Abstract
Bone is a complex endocrine organ that facilitates structural support, protection to vital organs, sites for hematopoiesis, and calcium homeostasis. The bone marrow microenvironment is a heterogeneous niche consisting of multipotent musculoskeletal and hematopoietic progenitors and their derivative terminal cell types. Amongst these progenitors, bone marrow mesenchymal stem/stromal cells (BMSCs) may differentiate into osteogenic, adipogenic, myogenic, and chondrogenic lineages to support musculoskeletal development as well as tissue homeostasis, regeneration and repair during adulthood. With age, the commitment of BMSCs to osteogenesis slows, bone formation decreases, fracture risk rises, and marrow adiposity increases. An unresolved question is whether osteogenesis and adipogenesis are co-regulated in the bone marrow. Osteogenesis and adipogenesis are controlled by specific signaling mechanisms, circulating cytokines, and transcription factors such as Runx2 and Pparγ, respectively. One hypothesis is that adipogenesis is the default pathway if osteogenic stimuli are absent. However, recent work revealed that Runx2 and Osx1-expressing preosteoblasts form lipid droplets under pathological and aging conditions. Histone deacetylase 3 (Hdac3) and other epigenetic regulators suppress lipid storage in preosteoblasts and/or control marrow adiposity. Establishing a better understanding of fat storage in bone marrow cells, as well as the osteoblast-adipocyte relationship within the bone marrow niche is necessary to understand the mechanisms underlying disease- and aging-related marrow fat storage and may lead to the development of new therapeutic targets for “fatty bone” and osteoporosis.
Keywords: Bone marrow mesenchymal/stromal cell, osteogenesis, osteoblast, adipogenesis, adipocyte, marrow fat, aging
INTRODUCTION
The Bone Marrow Microenvironment is Heterogeneous
Bone is a metabolically active tissue with complex physiological roles. Bone marrow is a semifluid component of bone that occupies the endosteal space. It consists of a diverse population of cell types including ones involved in bone development, hematopoiesis, tissue remodeling, and endocrine regulation. Many of the mature cell types present in bone marrow are derived from common progenitors. One multipotent progenitor, the bone marrow mesenchymal stem/stromal cell (BMSC), comprises a heterogeneous population of stem cells that can mature into cells of the chondrogenic, myogenic, osteogenic, and adipogenic lineages upon receiving the proper signals (1). BMSC retain their stem-like nature throughout development until triggered to differentiate by stressors and physiological needs. As BMSCs undergo differentiation, changes occur in their transcriptional profile, cellular metabolism, and morphology that are consistent with the respective lineage of commitment. During aging and in pathological conditions, the normal balance between osteogenic and adipogenic cell populations can shift toward the latter, thereby threatening bone health and integrity (2). Here we review the osteoblast-adipocyte relationship within the bone marrow and provide insights into how these two cell types can be better defined. Progress in this area will lead to new insights for preventing age- and pathology-related fractures and metabolic syndromes.
Commitment of BMSCs to Osteoblast or Adipocyte Differentiation
BMSCs are the multipotent progenitors responsible for maintaining the non-hematopoietic cell populations of the bone and bone marrow. The commitment of BMSC to osteogenesis is a tightly regulated process that is dependent on differing signaling mechanisms throughout development and during adulthood (Figure 1). Early regulators of osteogenesis in the BMSC population include Wnt/β-catenin signaling, bone morphogenetic proteins (BMPs), hedgehog proteins (i.e., sonic hedgehog, Indian hedgehog), endocrine hormones such as parathyroid hormone (PTH), epigenetic regulators, and various growth factors (3–5). The master osteoblastic transcription factors runt-related transcription factor 2 (Runx2) and Osterix 1 (Osx1/Sp7) are key and sequential players in the induction of osteogenic differentiation and shift the gene expression profile of BMSC to osteogenic genes that control type I collagen-based extracellular matrix (ECM) deposition (6). BMSC committed to osteogenesis continue to develop the genetic profile and morphology of the osteoblast, expressing genes such as alkaline phosphatase, osteoprotegerin, type I collagen, and later osteocalcin as these cells shift toward a terminal osteocyte morphology (7). In adults, the primary role of BMSC-derived osteoblasts is to produce and secrete osteoid and mineralization factors in a coupled fashion alongside the activity of osteoclasts (which resorb the bone matrix) to remodel the bone as the metabolic and structural needs of the body change. In cases where BMSC are not triggered to commit to osteogenesis, bone formation may become uncoupled from resorption and subsequent declines in bone mass and tissue integrity ensue (8).
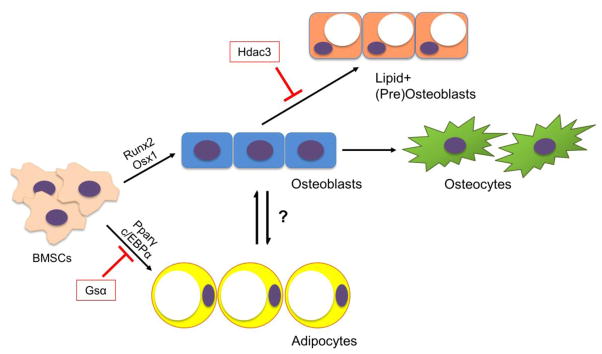
Figure 1.
Differentiation of BMSCs to osteogenic and adipogenic lineages. Bone marrow mesenchymal stem cells (BMSCs) are signaled to differentiate to osteoblasts by transcription factors such Runx2 and Osx1 or to adipocytes by Pparγ and c/EBPα. BMSC-derived osteoblasts can further differentiate into mature osteocytes or may become lipid-storing cell types. This diagram illustrates a potential lineage-switching mechanism between osteoblasts and adipocytes as well as a reduction of lipid storage within osteogenic cells by histone deacetylase 3 (Hdac3) and its associated cofactors.
BMSCs may alternatively be directed to adipogenesis under certain conditions. In fact, some consider the differentiation of BMSC to the adipocyte lineage to be a “default” pathway (9). The differentiation of the BMSC into a bone marrow adipocyte involves expression of key transcription factors: peroxisome proliferator-activated receptor γ (Pparγ) and CCAAT/enhancer-binding protein α (c/EBPα) (10). Bone marrow adipocytes characteristically store cytoplasmic lipid droplets and express a number of genes related to lipid storage (i.e., Cidec, Plin1), fatty acid metabolism (i.e., Fasn, lipases like ATGL and HSL), and adipocyte function (i.e., adiponectin, lepR) (11). While the roles of bone marrow adipocytes are still in question, there is evidence that these cells may serve as energy reservoirs for the bone (12). Additionally, marrow adipocytes interact with other bone cells in distinct niches where they may regulate the bone remodeling, repair and endocrine regulatory processes (13). As such, these lipid-storing cells are an important component to skeletal biology and may provide novel insights into the activity of osteoblasts and other BMSC-derived cell types.
Cellular Energetics of BMSC-Derived Osteoblasts and Adipocytes
Much like the varying energy needs of different tissues based on their physiological functions, BMSC undergo a shift in their cellular energetics profiles during the commitment to terminal differentiation (Figure 2). The mesenchymal progenitors primarily rely on glycolysis for energy metabolism, whereas the energy needs of these cells increase dramatically during osteogenesis (14). Importantly, increased glucose metabolism within BMSCs—which is facilitated by upregulated Glut1 transporter expression that precedes the expression of Runx2 in differentiating preosteoblasts—provides the energy necessary to produce the type 1 collagen that comprises the ECM (15). Mature osteoblasts also require a high yield of ATP from fuel sources in order to produce and deposit significant amounts of osteoid and, consequently, utilize fatty acid oxidation in addition to glucose metabolism to meet those energy needs (16). While the energetics of late-stage osteogenic cells are sufficient for their functions, the use of oxidative phosphorylation for a high energy yield can contribute to the production of excess reactive oxygen species that may cause cellular damage over time.
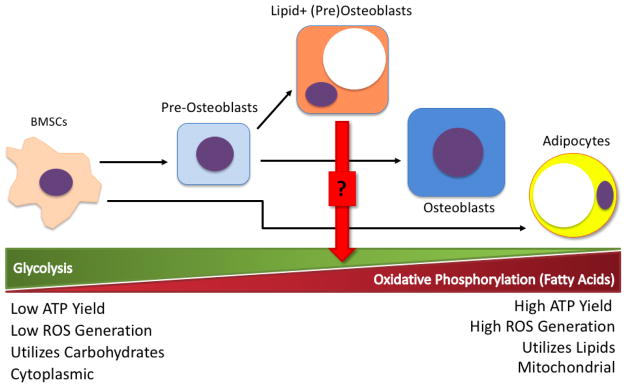
Figure 2.
Shift of cellular energetics profiles through BMSC differentiation. The progenitor BMSC population is highly glycolytic and dependent on glucose for energy metabolism. As BMSCs commit to osteogenesis, the energy demands of osteoid production require metabolic processes that generate more ATP—e.g., the metabolism of fatty acids for oxidative phosphorylation. Mature osteoblasts utilize a combination of glycolysis and oxidative phosphorylation to meet their energy needs, but this energetic balance must be tightly regulated to reduce reactive oxygen species (ROS) generation and subsequent cellular damage. In contrast to BMSCs, mature adipocytes generate much of their ATP from fatty acid oxidation and oxidative phosphorylation. The metabolic profile of the lipid-positive pre-osteoblast is still in question, but the intermediate morphology of this cell type suggests that it may use both energetic processes and may potentially store lipid droplets for fatty acid metabolism under high energy demand.
Bone marrow adipocytes tend to have different metabolic needs from osteogenic cell types, and this is also reflected in their cellular energetics. Rather than utilizing glucose metabolism for energy, these lipid-storing cell types are characterized by fatty acid metabolism and rely heavily on the lipolysis of their intracellular lipid stores to provide free fatty acids (FFA) for metabolism through oxidative phosphorylation (17). As a result of harnessing fatty acids as intracellular lipid droplets, marrow adipocytes may serve as important reservoirs for energy when other sources (i.e., glucose) are depleted. However, an excess of FFA produced by the metabolism of lipid droplets can have lipotoxic effects in BMSCs and osteoblasts by interrupting cellular functions and inducing cell death (18). The detrimental effects of this fatty acid metabolism on osteoblasts can be prevented with the inhibition of fatty acid synthesis (19). The intriguing relationship between fat-storing and bone-forming cells involves crosstalk at the metabolic level. The delicate balance between glycolysis and oxidative phosphorylation—particularly of lipid metabolites—in osteogenic and adipogenic cell types is an active area of interest that may provide novel insights into the maintenance of skeletal health.
Lipid Storage in Non-Adipogenic Skeletal Cells
While it is clear that both osteoblast progenitors and adipocytes reside in the bone marrow, their identities can be difficult to discern because lipid accumulation is often used a defining characteristic of adipocytes instead of lineage-defining transcription factors. A challenge in histology-based examination of bone marrow adiposity is that the lipid droplets in bone marrow cells consume much of the cellular volume, leaving little room to study other organelles, including the nucleus where lineage-directing transcription factors typically reside. However, the biochemical and metabolic pathways that create and dissolve lipid droplets are common to all cells and are not unique to adipocytes. The storage of intracellular lipid droplets in non-adipogenic lineage cells is not a novel concept—for example, lipid storage has been widely studied in hepatocytes of the liver as a consequence of increased alcohol consumption, but intracellular lipid storage in osteoblast-lineage cells is not currently recognized widely by the bone field despite growing evidence of this mechanism’s existence. Osteocytes have increased intracellular lipid deposition as a result of chronic alcohol consumption, which often precedes osteocyte apoptosis and osteonecrosis due to the disease (20). Chondrocytes, as well, were shown to contain intracellular lipids, the abundance of which increases with aging (21).
The storage of lipid droplets within osteoblast progenitors in the bone marrow is not as well-documented as other cell types, but there is recent evidence that BMSC-derived bone-building cells may store intracellular fat when their differentiation is challenged—similar to skeletal muscle (Figure 1) (22). Recent studies on the conditional deletion of the epigenetic regulator histone deacetylase 3 (Hdac3) in osteoblastic or chondroblastic cells (cells targeted by Osx1-Cre, Col2-Cre) revealed increases in the prevalence of lipid-containing, Runx2+ BMSC-derived osteoblasts that are hypothesized to contribute to high marrow fat observed in Hdac3-deficient models (23–25). An important aspect of these studies is that Runx2+ cells expressed lipid droplets when cultured in osteoblastic medium. Of interest, deletion of Hdac3-associated cofactors (e.g., Ezh2 and Zfp521) in osteoprogenitors also increased marrow adiposity (26,27). These data indicate that the Hdac3 cofactor complex controls energy homeostasis in osteoprogenitors like it does in the liver (28–30). Hdac3 levels decline in human bone with age (24), suggesting a novel potential mechanism that could contribute to marrow fat increases with aging and certain pathologies (i.e., aberrant glucocorticoid signaling, excess oxidized amino acid metabolites), as Hdac3 levels can decrease in these conditions as well (24,31–34).
Deletion or overexpression of other important osteoblast proteins in osteoprogenitor cells can also alter marrow adiposity. For example, conditional deletion of Cbfβ, a stabilizing co-factor for Runx transcription factors, in osteoprogenitors (with Osx1-Cre, Prxx1-Cre, or Col2-Cre) increases bone marrow adiposity (35), as does deletion of GNAS (Gsα) with Prxx1-Cre (36,37) or Vegf with Osx1-Cre (38). In contrast, transgenic animals overexpressing osteoanabolic agents such as Wnt10b (driven by the OCN promoter; (39)) or a gain-of-function form of Gsα do not have marrow adiposity (40).
Several groups performing fate-mapping studies with Osterix-Cre mice showed that cells in which the Cre is active contain lipid droplets, as defined by perilipin-positive rings, in vivo (41,42). The conclusions of these studies were that adipocytes express Osterix-Cre. However, an equally plausible conclusion is that osteoblasts are capable of forming lipid droplets when differentiation is challenged as it may be if one or both copies of Osx is deleted.
While considered potentially deleterious in the aforementioned studies, lipid storage within osteoblasts has also been proposed as a critical step for osteogenesis. Impairment of lipid droplet formation in BMSC with the drug triascin C caused a notable decrease in osteoblastic differentiation as measured by alkaline phosphatase and Von Kossa stain (43). Thus, while fat accumulation in osteoblasts is often associated with pathologies, the physiological roles of these lipid-storing cells still remains unclear and may vary depending on the stage of osteoblastic differentiation.
Despite the potential for lipid droplets to support osteoblast function, it is important to consider that excess marrow fat storage can compromise bone mineral density and tissue health if lipid deposition extends beyond supplying the energy needs of differentiating BMSC. Fatty tissue in the metaphyseal region of the bone marrow cavity tends to occupy space that could otherwise hold trabecular bone, and there is increasing evidence that lipid metabolism within the bone marrow can hinder bone formation (44,45). Lipolysis of adipocyte lipid droplets can release fatty acids such as palmitate, ultimately impairing osteoblast differentiation and function by interrupting β-catenin and Runx2 signaling mechanisms (18,19). Additionally, accumulation of free fatty acids can have lipotoxic effects on osteoblasts by inducing autophagic and apoptotic mechanisms that result in long-term bone loss (46).
The Identity of the Adipocyte-Osteoblast
Intracellular lipid deposition in osteoblasts raises concern regarding their distinction from marrow adipocytes as well as the purpose of lipid storage within bone. Though the molecular identity of lipid-storing, non-adipogenic cells in the bone marrow is still in early stages, there is speculation that these cells may represent an intermediate stage between osteogenic versus adipogenic differentiation endpoints. Because BMSC are the common progenitors for osteoblasts and adipocytes, the overlap in their morphologies suggests potential for transdifferentiation between cell types (rather than distinct terminal differentiation) under certain physiological conditions (47). The phenomenon of transdifferentiation has been observed in various tissues—from regenerative studies in the liver and eye to limb regeneration in amphibians—but a remaining question is whether committed unipotent osteoblasts may differentiate directly into marrow adipocytes (or vice versa) or whether an intermediate dedifferentiation step is required (48,49). There are also questions regarding the triggers for transdifferentiation, as microRNAs, loss of transcription factor signaling, and lineage specific growth conditions have been shown to influence lineage switching in committed cell types (50,51). Runx2 (Cbfa1) deficient calvarial progenitors cannot become osteoblasts but retain potential to become adipocytes and other mesenchymal lineages when cultured in conditions favoring their development (52). Alternatively, an intermediate cell type with both osteogenic and adipogenic properties may be a key contributor to marrow fat storage under pathological conditions. As described earlier, recent work has shown that Runx2-positive BMSC-derived osteoprogenitors can store lipid droplets and contribute to increased marrow adiposity in models of Hdac3 conditional deletion and aging (24). This phenomenon may be a result of incomplete lineage switching or another method of cell adaptation to changes within the bone marrow niche, but further characterization of BMSC-derived cell morphology and metabolism is needed to determine the identity of these lipid-positive bone cells. Such studies will require the ability to track the fate of single cells using lineage-defining transcription factors and care in accounting for cell culture conditions and media components. Indeed, a limitation of many published studies attempting to track cell fate and transdifferentiation is that heterogeneous cell populations and lineage-driving growth conditions (e.g., adipogenic or osteoblastic culture medium) are used to influence cell fate and conclusions are inferred to entire cell population. In a heterogeneous BMSC pool, there will be cells more inclined to become osteoblasts or adipocytes (or something else), but their potential is only achieved and observed under certain conditions. The observations of a cell population are insufficient to track individual cells or transdifferentiation.
Aging-Associated Marrow Fat
In vivo, marrow adiposity increases with age (45). In older individuals, the increase in marrow fat coincides with bone loss, but the physiological link between the two phenotypes is not easy to study in vivo (53). Bone marrow adipose tissue (MAT) can be classified into two groups—constitutive MAT (cMAT) and regulated MAT (rMAT)—that have unique formation patterns in addition to potentially unique roles (54). The former describes early MAT development in distal regions of the skeleton whereas the latter, rMAT, can form with age and tends to develop in the proximal regions of the bone (i.e., proximal tibia, femur, etc.) as it occupies the marrow distributed throughout the hematopoietic populations (55). The staining of murine tibia MAT with radio-opaque osmium tetroxide followed by micro-computed tomography (micro-CT; μCT) shows the accumulation of marrow fat that follows the formation patterns of cMAT (distal) and rMAT (proximal) that ultimately occupies most of the bone marrow cavity at 22 months of age (Figure 3). At the later stages of marrow fat accumulation, the low trabecular bone mass can put individuals at risk for osteopenia and the related comorbidities of fracture, increased healthcare costs, and even immortality.
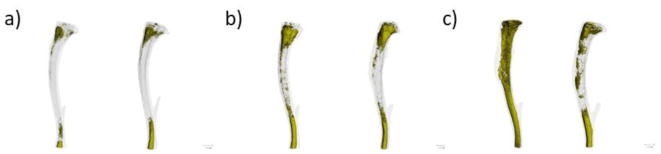
Figure 3.
Marrow fat accumulates with age in tibiae from female C57BL/6 mice. Osmium tetroxide-stained murine tibias imaged by micro-computed tomography show proximal and distal marrow adipose tissue at a) 4 months, b) 13 months, and c) 22 months of age. Each image is from a different mouse.
There are many factors involved in skeletal aging and downstream effects, making it difficult to define the hormonal, nutritional, epigenetic regulators and signaling mechanisms involved in the osteoblast-adipocyte biology. In general, after skeletal maturity, bone density decreases with age and is met with increased marrow adiposity, and the aged bone is at an increased risk for skeletal fracture (8,56). One major contributor to skeletal aging is elevated glucocorticoid signaling, which promotes lipid accumulation in a number of tissues and may be a key factor in inducing adipogenesis within the aging bone marrow (57–60). Finally, lifestyle—including reduced mechanical loading and diet—can influence the metabolic needs of the bone as well as alter the extracellular signals that dictate BMSC fate during aging. Load-bearing exercises can reduce bone marrow adiposity (61), but both high fat and calorically restricted diets induce bone marrow fat storage and consequent bone loss (11,62). Despite this evidence, whether these cellular responses to lifestyle and diet are efforts to store excess fuel sources in the form of lipid droplets remains unclear. The underlying mechanisms dictating BMSC differentiation with age are still an active area of research interest.
Summary and Future Directions
The diversity of the bone marrow mesenchymal stem cell population and its derivative cell types highlights the complexities of skeletal tissue. The heterogeneity of the bone marrow microenvironment supports the function of osteogenic, adipogenic, and chondrogenic cells, and BMSCs replenish these populations as the structural and metabolic needs of bone change.
BMSC commitment to osteogenesis is an important pathway during embryogenesis, skeletal growth, and bone remodeling to ensure proper tissue function. The generation of osteoblasts from BMSCs is dependent on tightly regulated signaling mechanisms (e.g., Wnt/β-catenin signaling), hormonal factors (e.g., PTH, glucocorticoids), epigenetic regulators (e.g., Hdac3), and the activity of specific transcription factors such as Runx2 and Osx1. BMSC-derived osteoblasts express genes and proteins related to producing osteoid (which transitions to calcified bone matrix), and this energy-demanding process tends to shift the energetic profile of these cells from predominantly glycolytic to a more complex metabolic profile that incorporates oxidative phosphorylation. The effects of aging on BMSC and osteoblastic bioenergetic profiles are not yet clear, but may shed light on the biological processes that contribute to increased MAT and decreased bone mass with age. Interestingly, BMSC-derived marrow adipocytes tend to utilize fatty acid oxidation but may impair osteoblast function and bone formation by doing so. These Pparγ+ cells characteristically store lipid droplets that may be used to meet energy demands, but recent evidence suggests that excess adipogenesis occurs at the expense of bone volume and that metabolism of adipocyte lipid droplets may hinder the function and survival of neighboring osteoblasts. Moreover, the fatty acids and adipokines (e.g., adiponectin) secreted by marrow adipocytes may drive further adipocyte differentiation (through Pparγ activation) and fatty acid metabolism in a feed-forward fashion. While marrow adipocytes have implications in bone endocrine functions and cellular signaling with osteogenic cell types within the bone, the identity of these cells (which is suggested to be unique from other adipose tissues) and the potential crosstalk with osteoblasts during aging is still in question.
There is increasing evidence that BMSC differentiation into distinct osteogenic, adipogenic, and chondrogenic populations requires intermediate steps and that transitional cell types may exist. Of interest for this review is the accumulation of lipid droplets in non-adipogenic cell types—a phenomenon that occurs in various soft tissues throughout the body during aging and certain pathologies. One argument for increased bone marrow lipid storage is that the cells positive for osteogenic markers may use lipid droplets as energy stores under energetically challenging conditions. Another plausible argument for osteogenic lipid storage is that adipogenesis is the default commitment pattern of BMSCs—as such, epigenetic regulators and signaling mechanisms dictated by the bone marrow microenvironment may downregulate adipogenesis in favor of bone formation and loss of function of these regulators (due to aging, stress, or pathological conditions, for example) can induce the reversion of osteogenic commitment. This “de-differentiation” step could be an intermediate step to lineage switching that may favor adipogenesis, but current techniques for lineage tracking of the cells in question limit these research endeavors. Lastly, an emerging school of thought in bone marrow fat research suggests that osteoblasts and adipocytes may undergo the phenomenon known as transdifferentiation, or a switch between the morphologies of these terminal cell types in lieu of an intermediate de-differentiation step. Despite recent evidence, it is still difficult to confirm whether observations of transdifferentiation are real or due to the conditions in which the BMSCs are cultured, as the available substrates and fuel sources in culture media can influence the commitment of these cells to certain lineages.
The dynamic between bone marrow osteoblasts and adipocytes changes throughout life and influences bone density and function with age. Marrow fat accumulation increases with age, contributing to osteopenia by decreasing trabecular bone mass. While there are some therapies—for example, bisphosphonates to slow bone resorption and PTH to stimulate new bone growth—in use to treat osteoporotic bone, the long-term administration of these drugs can cause adverse events (63,64). Moreover, existing measures to prevent and treat osteoporosis do not target the marrow fat that contributes to fracture risk and metabolic dysfunction. As such, a better understanding of the mechanisms influencing lipid storage in BMSC-derived cells and bone marrow adiposity can provide novel insights into preventing the decreased bone mass and increased fat deposition with age. Given the recently reported differences in the bioenergetic phenotypes of osteoblasts as compared to adipocytes (65), it will likely be critical to determine the relative importance of cellular energetic profile as compared to lineage commitment in the process of osteoblast versus adipocyte differentiation, particularly with aging.
Acknowledgments
The authors are supported by grants from the National Institute on Aging (P01-AG 036675), the National Institute for Arthritis, Musculoskeletal and Skin Diseases (T32 AR056950), the American Diabetes Association (1-16-JDF-062), and the National Science Foundation (CMMI 1727949).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. European Cells and Materials. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Liu X, Zuo B, Zhang L. The Role of Bone Marrow Microenvironment in Governing the Balance between Osteoblastogenesis and Adipogenesis. Aging Dis. 2016;7:514–525. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 4.D’Alimonte I, Lannutti A, Pipino C, Di Tomo P, Pierdomenico L, Cianci E, Antonucci I, Marchisio M, Romano M, Stuppia L, Caciagli F, Pandolfi A, Ciccarelli R. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013;9:642–654. doi: 10.1007/s12015-013-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhami MD, Rashid H, Chen H, Javed A. Runx2 activity in committed osteoblasts is not essential for embryonic skeletogenesis. Connect Tissue Res. 2014;55(Suppl 1):102–106. doi: 10.3109/03008207.2014.923873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2010;36:25–32. doi: 10.1002/biof.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu B, Wang CY. Osteoporosis: The Result of an ‘Aged’ Bone Microenvironment. Trends Mol Med. 2016;22:641–644. doi: 10.1016/j.molmed.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tencerova M, Kassem M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front Endocrinol (Lausanne) 2016;7:127. doi: 10.3389/fendo.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardouin P, Rharass T, Lucas S. Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front Endocrinol (Lausanne) 2016;7:85. doi: 10.3389/fendo.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J Bone Miner Res. 2018 doi: 10.1002/jbmr.3408. [DOI] [PubMed] [Google Scholar]
- 12.Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem. 2012;45:874–879. doi: 10.1016/j.clinbiochem.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig. 2016;28:21–38. doi: 10.1515/hmbci-2016-0012. [DOI] [PubMed] [Google Scholar]
- 14.Fillmore N, Huqi A, Jaswal JS, Mori J, Paulin R, Haromy A, Onay-Besikci A, Ionescu L, Thebaud B, Michelakis E, Lopaschuk GD. Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival. PLoS One. 2015;10:e0120257. doi: 10.1371/journal.pone.0120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Lezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161:1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shum LC, White NS, Mills BN, Bentley KL, Eliseev RA. Energy Metabolism in Mesenchymal Stem Cells During Osteogenic Differentiation. Stem Cells Dev. 2016;25:114–122. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F, Heeren J, Scheja L, Niemeier A. Quantification of Bone Fatty Acid Metabolism and Its Regulation by Adipocyte Lipoprotein Lipase. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–116. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 19.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14:982–991. doi: 10.1111/j.1582-4934.2009.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurel DB, Pallu S, Jaffre C, Fazzalari NL, Boisseau N, Uzbekov R, Benhamou CL, Rochefort GY. Osteocyte apoptosis and lipid infiltration as mechanisms of alcohol-induced bone loss. Alcohol Alcohol. 2012;47:413–422. doi: 10.1093/alcalc/ags057. [DOI] [PubMed] [Google Scholar]
- 21.Collins DH, Ghadially FN, Meachim G. Intra-Cellular Lipids of Cartilage. Ann Rheum Dis. 1965;24:123. doi: 10.1136/ard.24.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 2016;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5:e11492. doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, Khosla S, Oursler MJ, Westendorf JJ. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J Bone Miner Res. 2016;31:116–128. doi: 10.1002/jbmr.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpio LR, Bradley EW, McGee-Lawrence ME, Weivoda MM, Poston DD, Dudakovic A, Xu M, Tchkonia T, Kirkland JL, van Wijnen AJ, Oursler MJ, Westendorf JJ. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling. Sci Signal. 2016;9:ra79. doi: 10.1126/scisignal.aaf3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudakovic A, Camilleri ET, Xu F, Riester SM, McGee-Lawrence ME, Bradley EW, Paradise CR, Lewallen EA, Thaler R, Deyle DR, Larson AN, Lewallen DG, Dietz AB, Stein GS, Montecino MA, Westendorf JJ, van Wijnen AJ. Epigenetic Control of Skeletal Development by the Histone Methyltransferase Ezh2. J Biol Chem. 2015;290:27604–27617. doi: 10.1074/jbc.M115.672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F, Baron R. Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol. 2014;34:3076–3085. doi: 10.1128/MCB.00185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, Zhang D, Graham MJ, Unterman TG, Shulman GI, Sztalryd C, Bennett MJ, Ahima RS, Birnbaum MJ, Lazar MA. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 2012;18:934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, Everett LJ, Nabel CS, Li Y, Selvakumaran V, Won KJ, Lazar MA. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. 2013;52:769–782. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Zhang N, Huang X, Xu J, Fernandes JC, Dai K, Zhang X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013;4:e832. doi: 10.1038/cddis.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 33.Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, Xu J, Hill WD, Johnson MH, Hunter M, Pierce JL, Yu K, Hamrick MW, Isales CM. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J Bone Miner Res. 2017;32:2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M, Wang Y, Shao JZ, Wang J, Chen W, Li YP. Cbfbeta governs osteoblast-adipocyte lineage commitment through enhancing beta-catenin signaling and suppressing adipogenesis gene expression. Proc Natl Acad Sci U S A. 2017;114:10119–10124. doi: 10.1073/pnas.1619294114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M, Saeed H, Chen M, Weinstein LS, Pajevic PD, Kronenberg HM, Wu JY. Loss of Gsalpha early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J Bone Miner Res. 2014;29:2414–2426. doi: 10.1002/jbmr.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 40.Riminucci M, Robey PG, Saggio I, Bianco P. Skeletal progenitors and the GNAS gene: fibrous dysplasia of bone read through stem cells. J Mol Endocrinol. 2010;45:355–364. doi: 10.1677/JME-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rendina-Ruedy E, Guntur AR, Rosen CJ. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6:250–258. doi: 10.1080/21623945.2017.1356505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcu F, Bogdan F, Mutiu G, Lazar L. Histopathological study of osteoporosis. Rom J Morphol Embryol. 2011;52:321–325. [PubMed] [Google Scholar]
- 45.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 46.Gunaratnam K, Vidal C, Boadle R, Thekkedam C, Duque G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open. 2013;2:1382–1389. doi: 10.1242/bio.20136700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao B, Yang L, Luo ZJ. Transdifferentiation between bone and fat on bone metabolism. Int J Clin Exp Pathol. 2014;7:1834–1841. [PMC free article] [PubMed] [Google Scholar]
- 48.Ullah M, Sittinger M, Ringe J. Transdifferentiation of adipogenically differentiated cells into osteogenically or chondrogenically differentiated cells: phenotype switching via dedifferentiation. Int J Biochem Cell Biol. 2014;46:124–137. doi: 10.1016/j.biocel.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Seong S, Kim K, Kim I, Jeong BC, Kim N. Downregulation of Runx2 by 1,25-Dihydroxyvitamin D(3) Induces the Transdifferentiation of Osteoblasts to Adipocytes. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal. 2012;2012:793823. doi: 10.1100/2012/793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi H, Gao Y, Ueta C, Yamaguchi A, Komori T. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem Biophys Res Commun. 2000;273:630–636. doi: 10.1006/bbrc.2000.2981. [DOI] [PubMed] [Google Scholar]
- 53.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98:935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab. 2016;27:392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, Nelson T, Katz G, Berry R, Church CD, Doucette CR, Rodeheffer MS, Macdougald OA, Rosen CJ, Horowitz MC. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014;537:123–139. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 57.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, Cooper MS, Seibel MJ. Endogenous Glucocorticoids and Bone. Bone Res. 2013;1:107–119. doi: 10.4248/BR201302001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sciences. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 60.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 61.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 62.Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, Sulston RJ, Burr AA, Das AK, Simon BR, Mori H, Bree AJ, Schell B, Krishnan V, MacDougald OA. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia. Endocrinology. 2016;157:508–521. doi: 10.1210/en.2015-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reginster JY, Neuprez A, Beaudart C, Lecart MP, Sarlet N, Bernard D, Disteche S, Bruyere O. Antiresorptive drugs beyond bisphosphonates and selective oestrogen receptor modulators for the management of postmenopausal osteoporosis. Drugs Aging. 2014;31:413–424. doi: 10.1007/s40266-014-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Appelman-Dijkstra NM, Papapoulos SE. Modulating Bone Resorption and Bone Formation in Opposite Directions in the Treatment of Postmenopausal Osteoporosis. Drugs. 2015;75:1049–1058. doi: 10.1007/s40265-015-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guntur AR, Gerencser AA, PTL, DeMambro VE, Bornstein SA, Mookerjee SA, Maridas DE, Clemmons DE, Brand MD, Rosen CJ. Osteoblast like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018 doi: 10.1002/jbmr.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]