Abstract
Objective
The objective of this study was to report the tolerability and toxicity of a regimen consisting of intravenous (IV) docetaxel and intraperitoneal (IP) cisplatin and paclitaxel with granulocyte colony-stimulating factor support.
Methods
We conducted a retrospective cohort study of patients with surgical stage II-IV epithelial ovarian, fallopian tube or primary peritoneal carcinoma treated with an outpatient IP chemotherapy regimen consisting of docetaxel 75mg/m2 IV and cisplatin 75mg/m2 IP day 1 followed by paclitaxel 60mg/m2 IP day 8 every 21 days. Grade 3 and 4 toxicity, dose delays and reductions, port complications, and tolerability are reported. Outcomes, including response rate, progression-free survival (PFS), overall survival (OS) are also reported.
Results
60 patients received this IP regimen. Most common toxicities included neutropenia (47%), gastrointestinal (28%) and anemia (25%). Most patients (85%) experienced no IP port complications. Dose delay or reduction was required in 30% of patients. Two-thirds completed all prescribed cycles, with 80% of total planned cycles completed. Complete response was achieved for 88%, and 43% are currently without evidence of disease. Median PFS for all patients was 25.5 months (95% CI 20.4-30.5) while OS for all patients was 56.8 months (95% CI 47.7-65.9 months). For the 44 patients with stage III disease, median PFS was 22.1 months (95% CI 16.3-28.0 months), while median OS was 56.8 months (95% CI 47.3-66.3 months).
Conclusions
This docetaxel-based IP chemotherapy regimen demonstrates an improved tolerability profile compared to GOG172. Additional evaluations on alternative IP regimens remain warranted. Short follow-up time limits survival assessment, but results are encouraging.
Keywords: Docetaxel, intraperitoneal chemotherapy, ovarian cancer
Introduction
The American Cancer Society's annual projections for ovarian cancer include 22,440 new cases with 14,080 women expected to die from ovarian cancer within the United States [1]. Traditionally, advanced stage disease has been treated with primary cytoreductive surgery followed by intravenous (IV) platinum and taxane-based chemotherapy [2]. Alternative treatment regimens have included the use of both dose dense paclitaxel and platinum [3-5] as well as the use of a combination of both IV and intraperitoneal (IP) therapy [6-8].
Attention has been focused on IP administration of anti-neoplastic agents because of the intra-abdominal dissemination pattern of ovarian cancer, and has been evaluated in a series of trials by the Gynecologic Oncology Group (GOG) [6-8]. While the first two trials, GOG104 and 114, were considered positive trials, the improvement in overall survival was somewhat modest at the expense of added toxicity [6, 8]. However, GOG172 demonstrated the clear superiority of an IP and IV chemotherapy regimen when compared to an IV regimen alone in the primary treatment of stage III optimally-cytoreduced epithelial ovarian or primary peritoneal carcinoma [7]. GOG172 reported a 23.8 month progression-free survival (PFS) and 65.6 month overall survival (OS) for patients receiving IP chemotherapy as compared to 18.3 month PFS and 49.7 month OS for patients in the IV treatment group. These trial results prompted a US National Cancer Institute (NCI) Clinical Announcement in 2006 encouraging IP chemotherapy as the preferred method of treatment for these women [9]. Importantly, the rates of both grade 3 and 4 leukopenia (76% vs. 64 %, p <0.001) and neurologic events (19% vs. 9%, p =0.001) were more common in the patients receiving the IV/IP regimen.
Despite this endorsement and the subsequent support from numerous other multidisciplinary professional societies, adoption of IP chemotherapy use has been slow. Notable barriers include the need for coordinated multidisciplinary care, high toxicity, catheter related issues, inpatient administration, and limited access to facilities with experience providing such therapy [9]. Catheter-related complications and patient unwillingness to continue therapy are also potential obstacles. Secondary to side effects, only 42% of the patients receiving the IP arm in GOG172 were able to complete all assigned cycles [7]. Other studies have demonstrated similar findings [11].
Most recently, the GOG reported preliminary findings from GOG252 (NCT01167712), a phase III trial evaluating a modification of the GOG172 regimen with a lower dose of IP cisplatin versus two dose dense, or weekly paclitaxel regimens, one with IV carboplatin, and the second with IP carboplatin, all in combination with bevacizumab induction and maintenance [12]. Preliminary data demonstrated no survival benefit for either of the IP therapy arms with median PFS survival ranging from 26.8 to 28.7 months for the three arms. Moreover, Wright and colleagues utilized the MarketScan database and noted that IP therapy was used in only 15% of patients and that in the absence of a confirmatory trial in the US, dose dense paclitaxel appears to be increasing. However, two reports from ASCO 2016 note continued utility of IP regimens in ovarian cancer patients traditionally excluded from IP trials, namely those having a suboptimal cytoreduction as well as following an interval cytoreduction [13, 14].
In an effort to reduce toxicity and increase rates of therapy completion, modifications of the GOG172 regimen have been reported [10, 11]. We adopted a modified outpatient regimen consisting of IV docetaxel 75mg/m2, IP cisplatin with a fixed dose of 75mg/m2 and IP paclitaxel 60mg/m2 as our primary IP therapy regimen. Compared to paclitaxel, docetaxel has been shown to have a different side effect profile with less neuropathy at the expense or more neutropenia and without a detriment in quality of life for patients, all without sacrificing PFS or OS [15]. The objectives for this study are to report: 1) our docetaxel-based IP regimen protocol; 2) toxicity and tolerability of this regimen; 3) early outcomes including response rate and PFS.
Materials and Methods
After institutional review board (IRB) approval was obtained, a retrospective cohort study was performed on 98 patients who received IP chemotherapy between January 1, 2008 and April 30, 2014 at a single institution. Patients were included if they received at least one cycle of the defined regimen. Patients enrolled on a clinical trial for either primary therapy (N=20) or recurrent disease (N=13) were excluded. Alternative IP-based regimens were also excluded (N=5).
Eligible patients included those with stages II-IV epithelial ovarian, fallopian tube, and peritoneal carcinoma. Patients who received this regimen as primary adjuvant therapy (N=52) or following interval debulking surgery (N=8) were included. All patients had GOG performance status of 0-1 and adequate renal, hepatic, and bone marrow function prior to initiation of therapy.
Abstracted data included patient demographics, medical co-morbidities, and oncologic history including histology, grade, stage, and surgical details. Data was collected regarding the timing of IP port placement and related complications. Charts were reviewed for chemotherapy cycles planned and received, adverse events, and any treatment delays, dose reductions, or hospitalizations. Toxicity data regarding hematologic, gastrointestinal, metabolic, renal, neurological, infectious and embolic events was collected. Grading of toxicity was based on the National Cancer Institute Common Toxicity Criteria Version 2. Response to the treatment regimen was noted to be complete, partial, or progression as documented by the treating physician. Patients without a response were considered to have no progression free survival time. PFS and OS were calculated from the date of surgery. Recurrence was based on one of the following: Gynecologic Cancer Intergroup Criteria for Ca-125 from the nadir [16], radiographic imaging, or physical exam. Patients without evidence of progression were censored at the time of their last clinic visit. Disease status at time of last clinic visit was recorded. Patients known to be deceased were considered to have died from their cancer. When date of death was not documented in the patient's chart, public death records were utilized.
The modified regimen in this study is based on a proposed 21-day cycle with therapy on D1 and D8 for a total of 6 cycles. The details of the regimen are outlined in Table 1. The primary modifications include substitution of D1 docetaxel for paclitaxel as well as administration of IP cisplatin on D1 in an ambulatory setting. The dose of cisplatin was reduced to 75 mg/m2 from the 100 mg/m2 used in GOG172. All patients were given GCSF with pegfilgrastim 6mg subcutaneously on D8. Pre-treatment labs were routinely checked prior to D1 and D8 of treatment. Additional IV fluids were not routinely administered following cisplatin administration although they were added to the regimen if patients experienced significant renal dysfunction or dehydration.
Table 1. Outpatient docetaxel-based IP chemotherapy protocol.
Day 0
|
Day 1
|
Day 2-5
|
Day 8
|
The treating physician, prior to the initiation of IP therapy, determined the number of planned cycles of IP therapy. Dose reductions, delays and alterations were also made at the discretion of the treating physician. Dose alterations included omission of D8 treatment or failure to complete D1 therapy for any reason. IP cycles were considered completed once the patient received D1 of the cycle. If IP chemotherapy was stopped prior to completion of the planned number of cycles, the patient continued with IV therapy alone on a standard 21-day cycle to complete therapy as tolerated. Descriptive and summary statistics were determined and Kaplan-Meier estimates of survival were compared with the Log Rank test (SPSS version 22, IBM Corporation, Armonk, NY).
Results
Patient characteristics
Sixty patients received at least one cycle of IP therapy, and were included. Patients were predominantly white (78%) with a median age of 63 (range 29-77). Most patients, 38 (63%), had stage IIIC disease at the time of initial cytoreductive surgery. Nearly all patients were at least optimally debulked (97%) and 33% had no residual disease. Bowel resection was performed in 40% of patients. Papillary serous (69%) was the most common histology. A majority (60%) of patients had delayed placement of their IP catheter. Eight patients (13%) received at least one cycle of neoadjuvant IV chemotherapy. Complete patient and disease characteristics are depicted in Table 2.
Table 2. Patient demographics (N=60).
| Characteristic | Number (%) |
|---|---|
| Median age (range) | 63 (29-77) |
| Race | |
| White | 47 (78) |
| Black | 9 (15) |
| Other | 4 (7) |
| Median BMI (range) | 26.6 (16-44) |
| FIGO stage | |
| II | 13 (22) |
| IIIA | 4 (7) |
| IIIB | 2 (3) |
| IIIC | 38 (63) |
| IV | 3 (5) |
| Disease site | |
| Ovary | 53 (88) |
| Fallopian tube | 2 (3) |
| Peritoneal | 5 (8) |
| Histology | |
| Papillary serous | 42 (69) |
| Endometrioid | 5 (8) |
| Clear cell | 1 (2) |
| Mixed | 12 (20) |
| Debulking status | |
| No residual disease | 20 (33) |
| Optimal | 38 (64) |
| Suboptimal | 2 (3) |
| IP catheter placement | |
| Primary | 25 (40) |
| Delayed | 35 (60) |
Toxicity and tolerability
Table 3 lists the incidences of grade 3 or 4 toxicity. 18% of patients received therapy without experiencing significant toxicity. Neutropenia (47%), gastrointestinal (28%) and anemia (25%) were most frequently encountered. Other commonly observed toxicities included neurological (17%) and renal (10%). Thrombocytopenia, metabolic derangements, thromboembolic events and infection were all less frequent. Four patients (7%) experienced neutropenic fever. Twenty-three hospitalizations were documented among 18 patients during IP therapy. One patient was hospitalized for an IP port infection following cessation of IP therapy. No patients suffered death as a result of toxicity.
Table 3. Toxicity.
| Toxicity | Number (%) |
|---|---|
| Anemia | 15 (25) |
| Neutropenia | 28 (47) |
| With fever | 4 (7) |
| Thrombocytopenia | 2 (3) |
| Neurological | 10 (17) |
| Function | 6 (10) |
| ADL | 4 (7) |
| Renal | 6 (10) |
| Gastrointestinal | 17 (28) |
| Metabolic | 3 (5) |
| Thromboembolic event | 4 (7) |
| Infection | 2 (3) |
| Fatigue | 1 (2) |
Complications related to the IP port were relatively uncommon with most patients (85%) experiencing no IP port-related complications. Infection (8%) was the most common port reported toxicity, although only one patient required removal. The remaining infections were treated with oral antibiotics. Other complications included two port fractures (3%), one port that failed to function (2%) and one port occluded with a thrombus (2%). One patient suffered a bowel injury during laparoscopic placement and required laparotomy to repair.
Efficacy
Table 4 demonstrates the tolerability of therapy. Of 328 total IP cycles prescribed, patients were able to complete 80%. Two-thirds of patients were able to complete all prescribed IP cycles. Six IP cycles were prescribed for 45 patients (75%). 56% of those patients completed all six, and median number of IP cycles received was four. For the patients unable to complete all prescribed IP cycles, only two were unable to complete at least six cycles of IV chemotherapy.
Table 4. IP cycle administration and tolerability.
| Measurement | Number (%) |
|---|---|
| Tolerability | |
| Cycles prescribed | 328 |
| Cycles completed | 261 (80) |
| Patients completing all IP cycles | 39 (65) |
| Dose delay, reduction or alteration | |
| Delay >1 week (total) | 13 |
| Reduction | 6 (10) |
| Alteration (total) | 38 |
| Reason for stopping therapy | |
| Completion | 39 (65) |
| Toxicity | 20 (33) |
| Response plateau | 1 (2) |
| Response | |
| Complete | 53 (88) |
| Partial | 4 (7) |
| Progression | 3 (5) |
| Status | |
| No evidence of disease | 26 (43) |
| Alive with disease | 14 (23) |
| Deceased | 16 (27) |
| Unknown | 4 (7) |
Delay in therapy greater than one week was required in 12 patients (20%), with one patient requiring two separate delays. Six patients (10%) required dose-reduction due to toxicity, most commonly from renal dysfunction. Thirty-eight dose alterations were made in 25 patients (42%), most frequently due to neutropenia (18 total alterations). Less commonly observed indications for alteration included renal (7 alterations) and gastrointestinal (5 alterations). IP therapy was stopped due to toxicity in 33% of patients. No patients stopped due to progression; however one stopped due to a plateau in response as measured by her Ca-125 level. Nearly all patients (88%) demonstrated a complete response to therapy, and only three patients (5%) had progressive disease during therapy. At the time of data analysis, 43% of patients were without evidence of disease, 23% were alive with disease, 27% were deceased and the status of 7% was unknown. Median PFS was 25.5 months (95% CI 20.4-30.5), while OS was 56.8 months (95% CI 47.7-65.9 months) (Data Not Shown). When considering the 44 patients with stage III disease, median PFS was 22.1 months (95% CI 16.3-28.0 months) and is depicted in Figure 1, while median OS was 56.8 months (95% CI 47.3-66.3 months) and is depicted in Figure 2.
Figure 1.
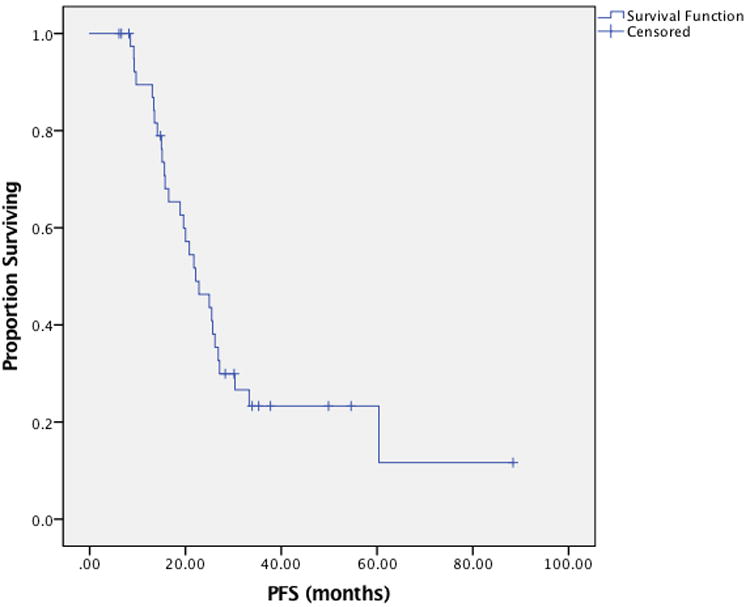
Progression-free survival among patients with stage III ovarian cancer treated with docetaxel based intraperitoneal regimen.
Figure 2.
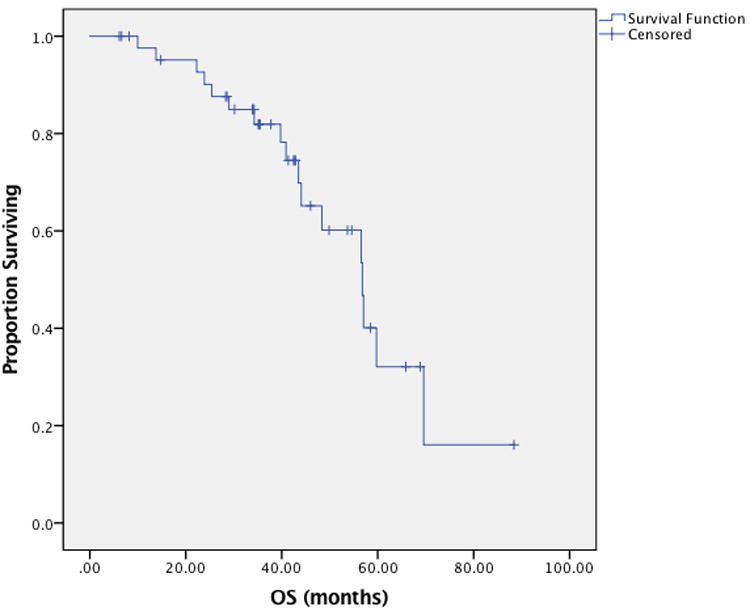
Overall survival among patients with Stage III ovarian cancer treated with docetaxel based intraperitoneal regimen.
Discussion
Following the publication of GOG172, which demonstrated an improvement in OS of 16 months for patients that received any IP chemotherapy compared to IV therapy alone, the utilization of IP chemotherapy received increased attention; however, patients receiving IP therapy experienced greater toxicity. In an attempt to limit potential toxicity, we adopted a docetaxel-based regimen of IP chemotherapy designed to improve tolerability and transition the regimen to an ambulatory setting, while still preserving the survival benefit.
We previously described our early experiences in a multi-institutional study with this regimen, although patients previously may have received cisplatin 100mg/m2 IP and amifostine administration was omitted [10]. This is based on published data, demonstrating similar efficacy but improved neurotoxicity and quality of life scores in women with advanced epithelial ovarian cancer [15, 17]. However, docetaxel was also associated with higher rates of neutropenia and neutropenic complications [15, 17]. Recognizing the high rate of neutropenia in GOG172, the substitution of an agent with higher bone marrow suppression justified the addition of primary prophylactic GCSF. This limited neuropathic toxicity to 17% while also minimizing neutropenic complications. Per institutional protocol, these patients received their pegfilgrastim injections on D8 following their paclitaxel infusions, a practice demonstrated to be safe in gynecologic cancer patients [18].
Since the start of 2008, 60 of our patients with advanced ovarian, fallopian tube or primary peritoneal carcinoma have received at least one cycle of this outpatient docetaxel-based IP chemotherapy regimen. We demonstrated excellent tolerability of this regimen among a broad population of patients including those with stage 2, 3, and 4 disease, suboptimally debulked patients, and those treated with neoadjuvant chemotherapy. As part of the treatment protocol, we also checked hematologic parameters prior to the day 8 infusion, although this approach was not utilized in GOG172. Neutropenia was responsible for half the instances where patients missed D8 treatment, and was the most common reason for dose alteration. This conservative approach of utilizing results from D8 labs prior to treatment may have contributed to the improvements in safety and tolerability of therapy compared to GOG172; however, it may have resulted in unnecessary treatment modifications, as an abnormal lab value was primarily responsible for two-thirds of the instances where D8 treatment was missed. Based on the data presented here, we have further modified our protocol to eliminate routine hematologic monitoring prior to D8 paclitaxel. The incidence of neutropenia appears reasonable when considering just 14% of neutropenic patients experienced the comorbidity of fever.
Major side effects of platinum-based chemotherapy include renal, metabolic and gastrointestinal symptoms [19]. The IP therapy arm in GOG172 experienced a significant increase in gastrointestinal (46% vs. 24%) and metabolic toxicities (27% vs. 7%), in addition to an increase in renal toxicity (7% vs. 2%) [7]. We addressed these potential toxicities by reducing the IP cisplatin dose from 100mg/m2 to 75mg/m2, and using an aggressive anti-emetic regimen of daily dexamethasone on D0 and D2-5 in addition to dexamethasone, diphenhydramine, fosaprepitant, palonosetron, and ranitidine on D1 and D8. The combination of anti-emetics used was aimed at preventing both acute and delayed emesis. Severe gastrointestinal and metabolic toxicities were observed in just 28% and 5% of patients, respectively, rates comparable to those observed in the IV therapy arm of GOG172 [7].
Overall, toxicity appeared to be favorable with thrombocytopenia, thromboembolic events and infection observed in 7% or fewer patients. Other infrequent toxicities included hearing loss and fatigue or weakness. Ten percent suffered severe renal toxicity. No patients experienced death as a result of complications from treatment. Hospitalizations were infrequent, occurring just 23 times total. Half of those occurred during or after cycle 1, and intractable nausea and emesis was the most common cause. Low rates of complications early in IP therapy may prevent conversion to less demanding IV therapy. Likewise, dose delays (13 total occurrences) and reductions (10% of patients) were infrequent, and are felt to be a direct result of the changes made to our regimen. Comparable statistics are not available from GOG172.
Treatment with IP chemotherapy carries a unique set of complications due to the presence of an intraperitoneal catheter. Comparable to GOG172, the IP port was placed during the debulking surgery in 40% of patients. During the initial adoption, delayed placement was preferred. This pattern has changed with time, and placement during the primary debulking surgery is now the preferred method at our institution. IP port complications were rare, and led to just 3 patients (5%) discontinuing IP therapy. This compares favorably to GOG172, which found that 34% of patients discontinued IP therapy mainly due to catheter-related complications [20]. As seen in multiple other studies, infection was the most frequently observed complication [21].
IP therapy was tolerated remarkably well by our patients. The completion rate of IP cycles prescribed was 80%, and 65% of patients were able to complete all prescribed IP cycles. A smaller joint institutional study, including our institution, performed in 2009 examined outcomes from a similar outpatient regimen and reported that 71% of patients had to discontinue therapy prior to completion [10]. Here, toxicity was short lived and did not typically prevent patients from completing IP therapy. This led to excellent response rates, with 88% of patients having complete response and just 5% experiencing progression. GOG172 utilized second-look laparotomy as an option to determine pathologic response, and found that 57% of patients experienced a complete response within eight weeks. Whereas GOG172 was a large multicenter phase 3 randomized control trial, our current study is limited to a single institutional experience. Other potential biased include its retrospective design, small patient population, relatively short follow-up and potential confounding, which may all impact our results. Moreover, physician bias may help explain decisions to enroll in patients on a clinical trial versus the use of the outpatient docetaxel regimen. Nonetheless, during the study period the described outpatient docetaxel regimen was the preferred regimen for patients not treated on a clinical trial and for the 44 patients with stage III disease, was associated with a median PFS of 22.1 months (95% CI 16.3-28.0 months) with median OS 56.8 months (95% CI 47.3-66.3 months).
In conclusion, this modified IP chemotherapy regimen is safe and well tolerated, which resulted in reduced rates of severe toxicity compared to GOG172, no treatment-related patient deaths, and promising preliminary survival data. As noted by more recent publications, data still supports the use of IP chemotherapy in patients with advanced ovarian cancer. By moving treatment to an outpatient setting and improving the toxicity profile, we anticipate our ability to treat patients will improve, as will our institutional survival rates. Further investigation into this docetaxel-based regimen is warranted.
Acknowledgments
Funding: Funding support was provided in part by NIH 3P30CA013148-43S3 and 5K12HD0012580-15 to CAL & KSB
Footnotes
Presented as a poster presentation at the Society of Gynecologic Oncology Annual Meeting on Women's Cancer, Chicago, IL, 2015
Disclosure statement: The authors of this paper have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. The New England journal of medicine. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. The New England journal of medicine. 2016;374:738–48. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–6. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 5.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 6.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. The New England journal of medicine. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. The New England journal of medicine. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 8.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 9.Trimble EL, Christian MC. National Cancer Institute-United States strategy regarding intraperitoneal chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2008;18(1):26–8. doi: 10.1111/j.1525-1438.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 10.Berry E, Matthews KS, Singh DK, Buttin BM, Lurain JR, Alvarez RD, et al. An outpatient intraperitoneal chemotherapy regimen for advanced ovarian cancer. Gynecologic oncology. 2009;113:63–7. doi: 10.1016/j.ygyno.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Battelli C, Campo M, Buss MK, Awtrey CS, Konstantinopoulos PA. Safety and outcome of patients treated with a modified outpatient intraperitoneal regimen for epithelial ovarian, primary peritoneal or fallopian tube cancer. Chemotherapy. 2013;59:251–9. doi: 10.1159/000356758. [DOI] [PubMed] [Google Scholar]
- 12.Walker JL, Brady MF, DiSilvestro PA, Fujiwara K, Alberts DS, Zheng W, et al. A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube and primary peritoneal carcinoma NCI-supplied agent(s): Bevacizumab (NSC #704865, IND #7921) NCT01167712 a GOG/NRG trial (GOG 252) 2016 [Google Scholar]
- 13.Hasegawa K, Shimada M, Takeuchi S, Fujiwara H, Imai Y, Iwasa N, et al. Multicenter phase II study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in patients with suboptimally debulked epithelial ovarian or primary peritoneal carcinoma. J Clin Oncol. 2016;34 suppl; abstr 5504. [Google Scholar]
- 14.Mackay H, Gallagher CJ, Parulekar WR, Ledermann JA, Armstrong DK, Gourley C, et al. OV21/PETROC: A randomized Gynecologic Cancer Intergroup (GCIG) phase II study of intraperitoneal (IP) versus intravenous (IV) chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer (EOC) J Clin Oncol. 2016;34 doi: 10.1093/annonc/mdx754. suppl; abstr LBA5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 16.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 17.Vasey PA, Atkinson R, Coleman R, Crawford M, Cruickshank M, Eggleton P, et al. Docetaxel-carboplatin as first line chemotherapy for epithelial ovarian cancer. Br J Cancer. 2001;84:170–8. doi: 10.1054/bjoc.2000.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitworth JM, Matthews KS, Shipman KA, Numnum TM, Kendrick JE, Kilgore LC, et al. The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecologic oncology. 2009;112:601–4. doi: 10.1016/j.ygyno.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecologic oncology. 2006;100:27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Helm CW. Ports and complications for intraperitoneal chemotherapy delivery. BJOG. 2012;119:150–9. doi: 10.1111/j.1471-0528.2011.03179.x. [DOI] [PubMed] [Google Scholar]


