Abstract
Background
The Glasgow Coma Scale (GCS) is an essential coma scale in critical care for determining the neurologic status of patients and for estimating their long-term prognosis. Similarly, cerebral autoregulation (CA) monitoring has shown to be an accurate technique for predicting clinical outcomes. However, little is known about the relationship between CA measurements and GCS scores among neurological critically ill patients. This study aimed to explore the association between non-invasive CA multimodal monitoring measurements and GCS scores.
Methods
Acutely comatose patients with a variety of neurological injuries admitted to a neurocritical care unit were monitored using near-infrared spectroscopy (NIRS) based multimodal monitoring for up to 72 hours. Regional cerebral oxygen saturation (rScO2), cerebral oximetry index (COx), GCS, and GCS motor data were measured hourly. COx was calculated as a Pearson correlation coefficient between low-frequency changes in rScO2 and mean arterial pressure (MAP). Mixed random effects models with random intercept was used to determine the relationship between hourly NIRS-based measurements and GCS or GCS motor scores.
Results
A total of 871 observations (hours) were analyzed from 57 patients with a variety of neurologic conditions. Mean age was 58.7 ± 14.2 years and the male to female ratio was 1:1.3. After adjusting for hemoglobin and PaCO2, COx was inversely associated with GCS ( =−1.12, 95%CI=−1.94 to −0.31, P=0.007) and GCS motor score ( =−1.06, 95%CI=−2.10 to −0.04, P=0.04). In contrast rScO2 was not associated with GCS ( =−0.002, 95%CI=−0.01 to 0.01, P=0.76) or GCS motor score ( =−0.001, 95%CI=−0.01 to 0.01, P=0.84).
Conclusions
This study demonstrated that fluctuations in GCS scores are inversely associated with fluctuations in COx; as COx increases (impaired autoregulation), more severe neurological impairment is observed. However, the difference in COx between high and low GCS is small and warrants further studies investigating this association. CA multimodal monitoring with COx may have the potential to be used as a surrogate of neurologic status when the neurologic examination is not reliable (i.e. sedation and paralytic drug administration).
Keywords: Glasgow coma scale, near infrared spectroscopy, cerebral autoregulation, multimodal monitoring
Introduction
The Glasgow Coma Scale (GCS) is the most commonly used and well validated coma scale to rapidly assess the patient’s neurological status.(1) This scale was created by Jennett & Teasedale in the early 1970’s and has been the gold standard of continuous neurological assessment for critically ill patients.(2) It is not only reliable, but simple to use at the time of injury and for subsequent patient monitoring.(1,3) More importantly, there is strong evidence supporting the use of post-resuscitation admission GCS score as a predictor of long-term prognosis of patients with acute traumatic brain injury (TBI) and other neurological illnesses including aneurysmal subarachnoid hemorrhage (aSAH), intracerebral hemorrhage, and ischemic stroke.(3–7) GCS is composed of three different components assessing different responses including best verbal, best eye, and best motor responses. The motor component is more descriptive than the total GCS score and has shown to have similar predictive value as the full GCS score for patients with acute TBI, ICH, and ischemic stroke.(8–12)
Near-infrared spectroscopy (NIRS) monitoring is a developing technique that allows real-time monitoring of regional cerebral oxygen saturation (rScO2),(13) which is a clinically acceptable surrogate of CBF and can be utilized to monitor dynamic cerebral autoregulation (CA) at the bedside.(14) NIRS-derived measurements of CA have been validated in comatose patients against TCD derived measurements of CA.(15) Further, NIRS is a practical monitoring device because it is non-invasive, it allows for prolonged continuous monitoring, and it does not require technical skills for maintenance. There is current interest in understanding the relationship between GCS and rScO2;(16–19) however, only one study observed acceptable agreement between rScO2 and GCS measured on admission.(17) Furthermore, the relationship between NIRS derived indices of CA with GCS has not been studied. Whether fluctuations in GCS during the acutely ill period are associated with alterations in NIRS-based measurements of CA is not known. This potential application would have direct implications for monitoring neurological status when neurological examination is not reliable (i.e. sedation and paralytic drug administration). This study aimed to explore the association between hourly GCS and GCS motor scores and NIRS-based bedside measurements during the first 72 hours after coma onset.
Methods
This is a prospective study that was performed in the Neuroscience Critical Care Unit (NCCU) at The Johns Hopkins Hospital between March of 2013 and December of 2015. Adult comatose patients with acute neurological injury were included in the study. Coma was defined as GCS ≤ 8, not due to aphasia or sedation. Patients were monitored for up to three consecutive days with NIRS at the bedside. All procedures received the approval of The Johns Hopkins Medical Institutions Review Board and the written informed consent was not required because of the low risk and conventional nature of this type of non-invasive neuromonitoring.
Data Collection
General information included demographics, medical history, brain imaging data, laboratory results, and outcomes. GCS and its component GCS motor score, MAP (mean arterial pressure), rScO2 and COx (cerebral oximetry index) values were recorded hourly for each day of monitoring. COx is a CA index derived from the correlation between rScO2 and MAP. There were two values of both rScO2 and COx for the two probes placed on both the right and left sides of the forehead. Routinely, neurocritical care patients are kept on minimal or no sedation to allow for neurological exams assessments. GCS was recorded hourly and sedation was suspended for a duration that ensured elimination of pharmacodynamic effects prior to bedside assessments.
NIRS-Based Autoregulation Monitoring
Within 24 hours of admission to the NCCU, rScO2 monitoring was begun using two NIRS sensors (Invos 5100, Medtronic/Covidien, Boulder, CO) placed bilaterally on the patient’s forehead. Simultaneously, a DT9800 data acquisition module (Data Translation Inc, Marlboro, MA) processed the analog MAP signals from the patient’s hemodynamic monitor. Both of these signals and the raw digital NIRS signals were analyzed using the ICM+ software (University of Cambridge, Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus), as described previously.(20) In order to obtain the slow vasogenic waves mediating CA, the arterial blood pressure and NIRS signals were filtered as non-overlapping 10-second mean values that were time-integrated. Averaged COx within each 10-second window was collected as 30 data points to monitor each COx in a 300-second window. A continuous, moving Pearson’s correlation coefficient between changes in MAP and rSO2 was calculated rendering the variable, COx. A COx value approaching zero indicates functional autoregulation (i.e., no correlation between rSO2 and ABP), whereas a COx value approaching 1 indicates impaired autoregulation. Each monitoring file was examined for artefact at a time scale of one hour. If any apparently irregular or artefactual recordings were present, the section was excised. The CO2 values were collected as the closest ones to the time of monitoring and an average was then calculated. In our neurocritical care unit the CO2 is at least measured daily if no further changes are made to the ventilator. If there are any adjustments made to the ventilator, then a new arterial blood gas is obtained. Hb was also collected as the closest one to the time of monitoring. In our neurocritical care unit Hb is at least measured daily if the value is stable compared to prior days.
Statistical Analysis
Descriptive characteristics of the sample were analyzed by using the statistical software Stata version 13.0 (Stata Corp, College Station, TX). We calculated the average of NIRS-based measurements of both right and left sides for each hour and if only one side was recorded, that was the value used. Mixed random effects models with random intercept were used to determine the relationship between NIRS-based measurements (rcSO2 and COx) and GCS scores extracted each hour from the patients. Mixed random effects models are the ideal methods to estimate within-patient associations by using clustered data.(21–24) The relation was adjusted for hemoglobin and carbon dioxide arterial pressure (pCO2). A P value of < 0.05 was considered as significant.
Results
Patient Characteristics
A total of 57 patients were included in the study. The summary of patient characteristics can be found in Table 1. 43.9% were males with a mean age of 58.7 ± 14.2 years. Of the patients studied, a majority had ICH (43.8%), 24.5% had SAH, 12.3% TBI, 7% ischemic stroke, 7% status epilepticus, and 1.8% with meningitis, ventriculitis, and cardiac arrest. 29.8% of patients had a left or right frontal lobe lesion. There was 871 hours of NIRS monitoring and GCS data available for analysis. The median duration of monitoring for each patient was 28 hours (IQR: 15 – 47).
Table 1.
Summary of clinical and monitoring information of the patients included in this study
| Variable | Value |
|---|---|
| Age, years (± SD) | 58.7 ± 14.2 |
| Gender, M / F, n | 25 / 32 |
| Race, n (%) | |
| African American | 29 (50.9%) |
| White | 24 (42.1%) |
| Hispanic | 3 (5.3%) |
| Asian | 1 (1.8%) |
| Etiology, n (%) | |
| Intracerebral hemorrhage | 25 (43.8%) |
| Aneurysmal subarachnoid hemorrhage | 14 (24.5%) |
| Traumatic brain injury | 7 (12.3%) |
| Ischemic stroke | 4 (7%) |
| Status epilepticus | 4 (7%) |
| Meningitis | 1 (1.8%) |
| Ventriculitis | 1 (1.8%) |
| Cardiac arrest | 1 (1.8%) |
| Frontal lobe lesion, n (%) | |
| Left | 7 (12.3%) |
| Right | 10 (17.5%) |
| Midline shift at pineal gland, mm (± SD) | 3.2 ± 5.4 |
| Hemoglobin during monitoring, g/mL (± SD) | 10.1 ± 1.8 |
| Mean pCO2 during monitoring, mmHg (± SD) | 38.9 ± 7.8 |
| Median duration of monitoring, hours, median (IQR) | 28 (15 – 47) |
| GCS score, median (IQR) | 7 (4 – 8) |
| GCS motor component, median (IQR) | 4 (2 – 5) |
| Mean COx (± SD) | 0.06 ± 0.15 |
Abbreviations: SD, standard deviation; M, male; F, female; IQR, interquartile range; GCS, glasgow coma scale
Cerebral autoregulation and GCS
An inverse relationship between COx and GCS as well as GCS motor score was observed. There was a decrease in COx (intact autoregulation) when GCS improves and an increase in COx (impaired autoregulation) when GCS worsens. This inverse relationship between COx and GCS score can be seen clearly in Figure 1. COx was significantly inversely associated with GCS ( =−1.12, 95%CI=−1.94 to −0.31, P=0.007) and GCS motor scores ( =−1.06, 95%CI=−2.10 to −0.04, P=0.04) after adjusting for hemoglobin and PaCO2.
Figure 1.
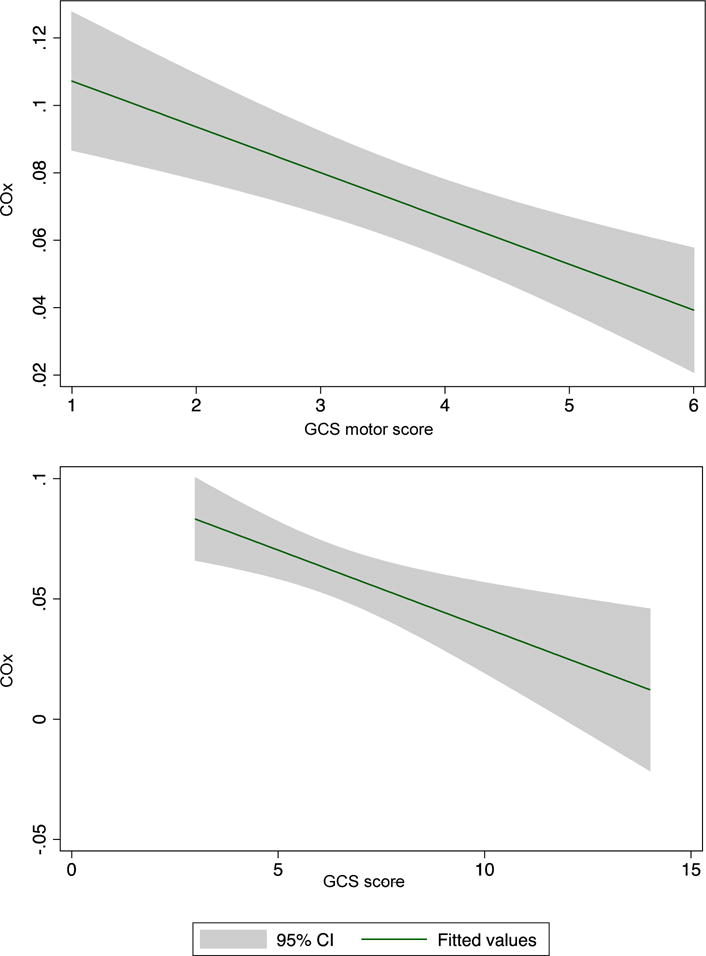
Pooled COx values of all patients, grouped by GCS score showing the inverse linear relationship between COx and (a) GCS and (b) GCS motor. CI indicates confidence interval; COx, cerebral oximetry index; GCS, Glasgow Coma Scale
Cerebral oximetry and GCS
In contrast, rScO2 was not associated with GCS ( =−0.002, 95%CI=−0.01 to 0.01, P=0.76) or GCS motor score ( =−0.001, 95%CI=−0.01 to 0.01, P=0.84).
Discussion
In this prospective cohort of 57 acutely brain injured comatose patients, we found that there was a linear, inverse association between COx and GCS scores. As patients’ neurological exam worsens and GCS scores decrease, COx values also worsen or increased. These results may support that increasing severity of neurological injury is associated with more impaired cerebral autoregulation whereby cerebral blood flow is perfusion pressure dependent. These results support the potential use of non-invasive CA monitoring derived from NIRS to provide a tool that reflects fluctuations of GCS score when the neurological examination is not feasible or unreliable (i.e. heavy sedation or paralysis administration).
GCS (and the GCS motor score) provides a strong prognostic value for neurologically critically ill patients. Decisions such as daily management plans are reliant on an accurate determination of the neurologic function that may be difficult in the presence of sedated patients.(25) In instances such as refractory intracranial hypertension with poor intracranial compliance controlled with heavy sedation like propofol and/or pentobarbital, stopping sedation to obtain a neurologic examination can lead to increase of intracranial pressure. Similarly, in patients with severe ARDS and severe hypoxemia, stopping sedation to foster neurological examination can lead to worsening oxygenation(26), further compounding the consequences.(27) Nevertheless, to prevent secondary injury and worsening of neurologic function, a method is needed to accurately assess neurologic examination. In those instances where the neurologic function cannot be assessed, CA using multimodal monitoring with NIRS can be used as a surrogate of a patient’s neurological state.
In our study, the variation of COx was observed to be less than observed in patients studied under different clinical conditions, such as patients studied using NIRS undergoing cardiac bypass. However, in patients with intracranial injury secondary to aneurysmal subarachnoid hemorrhage, the cut-off for delayed cerebral ischemia was found to be 0.1, a more subtle alteration in COx. Experimental studies in animals with induced coma have found that the CA plateau extends over a wider CPP range compared to awake animals and, consequently, the CA indices are more negative, have less variability and are closer to zero.(28) By lowering the brain’s oxygen demand, the cerebral vessels constrict at normal MAP and thereby have a greater vasodilatory reserve to handle a low MAP resulting in a plateau for CA which extends over a wider CPP range. Compared to studies of patients undergoing CPB, we would not expect the cerebrovascular responsivity in comatose patients to yield identical COx thresholds for autoregulation. The physiology of the former is impacted by exposure to volatile anesthesia rather than neurological injury, the latter state in which the anesthetics specifically vasodilate cerebral blood vessels.(29) These patients also have a lower hematocrit and possibly more atherosclerosis that may affect the limits of CA. The difference of variability of COx in our study compared with other studies can also perhaps be explained due to the little variation of rSO2 seen in our patients who, contrary to patients undergoing CABG, are not exposed to rapid changes of blood pressure or cerebral perfusion.(15,30–33)
Multimodal monitoring of CA with NIRS monitoring is able to continuously calculate a cerebral autoregulation index, COx. (34) In this study, we found that GCS score fluctuations are inversely related with COx fluctuations, meaning that higher COx values are associated with decreasing or worsening GCS scores. High COx values indicate impaired CA and poor outcomes.(15) Some pathophysiological mechanisms can be proposed to explain the relationship between GCS and COx such as the decreased cerebral uptake of oxygen observed in coma that is clearly reflected in the NIRS measurements,(35) disturbances in the metabolism and hemodynamic parameters may also explain the relation between the neurologic deterioration and impaired CA.(36) Impaired CA defined by other CA indices and its association with long term outcomes has also been studied. Impaired CA defined by the pressure reactivity index (PRx) and mean velocity index (Mx) have been shown to be significantly associated with worse 3-month outcomes defined by modified Rankin Scale in patients with severe TBI and non-traumatic brain injury.(18) In patients with aneurysmal subarachnoid hemorrhage, impaired CA determined by PRx has also been associated with 6 month functional outcomes.(37)
Other investigations have assessed the relation between rScO2 (not autoregulation) and GCS score. Demet et al.(16) showed that there was a non-significant correlation between GCS and rSO2 in ischemic stroke. Dunham et al.(19) demonstrated a significant relationship between rSO2 of < 60 and GCS score of 3 – 4 (P<0.001). The non-significance for rScO2 observed in this study could be explained by the other multiple factors affecting the brain cerebral metabolism and oxygen demand (i.e., seizures, burst suppression, local hypoxemia). (38) It may also be that cerebral oximetry by itself is not a robust indicator of neurologic function. Regardless, those prior studies did not perform analysis between GCS and COx as performed in this study.
The major strength of this study is the continuous and prolonged duration of CA monitoring (72 hours) to assess neurologic fluctuations. It is, on the other hand, important to mention the limitations of this study. First, the heterogeneity of patient etiologies; due to the fact that lumping together different patients with different conditions may make it difficult to accurately distinguish if all conditions yield the given result. Additionally, our conclusions are limited due to a small number of patients (N=58), but the large number of both GCS scores and NIRS observations (n = 871) increase the statistical power of this study. We also did not include other covariates (i.e., sedation) in the multivariable model, however, the hourly neurologic assessments in our neurocritical care unit are performed typically without sedation or following cessation of continuous light sedation to maximize neurologic function. Furthermore, in this study we only included patients who were comatose due to their primary neurologic injury and not secondary to sedation. Other limitations include the uncertain effect of frontal lesions on the accuracy of the NIRS readings. Patient movement may cause artefactual recordings, which may go unnoticed during visual inspection and elimination of these artefacts. This has the potential to reduce correlation between GCS and COx.
CONCLUSION
This study demonstrated that fluctuations in GCS scores are inversely associated with fluctuations in COx; as COx increases (impaired autoregulation), more severe neurological impairment is observed. However, the difference in COx between high and low GCS is small and warrants further studies investigating this association. CA multimodal monitoring with COx may have the potential to be used as a surrogate of neurologic status when the neurologic examination is not reliable (i.e. sedation and paralytic drug administration).
Acknowledgments
Sources funding: Dr. Hogue is the PI on an NIH-sponsored clinical study (R01 HL 92259). Dr. Rivera-Lara is the PI on an American Academy of Neurology/American Brain Foundation, Covidien/Metronic and Ornim grant.
Disclosures: Dr. Hogue receives research funding from Medtronic/Covidien, Dublin, IR, and he serves as a consultant to Medtronic/Covidien and Ornim Medical, Inc., Foxborough, MA.
References
- 1.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. The Lancet Neurology. 2014;13:844–54. doi: 10.1016/S1474-4422(14)70120-6. [DOI] [PubMed] [Google Scholar]
- 2.Fischer J, Mathieson C. The history of the Glasgow Coma Scale: implications for practice. Critical care nursing quarterly. 2001;23:52–8. doi: 10.1097/00002727-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, Marmarou A, Steyerberg EW. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. Journal of neurotrauma. 2007;24:329–37. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 4.Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, Mushkudiani NA, Choi S, Maas AI. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. Journal of neurotrauma. 2007;24:270–80. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- 5.Starke RM, Komotar RJ, Otten ML, Schmidt JM, Fernandez LD, Rincon F, Gordon E, Badjatia N, Mayer SA, Connolly ES. Predicting long-term outcome in poor grade aneurysmal subarachnoid haemorrhage patients utilising the Glasgow Coma Scale. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009;16:26–31. doi: 10.1016/j.jocn.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2001;32:891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 7.Navarrete-Navarro P, Rivera-Fernandez R, Lopez-Mutuberria MT, Galindo I, Murillo F, Dominguez JM, Munoz A, Jimenez-Moragas JM, Nacle B, Vazquez-Mata G. Outcome prediction in terms of functional disability and mortality at 1 year among ICU-admitted severe stroke patients: a prospective epidemiological study in the south of the European Union (Evascan Project, Andalusia, Spain) Intensive care medicine. 2003;29:1237–44. doi: 10.1007/s00134-003-1755-6. [DOI] [PubMed] [Google Scholar]
- 8.Majdan M, Steyerberg EW, Nieboer D, Mauritz W, Rusnak M, Lingsma HF. Glasgow coma scale motor score and pupillary reaction to predict six-month mortality in patients with traumatic brain injury: comparison of field and admission assessment. Journal of neurotrauma. 2015;32:101–8. doi: 10.1089/neu.2014.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handschu R, Haslbeck M, Hartmann A, Fellgiebel A, Kolominsky-Rabas P, Schneider D, Berrouschot J, Erbguth F, Reulbach U. Mortality prediction in critical care for acute stroke: Severity of illness-score or coma-scale? Journal of neurology. 2005;252:1249–54. doi: 10.1007/s00415-005-0853-5. [DOI] [PubMed] [Google Scholar]
- 10.Singh B, Murad MH, Prokop LJ, Erwin PJ, Wang Z, Mommer SK, Mascarenhas SS, Parsaik AK. Meta-analysis of Glasgow coma scale and simplified motor score in predicting traumatic brain injury outcomes. Brain injury. 2013;27:293–300. doi: 10.3109/02699052.2012.743182. [DOI] [PubMed] [Google Scholar]
- 11.Reith FCM, Lingsma HF, Gabbe BJ, Lecky FE, Roberts I, Maas AIR. Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury. Injury. 2017;48:1932–43. doi: 10.1016/j.injury.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Kouloulas EJ, Papadeas AG, Michail X, Sakas DE, Boviatsis EJ. Prognostic value of time-related Glasgow coma scale components in severe traumatic brain injury: a prospective evaluation with respect to 1-year survival and functional outcome. International journal of rehabilitation research Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2013;36:260–7. doi: 10.1097/MRR.0b013e32835fd99a. [DOI] [PubMed] [Google Scholar]
- 13.Sen AN, Gopinath SP, Robertson CS. Clinical application of near-infrared spectroscopy in patients with traumatic brain injury: a review of the progress of the field. Neurophotonics. 2016;3:031409. doi: 10.1117/1.NPh.3.3.031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigl W, Milej D, Janusek D, Wojtkiewicz S, Sawosz P, Kacprzak M, Gerega A, Maniewski R, Liebert A. Application of optical methods in the monitoring of traumatic brain injury: A review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:1825–43. doi: 10.1177/0271678X16667953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, Healy R, Radziq B, Palmisano C, Mirski MA, Ziai W, Hogue C. Validation of Near-Infrared Spectroscopy for Monitoring Cerebral Autoregulation in Comatose Patients. Neurocritical care. 2017;27:362–369. doi: 10.1007/s12028-017-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demet G, Talip A, Nevzat U, Serhat O, Gazi O. The evaluation of cerebral oxygenation by oximetry in patients with ischaemic stroke. Journal of postgraduate medicine. 2000;46:70–4. [PubMed] [Google Scholar]
- 17.Dunham CM, Sosnowski C, Porter JM, Siegal J, Kohli C. Correlation of noninvasive cerebral oximetry with cerebral perfusion in the severe head injured patient: a pilot study. The Journal of trauma. 2002;52:40–6. doi: 10.1097/00005373-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B, Reinhard M, Lezaic V, McLeod DD, Weinhold M, Mattes H, Klingelhofer J. Autoregulation monitoring and outcome prediction in neurocritical care patients: Does one index fit all? Journal of clinical monitoring and computing. 2016;30:367–75. doi: 10.1007/s10877-015-9726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunham CM, Ransom KJ, Flowers LL, Siegal JD, Kohli CM. Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. The Journal of trauma. 2004;56:482–9. doi: 10.1097/01.ta.0000114537.52540.95. discussion 9-91. [DOI] [PubMed] [Google Scholar]
- 20.Budohoski KP, Czosnyka M, Smielewski P, Varsos GV, Kasprowicz M, Brady KM, Pickard JD, Kirkpatrick PJ. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:449–56. doi: 10.1038/jcbfm.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuhaus JM, McCulloch CE. The effect of misspecification of random effects distributions in clustered data settings with outcome-dependent sampling. The Canadian journal of statistics = Revue canadienne de statistique. 2011;39:488–97. doi: 10.1002/cjs.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhaus JM, McCulloch CE, Boylan R. Estimation of covariate effects in generalized linear mixed models with a misspecified distribution of random intercepts and slopes. Statistics in medicine. 2013;32:2419–29. doi: 10.1002/sim.5682. [DOI] [PubMed] [Google Scholar]
- 23.Lidon-Moyano C, Fu M, Perez-Ortuno R, Ballbe M, Sampedro-Vida M, Martin-Sanchez JC, Pascual JA, Fernandez E, Martinez-Sanchez JM. Assessment of salivary cotinine concentration among general non-smokers population: Before and after Spanish smoking legislations. Cancer epidemiology. 2017;51:87–91. doi: 10.1016/j.canep.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Eggers KM, Lindahl B, Venge P, Lind L. Predictors of 10-year changes in levels of N-terminal pro B-type natriuretic peptide and cardiac troponin I in the elderly. International journal of cardiology. 2018;257:300–5. doi: 10.1016/j.ijcard.2017.10.095. [DOI] [PubMed] [Google Scholar]
- 25.Stocchetti N, Pagan F, Calappi E, Canavesi K, Beretta L, Citerio G, Cormio M, Colombo A. Inaccurate early assessment of neurological severity in head injury. Journal of neurotrauma. 2004;21:1131–40. doi: 10.1089/neu.2004.21.1131. [DOI] [PubMed] [Google Scholar]
- 26.Shah FA, Girard TD, Yende S. Limiting sedation for patients with acute respiratory distress syndrome - time to wake up. Current opinion in critical care. 2017;23:45–51. doi: 10.1097/MCC.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livingston BM, Mackenzie SJ, MacKirdy FN, Howie JC. Should the pre-sedation Glasgow Coma Scale value be used when calculating Acute Physiology and Chronic Health Evaluation scores for sedated patients? Scottish Intensive Care Society Audit Group Critical care medicine. 2000;28:389–94. doi: 10.1097/00003246-200002000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Donegan JH, Traystman RJ, Koehler RC, Jones MD, Jr, Rogers MC. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. The American journal of physiology. 1985;249:H421–9. doi: 10.1152/ajpheart.1985.249.2.H421. [DOI] [PubMed] [Google Scholar]
- 29.Summors AC, Gupta AK, Matta BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesthesia and analgesia. 1999;88:341–5. doi: 10.1097/00000539-199902000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, Neufeld KJ, Gottesman R, Adachi H, Hogue CW. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. British journal of anaesthesia. 2014;113:1009–17. doi: 10.1093/bja/aeu319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori D, Ono M, Rappold TE, Conte JV, Shah AS, Cameron DE, Adachi H, Everett AD, Hogue CW. Hypotension After Cardiac Operations Based on Autoregulation Monitoring Leads to Brain Cellular Injury. The Annals of thoracic surgery. 2015;100:487–93. doi: 10.1016/j.athoracsur.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, Hogue CW., Jr Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. The Journal of thoracic and cardiovascular surgery. 2014;147:483–9. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera-Lara L, Zorrilla-Vaca A, Geocadin R, Ziai W, Healy R, Thompson R, Smielewski P, Czosnyka M, Hogue CW. Predictors of Outcome With Cerebral Autoregulation Monitoring: A Systematic Review and Meta-Analysis. Critical care medicine. 2017;45:695–704. doi: 10.1097/CCM.0000000000002251. [DOI] [PubMed] [Google Scholar]
- 34.Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. Journal of clinical monitoring and computing. 2012;26:279–87. doi: 10.1007/s10877-012-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derr R, Zieve L. Decreased cerebral uptake of oxygen in coma—a consequence of decreased utilization of ATP. Journal of neurochemistry. 1973;21:1555–7. doi: 10.1111/j.1471-4159.1973.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 36.Brodersen P. Cerebral blood flow and metabolism in coma following cardiac arrest. Revue d’Electroencéphalographie et de Neurophysiologie Clinique. 1974;4:329–33. doi: 10.1016/s0370-4475(74)80019-5. [DOI] [PubMed] [Google Scholar]
- 37.Rasulo FA, Girardini A, Lavinio A, De Peri E, Stefini R, Cenzato M, Nodari I, Latronico N. Are optimal cerebral perfusion pressure and cerebrovascular autoregulation related to long-term outcome in patients with aneurysmal subarachnoid hemorrhage? Journal of neurosurgical anesthesiology. 2012;24:3–8. doi: 10.1097/ANA.0b013e318224030a. [DOI] [PubMed] [Google Scholar]
- 38.Lewis SB, Myburgh JA, Thornton EL, Reilly PL. Cerebral oxygenation monitoring by near-infrared spectroscopy is not clinically useful in patients with severe closed-head injury: a comparison with jugular venous bulb oximetry. Critical care medicine. 1996;24:1334–8. doi: 10.1097/00003246-199608000-00011. [DOI] [PubMed] [Google Scholar]


