Abstract
Background:
Models of power delivery within an intact organism have been limited to ionizing radiation and to some extent sound & magnetic waves for diagnostic purposes. Traditional electrical power delivery within intact human body relies on implanted batteries that limit the amount and duration of delivered power. The efficiency of current battery technology limits substantial demand to be met; such as continuous operation of an implantable artificial heart pump within a human body.
Methods:
Fully implantable, miniaturized FREE-D system compatible with any type of ventricular assist device (VAD) has been tested in a swine model (HVAD) for up to three hours. Key features of the system, the use of high quality factor (Q) resonators together with an automatic tuning scheme, were tested over an extended operating range. Temperature changes of implanted components were measured to address safety and regulatory concern of the FREE-D system in terms of specific absorption rate (SAR).
Results:
Dynamic power delivery using adaptive tuning technique kept the system operating at maximum efficiency, dramatically increasing the wireless power transfer within a one meter diameter. Temperature rise in the FREE-D system never exceeded the maximum allowable temperature deviation of 2 °C (but remained below body temperature) for an implanted device within the trunk of the body at 10 cm (25% efficiency) and 50 cm (20% efficiency) with no failure.
Conclusions:
Large operating range of FREE-D system extends the use of VAD for nearly all patients without being affected by the depth of the implanted pump. Our in-vivo results of FREE D system may offer a new perspective on quality of life for patients supported by implanted device.
Introduction
There are various biological phenomena that can be altered or enhanced using electrical stimuli, some of these are endogenous and others are exogenous; as in the case of pacemakers that use batteries as a power source. Biological organisms are constantly exposed to variety of external electromagnetic forces, however delivery of such forces within the body for utilization is very limited. Exploiting the revolution in digital microelectronics for sensing and stimulating deep seated human organs is hampered since the battery technology does not obey rules such as the Moore’s law governing processing power and has remained unchanged in its size constraints for the last few decades. Meanwhile, considerable progress has been made in understanding organ failure such as end stage heart failure, which can be successfully addressed by implantation of artificial heart pumps (Left Ventricular Assist devices or Total Artificial Hearts), which essentially supplant the pumping action of the human heart. Such pumps require constant high electrical energy for safe and effectual functioning needing constant electrical supply unmet by existing battery technology. The only way these pumps function uninterrupted is by connecting them to an external electrical power source across their pierced skin that carries the electrical wire; Violating the natural barrier of skin leads to infection and contamination. Another unintended consequence of powering in this manner is making the person supported by such device to lead life tethered to an electrical power source, thus hampering quality of life & negating the benefit offered by the artificial heart pump also known as ventricular assist device (VAD).
The greatest challenge for VADs adopting wireless power, however, is that VADs require 5–25W of power – a huge amount for an implanted device. Transcutaneous Energy Transfer system (TETS), which have been tried before1,2 presented significant limitations for practical use despite their successful applications shown in the early generation of VADs and artificial hearts3: Intolerance to misalignment between the external transmit coil and the implanted receive coil; high dependence on the separation distance between transmit coil and receive coil, at most 10 – 20 mm, which is not sufficient range for all patients, considering VAD patients vary drastically in size and body type. The inflexible range and misalignment between the coils diminish the practicality of the system. These systems, however were remarkable in reducing overall infection rate in these very sick group of patients.
Unlike TETS which uses inductive power transfer, our previously developed Free-range Resonant Electrical Energy Deliver (FREE-D) system utilizes magnetic resonance power transfer, which can lead to improved efficiency across greater distances if proper tuning mechanisms are employed4. Performance and characteristics of FREE-D system for both short and long-range have been investigated and refined throughout extensive in-vitro studies using HVAD (Medtronic, Minneapolis, MN) and Heartmate 2, HeartMate 3 (Abbott, Chicago, IL), Jarvik 2000 (Jarvik Heart Inc, New York, NY) and experimental TAH systems in terms of the relation among pump flow, pump speed, pump power and temperature4–7. Demo videos from our previous in-vitro work showing FREE-D system being able to rapidly adapt to various distances and misalignment between resonators and run Heartmate 2 within portable configuration are online6.
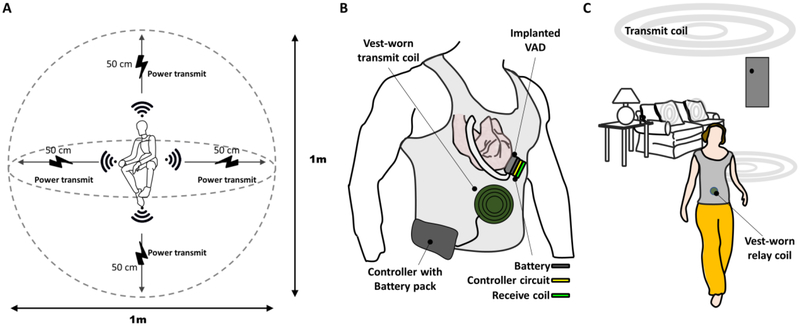
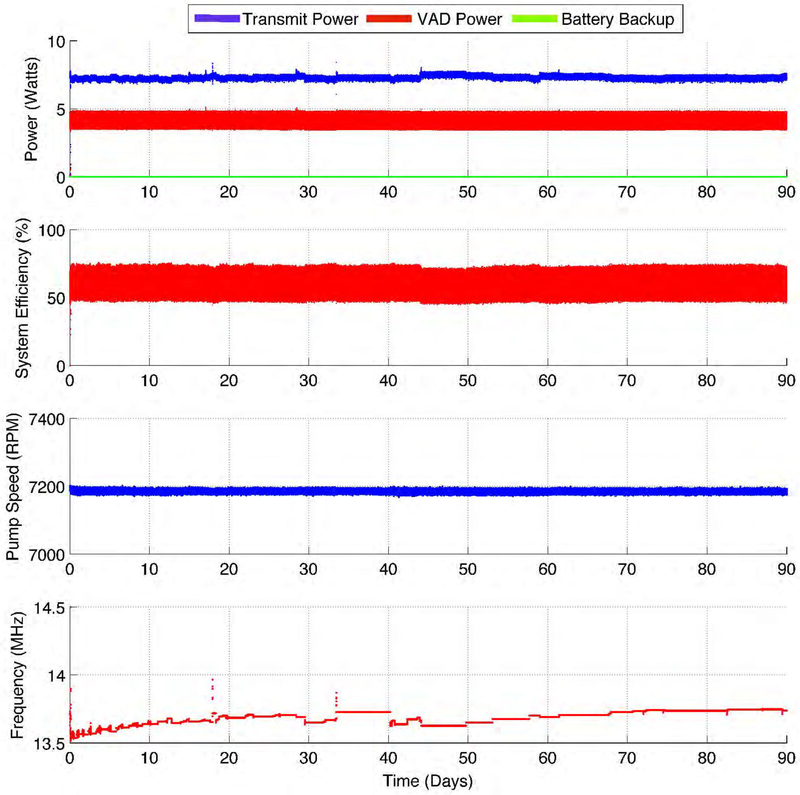
In this work, we present in-vivo results of the FREE-D system working across short range of 10 cm and long-range of 50 cm distance, which essentially signifies its effective three-dimensional working space as 1 meter – diameter sphere in respect to the VAD patient at the center (Figure 1A), showing the step toward the vision of the FREE-D system for improving VAD patients’ quality of life (Figure 1B and 1C). The FREE-D system, proven to run continuously for 90 days in-vitro without any interruptions or faults (Figure 2), was successfully operated within the maximum allowable temperature rise of 2 °C in-vivo, which accords to the Food and Drug Administration (FDA) and the Federal Communications Commission (FCC) requirements8.
Figure 1. Vision of FREE-D system for patients with LVAD.
(A) Conceptual schematic showing extendable range of wireless power transfer in three-dimensional space within a long-range configuration. (B) Artistic representation of FREE-D system transferring wireless power from the vest-worn transmit coil to the implanted receive coil for nearly any angular orientation. (C) Artistic representation of specific scenarios where the long-range FREE-D system can significantly improve VAD patients’ quality of life with transmit coils installed throughout the patient’s home and around the patient’s household items.
Figure 2. Long-term continuous operation of the FREE-D system.
The system was continuously run in bench top preparation for 90 days without any interruptions or faults. Power consumption, system efficiency, and pump speed were all stably maintained.
Materials and Methods
Theoretical basis of FREE-D operation
The FREE-D system consists of two magnetically coupled coils, transmit and receive coil, producing high quality factor (Q) resonances (see Figure S1 and S2 in the Supplementary Material). Each coil contains two elements, a single-turn drive loop and a multi-turn coil, which can be configured either combined or in a separated fashion. Alternating current (AC) signal delivered to the transmit coil induces oscillating magnetic field dispersed in all directions. The magnetic field that oscillates at a specific resonant frequency induces AC signal in the receive coil, which is then converted into a direct current (DC) voltage by a rectifier connected to it to power the implanted system controller. With varying distance or orientation between the transmit and receive coil, the specific resonant frequency at which two coils efficiently exchange energy by sharing magnetic field changes. The dynamic tuning scheme, one of the key features that distinguishes the FREE-D system from prior inductive coupling technology, enables the system to rapidly adapt to these variations and select the ideal resonant frequency, achieving the maximum operating efficiency5,9,10 (see Dynamic Power Delivery section in Supplementary Material for further details). High efficiency is extremely important for regulatory compliance of wirelessly powered implanted medical devices. Higher efficiency implies lower transmit power levels for the same amount of power delivered to the VAD, which means lower field strengths and safer operating conditions for humans around the FREE-D system. The system-level block diagram of the FREE-D system shown in Figure S3 in the Supplementary Material outlines the design considerations for both the hardware and software associated with each element. Animation showing theoretical concept of FREE-D system is online6.
Animal Preparation
The Yale University Institutional Animal Care and Use Committee granted approval of all experimental protocols. Eight Yorkshire pigs (both male and female; 41 – 50 kg) were anesthetized based on a refined protocol that significantly improved outcomes compared to the standard swine anesthetic regimen by properly maintaining cardiac function without ventricular fibrillation during entire period of in-vivo experiments11
Surgery and Instrumentation
HeartWare HVAD was used for all eight in-vivo experiments. A median sternotomy was used for the surgical approach, which was performed without the use of cardiopulmonary bypass. Teflon pledgeted sutures were placed radially around the left ventricular apex and the inflow cannula tip was inserted and seated at the apex. The outflow graft was connected to ascending aorta in a standard fashion. FREE-D receiver which contains controller circuit and backup battery was mounted directly beneath the HVAD for optimal heat dissipation. We have previously demonstrated an implantable controller with an extremely small foot print which is capable of producing an artificial pulse timed with patient ECG12,13. They are integrated in a compact design having a small footprint of 40–50 mm diameter14. Further details for transmitter & receiver design, pressure, flow, and ECG measurement are described in the Supplementary Material.
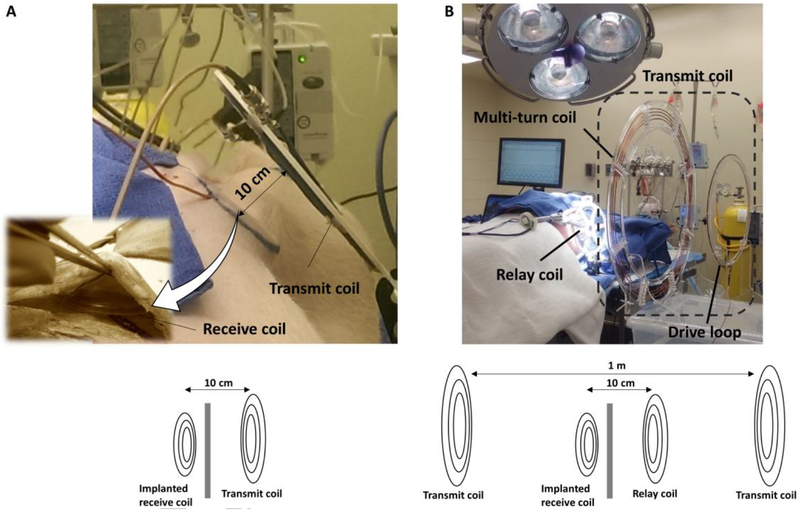
After implantation, an external power supply was used to startup the VAD due to the high initial power consumption required by the magnetically levitated motor. After initial power-up, the receive coil was placed beneath the skin as shown in Figure 3A. The external transmit coil directly connected to the transmitter was held 10 cm away from the surface of the skin for short-range configuration and 50 cm for long-range configuration within an additional external coil as a relay coil placed 10 cm away from the skin, as shown in Figure 3. Temperature, pressure and flow sensors were installed around the receive coil and around the animal’s heart to analyze any impact that the wireless power may potentially have on the cardiac function of the animal. A FLIR E4 thermal camera (FLIR Systems AB, Sweden) was also in place to monitor the temperature of the animal and the coils.
Figure 3. In-vivo FREE-D system configurations.
(A) Short-range configuration where the external transmit coil directly connected to the FREE-D transmitter stand 10 cm away from the implanted receive coil beneath the animal skin. (B) Long-range configuration where the large external transmit coil directly connected to the FREE-D transmitter stand 50 cm away from the implanted receive coil with an additional external coil placed between them as a relay coil, 10 cm away from the skin.
The average pump speed was measured by back emf (electromagnetic field) from the motor controller, and the average pump power was measured by voltage and current sense amplifiers on the receiver circuit. Thermocouples were placed inside the skin above the receive coil, directly above the receive coil, directly below the receive coil, and core temperature. The maximum temperature rise during wireless power operation was measured at each position.
Statistics
Power efficiency was measured in real-time for all eight experiments during the entire duration of each experiment. Averaging power efficiency variations measured from each experiment, four data set for short- and four data set for long-range configuration were considered in the analysis. Two-sample t-test was used at significance level (p value) of 0.01.
Results
Preclinical applications in-vivo
For every experiment, HVAD was successfully implanted, and the pigs remained in stable condition on wirelessly powered HVAD support for the full procedure duration. Pump speed was between 1700 and 2500 RPM in-vivo experiments to produce pump flow between 2–4 L/min. Experiments were performed with anesthetized swine where the heart continued to beat during entire procedure.
In the short-range configuration, the external transmit coil and the implanted receive coil beneath the animal skin were 10 cm apart with the FREE-D transmitter directly connected to the external transmit coil (Figure 3A). In the long-range configuration, the external transmit coil connected to the FREE-D transmitter is 50 cm apart from the implanted receive coil within an additional external coil placed 10 cm away from the skin of the animal just like the short-range configuration, acting as a relay coil (Figure 3B).
After all FREE-D system components and measurement probes were in place, the wireless power was enabled using a combination of frequency tracking, adaptive impedance matching, and power tracking. In every experiment, the wireless power successfully powered the HVAD without any contribution from the backup battery power.
The mean and standard deviation for time duration, animal weight, pump speed, and pump power for all eight experiments were 125.5 ± 33.1 min, 46.9 ± 3.1 kg, 2210 ± 230.0 RPM, and 2 ± 0.3 W. The time duration refers to the length of time that the HVAD was powered by wireless power.
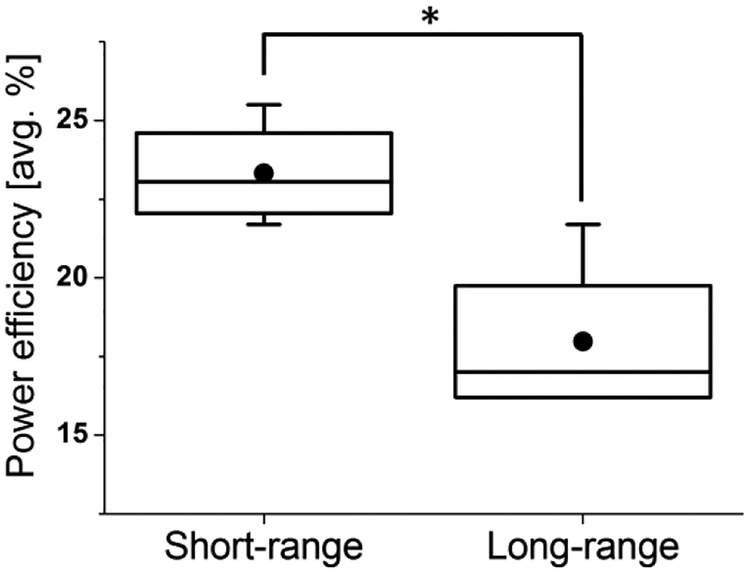
The system efficiency, which represents power delivered to the VAD divided by power input to the transmitter, was significantly higher in the short-range configurations, indicating better efficiency (Figure 4 and Figure 5). However, the long-range configuration achieved a 10x improvement in range with only a 7.5% efficiency reduction. Also, the efficiency can be correlated to the pump speed and power level, which varied between each experiment. For lower power levels, the efficiency was lower because the static power consumption to perform adaptive tuning consumes approximately 0.7W. Compared to humans, the pigs do not require as high of a blood flow rate, and consequently the power level was much lower for the animal experiments.
Figure 4. Comparison of power efficiency between short- and long- range configuration.
The average system efficiency was significantly higher for short-range configuration compared to the long-range configuration (p<0.01). Dot and horizontal bar inside the box indicates mean and median value respectively.
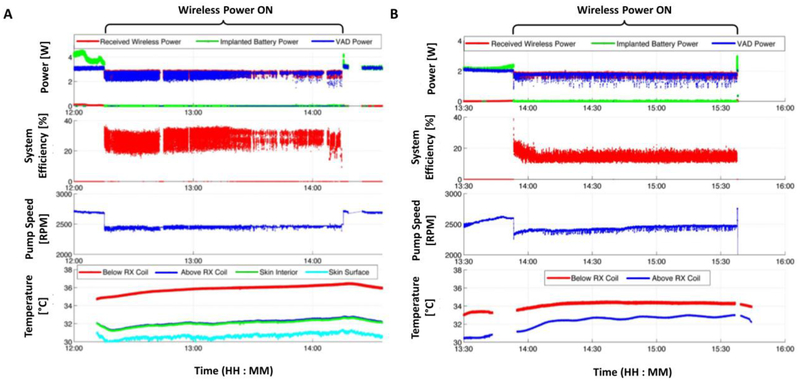
Figure 5. In-vivo FREE-D system performance measurements for short- and long-range configuration.
For both experiments, the adaptive tuning techniques allow for the system to maintain seamless wireless power delivery, without any backup battery assist. The VAD power and system efficiency fluctuations were attributed to systolic and diastolic cycles or expansions and contractions of the animals’ chest while they breathe. Measurements of wireless power delivered to the receiver, backup battery power, load power, pump speed, wireless power transfer efficiency, and temperature rise during (A) short- and (B) long- range configuration in-vivo experiment.
Effect on local and body temperature
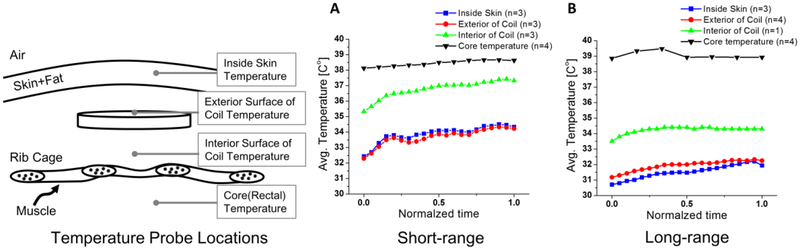
The temperature measurements show that the heat generated by the FREE-D system never exceeded the maximum allowable temperature deviation of 2 ° C for an implanted device inside the trunk of the body (Figure 5 and Figure 6). The greatest temperature rise occurred above the receive coil. This result can be understood by considering that closer to the surface of the skin, the natural air flow helps regulate temperature. Similarly, closer to the inside of the body, blood flow and thermoregulation helps maintain a more constant temperature as blood carries heat away from the coil. Directly above the receive coil, both of these thermoregulating mechanisms would be minimal, resulting in the greatest temperature rise. For example, the temperature below the coil was 33.45 C before wireless power was enabled. Over the next 45 minutes, the temperature below the coil rose to a maximum of 34.46° C and maintained a relatively stable temperature for the duration of the experiment until wireless power was disabled and the coil temperature returned to its initial value. The temperature probe above the receive coil (closer to the surface of the skin) started at 31.18° C and rose to a maximum temperature of 32.94 ° C. The oscillations of this temperature measurement were attributed to the temperature control of the room itself. At the end of the experiment, the HVAD was turned off and the experiment concluded.
Figure 6. Average temperature changes at four different locations between (A) short- and (B) long- range configuration.
Thermocouples were placed at four different locations, inside the skin above the receive coil, directly above the receive coil, directly below the receive coil, and core temperature. The greatest temperature rise occurred at directly above the receive coil. All temperature measurements never exceeded the maximum allowable temperature deviation of the body, 2 °C, for an implanted device inside the trunk.
Temperature rise in the FREE-D system could be predominantly caused by thermal conduction and thermal radiation. Current flowing through the receive coil dissipated as heat across the small resistance presented by the turns in the coil, and heat conductively transferred to the body. Thermal radiation caused by the RF energy from the wireless power transfer could also generate heat across the conductive tissues inside the body. However, the key result that has been repeatedly demonstrated through the animal experiments was that the FREE-D system does not generate temperature rise inside the body.
While the wireless power was enabled during the long-range experiment, the battery power never contributed to the load. The VAD power fluctuated due to the animal’s healthy heart changing mechanical load presented to the VAD every systolic and diastolic cycle. The efficiency also changed not only because of the changing load power, but also because of the animals’ chest expansion and contraction while they breathe, thus the distance between the coils was constantly changing by a couple of centimeters. The dynamic tuning techniques allowed for the system to maintain seamless wireless power delivery, without any backup battery assist.
We could successfully supply the wireless power on the implanted device on animals at a distance of 50 cm away in either right or left side, accomplishing one meter distance wireless power delivery.
Discussion
These results show that the FREE-D system can transfer wireless power up to a meter distance in all directions, which can lead to improvement in quality of life of patients with an implanted device.
To extend the range of the wireless power from the transmit coils directly connected to the transmitter to the implanted receive coil, the FREE-D system consists of external coils between them as relay coils, with the one near the skin, for example in a form of the vest-worn (vest-coil, Figure 1B). Other relay coils or transmit coils can ultimately be hidden inside wall, floors, couches, tables and beds. Although it may not be practical for the patient to completely remove the vest-coil everywhere inside the household because of technological limitations in transmitting power from a large transmit coil to a small, implanted receive coil, the unique advantage for the patient is that the bulky transmitter circuit does not need to be carried around, allowing the patient to experience maximal mobility throughout their household – unconstrained by heavy body-worn batteries and circuitry. The patient may be able to move freely throughout his home, while receiving power from large transmit coils placed strategically around his home (Figure 1C) akin to accessing wifi for internet. With external coils placed beneath the patient’s bed, the patient may be able to remove the vest-coil while sleeping at night. For outside activities, patients simply go out with vest-coil connected to body-worn batteries and circuitry. Therefore, FREE-D technology will work just like how the current WiFi signal works for accessing internet. As the signal is strongest in and around the house (porch, backyard, garden area) there is seamless and uninterrupted access to WiFi. As one drives away from the home, the signal will become weak and fade away, making one to rely on the cellphone towers to allow data access. However, in case of the VAD, one would simply carry the vest based portable coil and its batteries to have freedom of moving around (just as the current VAD patients rely on batteries when outside of the house in a restaurant or shopping or recreational areas). The vest based system can be externally powered via car charger or any wall outlet if needed.
In our design considerations, we did an informal survey of all household chores that would need full freedom from an external vest coil. Such tasks included daily routine hygiene tasks such as showers and bath. And recreational activity such as swimming or physical activity leading to excess body sweat. When considering all such activities in a healthy adult, they rarely exceed one hour (data not presented). In view of this we feel an implanted battery time of 90 minutes to 120 minutes should give a good balance between total freedom of movement and conserving the implanted battery life to prevent repeated replacements. With nominal power consumption less than 5 watt, the size of implanted battery can be as small as approximately 50–60 mm in width and height and 5 mm in thickness (Figure S4) for two-hour use.
The FREE-D system uses the 13.56 MHz industrial, scientific and medical (ISM) frequency band. At this frequency and at the power level of approximately 1–5 W in the in-vivo experiments, the primary safety and regulatory concern was the specific absorption rate (SAR). SAR limits are in place to limit the extent that RF energy can cause tissue heating7,15,16.
However, proper techniques for measuring SAR in-vivo have not yet been established at these sub-30 MHz frequency bands. A related metric acknowledged by both the FDA and FCC requires that the maximum temperature rise from induced RF energy inside the trunk of the body does not exceed 2 °C. Therefore, in this work we relied on direct temperature measurements to demonstrate that the FREE-D system operates within the maximum allowable temperature rise of 2 °C in its current embodiment.
Prior studies on fully implantable systems have shown significant reduction of overall infection rate1,2. This is in spite of the fact that a very sick cohort of patients was selected for both these studies. A more likely inference from these is that eliminating an externally communicating driveline does have impact on overall infection rates in recipients of ventricular assist device and total artificial hearts. Over the years there have been significant discussions in the VAD community towards achieving a goal of reduced adverse events by eliminating drivelines for power delivery. We sincerely hope that the current investigation will be able to swing the pendulum in favor of eliminating driveline for the future designs of pumps17,18.
Supplementary Material
Acknowledgments
Disclosure statement: The authors have no conflicts of interest to disclose. This work was supported by the following funding sources: NIH/NHLBI, 1R21HL118611–0.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Banayosy A, Arusoglu L, Kizner L, et al. Preliminary experience with the LionHeart left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg. 2003;75:1469–1475. [DOI] [PubMed] [Google Scholar]
- 2.Dowling RD, Gray LA, Etoch SW, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg. 2004;1271:131–141. [DOI] [PubMed] [Google Scholar]
- 3.Knecht O, Bosshard R, Kolar JW. High-Efficiency Transcutaneous Energy Transfer for Implantable Mechanical Heart Support Systems. IEEE T Power Electr. 2015;30:6221–6236. [Google Scholar]
- 4.Sample AP, Meyer DA, Smith JR. Analysis, Experimental Results, and Range Adaptation of Magnetically Coupled Resonators for Wireless Power Transfer. IEEE T Ind Electron. 2011;58:544–554. [Google Scholar]
- 5.Waters BH, Smith JR, Bonde P. Innovative Free-Range Resonant Electrical Energy Delivery System (FREE-D System) for a Ventricular Assist Device Using Wireless Power. ASAIO J. 2014;60:31–37. [DOI] [PubMed] [Google Scholar]
- 6.Wang JX, Smith JR, Bonde P. Energy transmission and power sources for mechanical circulatory support devices to achieve total implantability. The Annals of thoracic surgery. 2014;97:1467–74. [DOI] [PubMed] [Google Scholar]
- 7.Christ A, Douglas MG, Roman JM, et al. Evaluation of Wireless Resonant Power Transfer Systems With Human Electromagnetic Exposure Limits. IEEE T Electromagn C. 2013;55:265–274. [Google Scholar]
- 8.US FOOD AND DRUG ADMINISTRATION, et al. Guidance for industry: Guidance for the submission of premarket notifications for magnetic resonance diagnostic devices. 1998.
- 9.Sample AP, Waters BH, Wisdom ST, et al. Enabling Seamless Wireless Power Delivery in Dynamic Environments. P IEEE. 2013;101:1343–1358. [Google Scholar]
- 10.Waters BH, Sample AP, Smith JR. Adaptive Impedance Matching for Magnetically Coupled Resonators. Prog Electromagn Res. 2012;694–701. [Google Scholar]
- 11.Goodrich JA, Bouwmeester JC, Letzen BS, et al. Refinement of an anesthesia protocol fora porcine model for a FREE-D powered ventricular assist device. J Invest Surg. 2015;28:60. [Google Scholar]
- 12.Bouwmeester JC, Park J, Geirsson A, et al. Quantification of Pulsed Operation of Rotary Left Ventricular Assist Devices with Wave Intensity Analysis. ASAIO J. 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouwmeester JC, Park J, Valdovinos J, et al. Wave Intensity Analysis of Right Ventricular Function during Pulsed Operation of Rotary Left Ventricular Assist Devices. ASAIO J. 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgari SS, Bonde P. Implantable physiologic controller for left ventricular assist devices with telemetry capability. J Thorac Cardiovasc Surg. 2014;147:192–202. [DOI] [PubMed] [Google Scholar]
- 15.Christ A, Douglas M, Nadakuduti J, et al. Assessing human exposure to electromagnetic fields from wireless power transmission systems. P IEEE. 2013;101:1482–1493. [Google Scholar]
- 16.Kyriakou A, Christ A, Neufeld E, et al. Local tissue temperature increase of a generic implant compared to the basic restrictions defined in safety guidelines. Bioelectromagnetics. 2012;33:366–374. [DOI] [PubMed] [Google Scholar]
- 17.Valdovinos J, Bouwmeester JC and Bonde P Preliminary Design and Testing of a Cavo-Arterial Pump Utilizing Axial Magnetic Couplings. Journal of Medical Devices. 2018;12: 011001. [Google Scholar]
- 18.Letzen B, Park J, Tuzun Z, & Bonde P Design and Development of a Miniaturized Percutaneously Deployable Wireless Left Ventricular Assist Device: Early Prototypes and Feasibility Testing. ASAIO J. 2018;64:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.