Abstract
Introduction:
Aortic root thrombosis (ART) is a recently recognized complication of continuousflow left ventricular assist device (CF-LVAD) therapy. However, little is known about the incidence or clinical significance of this complication. The aim of our study was to systematically evaluate the incidence and significance of ART on CF-LVAD support.
Methods:
We retrospectively reviewed all patients who underwent HeartMate II or HeartWare HVAD CF-LVAD implantation from April 2004 through June 2016 at Columbia University Medical Center. Echocardiography studies were systematically reviewed to identify patients who developed ART. Study outcomes included post-ART survival on CF-LVAD support, stroke, pump thrombosis, and clinically significant myocardial infarction (MI).
Results:
The study cohort consisted of 436 CF-LVAD patients with 21 patients (4.8%) diagnosed with confirmed ART at a median time of 22 days (IQR 3 – 56 days) following CFLVAD implantation. Involvement of the non-coronary cusp was the most common location of ART (n=15, 71.4%) and concomitant RV failure occurred in 14 patients (66.7%). Actuarial survival at 1 and 2 years following the diagnosis of ART of 73.8% and 44.3%, respectively. ART was associated with a high rate of complications including stroke (28.6%, 0.337 EPPY) and clinically significant myocardial infarction (28.6%, 0.337 EPPY).
Conclusion:
Aortic root thrombosis is not uncommon following CF-LVAD implantation and is associated with significant morbidity and mortality in CF-LVAD patients. Given the early occurrence and high incidence of stroke and MI in patients who develop ART, surveillance and treatment strategies should be implemented to address this potentially devastating complication.
Introduction
Continuous-flow left ventricular assist devices (CF-LVADs) are increasingly being implanted to treat end-stage heart failure and have demonstrated clear survival benefit over optimal medical therapy and 1st generation pulsatile LVADs.1 Unfortunately complications of device support limit the benefits of this technology, leading to recurrent hospitalizations, significant morbidity and early mortality.2 Pump thrombosis has been recognized as a major complication of CF-LVAD therapy which frequently necessitates device exchange in eligible patients to restore forward flow and prevent embolic stroke.3 In the MOMENTUM-3 trial, the HeartMate 3 LVAD was shown to dramatically reduce the rate of suspected pump thrombosis to approximately 1%.4 Despite the low rate of pump thrombosis, the stroke rate remained above 10% at two years, suggesting alternative mechanisms for stroke in CF-LVAD patients.
Aortic root thrombosis has been recently recognized as a complication of CF-LVAD support. 5–8 The CF-LVAD delivers constant flow to the aortic root and this coupled with decreased preload in the native LV may lead to decreased excursion or even complete closure of the aortic valve (AV), particularly at high pump speeds. The resultant stasis in the aortic root forms a nidus for clot formation which can lead to devastating consequences including systemic embolization and stroke or even obstruction of coronary flow resulting in acute myocardial infarction (MI). However, the prevalence, clinical predictors, and outcomes of this unique thrombotic complication remain largely unknown as the available data is restricted to case reports. The purpose of this study is to systematically evaluate the prevalence, potential consequences, and optimal management strategies of aortic root thrombosis in CF-LVAD patients.
Methods
Study Population
This study analyzes patients who underwent Heartmate II (HM II, Thoratec, Inc, Pleasanton, California) or HeartWare HVAD CF-LVAD (Heartware Inc, Framingham MA) implantation at Columbia University Medical Center between April 2004 and June 2016. We included adult patients (age ≥ 18) who underwent CF-LVAD implantation for bridge-to-transplantation or destination therapy. Patients who received total artificial hearts or investigational CF-LVADs were excluded. Patient characteristics at the time of CF-LVAD implantation were collected, including patient demographics, comorbid conditions, and laboratory values. Pre- and post- operative echocardiography studies and operative reports were reviewed to identify patients with suspected ART on CF-LVAD support. All echocardiographic images of patients with suspected ART were then reviewed by a single advanced echocardiography trained cardiologist with expertise in reviewing LVAD patients in order to confirm the presence of ART and to determine clot characteristics systematically. Study outcomes included post-ART survival on CF-LVAD support, stroke, pump thrombosis, myocardial infarction. MI following discovery of the ART was defined as troponin I > 50.0 ng/mL with the upper limit of normal being 0.03 ng/mL. RV failure was defined as the requirement for prolonged (> 7 days) use of inhaled pulmonary vasodilators and inotropes and/or placement of right sided mechanical circulatory support.
Statistical Analysis
Continuous variables are reported as medians with interquartile range and compared using Mann-Whitney U test. Categorical variables are reported as frequencies and compared using chi-square or Fisher’s exact test where appropriate. Survival on LVAD support following diagnosis of ART was calculated using Kaplan-Meier estimates, censoring for cardiac transplantation. All statistical analyses were performed with the use of IBM SPSS Statistics software, version 23.0 (IBM Corp, Armonk, NY).
Results
Clinical Characteristics of Patients with ART
The study population consistent of 436 CF-LVAD patients with a median follow-up duration of 10.6 months (IQR: 4.6 – 22.3). Of these, 21 (4.8%) patients had confirmed ART. Baseline characteristics of the patients with ART compared with the patients without diagnosed ART are presented in Table 1. The only statistically significant difference between patients with and without ART was the strategy at device implantation with a higher percentage of patients with ART having LVAD implantation as destination therapy (66.7% vs. 39.8%, p=0.021). Age, gender, and device type did not have an effect on development of ART. 6 of 21 patients (28.6%) who developed ART had a history of prior sternotomy that preceded primary LVAD implantation.
Table 1.
Baseline Characteristics of CF-LVAD Patients with or without Aortic Root Thrombosis
| Variable | ART (n=21) | No ART (n=415) | p-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 63.6(52.4–68.6) | 59.6(48.0–67.1) | 0.407 |
| Female gender | 2 (9.5%) | 92 (22.2%) | 0.274 |
| Race / Ethnicity | 0.873 | ||
| White | 10 (47.6%) | 233 (56.1%) | |
| Black | 7 (33.3%) | 114 (27.5%) | |
| Hispanic | 3 (14.3%) | 46(11.1%) | |
| Other | 1 (4.8%) | 22 (5.3%) | |
| BMI, kg/m2 | 25.3 (23.0–38.1) | 25.9(22.6–30.1) | 0.754 |
| BSA, m2 | 1.91 (1.76–2.02) | 1.95 (1.78–2.13) | 0.443 |
| Device Type | 0.849 | ||
| Heartmate II | 19 (90.5%) | 370 (89.2%) | |
| Heartware | 2 (9.5%) | 45 (10.8%) | |
| Etiology of HF | 0.282 | ||
| Ischemic | 7 (33.3%) | 188 (45.3%) | |
| Non-Ischemic | 14 (66.7%) | 227 (54.7%) | |
| Strategy | 0.021 | ||
| BTT | 7 (33.3%) | 250 (60.2%) | |
| DT | 14 (66.7%) | 165 (39.8%) | |
| Aortic Valve | 0.264 | ||
| Native | 15 (71.4%) | 328 (79.0%) | |
| Native, s/p repair with LVAD | 4(19.0%) | 75 (18.1%) | |
| Native, s/p bio-AVR with LVAD | 0 (0.0%) | 3 (0.7%) | |
| s/p bio-AVR (old) | 1 (4.8%) | 6(1.4%) | |
| s/p mechanical-AVR (old) | 1 (4.8%) | 3 (0.7%) | |
| WBC count | 8.3 (7.0–9.3) | 8.0(6.4–10.3) | 0.725 |
| Hemoglobin | 11.2(9.0–12.7) | 11.1 (9.6–12.6) | 0.936 |
| Platelet | 200(189–238) | 190(148–244) | 0.301 |
| Creatinine | 1.34(1.04– 1.50) | 1.34(1.00– 1.70) | 0.855 |
| Albumin | 3.4 (3.0–3.7) | 3.6 (3.2–4.0) | 0.140 |
| Bilirubin (total) | 1.0 (0.8 −2.1) | 1.0 (0.7–1.7) | 0.531 |
| LVAD Risk Scores | |||
| HMRS | 1.70(1.12–2.30) | 1.48 (1.01 – 1.98) | 0.245 |
| MELD-Xi | 14.7(12.6–17.9) | 14.2(11.5–17.4) | 0.709 |
Pre-existing Aortic Valve Pathology
A total of 6 patients had aortic valve interventions either prior to or during LVAD implantation. One patient had a bioprosthetic AV with normal function and trace aortic regurgitation that pre-dated LVAD implant and was not intervened upon during primary LVAD implantation. One patient had a history of mechanical AV for which a patch closure was performed at the time of primary LVAD implantation. Four patients had Park’s stich repairs due to greater than mild aortic regurgitation at the time of primary LVAD implantation in accordance with the practice of our center. The 14 remaining patients did not have any additional AV interventions or pathology. No patient had significant aortic stenosis at the time of implantation or aortic root thrombosis diagnosis.
Clinical Presentation of ART in LVAD Patients
Median time to ART diagnosis was 22 days after CF-LVAD implantation (IQR 3 – 56 days), with 71.4% (n=15) developing ART within 30 days following CF-LVAD insertion (Figure 1). Table 2 describes the clinical characteristics and individual outcomes of ART patients in the study. Involvement of the non-coronary cusp was the most common location of ART (n=15, 71.4%). Concomitant RV failure was common and occurred in 14 patients with ART (66.7%) with 6 patients (28.6%) requiring surgical placement of right-sided ventricular assist device. Fifteen of 17 patients (88.2%) who had an echocardiogram pre-dating the index study showed a closed AV. Median lactate dehydrogenase (LDH) level at the time of ART diagnosis was 757 U/L (IQR 344 – 1017). Median LDH values of ART patients with concomitant device thrombosis or MI was 1014.5 U/L (IQR 554 – 1219) compared with ART patients with no device thrombosis or MI whose median LDH was 373 U/L (IQR 331 – 899, p=0.0783). LDH values of individual patients on the day of diagnosis of ART along with their complications is depicted in Figure 2.
Figure 1.
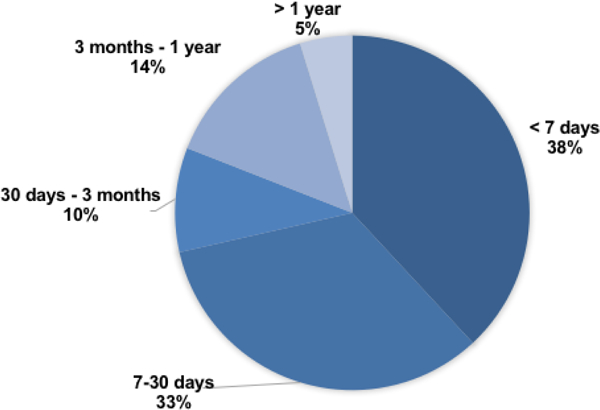
Time from CF-LVAD Implantation to Aortic Root Thrombosis diagnosis
Table 2.
Patient Level Characteristics of Aortic Root Thrombosis on CF-LVAD Support
| Patient number |
Device type | Days to Diagnosis | AV Pathology/ Intervention |
Location of Thrombus (Cusp Involvement) | Index AV Opening | Speed at Diagnosis | Ml | Pump Thrombus |
Stroke | RV failure | aPTT | INR | Days to Outcome | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HM2 | 26 | Park stich | All 3 | No | 8600 | No | Yes | Yes | No | none | 2.1 | 508 | Death |
| 2 | HM2 | 1018 | Mechanical AVR closure | N/A | No | 8800 | No | Yes | No | No | 54.6 | 1.9 | 103 | Transfer |
| 3 | HM2 | 5 | No | NCC | Yes, 1:1 | 8600 | No | No | No | Yes | 31.3 | 1.1 | 82 | Transplant |
| 4 | HM2 | 2 | No | NCC | No | 8400 | No | No | No | Yes | 46.3 | 1.7 | 159 | Transplant |
| 5 | HM2 | 2 | No | All 3 | No | 8400 | No | No | No | Yes(RVAD) | 34.1 | 1.7 | 135 | Transplant |
| 6 | HM2 | 56 | Park stich | NCC | No | 8800 | No | No | Yes | No | 49.7 | 1.8 | 108 | Transplant |
| 7 | HM2 | 190 | No | NCC, LCC | Partial | 10000 | No | Yes | No | No | none | 1.1 | 646 | Death |
| 8 | HM2 | 22 | No | NCC, LCC | No | 8400 | Yes | No | No | Yes | none | 2.7 | 150 | Transplant |
| 9 | HM2 | 3 | No | NCC | No | 8600 | Yes | No | No | Yes | 35.9 | 1.8 | 1390 | Alive on Support |
| 10 | HM2 | 4 | Pre-existing Bio-AVR |
NCC | No | 8800 | No | No | No | Yes | 42.8 | 1.5 | 1319 | Alive on Support |
| 11 | HM2 | 3 | No | LCC | Partial | 9200 | Yes | No | Yes | Yes | 33.9 | 1.3 | 40 | Death |
| 12 | HM2 | 3 | No | NCC | No | 8600 | No | No | No | Yes | 44.7 | 1.5 | 411 | Transplant |
| 13 | HM2 | 209 | No | LCC | No | 8990 | No | No | Yes | Yes | 180 | 1.1 | 759 | Death |
| 14 | HVAD | 3 | No | LCC | No | 2400 | Yes | No | No | Yes(RVAD) | 39.4 | 1.5 | 73 | Transplant |
| 15 | HVAD | 71 | Park stich | NCC, LCC | No | 2500 | Yes | No | Yes | No | 58.3 | 2.9 | 169 | Death |
| 16 | HM2 | 26 | No | LCC | Partial | 8200 | No | No | Yes | Yes(RVAD) | 90.9 | 1.2 | 38 | Death |
| 17 | HM2 | 29 | No | NCC | No | 9000 | No | No | No | Yes(RVAD) | 47.8 | 1.4 | 75 | Death |
| 18 | HM2 | 12 | No | All 3 | No | 9000 | Yes | No | No | Yes(RVAD) | 40.4 | 2.4 | 33 | Transplant |
| 19 | HM2 | 274 | No | N/A | No | 9200 | No | Yes | No | No | 30.3 | 1.1 | 82 | Transplant |
| 20 | HM2 | 10 | No | NCC | Yes, 1:1 | 8600 | No | No | No | No | 46.7 | 2.9 | 186 | Alive on Support |
| 21 | HM2 | 22 | Park stich | All 3 | No | 8800 | No | No | No | Yes | 36 | 1.4 | 435 | Alive on Support |
Figure 2.
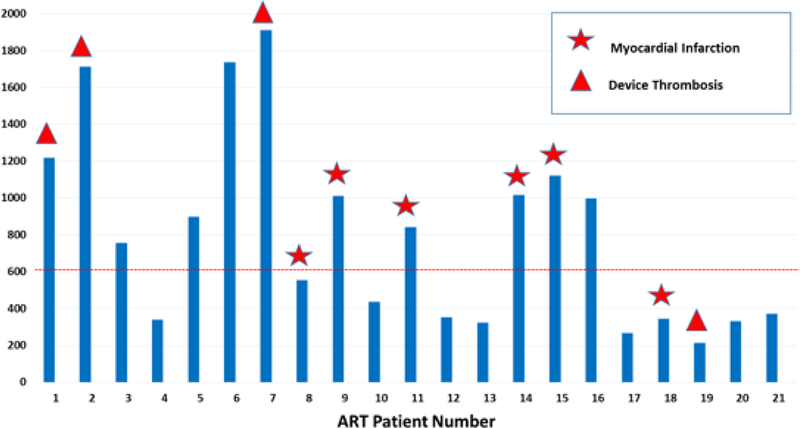
Lactate Dehydrogenase (LDH) levels on the day of Aortic Root Thrombosis diagnosis (star: patients with myocardial infarction, triangle: patients with pump thrombosis)
Anti-coagulation at the time of diagnosis of ART
Anti-coagulation was adequate (INR ≥ 2.0 or aPTT ≥ 60) in one-third of patients (n=7) at the time of diagnosis of ART. Of the 14 patients with sub-therapeutic markers of anticoagulation recorded on the day of diagnosis, 8 patients were diagnosed with ART within the first 5 days of their post-operative course when anti-coagulation is being initiated and gradually uptitrated as tolerated. The following reasons were documented for subtherapeutic anticoagulation in the remaining 6 patients: bleeding post-operative day 1 following outflow cannula bend relief repair (patient 2), early after acute stroke (patient 6), recent/active gastrointestinal bleeding (patient 7), off anti-coagulation due to recent subdural hematoma requiring burr hole decompression (patient 19), off anti-coagulation due to significant retroperitoneal bleed (patient 21), post-operative day 2 from surgical RVAD implantation with bloody chest tube output (patient 17).
Management of ART
In regards to management of ART, 15 patients (68.8%) had the speed of their LVAD devices increased following discovery of ART and 12 patients (54.5%) had their anti-coagulation regimen intensified. Furthermore, 2 patients underwent aortic root thrombectomy as a concomitant procedure when a surgical procedure was performed for an alternative reason. The first patient developed severe RV failure early in their post-operative course following implantation of a HeartMate II LVAD and were found to have extensive aortic root thrombus formation measuring 4.5 cm in diameter that was thought to be contributing to the RV failure. When the patient was taken back to the operating room on post-operative day 2 for RVAD insertion, the decision was made to perform concomitant thrombectomy. The second patient underwent HeartMate II implantation with post-operative course complicated by de novo severe aortic insufficiency as well as ART complicated by myocardial infarction, RV failure. Approximately 1 month after primary device implantation, the patient underwent bovine pericardial patch closure of the AV, tricuspid valve replacement, and aortic root thrombectomy. Operative findings demonstrated organized thrombi on the non- and left-coronary cusps occluding the left main coronary orifice with organized thrombus filling the left main.
Clinical Outcomes of Patients with ART
At the end of the study period, 6 patients were ongoing on support, 7 patients died and 8 patients were successfully transplanted. Actuarial survival at 1 and 2 years following diagnosis of ART was 73.8% and 44.3% respectively (Figure 3). In patients who were not diagnosed with ART during the study period, post-LVAD survival at 1 and 2 years was 84.7% and 76.5%, respectively. 6 (28.6%) out of the 21 patients developed a clinically significant acute MI following discovery of the ART (0.337 EPPY). Peak troponin levels of the clinically significant MIs during ART were the following: 63.3 ng/mL(patient 8), 50.4 ng/mL(patient 9), 239.9 ng/mL(patient 11), 65.2 ng/mL(patient 14), 90.9 ng/mL(patient 15), and 211.8 ng/mL(patient 18). Stroke also occurred in 6 (28.6%) out of the 21 patients (0.337 EPPY). Stroke incidence in CF-LVAD patients at our institution during the study period with no ART was 13.7% (0.096 EPPY).
Figure 3.
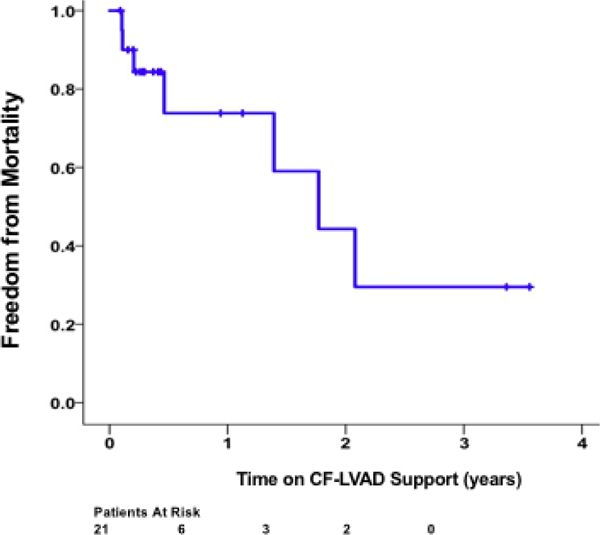
Actuarial survival of patients following diagnosis of aortic root thrombosis
Discussion
This study represents the first in-depth examination of patients who developed aortic root thrombosis during CF-LVAD support. Our principal findings are the following: (1) ART occurred in approximately 5% of the patients who underwent Heartmate II and HeartWare HVAD device during the study period; (2) ART develops most commonly in the early postoperative period (3) ART occurs more frequently in CF-LVAD patients with i.) RV failure ii.) closed aortic valves iii.) destination therapy indication; (4) ART is associated with an increased risk of stroke and poor outcomes in particular in CF-LVAD patients. Taken together, ART formation is not uncommon following CF-LVAD implantation and is associated with significant morbidity and early mortality.
The prevalence of ART was approximately 5% in our study, which is lower than pump thrombosis rates reported in patients supported with Heartmate II or HVAD LVADs9. However, this 5% rate is likely an underestimate of the true incidence of ART since patients are not routinely monitored for this complication. Moreover, the sensitivity of the transthoracic echocardiogram may not be sufficient enough to visualize aortic root thrombosis especially in patients with prior aortic valve interventions. Indeed, ART in 5 of the 21 patients in our study were initially diagnosed with transesophageal echocardiogram. Another challenge in the diagnosis of this complication is lack of established biomarkers for ART in contrast to pump thrombosis which characteristically leads to elevation in LDH or plasma-free hemoglobin. Similar to prior case reports, our study found that the non-coronary cusp was the most common location for ART which is likely due to higher stasis in this location as the result of a lack of coronary flow in this cusp (Figure 4).
Figure 4.
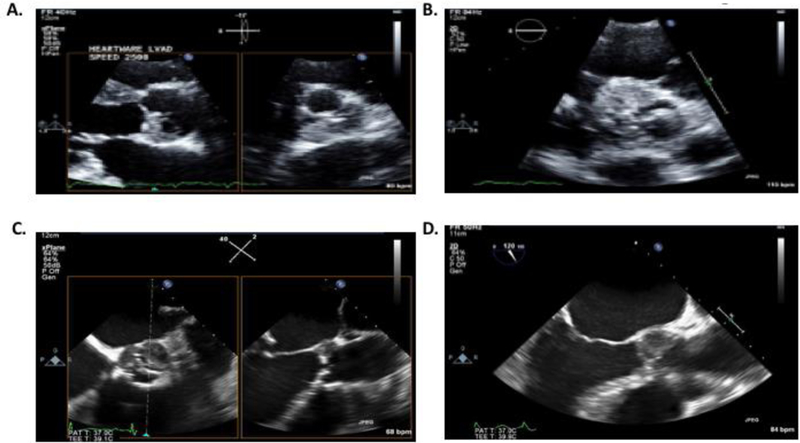
A.) Biplane trans-thoracic echo image at the aortic valve level showing aortic root thrombus on the non-coronary cusp B.) Trans-thoracic short-axis echo image of the aortic root thrombus localized primarily in the right and non-coronary cusps C.) Biplane Trans-esophageal echo demonstrating aortic root thrombosis in left- and non-coronary cusps D.) Transesophageal echo at mid-position and 120 degrees demonstrating aortic root thrombosis in the non-coronary cusp
Although the exact mechanisms responsible for development of ART remain unknown; patient, physician and device related factors are likely to contribute to this complication. Mechanical unloading with CF-LVAD decreases flow through the aortic valve and leads to decreased excursion of the leaflets or even complete aortic valve closure. The resultant stasis in the aortic root may create a milieu that favors clot formation. Indeed, a closed aortic valve was observed in 15 of 17 (88.2%) patients who had an echocardiogram predating the index study. Similarly, RV failure may reduce LV filling and decrease flow through the aortic valve which can further precipitate clot formation in the aortic root. Concomitant RV failure was present in 14 patients with ART (66.7%) with 6 patients (28.6%) requiring surgical placement of right-sided ventricular assist device. Moreover, device outflow graft orientation, in particular the angle at which the graft enters the aorta, has been shown to effect fluid dynamics of the proximal aorta and further impact stasis along the aortic root10. Insufficient anti-coagulation is also likely to play a role in the genesis of ART with two-thirds of patients in our study having markers of subtherapeutic anti-coagulation at the time of ART diagnosis. However, since ART generally develops in the early post-operative period, use of therapeutic anticoagulation must be carefully weighed against the risk of causing or exacerbating post-operative bleeding. Recognition of the risk of ART is particularly pertinent in current era as protocols utilizing lower anti-coagulation thresholds are being tested for new generation continuous-flow devices with minimal risk of pump thrombosis.11
Our study suggested that ART has significant consequences with nearly half of patients developing clinically significant MI and/or stroke. Despite a left ventricle that is relatively protected from hemodynamic collapse due to the LVAD, coronary flow obstruction caused by ART can still have deleterious effects via two primary mechanisms. First reduced coronary flow can cause RV infarction and failure. Second, it can precipitate ventricular arrhythmias which also can lead to RV failure and underfilling of the LV. While RV failure can be ultimately managed by placement of percutaneous or surgical RVAD, disabling stroke is generally irreversible and may impact transplant eligibility as well as patient outcomes. It is important to note that ART may represent a novel mechanism for the development of stroke in CF-LVAD patients, which remains a challenge even in patients with new generation devices which dramatically reduce the rates of pump thrombosis4.
A number of management strategies can be employed to minimize risk of ART and its associated complications. Given the ART risk in patients with closed aortic valves, we perform routine echocardiography in early post-implantation period to optimize pump speed and allow for AV opening. Patients with post-implant RV failure are typically at higher risk and should be screened for AV opening and presence of ART. Once ART is detected, serial cardiac biomarkers and ECGs should be obtained to rule out clinically significant myocardial infarction. Coronary angiography is typically avoided due to risk of distal embolization. Frequent neurological assessment should also be performed in patients with ART due to the risk of embolization to cerebral vessels. We typically increase the pump speed at the time of ART diagnosis in an effort to close the AV and theoretically limit the risk of distal embolization. We also intensify anticoagulation in absence of significant bleeding. We do not advocate for routine thrombectomy given the associated surgical risks. Due to the high morbidity and mortality associated with ART, we suggest that patients listed for transplant should be considered for an upgrade to a higher priority status for organ allocation if diagnosed with this complication. Given the paucity of data on this complication to guide management, we advocate for a multidisciplinary approach emphasizing shared decision making to address these patients on a case-by-case basis.
Limitations:
This is a retrospective study of a large academic institution with limitations inherent to this study design. First, the number of ART cases were limited which precluded utilization of advanced statistical models to identify risk factors associated with this complication. Second, our institution did not have a specific protocol for echocardiographic surveillance of ART at the time of our study, and therefore discovery of this complication occurred only during studies performed for other purposes. However, if anything, this may lead to an underestimation of the number of patients who develop this complication. Notably we limited our study population to only patients that were implanted with FDA approved devices at the time of our study. While the relative number of HeartMate II to HVAD devices actually mirrors how our institution utilized the devices during the study period, we did not account for the notable differences in these two devices. Notwithstanding these limitations, we feel the frequency of this complication and the magnitude of increased morbidity and mortality associated with it warrant further investigation in prospective and multi-center studies.
Conclusions:
Our findings demonstrate that ART is not uncommon in CF-LVAD patients and associated with significant morbidity and mortality. Early recognition of this complication may impact management and lead to improved outcomes in affected individuals. Future research will establish whether speed algorithms allowing for intermittent aortic valve opening may decrease the likelihood of developing this complication. These findings warrant further investigation of ART in larger multi-center studies.
Acknowledgments
Financial Support: This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Footnotes
Disclosures:
Dr. Topkara is supported by National Institutes of Health Grant No. UL1TR001873. Dr. Garan is supported by National Institutes of Health Grant No. KL2TR001874, has previously received honoraria from Abiomed (Danvers, MA) and is now an unpaid consultant for Abiomed. Dr. Naka has received consulting fees from St. Jude Medical (St. Paul, MN). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Industry Relationships: Dr. Naka received consulting fees from Abbot. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. [DOI] [PubMed] [Google Scholar]
- 2.Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61(2):153–163. [DOI] [PubMed] [Google Scholar]
- 3.Levin AP, Saeed O, Willey JZ, et al. Watchful Waiting in Continuous-Flow Left Ventricular Assist Device Patients With Ongoing Hemolysis Is Associated With an Increased Risk for Cerebrovascular Accident or Death. Circ Heart Fail. 2016;9(5). [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR, Goldstein DJ, Uriel N, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N Engl J Med. 2018;378(15):1386–1395. [DOI] [PubMed] [Google Scholar]
- 5.Demirozu ZT, Frazier OH. Aortic valve noncoronary cusp thrombosis after implantation of a nonpulsatile, continuous-flow pump. Tex Heart Inst J. 2012;39(5):618–620. [PMC free article] [PubMed] [Google Scholar]
- 6.Crestanello JA, Orsinelli DA, Firstenberg MS, Sai-Sudhakar C. Aortic valve thrombosis after implantation of temporary left ventricular assist device. Interact Cardiovasc Thorac Surg. 2009;8(6):661–662. [DOI] [PubMed] [Google Scholar]
- 7.Fried J, Han J, Naka Y, Jorde UP, Uriel N. Myocardial infarction after left ventricular assist device implantation: clinical course, role of aortic root thrombus, and outcomes. J Heart Lung Transplant. 2014;33(1):112–115. [DOI] [PubMed] [Google Scholar]
- 8.Barrick BP, Smeltz A, Ganesh A, Arora H, Kumar PA. Aortic Valve Thrombus in a Patient With an Extracorporeal Left Ventricular Assist Device: The Dilemma of Management. Journal of Cardiothoracic and Vascular Anesthesia. 2016;30(1):196–199. [DOI] [PubMed] [Google Scholar]
- 9.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376(5):451–460. [DOI] [PubMed] [Google Scholar]
- 10.Callington A, Long Q, Mohite P, Simon A, Mittal TK. Computational fluid dynamic study of hemodynamic effects on aortic root blood flow of systematically varied left ventricular assist device graft anastomosis design. J Thorac Cardiovasc Surg. 2015;150(3):696–704. [DOI] [PubMed] [Google Scholar]
- 11.Netuka I, Ivak P, Tucanova Z, et al. Evaluation of low-intensity anti-coagulation with a fully magnetically levitated centrifugal-flow circulatory pump-the MAGENTUM 1 study. J Heart Lung Transplant. 2018;37(5):579–586. [DOI] [PubMed] [Google Scholar]


