Abstract
Cocaine administration has been shown to produce immediate positive (rewarding) and subsequent negative (anxiogenic) effects in humans and animals. These dual and opposing affective responses have been more difficult to demonstrate with administration of methamphetamine (meth). While animal studies have reliably demonstrated the positive reinforcing effects of the drug, reports of negative aftereffects following acute exposure have been few in number and contradictory in nature. The current research was devised to assess the effects of acute meth using a runway model of self-administration that is uniquely sensitive to both the positive and negative effects of a drug reinforcer in the same animal on the same trial. Male rats were allowed to traverse a straight alley once a day for 16 consecutive days/trials where entry into the goal box resulted in a single IV injection of meth (0.25, 0.5 or 1.0 mg/kg/inj.). The chosen doses were confirmed to be psychoactive as they produced dose-dependent increases in motoric/locomotor activation in these same subjects. The results demonstrated a U-shaped dose-response curve for the reinforcing effects of meth in that the intermediate dose group (0.5 mg/kg) produced the strongest approach behavior in the runway. Unlike other psychomotor stimulants, like cocaine, animals running for IV meth exhibited no evidence of any significant approach-avoidance behaviors reflective of the drug’s negative anxiogenic effects. These results suggest that the abuse potential for meth is likely higher than for other shorter-acting psychomotor stimulants and reaffirms the utility of the runway procedure as a screen for a substance’s abuse potential.
Keywords: methamphetamine, reward, operant behavior, runway, drug self-administration
1. Introduction
Acute administration of psychomotor stimulant drugs is well known to produce both positive and negative effects. For example, human users of cocaine describe an initial state of energy and euphoria followed in time by a “crash” that is characterized by feelings of anxiety craving and agitation (e.g., Anthony et al., 1989; Gawin and Ellinwood, 1988; Williamson et al., 1997). These dual positive and negative effects of the drug have also been observed in animal studies where cocaine is readily self-administered (Ettenberg et al., 1982; Foltin and Fischman, 1994; Goeders, 1988; Roberts et al., 1977; Wolverton, 1992), produces conditioned place preferences (Bardo, et al., 1995; Ettenberg, 2004; Mueller and Stewart, 2000; Mucha et al., 1982; Tzschentke, 1998), and reduces the threshold for rewarding brain stimulation (Ahmed et al. 2002; Radke et al., 2016). In contrast, cocaine has also been shown to produce enhanced anxiety in a variety of tests including the elevated plus maze (Rogerio and Takahashi, 1992; Yang et al., 1992; Ben-Shahar et al., 2008), acoustic startle test (Willick and Kokkinidis, 1995), and in conditioned taste aversion studies (Goudie et al., 1978). In our own laboratory, animals demonstrate preferences for a place paired with the immediate effects of IV cocaine, but exhibit aversions for a place paired with the drug effects present 15-min after drug administration (e.g., Ettenberg et al., 1999; Knackstedt et al., 2002; see also Jhou et al., 2013). On the basis of these findings, it seems reasonable to conclude that the motivation of organisms to self-administer the drug – i.e., the drug’s “abuse potential” -- likely reflects the relative magnitude of the positive versus the negative impact of drug administration. Consistent with this view, we recently reported that animals exhibiting a greater positive than negative response to acute cocaine (as measured in a place conditioning paradigm) were at the greatest risk for subsequent escalated cocaine self-administration, a presumed indicator of cocaine addiction (Ettenberg et al., 2015).
The current study was devised to extend our investigation to the putative positive and negative effects of self-administered methamphetamine (“meth”). The euphoric effects of meth are reflected in the fact that it is widely abused both nationally and globally. In the 2016 World Drug Report, the United Nations Office on Drug and Crime reported that there were an estimated 35.7 million human users worldwide, with those numbers rapidly growing each year based upon the substantial increase in the amount of meth that has been seized around the world (UNODC, 2016). Human meth users describe the drug’s strong euphoric effects typically coupled with increased energy, heightened curiosity, amplified feelings of attentiveness, and reduced anxiety (Barr et al., 2006; Cruickshank and Dyer, 2009; Newton et al., 2005; Paneka et al., 2013; Perez-Reyes et al., 1991). Like cocaine, meth is also self-administered by animals (Collins et al., 1984; Cornett and Goeders, 2013; Kucerova et al., 2012), produces conditioned place preferences (Berry et al., 2012; Cherng et al., 2007), and significantly decreases the brain stimulation reward threshold (Sarkar et al., 1995). With respect to the drug’s negative effects, while human meth users report agitation, drug craving and heightened anxiety after drug use, such data are drawn from the self-reports of drug-dependent chronic meth users (e.g., Cruickshank and Dyer, 2009; Harro, 2015; Hellem, 2016; Su et al., 2017) and hence do not address the question of whether the motivation to seek the drug after an initial acute drug exposure is due to the greater magnitude or salience of the drug’s positive relative to negative effects.
While animal research should be able to more clearly answer this question, attempts to measure the anxiogenic/aversive effects of acute meth have produced contradictory and inconclusive results. Some studies employing an elevated plus maze show meth to be anxiogenic in that treated animals increased their latency to enter or spend time in the open arms of the apparatus (e.g., Beirami et al., 2017; Miladi-Gorji et al., 2015; Pometlová et al., 2016), while other studies have reported the exact opposite result and have suggested that the acute administration of the drug is anxiolytic (e.g., Etaee et al., 2017). In other work, dose-dependent decreases in social interactions have been observed after meth administration and hence may be another indicator of an anxiogenic response in rats (Šlamberová et al., 2010). While meth administration has been shown to produce conditioned place preferences for environments paired with the drug (Berry et al., 2012; Zakharova et al., 2009), it can also produce conditioned taste aversions to a novel taste associated with meth administration (Awasaki et al., 2011). Hence, while the positive rewarding effects of acute meth are well documented, whether or not meth administration produces subsequent aversive/anxiogenic consequences remains unclear.
In this context, the current study was devised to examine the effects of meth using a behavioral model developed in our laboratory that is sensitive to both the positive and negative actions of the drug in the same animal on the same trial. In the operant runway model of IV drug self-administration animals quickly learn to traverse a long straight-arm runway once a day for an injection of drug reinforcer upon goal-box entry (Ettenberg, 2009). In this model, the positive incentive properties of the reinforcer are reflected by the latency with which animals leave the start box once the start door is raised. For example, cocaine-reinforced animals exhibit progressively faster start latencies as testing proceeds. However, over trials, these animals develop an ambivalence about entering the goal-box as reflected in an increased frequency of approach-avoidance behaviors in which animals run quickly to the goal box entry, then stop and retreat back toward the start box. These approach-avoidance retreats have been shown to result from mixed positive (rewarding) and negative (anxiogenic) associations that the animals’ form with the cocaine-associated goal box (Ettenberg, 2004). Thus, on the same trial one can observe short, fast start latencies, as well as numerous approach-avoidance retreats. An additional advantage to this method is that, unlike traditional lever-press self-administration methods where the animal is essentially working to retain its drugged state, the runway procedure examines the motivation of non-drugged animals to seek their drug reinforcer each day – i.e., all the behavioral dependent measures are obtained before the drug reinforcer is delivered. Hence the data are devoid of potentially confounding nonspecific motoric effects caused by administration of a stimulant drug reinforcer. The test, therefore, has strong translational power in that human motivation to seek a drug reinforcer can also be assessed by the person’s willingness to return to a place where he/she had previously obtained the reinforcer. The current study, therefore, employed the operant self-administration runway as a means of: a) examining the motivation of animals to seek IV meth, b) assessing the drug’s putative positive and anxiogenic properties, and c) to thereby assess its abuse potential relative to other psychomotor stimulant drugs of abuse previously tested using the same procedure.
2. Materials and methods
2.1. Subjects
Thirty male Sprague-Dawley rats weighing 270–320g at the beginning of the experiment (Charles River Laboratories, Hollister, CA) served as subjects. The animals were housed in standard rat cages located within a temperature-controlled (22°C) vivarium maintained on a 12-hour light and dark cycle (lights on at 8:00am). Food and water were available ad libitum throughout the course of the experiment. Upon arrival, the rats experienced a week of gentle handling prior to the start of the study. All treatments and methods adhered to the guidelines provided in the National Institute of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California at Santa Barbara’s Institutional Animal Care and Use Committee (IACUC).
2.2. Surgery
Immediately prior to surgery, each subject received a subcutaneous (SC) injections of the non-opiate analgesic, meloxicam (2.0 mg/kg) and the opiate antagonist, buprenorphine HCL (0.05 mg/kg), to reduce post-operative pain. Subjects were then individually implanted with a chronic indwelling intravenous (IV) catheter placed into the right jugular vein where it was secured in place with silk sutures. Surgery was conducted under deep anesthesia produced by an intramuscular (IM) injection of ketamine/xylazine (56.25 and 7.5 mg/kg respectively). Each IV catheter consisted of 13 mm of silastic tubing (0.3 mm inner diameter and 0.64 mm outer diameter). Once secured in place, the open end of the catheter was passed subcutaneously to a threaded 22-gauge guide cannula (Plastics One) that exited through a 2.0 mm hole on the animal’s back. While still anesthetized, each subject received a 3.0 ml injection of 0.9% physiological saline for hydration The guide cannula was held in place by affixing it using dental acrylic to a 1.0 cm square of Mersiline mesh (Bard) positioned flat and sub-dermally on the animal’s back. Each catheter was flushed daily with infusions of the antibiotics cefazolin and gentamicin (0.1 ml IV each) followed by an infusion of heparinized saline (6.25 IU, 0.1 ml IV) to prevent against infection and to ensure patency. Catheter patency was assessed during the course of the experiment by administering a 2.0 mg/kg/0.1ml IV injection of the fast-acting barbiturate, methohexital (Brevital), which caused the animals to momentarily lose their righting reflex. Four animals in this study had to be removed from the data analyses due to failure of the Brevital test midway through the experiment.
2.3. Drugs
The methamphetamine (Sigma-Aldrich) was dissolved in 0.9% physiological saline and sterile filtered through a 0.22μm filter (ThermoScientific). The drug reinforcer was administered in doses of 0.25, 0.5, or 1.0 mg/kg IV in a volume of 0.1 ml over a period of 4.3 s. The selected range of doses was chosen to mimic aspects of human use on a dose/body weight basis and to be comparable to those reported for locomotor activation in rats following IV administration of the drug (e.g., Rivière et al., 1999; Segal and Kuczenski, 2006). The selection of doses that produce locomotor activation was based on the observation that the locomotor response to psychomotor stimulants, including methamphetamine, has been shown to be predictive of drug reward as measured by subsequent IV self-administration (e.g., Piazza et al., 1989, 1990; Vezina, 2004; Gancarz et al., 2011).
2.4. Runway apparatus
Behavioral testing was conducted in two identical straight-arm runways (155 cm long × 15 cm wide × 40 cm high). On one end of each runway was a start box (24cm × 25 cm × 40 cm) and on the opposite end an identically-sized goal box. Two retractable doors separated the middle section of the runway from both the start and goal boxes. The floors of the apparatus consisted of 3.0 mm diameter steel rods oriented perpendicular to the walls and spaced 1.2 cm apart. Thirteen pairs of infrared photodetector-emitter beams were situated 16 cm apart along the interior length of the runway. These photocells fed information to a laptop computer running AnyMaze software that tracked the movements and location of subjects in real time throughout each trial. This software also controlled the opening of the start box door, as well as the closing of the goal box door and activation of a syringe pump that delivered the meth reinforcer to a subject upon its entry into the goal box. Suspended above each runway were two magnetic rails that ran in parallel down the length of each apparatus, and between them there was a flow-through plastic swivel attached to a Plexiglas disc that prevents the swivel from falling through the gap between the rails. A magnet aligned to the bottom of the disc was arranged with the opposite polarity to that of the magnetic rails thereby allowing the entire swivel to float a few cm above the rails. This permitted subjects to move freely throughout the apparatus with minimal friction or resistance. Polyethylene-50 tubing ran from the flow-through swivel to the guide cannula on each subject’s catheter, with the other end attached to the drug delivery syringe. A more detailed description of the runway apparatus can be found in Geist and Ettenberg (1990).
2.5. Locomotor Activity Apparatus
To assess the ambulatory behavior of the animals, subjects were individually placed into one of 12 identical Plexiglas chambers each measuring 20 cm L × 40 cm W × 20 cm H (Kinder Scientific, San Diego, CA). A series of infrared photodetector-emitter pairs were embedded in the walls 8 cm above the floor of the apparatus, with 15 along either side of the long axis and 7 along the narrow axis of the apparatus. Any movement within the chamber produced interruptions in the photobeams that were then recorded by a desktop computer running custom software (Kinder Scientific).
2.6. Procedures
Behavioral testing commenced one week after IV catheterization and consisted of 17 days of runway testing (one day of habituation to the apparatus followed by 16 days of methamphetamine-reinforced runway trials). Approximately one week following completion of the runway phase of the study, the effects of the selected doses of methamphetamine were examined on the locomotor activity of the subjects during a single test session.
2.6.1. Runway
The runway procedure employed here has been developed in and previously used by our laboratory for the study of a wide range of drug reinforcers (i.e., see review by Ettenberg, 2009). Each rat was randomly assigned to one of three groups corresponding to a low, medium and high dose of meth (0.25, 0.5, or 1.0 mg/kg/inj, respectively). Animals were permitted to acclimate to the apparatus during a single habituation trial consisting of 10 min exposure to the apparatus during which the subjects were individually permitted to wander throughout and explore the runway (with the exception of the goal box the door to which was closed). On the following day, formal testing and data collection began. Each animal was connected to the drug delivery system, and placed into the start box where, after 5 sec, the start door opened, and the trial commenced. Upon entry into the goal box, the goal door closed behind the animal (to prevent retracing) and an IV infusion of methamphetamine was automatically administered. Each animal then remained in the goal box for 5-min after which it was disconnected from the drug delivery system and returned to its home cage.
Data collection on each trial entailed three dependent variables: start latency, run time, and approach-avoidance retreat frequency. Start latency was defined as the time that it took the rat to leave the goal box once the start door opened; run time was total time that it took the rat to enter the goal box after leaving the start box; and the “retreats” were defined by a change in the direction that the animal was moving – i.e., moving in the direction of the goal box (approach) for a distance of at least three photobeams (48 cm) and then stopping and reversing its direction back toward the start box (avoidance) by the distance of at least two photobeams (32 cm). By testing subjects on only one trial a day, these behavioral measures were collected in non-drugged subjects and hence were unaffected by any potential confounding non-specific or motoric side effects of the drug itself. In this context, start latencies and run times provide an index of the subject’s motivation to seek the reinforcer, while the development of approach-avoidance retreat behaviors is reflective of dual positive + negative associations that animals develop about goal box entry due to mixed and opposing effects of the drug reinforcer (e.g., see reviews by Ettenberg 2004, 2009).
2.6.2. Locomotor Activity
One week following the completion of runway testing, a locomotor activity test was conducted to ensure that the doses employed in the current study were behaviorally effective (i.e., produced locomotor activation). Each subject was placed individually into an assigned locomotor chamber for 30 min of baseline testing after which subjects were removed, injected with their corresponding IV dose of methamphetamine, and immediately replaced into the apparatus for an additional 60 min test session. The total distance traveled (cm) by each subject was recorded during both the baseline and test segments of the session for statistical analyses.
3. Results
3.1. Runway
The group means (±SEM) for each of the three dependent measures (start latency, run time, and retreat frequency) during the course of the 16-days/trials of the runway test are depicted in Figure 1. Final group sizes were n = 10, 10, 6, respectively for the low, medium and high doses of methamphetamine. Individual two-factor (Group × Trial) Analyses of Variance (ANOVA) were computed for each of the three measures shown in the figure. Analysis of the mean start latencies of the three groups revealed a significant main effect of Trials [F(15,345) = 3.848, p < .001]; i.e., when averaged across all subjects, animals left the start box faster as trials progressed. This effect was the same across groups as there was no significant main effect of Group nor a significant Group × Trial interaction (p > 0.05). The ANOVA computed on run times also revealed significant main effects for Trials [F(15,330)) = 4.594, p < .001], Group [F(2,22) = 3.905, p < .04], as well as a significant Group × Trial interaction [F(30,330) = 2.141, p < .002]. As the figure illustrates, the interaction stems from the fact that group differences appearing during the first half of trials were no longer present during the remaining week of testing (middle panel of Figure 1). These initial differences in group performance reflected an inverted-U-shaped dose-response curve in that the fastest runway behavior was exhibited by the medium dose group with both the low dose and high dose groups taking the longest to enter the goal box. Post hoc analyses (one-tailed independent-group t-tests) computed on the average run times exhibited by the three groups during the first seven days of testing, confirmed that while the high and low doses were not significantly different from one another (p>.05) the 0.5 mg/kg group entered the goal box sooner than either the 0.25 or the 1.0 mg/kg groups (respectively, t(18) = 1.73, p=0.05, and t(14) = 3.42, p<.003). Finally, the frequency of approach-avoidance retreats exhibited by the three groups was low (averaging less than 1 retreat per animal per trial over the course of the 16 days of testing), a result reflected in the fact that the ANOVA identified no significant main effects of Trial or Group, nor a Group × Trial interaction.
Figure 1.
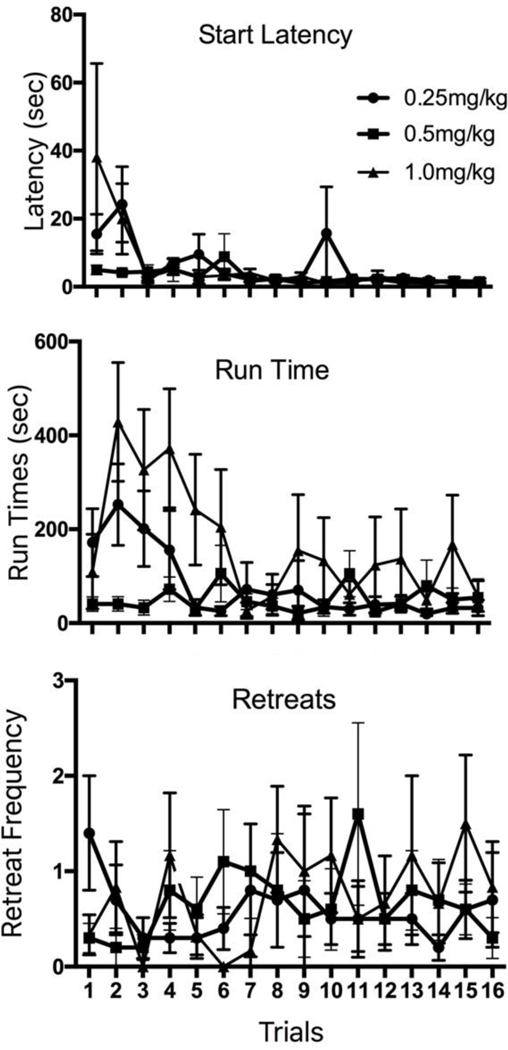
Runway behavior of rats working for IV methamphetamine. The data depict mean (±SEM) start latencies (top graph), run times (middle graph) and retreat frequency (bottom graph) of animals running a straight alley once each day for single daily infusions of IV methamphetamine (0.25, 0.5, or 1.0 mg/kg).
3.2. Locomotor activity test
A two-factor Group × Time ANOVA computed on the data derived from the 30-min baseline confirmed a highly reliable main effect of Time (F,5,115) = 50.19, p <.001) as animals habituated to the apparatus. However, there was neither a Group × Time Interaction nor a main effect of Group (p >.05) indicating that all animals performed equivalently as the trial progressed. The locomotor behavior of the subjects after IV administration of meth is depicted in Figure 2. The figure shows the mean (±SEM) distance traveled (cm) during each 5-min bin of a 60-min test. session immediately following drug administration. For comparison, the mean (±SEM) distance traveled during the last 5-min of baseline are included at the far left of the figure. Meth administration produced a dose-dependent increase in responding as confirmed by computation of a two-factor (Group × Time) ANOVA on the data derived from the 60-min test session. The ANOVA revealed a significant main effect of Time [F(11,253) = 22.27, p < .001] and while there was no main effect of Group [F(2,23) = 1.05, p > .05] there was a reliable Group × Time interaction [F(22,253) = 1.66, p = .035) reflecting the fact that the high dose produced elevated responding for a longer duration than either of the other two doses (see Figure 2).
Figure 2.
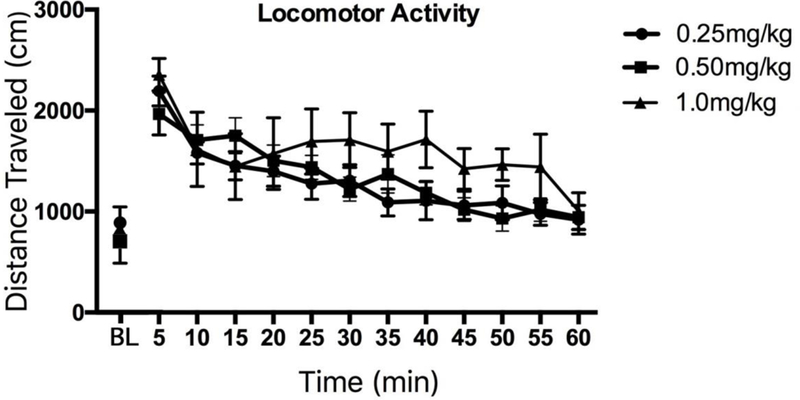
Methamphetamine-induced locomotor activity. Mean (±SEM) ambulatory behavior over a 60-min test session produced by administration of 0.25, 0.5, and 1.0 mg/kg IV methamphetamine. The data are presented as distance traveled (cm) during each 5-min bin. The disconnected scores on the far bottom left of the figure reflect the mean (±SEM) distance traveled during the final 5-min of the preceding baseline period prior to drug administration.
4. Discussion
Animals traversing a straight alley and entering a goal box once a day for a single IV infusion of methamphetamine demonstrated a progressively stronger motivation to seek the drug as testing progressed. This conclusion is based upon the observations of faster start latencies and run times over the course of daily trials. These data are consistent with prior reports that animals will develop preferences for places associated with meth administration (e.g., Berry et al., 2012; Cherng et al., 2007) and will self-administer the drug in traditional operant lever-press models of drug reinforcement (e.g., Collins et al., 1984; Cornett and Goeders, 2013; Kucerova et al., 2012). Unlike the conditions of traditional operant self-administration, in the runway model the animals are tested each day in a non-drugged state, and hence are unaffected by any nonspecific motoric actions of the drug reinforcer. Thus, the start latencies and run times provide drug-free indices of the subjects’ motivation to return to a place associated with prior meth administration. The procedure, therefore, combines aspects of both operant (subjects must run to earn the reinforcer) and conditioned place preference methodologies in the same animals on the same trial.
Long classified as a psychomotor stimulant, meth has been shown to produce locomotor activation in animal studies (e.g., Segal and Kuczenski, 2006; Wallace et al., 1999; Zakharova et al., 2009) and all three doses of meth produced locomotor activation with the highest dose (1.0 mg/kg) producing the longest duration of action (see Figure 2). The absence of a significant main effect of “Group” in the ANOVA, is likely due to a ceiling effect during the initial 15–20 minutes of the test when animals were locomoting between 1700 and 2500 cm in each 5-min period. An unexpected finding, however, was that subjects appeared to be most motivated to run for the intermediate dose (0.5 mg/kg) of meth, which produced the fastest start latencies and run times. The data therefore suggest an inverted U-shaped dose-response curve with all three doses producing evidence of reinforcement but the middle dose having the strongest behavioral impact. No comparable data exist for the conditioned place preference test (the most comparable test to the runway) since such tests have always been conducted with an IP as opposed to an IV route of administration.
The progressive changes in start latency and run time in the low and high dose groups can be accounted for if the subjects required multiple exposures to the goal box before learning an association between the goal box and the drug. In that scenario, as subjects learn that goal box entry is paired with IV meth administration, their approach behavior strengthens. An alternative explanation is based upon reports that repeated single daily injections of meth can produce a sensitized/enhanced behavioral response to the drug that parallels changes in the function of the underlying substrates at which the drug acts (e.g., Jedynak et al., 2012; Nishikawa et al., 1983; Ota et al., 2015; Yamada et al., 1988). The changes in start and run times over trials would therefore be explained by sensitized changes (i.e., increases) in the rewarding value of the meth as trials progressed. While this remains a viable explanation for the current results, it does not, of course, account for why the intermediate dose group ran quickly from the outset. Hence, the authors maintain that the most parsimonious explanation for group differences in runway behavior was due to the relative rewarding impact of the goal box experience – i.e., that the intermediate dose was more rewarding to the subjects than either of the other two doses.
As indicated in the Introduction of this paper, the presence or absence of aversive/anxiogenic effects after acute administration of meth has produced contradictory and inconsistent results (e.g., Beirami et al., 2017; Etaee et al., 2017; Pometlová et al., 2016). An examination of the subjects’ retreat behavior suggests that, unlike that which we have previously observed in animals running for IV cocaine (e.g., Ettenberg and Geist, 1991; 1993; Guzman and Ettenberg, 2007; Knackstedt et al., 2002), rats running for meth did not exhibit signs of any significant aversive consequences of the drug administration. This is reflected in the extremely low frequency of approach-avoidance behaviors (averaging a little over one retreat per group per trial), hence there is little evidence for the development of mixed positive + negative associations with goal box entry (e.g., see Ettenberg, 2004; 2009). If one presumes that the motivation to re-seek a drug after an initial exposure is a function of the organism’s perceived relative reward versus aversive effects of the drug, then the current results would suggest that meth has relatively high abuse potential.
On the surface, the retreat data would seem to be in contradiction to the reports of human chronic users of the drug who describe a “crash” that follows on the heels of the drug-induced “high” (e.g., Cruickshank and Dyer, 2009; Harro, 2015; Hellem, 2016; Su et al., 2017). However, these human subject studies invariably describe the affective responses of drug-dependent individuals withdrawn from meth and therefore do not address the question of whether or not the initial acute exposures to the drug have such effects. Of course, one might argue that the negative effects of methamphetamine might have been observed had additional higher doses of drug been examined in the runway. While this certainly is a possibility there is no a priori reason to conclude that increasing the dose of methamphetamine would have produced results any different from those reported here. Indeed, the high dose employed here was four-fold higher than the low dose. Additionally, the approach for selecting the doses in the current study (based on locomotor activation and i.v. self-administration) was precisely the same as that which we employed in numerous previous studies with other drugs of abuse, yet only cocaine produced evidence of approach-avoidance behavior with respect to goal-box entry (see review by Ettenberg 2009). These differences in drug effects may be accounted for by the fact that meth has an extremely long duration of action which serves to heighten the perceived rewarding impact relative to any subsequent anxiogenic consequences. For example, when the subjective responses of human users were directly compared for cocaine versus meth, the positive euphoric effects of cocaine peaked and then declined rapidly while the effects of meth tended to rise more slowly, but remained elevated longer (Newton et al., 2005). Indeed, while the plasm half-life of IV cocaine in rats has been reported to be approximately 18 min (Barbieri et al., 1992), comparable doses of IV meth have a half-life in excess of 63 min (Rivière t al., 1999). A similar phenomenon has also been observed in human subjects where IV meth has been reported to have a half-life of 12 h (Cook et al.,1993) compared to a cocaine half-life of just over 1.5 h (Perez-Reyes, 1994). Meth’s rapid onset of positive effects paired with its long duration of action, and the consequent delayed onset of any adverse consequences, are thought to be key factors contributing to the high abuse potential of the drug (National Institute on Drug Abuse [NIDA], 2013). In the runway, these same factors reduce the likelihood of the subjects associating the goal box with anything but the immediate positive rewarding actions of the drug, and hence no ambivalence about goal box entry develops. Indeed, IV heroin --- which also has a long duration of action – produced a behavioral profile in the runway similar to that observed here with methamphetamine (Ettenberg and Geist, 1993), and the abuse potential of heroin is significant, as evidenced by the growing national “epidemic” of heroin addiction worldwide. This is, of course, consistent with the view that the runway model can serve as a useful behavioral screen for the abuse potential of psychoactive substances (Ettenberg, 2005).”
Highlights.
Rats learned to traverse a runway for daily i.v. infusions of methamphetamine
The motivation to seek meth was reflected in faster start latencies and run times
There was no evidence of aversive/anxiogenic actions of the drug
Strong positive without aversive effects reflect meth’s high abuse potential
The runway model provides an effective screen for a substance’s abuse potential
Acknowledgments
The authors acknowledge the assistance of Alex Murphy, James McCann and Jacob Krug in the collection of the data. The work described herein was funded by a research grant from the National Institute on Drug Abuse (DA-033370) awarded to Dr. Aaron Ettenberg.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest with respect to the current study.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A, 2002. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat. Neurosci 5, 625–626. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Tien AY., Petronis KR, 1989. Epidemiologic Evidence on Cocaine Use And Panic Attacks. Am. J. Epidemiol 129, 543–549. doi:10.1093/oxfordjournals.aje.a115166 [DOI] [PubMed] [Google Scholar]
- Awasaki Y, Nojima H, Nishida N, 2011. Application of the conditioned taste aversion paradigm to assess discriminative stimulus properties of psychostimulants in rats. Drug Alcohol Depend 118, 288–294. doi:10.1016/j.drugalcdep.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Barbieri EJ, Ferko AP, Digregorio G, Ruch EK, 1992. The presence of cocaine and benzoylecgonine in rat cerebrospinal fluid after the intravenous administration of cocaine. Life Sci 51, 1739–1746. doi:10.1016/0024-3205(92)90303-7 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ, 1995. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci. Biobehav. Rev 19, 39–51. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte TJ, 2006. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci 31, 301–13. [PMC free article] [PubMed] [Google Scholar]
- Beirami E, Oryan S, Tamijani SM, Ahmadiani A, Dargahi L, 2017. Intranasal insulin treatment alleviates methamphetamine induced anxiety-like behavior and neuroinflammation. Neurosci. Lett 660, 122–129. doi:10.1016/j.neulet.2017.09.026 [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus E, Waldroup SA, Ettenberg A, 2008. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog. Neuropsychopharmacol. Biol. Psychiatry, 32, 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JN, Neugebauer NM, Bardo MT, 2012. Reinstatement of methamphetamine conditioned place preference in nicotine-sensitized rats. Behav. Brain Res 235, 158–65. doi: 10.1016/j.bbr.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng C, Tsai C, Tsai Y, Ho M, Kao S, Yu L, 2007. Methamphetamine-disrupted sensory processing mediates conditioned place preference performance. Behav. Brain Res 182, 103–108. doi:10.1016/j.bbr.2007.05.010 [DOI] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR, 1984. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 82, 6–13. [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hil l J.M., Pugh DE, Patetta PK, Sadler BM,, White WR, Perez-Reyes M,1993. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab. Dispos. 21, 717–23. [PubMed] [Google Scholar]
- Cornett EM, Goeders NE, 2013. 96-hour methamphetamine self-administration in male and female rats: a novel model of human methamphetamine addiction. Pharmacol. Biochem. Behav 111, 51–57. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR, 2009. A review of the clinical pharmacology of methamphetamine. Addiction 104, 1085–1099. [DOI] [PubMed] [Google Scholar]
- Etaee F, Asadbegi M, Taslimi Z, Shahidi S, Sarihi A, Asl SS, Komaki A, 2017. The effects of methamphetamine and buprenorphine, and their interaction on anxiety-like behavior and locomotion in male rats. Neurosci. Lett 655, 172–178. doi:10.1016/j.neulet.2017.04.043 [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF, 1982. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78, 204–209. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD, 1991. Animal model for investigating the anxiogenic effects of cocaine. Pharmacol. Biochem. Behav 103, 455–461. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist T D., 1993. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol. Biochem. Behav 44, 191–198. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD, 1999. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav 64, 507–12. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, 2004. Opponent process properties of self-administered cocaine. Neurosci. Biobehav. Rev 27, 721–728. doi:10.1016/j.neubiorev.2003.11.009 [DOI] [PubMed] [Google Scholar]
- Ettenberg A, 2009. The runway model of drug self-administration. Pharmacol. Biochem. Behav 91, 271–277. doi:10.1016/j.pbb.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Fomenko V, Kaganovsky K, Shelton K, Wenzel JM, 2015. On the positive and negative affective responses to cocaine and their relation to drug self-administration in rats. Psychopharmacology (Berl) 232:2363–2375. doi: 10.1007/s00213-015-3873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, 1994. Cocaine self-administration research: treatment implications. NIDA Re.s Monogr 145, 139–162. [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L, Richards JB 2011. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav. Processes 86, 295–304. doi: 10.1016/j.beproc.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH Ellinwood EH, 1988. Cocaine and other stimulants: Actions, abuse, and treatment. N. Engl. J. Med 318, 1173–82. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A, 1990. A simple method for studying intravenous drug reinforcement in a runaway. Pharmacol. Biochem. Behav 36, 703–706. [DOI] [PubMed] [Google Scholar]
- Goeders NE, 1988. Intracranial cocaine self-administration. NIDA Res. Monogr, 88, 199–216. [PubMed] [Google Scholar]
- Goudie A, Dickins D, Thornton E, 1978. Cocaine-induced conditioned taste aversions in rats. Pharmacol. Biochem.Behavior 8, 757–761. doi:10.1016/0091-3057(78)90279-4 [DOI] [PubMed] [Google Scholar]
- Guzman D, Ettenberg A, 2007. Runway self-administration of intracerebroventricular cocaine: evidence of mixed positive and negative drug actions. Behav Pharmacol 18, 53–60. doi:10.1097/fbp.0b013e3280144ac9 [DOI] [PubMed] [Google Scholar]
- Harro J, 2015. Neuropsychiatric Adverse Effects of Amphetamine and Methamphetamine. Int Rev. Neurobiol 120, 179–204. doi: 10.1016/bs.irn.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Hellem TL, 2016. Review of Methamphetamine Dependence and Withdrawal Treatment: A Focus on Anxiety Outcomes. J. Subst. Abuse Treat 71, 16–22. doi: 10.1016/j.jsat.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Cameron CM, Robinson TE, 2012. Repeated methamphetamine administration differentially alters fos expression in caudate-putamen patch and matrix compartments and nucleus accumbens. PLoS One 7, :e34227. doi: 10.1371/journal.pone.0034227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S, 2013. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J. Neurosci 33, 7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A, 2002. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol. Biochem. Behav 72, 931–936. [DOI] [PubMed] [Google Scholar]
- Kucerova J, Pistovcakova J, Vrskova D, Dusek L, & Sulcova A (2012). The effects of methamphetamine self-administration on behavioural sensitization in the olfactory bulbectomy rat model of depression. Int. J. Neuropsychopharmacol 15, 1503–1511. doi.org/10.1017/S1461145711001684. [DOI] [PubMed] [Google Scholar]
- Miladi-Gorji H, Fadaei A, Bigdeli I, 2015. Anxiety Assessment in Methamphetamine –Sensitized and Withdrawn Rats: Immediate and Delayed Effects. Iran J. Psychiatry,10, 150–157. [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Kooy DV, Oshaughnessy M, Bucenieks P, 1982. Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243, 91–105. doi:10.1016/0006-8993(82)91123-4 [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J, 2000. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav. Brain Res, 115, 39–47. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2013. Research Report Series: Methamphetamine https://www.drugabuse.gov/publications/research-reports/methamphetamine/letter-director (accessed 27 June 2018).
- Newton TF, de La Garza RD 2nd, Kalechstein AD, Nestor L, 2005. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol. Biochem.Behav 82, 90–97. doi:10.1016/j.pbb.2005.07.012 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Mataga N, Takashima M, Toru M., 1983. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur. J. Pharmacol 88, 195–203. [DOI] [PubMed] [Google Scholar]
- Ota M, Ogawa S, Kato K, Wakabayashi C, Kunug i H., 2015. Methamphetamine-sensitized rats show augmented dopamine release to methylphenidate stimulation: a positron emission tomography using [18F]fallypride. Psychiatry Res 232, 92–7. doi: 10.1016/j.pscychresns.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM, 2013. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend 129, 167–79. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR, Myer,s M, Sihler K, Cook CE, 1994. Comparison in humans of the potency and pharmacokinetics of intravenously injected cocaethylene and cocaine. Psychopharmacology (Berl) 16, 428–432. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, White WR, McDonald SA, Hicks RE, Jeffcoat AR, Hill JM, Cook CE, 1991. Clinical Effects of Daily Methamphetamine Administration. Clin. Neuropharmacol 14, 352–358. doi:10.1097/00002826-199108000-00007 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Maccari S, Mormède P, Le Moal M, Simon H 1990. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav. Pharmacol 1, 339–345. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H 1989. Factors that predict individual vulnerability to amphetamine self-administration. Science 245, 1511.–. [DOI] [PubMed] [Google Scholar]
- Pometlová M, Yamamotová A, Nohejlová K, Šlamberová R, 2016. Can Anxiety Tested in the Elevated Plus-maze Be Related to Nociception Sensitivity in Adult Male Rats? Prague Med. Rep 117, 185–197. doi:10.14712/23362936.2016.19 [DOI] [PubMed] [Google Scholar]
- Radke AK, Zlebnik NE, Holtz NA, Carroll ME, 2016. Cocaine-induced reward enhancement measured with intracranial self-stimulation in rats bred for low versus high saccharin intake. Behav. Pharmacol 27(2–3 Spec. Issue):133–6. doi: 10.1097/FBP.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM, 1999. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J. Pharmacol. Exp. Ther 291, 1220–1226. [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC, 1977. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol. Biochem. Behav 6, 615–620. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN,1992. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol. Biochem. Behav 43, 631–633. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Kornetsky C, 1995. Methamphetamines action on brain-stimulation reward threshold and stereotypy. Exp. Clin. Psychopharmacol 3, 112–117. doi:10.1037//1064-1297.3.2.112 [Google Scholar]
- Segal DS, Kuczenski R, 2006. Human Methamphetamine Pharmacokinetics Simulated in the Rat: Single Daily Intravenous Administration Reveals Elements of Sensitization and Tolerance. Neuropsychopharmacology, 5, 941–955. doi:10.1038/sj.npp.1300865 [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Mikulecká A, Pometlová M, Schutová B, Hrubá L, Deykun K, 2010. The effect of methamphetamine on social interaction of adult male rats. Behav. Brain Res 214, 423–427. doi:10.1016/j.bbr.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Su H, Zhang J, Ren W, Xie Y, Tao J, Zhang X, He J, 2017. Anxiety level and correlates in methamphetamine-dependent patients during acute withdrawal. Medicine (Baltimore) 96:e6434. doi: 10.1097/MD.0000000000006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, 1998. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol 56, 61–672. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) (2016). World Drug Report 2016 United Nations, New York: http://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. [Google Scholar]
- Vezina P 2004. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev 27, 827–839. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV, 1999. Methamphetamine-Induced Neurotoxicity Alters Locomotor Activity, Stereotypic Behavior, and Stimulated Dopamine Release in the Rat. J. Neurosci 19, 9141–9148. doi:10.1523/jneurosci.19-20-09141.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, 1997. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend 44, 87–94. doi:10.1016/s0376-8716(96)01324-5 [DOI] [PubMed] [Google Scholar]
- Willick ML, Kokkinidis L, 1995. Cocaine enhances the expression of fear-potentiated startle: evaluation of state-dependent extinction and the shock-sensitization of acoustic startle. Behav. Neurosci 109, 929–938. [PubMed: 8554716] [DOI] [PubMed] [Google Scholar]
- Woolverton WL, 1992. Determinants of cocaine self-administration by laboratory animals. Ciba Found. Symp 166, 149–161. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kojima H, Yokoo H, Tsutsumi T, Takamuki K, Anraku S, Nishi S, Inanaga K, 1988. Enhancement of dopamine release from striatal slices of rats that were subchronically treated with methamphetamine. Biol. Psychiatry, 24, 399–408. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE, 1992. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol. Biochem. Behav 41, 643–650. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S, 2009. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav. Brain Res 198, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


