time, we determined time intervals where the hazard was proportional to establish mortality HRs (Table 3). Overall transplant-free survival was initially similar between the two surgical groups, but after 10 years post d-TGA repair, the ASO had better long-term transplant-free survival as compared to the atrial switch (adjusted mortality HR=0.07, 95% CI 0.01–0.52, p-value=0.009).
Figure 3.
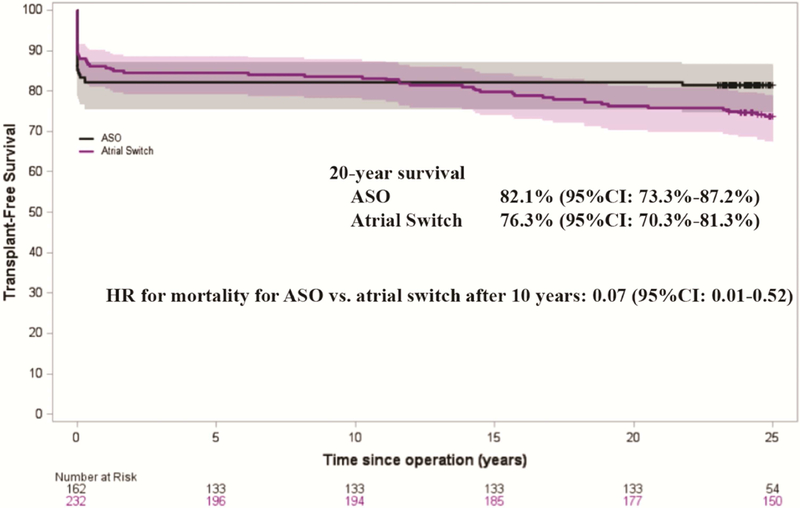
Kaplan-Meier curves for overall transplant-free survival of patients with detro-transposition of the great arteries are shown by operation group. For the time period after 10 years, mortality HR for ASO vs. atrial switch is displayed, adjusted for sex, transposition complexity, and surgical center. ASO = arterial switch operation, HR = hazard ratio, CI = confidence interval.
Table 3.
Effect of treatment strategy on overall survival of dextro-transposition of the great arteries patients during time periods after surgical correction that have proportional hazards.
| Unadjusted mortality HR (95%CI) |
p-value | Adjusted mortality HR (95%CI)* |
p-value | |
|---|---|---|---|---|
| ASO (vs atrial switch): | ||||
| Between 0 and 1 month | 1.39 (0.81–2.37) | 0.23 | 1.62 (0.93–2.85) | 0.10 |
| Between 1 month and 10 years | 0.45 (0.12–1.64) | 0.23 | 0.52 (0.14–1.90) | 0.32 |
| After 10 years | 0.06 (0.01–0.46) | 0.007 | 0.07 (0.01–0.52) | 0.009 |
HR = hazard ratio, CI = confidence interval, ASO = arterial switch operation
adjusted for sex, transposition complexity, and surgical center
In comparing the overall transplant-free survival by type of atrial switch, patients who underwent Mustard and Senning atrial switch procedures had similar long-term results. There was an initial difference in early mortality, with Mustard having a lower hazard of transplant/mortality within the first month after the operation (adjusted mortality HR=0.27, 95%CI 0.09–0.81, p-value=0.02). Beyond this early period there was no difference in overall transplant-free survival, with both atrial switch procedure groups experiencing a steady survival decline (Supplemental Figure 1, Supplemental Table 3).
Conditional Survival
Much of the mortality in this population was in-hospital mortality. For the ASO, in-hospital mortality was 21.6%; for the atrial switch, it was 12.9% (OR=1.87, 95% CI 1.19–2.93, p-value=0.007). Among those who survived to hospital discharge, 20-year transplant-free survival was 97.7% for those with ASO and 86.3% for those with atrial switch as shown in Figure 4 (p-value <0.001). Again, time intervals were established where the hazard of transplant/mortality remained proportional (Table 4). Within the first 10 years after the operation there was no significant difference between the transplant-free conditional survival of the two surgical groups. However, beyond 10 years the advantage of the ASO became clear, with an adjusted mortality HR of 0.07 (95% CI 0.01–0.52, p-value=0.009).
Figure 4.
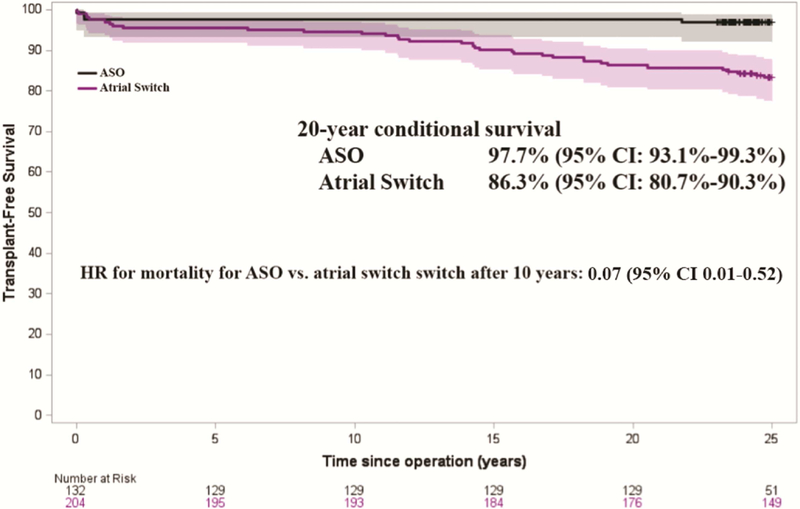
Kaplan-Meier survival curves for transplant-free survival of patients with d-transposition of the great arteries shown by operation group, conditional on survival to hospital discharge following initial operation. For the time period after 10 years, mortality HR of ASO vs. atrial switch is displayed, adjusted for sex, transposition complexity, and surgical center. ASO = arterial switch operation, HR = hazard ratio, CI = confidence interval.
Table 4.
Effect of treatment strategy on long-term mortality among dextro-transposition of the great arteries hospital survivors during time periods after surgical correction that have proportional hazards.
| Unadjusted mortality HR (95%CI) |
p-value | Adjusted mortality HR (95%CI)* |
p-value | |
|---|---|---|---|---|
| ASO (vs atrial switch): | ||||
| Between 0 and 10 years | 0.42 (0.12–1.51) | 0.18 | 0.47 (0.13–1.71) | 0.25 |
| After 10 years | 0.06 (0.01–0.47) | 0.007 | 0.07 (0.01–0.52) | 0.009 |
HR = hazard ratio, CI = confidence interval, ASO = arterial switch operation
adjusted for sex, transposition complexity, and surgical center
In examining the atrial switch outcomes by subtype, those with a Senning operation had significantly higher in-hospital mortality than those with a Mustard (16.5% vs. 6.5%, OR=2.82, 95%CI 1.19–6.67, p-value=0.02). Among survivors of these initial operations, patients had similar long-term outcomes, with transplant-free survival at 20 years of 87.6% for those with a Senning and 84.0% for those with a Mustard (p-value=0.99, Supplemental Figure 2). When comparing the conditional survival of the ASO to each individual atrial switch procedure the results were similar (Supplemental Table 4).
Cause of Death
There were 38 late deaths in our cohort, with four occurring in ASO patients and 34 in atrial switch patients (Table 5). The vast majority of these deaths (66%) were noted to have cardiac etiology. The low numbers of late deaths in the ASO group prohibited any cause of death comparisons between the two groups.
Table 5.
Late causes of death among d-TGA hospital survivors stratified by surgical operation
| Overall | ASO | Atrial Switch | |
|---|---|---|---|
| Cardiac failure/CHD related | 25 | 2 | 23 |
| Sepsis | 2 | 0 | 2 |
| Multiple System Organ Failure | 1 | 0 | 1 |
| Pulmonary Hypertension | 1 | 0 | 1 |
| Pneumonia/Other Respiratory | 3 | 1 | 2 |
| Neurologic | 1 | 0 | 1 |
| External cause of injury | 4 | 1 | 3 |
| Unknown | 1 | 0 | 1 |
d-TGA = dextro-transposition of the great arteries, ASO = arterial switch operation, CHD = congenital heart defect
COMMENT
In this study, which to our knowledge is the largest of its kind comparing surgical techniques in d-TGA patients with at least 20 years of follow-up, we found that long-term transplant-free survival among patients with d-TGA was superior in patients who received an ASO as compared to an atrial switch. This finding was true both with and without consideration of the in-hospital mortality associated with the initial operation. Prior survival modeling of the two groups of operations using mortality parameters from this era predicted that the overall survival curves would intersect around 30 years, beyond which ASO survival would be superior [21]. Our study reveals that this cross-over point actually took place around 10 years after the operation in the transitional era between the two procedures in the US. Our results are consistent with many other studies of smaller size or shorter duration of follow-up [10–14,16].
In the current era of the ASO, with extremely low in-hospital mortality, the 20-year survival of d-TGA patients likely approximates the >97% 20-year survival of the ASO hospital survivors shown here. The ASO is clearly the better long-term strategy, but in the 1980’s this outcome was not a certainty. The delay between the first successful ASO and its widespread use was primarily due to the concern for the high in-hospital mortality of the operation. ASO results improved throughout the 1980’s with the advent of the Lecompte maneuver, the neonatal ASO, and improvements in coronary implantation techniques [22]. Our study shows the gradual transition in d-TGA repair preference throughout the decade. Over half of the ASOs that were included in this study occurred in 1990 and 1991. However, despite the surgical innovations, our results reveal that during this time period the ASO, compared to atrial switch, remained a risk factor for early mortality. Greater than 20% of patients who underwent an ASO were not discharged from the hospital alive. This in-hospital mortality for the ASO is slightly higher than other reports from the same era [11,13]. In the current era, in-hospital mortality following ASO is considered a rare event. Villafañe et al. reported that the in-hospital mortality after ASO in the PCCC was only 2.9% from 2003–2007 [9], similar to other present-day reports consistently below 5% [11,13].
There are many factors which may contribute to the increased long-term mortality among the atrial switch cohort. The atrial baffles diverting venous inflow to the contralateral ventricle in the atrial switch procedure are prone to baffle leaks and obstructions. In addition, the process of atrial reconstruction predisposes these patients to atrial arrhythmias. Finally, the systemic right ventricular function deteriorates over time, also leading to tricuspid regurgitation [23]. These complications give rise to a high rate of re-interventions [4]. Our study agrees with prior estimates of overall long-term survival in atrial switch patients, with around 70–80% remaining alive 25 years after their operation [23]. Despite the difference in in-hospital mortality between the Senning and Mustard procedures, patients undergoing these operations in our study had similar overall survival to beyond 20 years.
Our study has several limitations in addition to those inherent of a retrospective cohort study. First, a proportion of hospital survivors did not contain adequate identifiers to be submitted to NDI. Our previous work with this linked registry demonstrated that younger patients were less likely to have adequate identifiers [20]. This limitation did not affect our ability to detect a difference in transplant-free survival, but it may have prohibited us from comparing causes of death. Second, in 1990 there was a rapid shift from the atrial switch to the ASO, a shift that resulted in more than half of the ASO group occurring in the last 2 years of our study. This timeframe somewhat limits our ability to compare exactly contemporaneous outcomes between groups. Third, there was limited data collection or ascertainment for some of our variables of interest such as coronary artery anatomy. However, we feel that this is unlikely to have affected our overall results as this potential misclassification would have been equally distributed between the two groups. Finally, the PCCC database does not collect information regarding long-term complications. Thus, we cannot assess whether there are differences in complications between the surgical groups other than mortality or transplant. Nevertheless, our study describes the late survival of a large nationally representative cohort of d-TGA patients who underwent surgery when the superiority of the ASO was uncertain.
Our study shows that among d-TGA patients, the survival into the third decade of life of those who underwent ASO is superior to that of atrial switch patients. This is particularly true among those who survived to discharge following the initial hospitalization. In the current era of the operation with low ASO perioperative mortality, the overall ASO survival to 20 years of age likely closely approximates the >97% 20-year survival of ASO hospital survivors reported here. As patients with ASO age and are exposed to the cardiovascular complications of aging, future efforts should be directed at continued surveillance of this cohort for coronary artery disease and sudden cardiac death.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yacoub M, Hosny H, Afifi A. Surgery for TGA in Developing Countries: The End of the Beginning. J Am Coll Cardiol 2017;69(1):52–55. [DOI] [PubMed] [Google Scholar]
- 2.Senning A Surgical correction of transposition of the great vessels. Surgery 1959;45(6):966–80. [PubMed] [Google Scholar]
- 3.Mustard WT. Successful Two-Stage Correction of Transposition of the Great Vessels. Surgery 1964;55:469–72. [PubMed] [Google Scholar]
- 4.Horer J, Herrmann F, Schreiber C, et al. How well are patients doing up to 30 years after a mustard operation? Thorac Cardiovasc Surg 2007;55(6):359–64. [DOI] [PubMed] [Google Scholar]
- 5.Jatene AD, Fontes VF, Paulista PP, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976;72(3):364–70. [PubMed] [Google Scholar]
- 6.Jatene AD, Fontes VF, Souza LC, et al. Anatomic correction of transposition of the great arteries. J Thorac Cardiovasc Surg 1982;83(1):20–6. [PubMed] [Google Scholar]
- 7.Stark J Transposition of the great arteries: which operation? Ann Thorac Surg 1984;38(5):429–31. [DOI] [PubMed] [Google Scholar]
- 8.de Leval MR. Lessons from the arterial-switch operation. Lancet 2001;357(9271):1814. [DOI] [PubMed] [Google Scholar]
- 9.Villafane J, Lantin-Hermoso MR, Bhatt AB, et al. D-transposition of the great arteries: the current era of the arterial switch operation. J Am Coll Cardiol 2014;64(5):498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricke TA, d’Udekem Y, Richardson M, et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg 2012;94(1):139–45. [DOI] [PubMed] [Google Scholar]
- 11.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013;127(3):331–9. [DOI] [PubMed] [Google Scholar]
- 12.Losay J, Touchot A, Serraf A, et al. Late outcome after arterial switch operation for transposition of the great arteries. Circulation 2001;104(12 Suppl 1):I121–6. [DOI] [PubMed] [Google Scholar]
- 13.Raissadati A, Nieminen H, Sairanen H, et al. Outcomes after the Mustard, Senning and arterial switch operation for treatment of transposition of the great arteries in Finland: a nationwide 4-decade perspective. Eur J Cardiothorac Surg 2017;52(3):573–80. [DOI] [PubMed] [Google Scholar]
- 14.Tobler D, Williams WG, Jegatheeswaran A, et al. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol 2010;56(1):58–64. [DOI] [PubMed] [Google Scholar]
- 15.Angeli E, Formigari R, Pace Napoleone C, et al. Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg 2010;38(6):714–20. [DOI] [PubMed] [Google Scholar]
- 16.Baruteau AE, Vergnat M, Kalfa D, et al. Long-term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or aortic arch obstruction. Interactive cardiovascular and thoracic surgery 2016;23(2):240–6. [DOI] [PubMed] [Google Scholar]
- 17.van Wijk SWH, van der Stelt F, Ter Heide H, et al. Sudden Death Due to Coronary Artery Lesions Long-term After the Arterial Switch Operation: A Systematic Review. The Canadian journal of cardiology 2017;33(9):1180–87. [DOI] [PubMed] [Google Scholar]
- 18.Moller JH, Hills CB, Pyles LA. A multi-center cardiac registry. A method to assess outcome of catheterization intervention or surgery. Progress in Pediatric Cardiology 2005;20(1):7–12. [Google Scholar]
- 19.Pyles LA, Hills CM, Larson VE, et al. Pediatric Cardiac Care Consortium: an instrument for evidence-based clinical decision support. J Cardiovasc Transl Res 2009;2(2):219–24. [DOI] [PubMed] [Google Scholar]
- 20.Spector LG, Menk JS, Vinocur JM, et al. In-Hospital Vital Status and Heart Transplants After Intervention for Congenital Heart Disease in the Pediatric Cardiac Care Consortium: Completeness of Ascertainment Using the National Death Index and United Network for Organ Sharing Datasets. J Am Heart Assoc 2016;5(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bull C, Yates R, Sarkar D, et al. Scientific, ethical, and logistical considerations in introducing a new operation: a retrospective cohort study from paediatric cardiac surgery. BMJ 2000;320(7243):1168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marathe SP, Talwar S. Surgery for transposition of great arteries: A historical perspective. Ann Pediatr Cardiol 2015;8(2):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuypers JA, Eindhoven JA, Slager MA, et al. The natural and unnatural history of the Mustard procedure: long-term outcome up to 40 years. Eur Heart J 2014;35(25):1666–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


