Abstract
Background
Carbapenem-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae (CR-HMKP) poses a significant public health challenge. We investigated its epidemiology and molecular characteristics in a tertiary care hospital in eastern China.
Methods
CR-HMKP were identified among 106 non-duplicated carbapenem-resistant K. pneumoniae isolates (from June 2013 to September 2017) using the string test. The pulsotype (PT) and sequence type (ST) of CR-HMKP isolates were determined using pulsed-field gel electrophoresis and multilocus sequence typing. Resistance determinants, capsular serotypes, and virulence genes were detected by PCR and sequencing. Representative isolates from each PT were selected, and their virulence phenotypes were established using the serum killing and Galleria mellonella lethality assays.
Results
Of the 106 isolates, 13 (12.3%) were CR-HMKP. Seven were positive for blaNDM-1 and shared the same genotype (PT5/ST1764); the others were positive for blaKPC-2, belonged to ST11, and were divided into four different PTs. The serotype of all blaNDM-1-positive isolates was K64, while that of blaKPC-2-positive isolates were K47 (N=4) and K64 (N=2). The NDM-1-producing HMKP isolates were positive for aerobactin, exhibited high serum resistance, and elicited significantly increased larval mortality compared with the other isolates. All patients had received invasive treatment prior to infection by NDM-1-producing HMKP. The infections occurred between July and August 2016 and were hospital-acquired.
Conclusions
NDM-1-producing HMKP ST1764 isolates were identified; this is the first report worldwide on an outbreak of nosocomial infection caused by these isolates. Effective surveillance and strict infection control strategies should be implemented to prevent CR-HMKP dissemination.
Keywords: Carbapenem-resistant, Hypervirulent, Hypermucoviscous, Klebsiella pneumoniae, NDM-1, Epidemiology, Molecular characteristics
INTRODUCTION
Klebsiella pneumoniae is an important opportunistic pathogen that causes a wide range of infections and exhibits increasingly frequent acquisition of antibiotic resistance, especially carbapenem resistance [1]. In recent years, a new variant designated hypervirulent (hypermucoviscous) K. pneumoniae (HMKP) has raised concerns worldwide, owing to its ability to cause severe and life-threatening infections in young immune-competent individuals. Remarkably, these infections are often complicated by other devastating disseminated infections, including endophthalmitis and meningitis [2]. Since HMKP was first identified in East Asia, the incidence of HMKP-associated infection has increased worldwide [3]. Due to the increased production of capsular polysaccharide, HMKP exhibits a distinct hypermucoviscous phenotype when grown on Columbia blood agar plates that can be detected using the string test [2]. In addition, it can secrete various siderophores such as aerobactin, salmochelin, yersiniabactin, and enterobactin. Of these, aerobactin accounts for >90% of the siderophore activity and plays a crucial role in the growth and survival of HMKP in human ascites fluid or serum and in vivo mouse infection models, suggesting that aerobactin is a crucial virulence factor of HMKP [4].
Since HMKP is susceptible to commonly used antimicrobial agents, the multidrug-resistant K. pneumoniae population that does not exhibit the hypermucoviscosity phenotype is defined as “classic” K. pneumoniae (cKP); it was previously thought that there was no overlap between HMKP and cKP [5]. Unfortunately, virulence and resistance could converge to produce strains that are able to cause severe and untreatable invasive infections, representing a major public health concern [6]. Furthermore, carbapenem-resistant HMKP (CR-HMKP) isolates are increasingly being detected worldwide, and in particular in China [3,7,8,9,10,11,12]. Thus, to better understand the incidence and characteristics of CR-HMKP strains, we conducted a retrospective study to investigate the clinical data, epidemiology, and molecular characteristics of CR-HMKP isolates, in a medical center in eastern China. In addition, for the first time, we observed an outbreak of nosocomial infection associated with New Delhi metallo-β-lactamase-1 (NDM-1)-producing HMKP.
METHODS
Strain identification and hypermucoviscosity phenotype detection
A total of 106 non-duplicated K. pneumoniae isolates (identified using VITEK 2 compact system [BioMérieux, Marcy-l'Étoile, France]) exhibiting a carbapenem-resistant phenotype (imipenem minimum inhibitory concentration [MIC] ≥4 µg/mL and/or ertapenem MIC ≥2 µg/mL) were consecutively collected between June 2013 and September 2017 at the Second Hospital of Anhui Medical University in Anhui, eastern China. The hypermucoviscosity phenotype of the carbapenem-resistant K. pneumoniae (CRKP) isolates was detected using the string test, as described previously [7]. Briefly, all the tested isolates were cultured overnight on Columbia blood agar plates (BioMérieux, Shanghai, China) at 37℃, and bacterial colonies were then stretched with an inoculation loop. The formation of a viscous string over five mm in length was defined as a positive result; CRKP isolates with a positive string test were defined as CR-HMKP.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of CR-HMKP isolates was initially performed using the VITEK 2 compact system. Subsequently, ampicillin, amikacin, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, levofloxacin, gentamicin, trimethoprim-sulfamethoxazole, tobramycin (National Institutes for Food and Drug Control, Beijing, China); ertapenem, imipenem (Merck Sharp & Dohme Corp, Hangzhou, China); meropenem (Sumitomo Pharmaceuticals, Suzhou, China); and piperacillin-tazobactam (Pfizer, New York, NY, USA). MICs were confirmed using the agar dilution method according to the recommendations and breakpoints proposed by the CLSI [13,14]. Tigecycline (Pfizer) and colistin (Sigma Aldrich, Shanghai, China) MICs were determined by the broth microdilution method, using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines and breakpoint [15]. The standard strains Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used for quality control.
Carbapenemase phenotypes and detection of resistance determinants
Both the modified Hodge test (MHT) and imipenem-EDTA double-disk synergy test (DDST) were performed to determine the carbapenemase phenotypes as described previously [16]. Carbapenemase-encoding genes (including blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48) and common extended-spectrum β-lactamase (ESBL)-encoding genes (including blaCTX-M, blaSHM, and blaTEM) were screened by PCR as described previously [17,18]. All positive PCR products were sequenced and compared with the published sequences in GenBank (www.ncbi.nlm.nih.gov/blast/).
Capsular polysaccharide serotyping and virulence genes
The capsular polysaccharide serotypes of the CR-HMKP were determined by PCR and sequencing of the K-serotype specific wzi allele, which is closely associated with K-type [19]. The PCR products were sequenced and assigned using an online database (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html), and then compared with the reference sequences in GenBank [20]. The corresponding capsular types were determined according to the criteria of ≥94% DNA identity for the same types and ≤80% identity for different types [21]. Fifteen virulence-associated genes, including aerobactin, allS, entB, fimH, iroB, iucA, kfuBC, magA, mrkD, rmpA, rmpA2, ureA, wabG, ybtS, and ycfM, were detected by PCR and sequenced using previously described primers [7,22].
Genotyping of CR-HMKP
The genotypes of the CR-HMKP isolates were determined by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). Briefly, following digestion with the XbaI restriction endonuclease (Takara, Dalian, China), the genomic DNA of the tested isolates and the reference strain Salmonella H9812 were separated on agarose gels using a CHEF Mapper XA PFGE system (Bio-Rad, Hercules, CA, USA). Next, the gel was stained, and dendrograms were constructed from the PFGE data using the BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium) with the Dice similarity coefficient. The same pulsotype (PT) was defined when the isolates shared >90% similarity. MLST was performed as described previously [23]. The number of alleles was determined and the sequence type (ST) was then assigned using the MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Serum killing and Galleria mellonella lethality assays
The virulence of the CR-HMKP isolates was assessed by serum killing and G. mellonella lethality assays. For references, the clinical hypermucoviscous K. pneumoniae strain KPN54798 (ST23, serotype K1, carbapenem-susceptible) was employed as the hypervirulent control (H-control), while the clinical non-hypermucoviscous K. pneumoniae isolate KPN49 (ST11, serotype K47, harboring blaNDM-1) was employed as the low-virulence control (L-control). The clinical and microbiological characteristics of the two reference strains are listed in Supplemental Data Table S1. For the serum killing assay, human blood was collected from five healthy volunteers, and the serum was then separated and stored at −80℃. The assays were performed and the results were expressed as previously described [21,24]. For the G. mellonella infection model, different concentrations of bacterial suspensions were diluted in 10 mM phosphate-buffered saline (PBS) and inoculated into the hemocoel of 20 larvae of G. mellonella (weighing 300±25 mg) (Kaide Ruixin Co., Ltd., Tianjin, China) at final concentrations of 1×103−107 colony-forming units (CFUs)/larva. Following injection, the larvae were incubated at 37℃ and monitored every 12 hours for 72 hours. Experiments were performed three times on separate occasions. The survival rates were recorded, and the 50% lethal dose (LD50) was determined as described previously [25]. Negative control groups included larvae that were inoculated with 10 µL of PBS.
Clinical information collection and ethics
The clinical information of patients infected with CR-HMKP was collected from electronic medical records. All patients were evaluated based on the US Centers for Disease Control and Prevention (CDC) criteria to determine whether the infections were due to CR-HMKP [26]. This retrospective study was approved by the ethical committee of the Second Hospital of Anhui Medical University, with waiver of informed consent (approval number PJ-YX2018-001).
Statistical analysis
The Shapiro-Wilk method was used to test normality. Normally distributed variables were summarized as mean±SD, and non-normally distributed variables were summarized as median and range. The serum killing assays data were summarized as mean±SE. Survival data were plotted using the Kaplan-Meier method and analyzed using log-rank tests with GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). The LD50 values were calculated using the Bliss method, and the results are expressed as log10 (lg) transformed values. A one-sample t-test was used to compare the data and was performed using SPSS v. 21.0 (IBM Corp., Armonk, NY, USA). A two-sided P<0.05 was considered statistically significant.
RESULTS
Clinical and demographic characteristics of the patients
Of the 106 non-duplicated CRKP isolates, 12.3% (13/106) were positive for the string test and identified as CR-HMKP. The patients with CR-HMKP isolates were distributed across several departments, and their median length of hospitalization was 36 days (range 6–174 days). Notably, 76.9% (10/13) of the patients had a history of a stay in the intensive care unit (ICU). Of these patients, seven (53.8%) were males, and their mean age was 55.3±18.6 years. All but one patient received invasive treatment prior to infection with CR-HMKP. Eleven patients exhibited a high fever symptom following infection with CR-HMKP; four of these patients subsequently developed septic shock and eventually died because of failure of the anti-infection treatment (Table 1). Notably, seven cases occurred between July and August 2016, and all these cases were assessed as hospital-acquired (HA) infections. Five of the patients had an ICU stay history in July 2016, and their inpatient bed positions were very close, while the other two patients were in adjacent beds during hospitalizations in the Department of Plastic Surgery (PS) in August 2016.
Table 1. Clinical and demographic characteristics of the patients with CR-HMKP isolates.
| No. Case* | Sex/Age (yr) | Ward | Admission (month-yr) | LOS/LOS in ICU (day) | Underlying disease | Invasive treatment† | Septic shock/Tmax (℃) | Antimicrobial treatment‡ | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| KPN11 | F/63 | NEU-ICU | 10-2013 | 42/42 | Encephalon injury, Pneumonia | Surgery, MV, CVC | No/39.8 | TGC, FOS | Death |
| KPN18 | F/80 | GS-ICU | 11-2013 | 36/11 | Esophageal cancer | MV | No/39.3 | TGC | Death |
| KPN19 | M/42 | ES-ICU | 11-2013 | 39/27 | Severe acute pancreatitis | MV, CVC | Yes/40.8 | MEM | Death |
| KPN34 | F/69 | EICU | 04-2015 | 12/0 | Pneumonia, Diabetes | MV | No/37.9 | TGC, MNO | Discharge |
| KPN53 | M/70 | HS-ICU | 05-2016 | 43/2 | Hepatocellular carcinoma | Surgery | No/38.5 | LVX, SCF | Discharge |
| KPN60 | F/38 | GO-ICU | 07-2016 | 18/2 | Postpartum hemorrhage | Surgery, MV | No/37.9 | LVX | Discharge |
| KPN63 | M/62 | GE-ICU | 07-2016 | 11/10 | Pneumonia | Surgery, CVC | Yes/39.0 | LVX, AMK | Death |
| KPN68 | M/11 | PS | 07-2016 | 49/0 | Scar contracture | Surgery | No/36.6 | LVX | Discharge |
| KPN69 | M/49 | GS-ICU | 07-2016 | 26/2 | Arterial aneurysm | Surgery | No/37.3 | LVX, FOS | Discharge |
| KPN72 | F/60 | EM-ICU | 07-2016 | 6/5 | Thermoplegia, MOF | MV, CVC | Yes/39.3 | LVX | Death |
| KPN74 | F/60 | NEP-PS | 06-2016 | 75/0 | CKD, Diabetes, Gangrene | Surgery, CVC | No/38.6 | LVX | Discharge |
| KPN100 | M/73 | GS-ICU | 06-2017 | 20/19 | Severe acute pancreatitis | MV, CVC | Yes/39.8 | TGC | Death |
| KPN104 | M/42 | ORT-ICU | 06-2017 | 174/7 | Pelvic fractures | Surgery, BC | No/39.0 | TGC, FOS, MEM | Discharge |
*All cases were assessed as hospital-acquired infections; †Invasive treatment prior infection with CR-HMKP; ‡Antimicrobial treatment after CR-HMKP was identified.
Abbreviations: AMK, amikacin; BC, bladder catheter; CKD, chronic kidney diseases; CR-HMKP, carbapenem-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae; CVC, central venous catheter; EICU, emergency intensive care unit; EM, emergency medicine department; ES, emergency surgery department; F, female; FOS, fosfomycin; GE, gastroenterology department; GO, gynecology and obstetrics department; GS, general surgery department; HS, hepatobiliary surgery department; ICU, intensive care unit; LOS, length of stay; LVX, levofloxacin; M, male; MEM, meropenem; MV, mechanical ventilation; NEP, nephrology department; NEU, neurosurgery department; ORT, orthopedics department; PS, plastic surgery department; SCF, cefoperazone-sulbactam; TGC, tigecycline; Tmax, maximal body temperature.
Antimicrobial susceptibility and resistance mechanism
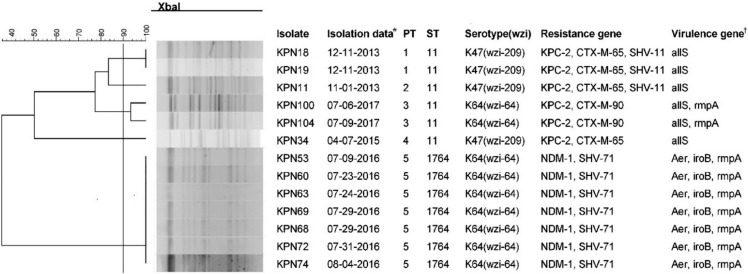
The CR-HMKP isolates were primarily isolated from secretion/pus (N=6, 46.2%), followed by sputum (N=4, 30.8%), blood (N=2, 15.4%), and puncture fluid (N=1, 7.7%). All were resistant to imipenem, ertapenem, and meropenem but were susceptible to tigecycline and colistin (Table 2). The carbapenemase phenotype tests revealed that seven isolates were DDST-positive, and the others were MHT-positive. Consistent with these phenotypes, seven isolates harbored blaNDM-1, while the remaining isolates harbored blaKPC-2. Additionally, half of the blaKPC-2-positive isolates co-harbored blaCTX-M-65 and blaSHV-11, while all blaNDM-1-positive isolates co-harbored blaSHV-71 (Fig. 1).
Table 2. Antimicrobial susceptibility testing result of CR-HMKP isolates.
| Isolate | SPE | MIC of antimicrobial agents (μg/mL) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMK | ATM | CAZ | CIP | COL | CRO | CZO | ETP | FEP | GEN | IPM | LVX | MEM | SXT | TGC | TOB | TZP | ||
| KPN11 | SP | > 256 | 64 | > 128 | 64 | > 32 | 1 | > 32 | > 256 | > 32 | 64 | 16 | > 32 | > 64 | > 32 | 2/38 | 0.50 | 32 | 512 |
| KPN18 | SP | > 256 | 32 | > 128 | > 128 | > 32 | 1 | > 32 | > 256 | > 32 | 64 | 8 | > 32 | > 64 | > 32 | 2/38 | 0.50 | 8 | 512 |
| KPN19 | BL | > 256 | 32 | > 128 | 128 | > 32 | 2 | > 32 | > 256 | > 32 | > 128 | 8 | > 32 | > 64 | > 32 | 2/38 | 0.50 | 16 | > 512 |
| KPN34 | SP | > 256 | 256 | > 128 | > 128 | > 32 | 2 | > 32 | > 256 | > 32 | 64 | 64 | > 32 | > 64 | > 32 | 2/38 | 0.25 | 64 | 256 |
| KPN53 | PU | > 256 | 1 | 1 | > 128 | 1 | 1 | > 32 | > 256 | > 32 | 16 | 0.5 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN60 | SE | > 256 | 1 | 2 | > 128 | 2 | 2 | > 32 | > 256 | > 32 | 16 | 0.5 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN63 | SP | > 256 | 1 | 2 | > 128 | 1 | 1 | > 32 | > 256 | > 32 | 16 | .0.5 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN68 | SE | > 256 | 2 | 1 | > 128 | 1 | 1 | > 32 | > 256 | > 32 | 16 | 1 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN69 | PU | > 256 | 1 | 2 | > 128 | 1 | 1 | > 32 | > 256 | > 32 | 16 | 0.5 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN72 | SE | > 256 | 2 | 2 | > 128 | 2 | 1 | > 32 | > 256 | > 32 | 16 | 1 | > 32 | 2 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN74 | SE | > 256 | 1 | 2 | > 128 | 1 | 2 | > 32 | > 256 | > 32 | 16 | 0.5 | > 32 | 1 | > 32 | 1/19 | 0.50 | 1 | 256 |
| KPN100 | PF | > 256 | > 512 | 64 | 64 | > 32 | 2 | > 32 | 128 | 16 | 8 | > 128 | 8 | 32 | 8 | 4/76 | 1 | > 128 | 128 |
| KPN104 | BL | > 256 | > 512 | 64 | 128 | > 32 | 2 | > 32 | 128 | 16 | 8 | > 128 | 16 | 64 | 8 | 4/76 | 1 | > 128 | 128 |
| RR (%) | 100.0 | 30.8 | 46.2 | 100.0 | 46.2 | 0.0 | 100.0 | 100.0 | 100.0 | 84.6 | 30.8 | 100.0 | 46.2 | 100.0 | 15.4 | 0.00 | 38.5 | 100.0 | |
Abbreviations: AMP, ampicillin; AMK, amikacin; ATM, aztreonam; BL, Blood; CAZ, ceftazidime; CIP, ciprofloxacin; COL, colistin; CRO, ceftriaxone; CZO, cefazolin; ETP, ertapenem; FEP, cefepime; GEN, gentamicin; IPM, imipenem; LVX, levofloxacin; MEM, meropenem; MIC, minimum inhibitory concentrations; PF, puncture fluid; PU, Pus; RR, resistant rate; SE, secretion; SP, sputum; SPE, specimen; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline; TOB, tobramycin; TZP, piperacillin-tazobactam.
Fig. 1. Pulse-field gel electrophoresis (PFGE) dendrograms, genotype, serotype, resistance and virulence genes of the CR-HMKP isolates. The isolates that exhibited PFGE dendrograms with more than 90% similarity are considered one pulsotype (PT). *The isolation date is listed as month-day-year; †Only partial results are shown. Of the other virulence genes tested, entB, iucA, kfuBC, mrkD, rmpA2, ureA, wabG, ybtS, and ycfM were detected in all isolates, and the magA gene was not detected in any of them.
Abbreviations: Aer, aerobactin; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; ST, sequence type.
Genotyping, capsular serotyping, and virulence-associated genes
Five PTs and two STs were identified among the CR-HMKP isolates. All blaNDM-1-positive isolates belonged to PT5 and were assigned to ST1764 (gapA_5, infB_3, mdh_1, pgi_1, phoE_9, rpoB_4, tonB_283), which is a three-locus variant of ST23 (gapA_2, infB_1, mdh_1, pgi_1, phoE_9, rpoB_4, tonB_12). The blaKPC-2-positive isolates belonged to ST11 and were divided into four PTs. Nine of the isolates (69.2%) belonged to capsular serotype K64 (wzi-64) and four (30.8%) to capsular serotype K47 (wzi-209). Of the virulence genes tested, entB, iucA, kfuBC, mrkD, rmpA2, ureA, wabG, ybtS, and ycfM were detected in all CR-HMKP isolates, and the detection rates for aerobactin, allS, iroB, and rmpA were 53.8% (7/13), 46.2% (6/13), 53.8% (7/13), and 69.2% (9/13), respectively. The magA gene was not detected in any of the isolates. Notably, all NDM-1-producing isolates were positive for the aerobactin gene (Fig. 1).
Serum killing assay
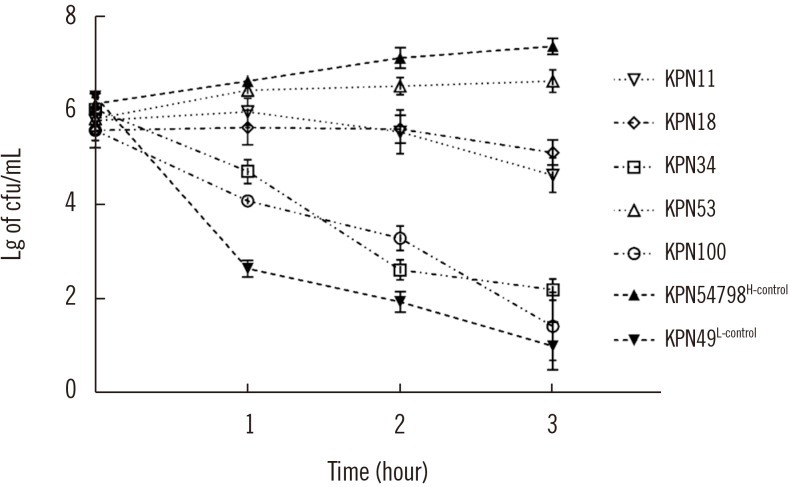
Five representative CR-HMKP isolates [KPN18(PT1), KPN11(PT2), KPN100(PT3), KPN34(PT4), and KPN53(PT5)] were selected based on PT, and their virulence phenotypes were established. The serum killing assay results demonstrated that KPN53 was highly resistant (Grade 5), KPN11 and KPN18 were intermediately sensitive (Grade 4), and KPN34 and KPN100 were serum-sensitive (Grade 1). Of the two reference strains, KPN54798H-control was highly resistant (Grade 6), and KPN49L-control was serum-sensitive (Grade 1; Fig. 2).
Fig. 2. Serum killing assays of the tested isolates. Data are presented as mean±SE, and log10-transformed values were utilized to normalize the data (N=3 for each isolate).
Abbreviations: H-control, hypervirulence control; L-control, low-virulence control; Lg, log10 -transformed values.
Galleria mellonella lethality assay
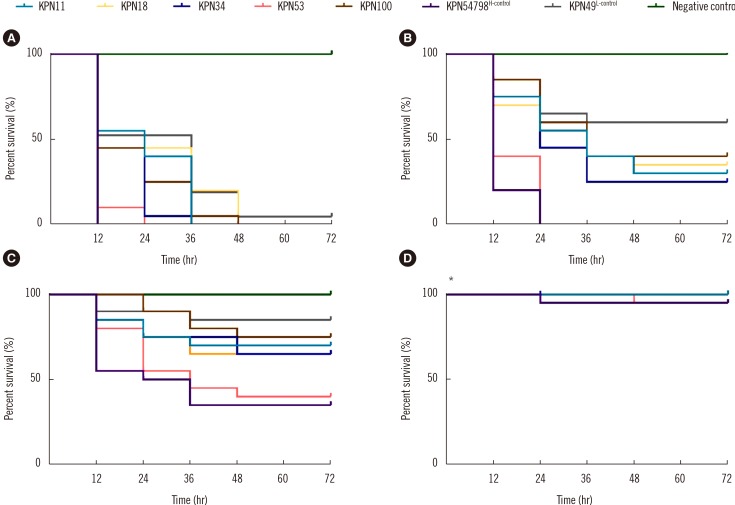
In the G. mellonella infection model, time- and dose-dependent deaths were observed for all tested isolates. At the dose of 1×107 CFU/larvae, 100.0% mortality was observed in all tested isolates except for the KPN49L-control. At the dose of 1×106 CFU/larvae, only KPN53 and KPN54798H-control resulted in 100.0% larval mortality at 24 hours post-infection, whereas KPN11, KPN18, KPN34, KPN100, and KPN49L-control killed 70.0%, 65.0%, 75.0%, 60.0%, and 40.0% of the larvae at this dose, respectively. The observed larval lethality rates due to KPN53 were higher than those of the other CR-HMKP isolates and KPN49L-control (P<0.01 by the log-rank test) (Fig. 3). Additionally, KPN53 had a lower lgLD50 than the other isolates except KPN54798H-control (P<0.05 by the one-sample t-test) (Table 3).
Fig. 3. Survival curves for G. mellonella larvae inoculated with 1×107 (A), 1×106 (B), 1×105 (C), and 1×104 (D) colony-forming units of the tested isolates, and the data shown are from a single representative experiment out of three repeats. *The curves of KPN11, KPN18, KPN34, KPN100, KPN49L-control and negative control are completely overlapping.
Abbreviations: H-control, hypervirulence control; L-control, low-virulence control.
Table 3. lgLD50 values of tested isolates in G. mellonella at 72 hours post-infection.
| Isolate | lgLD50* |
|---|---|
| KPN11 | 5.38 ± 0.06 |
| KPN18 | 5.51 ± 0.09 |
| KPN34 | 5.44 ± 0.03 |
| KPN53 | 4.95 ± 0.06† |
| KPN100 | 5.61 ± 0.10 |
| KPN54798H−control | 4.81 ± 0.11 |
| KPN49L−control | 5.97 ± 0.14 |
*Data are presented as mean±SD; †The lgLD50 value of KPN53 did not differ from that of KPN54798H-control (P=0.059 by the one-sample t-test) and is significantly lower than those of the other CR-HMKP isolates and KPN49L-control (P<0.05 by the one-sample t-test).
Abbreviations: H-control, hypervirulence control; L-control, low-virulence control; lgLD50, Log10 (50% lethal dose).
DISCUSSION
In this study, the prevalence rate of HMKP among CRKP isolates was 12.3%, which is higher than the 7.4% rate reported in a previous study from Hangzhou, eastern China in 2015, but similar to the results of another recent study from Wenzhou, eastern China in 2017 [7,9]. Our study and other reports indicated that the prevalence of HMKP among CRKP isolates in China is high, becoming a serious threat to public health.
The major mechanism of K. pneumoniae drug resistance to carbapenems is the expression of carbapenemases; the Klebsiella pneumoniae carbapenemase (KPC)-type carbapenemases are more prevalent than other types [27]. Similarly, most of the carbapenemases present in CR-HMKP isolates are KPC-2, and the NDM-type carbapenemase are rarely detected [11]. To the best of our knowledge, four NDM-producing HMKP isolates have been reported worldwide to date, which were distributed sporadically throughout Asia and the Middle East and belonged to different STs [12,28,29,30]. We found that 53.8% of the CR-HMKP isolates harbored blaNDM-1, shared the same PT, and belonged to ST1764. Hence, our findings expand the CR-HMKP molecular epidemiology database worldwide.
ST11 is the dominant KPC-producing K. pneumoniae clone in China [31]. In our study, most of the CR-HMKP isolates belonged to ST11 and were serotype K47 (wzi-209). Interestingly, a similar result was obtained in a recent study that described a fatal outbreak caused by KPC-2-producing HMKP ST11 isolates in a hospital in eastern China [8]. All the strains in that report were serotype K47 [8]. Thus, additional studies should be conducted to determine whether regional clonal dissemination of serotype 47 CR-HMKP belonging to ST11 has occurred.
Various virulence factors are associated with HMKP isolates. The magA gene was identified as the serotype K1 allele of the polymerase wzy gene in the cps gene cluster [32]. As we did not identify any serotype K1 isolates, the magA gene was not detected in any of the isolates. Conversely, two plasmid-carried genes, rmpA and rmpA2, which both contribute to the enhancement of capsular production, were detected in most of the CR-HMKP isolates in our study. Several studies have also demonstrated that nearly all HMKP isolates tested positive for the rmpA/rmpA2 genes [33,34].
Iron is essential for the survival of K. pneumoniae. As free iron in the host plasma is scarce, K. pneumoniae predominantly acquires iron via the secretion of siderophores, molecules that have a higher affinity for iron than the host transport proteins. In our study, the detection rates of the siderophore-associated genes (the aerobactin, iucA, iroB, ybtS, and entB genes) ranged from 53.8% to 100.0%. In contrast, the detection rates of siderophore-associated genes in cKP were <20.0%, except for the entB gene [5]. Of the siderophores secreted by HMKP, aerobactin is the most important virulence factor [4,35]. Our subsequent assays also indicated that KPN53 (the representative aerobactin-positive strain) was highly serum-resistant, and its lgLD50 value in G. mellonella model was significantly lower than that of the other isolates. According to a previous study, the LD50s of most clinical K. pneumoniae isolates in the G. mellonella model were 105 CFU/larva [36], higher than those observed for KPN53. Moreover, the capsular gene wabG, the type 1 and 3 fimbriae genes fimH and mrkD, and other virulence factors also contribute to the enhancement of the virulence potential of CR-HMKP isolates. In our data, the detection rates of the fimH, mrkD, ureA, wabG, and ycfM genes were >90.0%, which is consistent with a previous study [7].
Most patients infected by CR-HMKP have a history of ICU hospitalization and received invasive treatment [7,8], which was consistent with our data. However, the outcomes of patients infected by CR-HMKP are diverse. Gu et al. [8] and Zhang et al. [9] demonstrated that all patients with CR-HMKP infections died of septic shock. Another study noted that only one patient died of septic shock and multiple organ failure [7]. In our study, 46.2% of the patients died, while the rest were discharged. Notably, seven cases occurred between July and August 2016 and were assessed as HA infections, and the seven case-related NDM-1-producing HMKP isolates had identical genotypes. Thus, ours is the first report on an outbreak of a nosocomial infection associated with NDM-1-producing HMKP ST1764. Of these cases, their inpatient beds were positioned very closely, and this proximity suggests nosocomial dissemination of NDM-1-producing-HMKP, most likely because of contact propagation during medical activities. As this was a retrospective study, the main limitation is the insufficient data for explaining how NDM-1-producing HMKP isolates was transmitted between the ICU and PS wards. Therefore, to prevent further nosocomial transmission of CR-HMKP, strict infection control measures such as hand hygiene, contact isolation, active screening, and environmental surface disinfection should be implemented.
In summary, a high prevalence of HMKP was observed among the CRKP isolates in this study. All the CR-HMKP isolates produced carbapenemase, and NDM-1-producing HMKP ST1764 was identified among them. To the best of our knowledge, this is the first report worldwide on an outbreak of nosocomial infection caused by NDM-1-producing HMKP. These findings indicate that effective surveillance and the implementation of strict infection control strategies are needed to prevent the nosocomial dissemination of CR-HMKP.
Acknowledgements
This work was supported by grant 81673242 from the National Natural Science Foundation of China and grant 2016QK036 from the Planned Scientific Program of Health and Family Planning Commission of Anhui Province. We thank the physicians involved in collection of the clinical data.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article are reported.
SUPPLEMENTARY MATERIAL
Clinical and microbiological characteristics of the two reference strains
References
- 1.Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 2.Shon AS, Bajwa RPS, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, et al. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol. 2017;7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83:3325–3333. doi: 10.1128/IAI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Kreiswirth BN. Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect Dis. 2018;18:2–3. doi: 10.1016/S1473-3099(17)30517-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhan L, Wang S, Guo Y, Jin Y, Duan J, Hao Z, et al. Outbreak by hypermucoviscous Klebsiella pneumoniae ST11 isolates with carbapenem resistance in a tertiary hospital in China. Front Cell Infect Microbiol. 2017;7:182. doi: 10.3389/fcimb.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18:37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother. 2015;60:709–711. doi: 10.1128/AAC.02173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao B, Xiao X, Wang F, Zhou L, Zhang X, Zhang J. Clinical and molecular characteristics of multi-clone carbapenem-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in a tertiary hospital in Beijing, China. Int J Infect Dis. 2015;37:107–112. doi: 10.1016/j.ijid.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Arena F, Henrici De, D'Andrea MM, Cannatelli A, Fossati L, Di Pilato V, et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae with hypermucoviscous phenotype: a case report and literature review. Virulence. 2017;8:1900–1908. doi: 10.1080/21505594.2017.1286439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar C, Nabarro LE, Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Daniel JL, Doss C GP, et al. Draft genome sequences of three hypervirulent carbapenem-resistant Klebsiella pneumoniae isolates from bacteremia. Genome Announc. 2016;4:pii: e01081-16. doi: 10.1128/genomeA.01081-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. CLSI supplement M100. [Google Scholar]
- 14.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. CLSI Standard M07. [Google Scholar]
- 15.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 7.0. 2017. [Updated on Jan 2017]. http://www.eucast.org.
- 16.Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother. 2008;61:548–553. doi: 10.1093/jac/dkm535. [DOI] [PubMed] [Google Scholar]
- 17.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol. 2012;50:3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Pu S, Jia X, Xu X, Yang S, Shi J, et al. Multidrug resistance mechanisms of carbapenem resistant Klebsiella pneumoniae strains isolated in Chongqing, China. Ann Lab Med. 2017;37:398–407. doi: 10.3343/alm.2017.37.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51:4073–4078. doi: 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan YJ, Lin TL, Chen YH, Hsu CR, Hsieh PF, Wu MC, et al. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS One. 2013;8:e80670. doi: 10.1371/journal.pone.0080670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Liu PP, Wang LH, Wei DD, Wan LG, Zhang W. Capsular polysaccharide types and virulence-related traits of epidemic KPC-producing Klebsiella pneumoniae isolates in a Chinese university Hospital. Microb Drug Resist. 2017;23:901–907. doi: 10.1089/mdr.2016.0222. [DOI] [PubMed] [Google Scholar]
- 22.Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35:387–396. doi: 10.1007/s10096-015-2551-2. [DOI] [PubMed] [Google Scholar]
- 23.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang TT, Yang YS, Yeh KM, Chiu SK, Wang NC, Lin TY, et al. Quantification and comparison of virulence and characteristics of different variants of carbapenemase-producing Klebsiella pneumoniae clinical isolates from Taiwan and the United States. J Microbiol Immunol Infect. 2016;49:83–90. doi: 10.1016/j.jmii.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Mclaughlin MM, Advincula MR, Malczynski M, Barajas G, Qi C, Scheetz MH. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect Dis. 2014;14:31. doi: 10.1186/1471-2334-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei YF, Liu PP, Wan LG, Liu Y, Wang LH, Wei DD, et al. Virulence and genomic feature of a virulent Klebsiella pneumoniae sequence Type 14 strain of serotype K2 harboring blaNDM-5 in China. Front Microbiol. 2017;8:335. doi: 10.3389/fmicb.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compain F, Vandenberghe A, Gominet M, Genel N, Lebeaux D, Ramahefasolo A, et al. Primary osteomyelitis caused by an NDM-1-producing K. pneumoniae strain of the highly virulent sequence type 23. Emerg Microbes Infect. 2017;6:e57. doi: 10.1038/emi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei DD, Wan LG, Liu Y. Draft genome sequence of an NDM-1- and KPC-2-coproducing hypervirulent carbapenem-resistant Klebsiella pneumoniae strain isolated from burn wound infections. Genome Announc. 2018;6:pii: e00192-18. doi: 10.1128/genomeA.00192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66:307–312. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 32.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL. The function of wzy_K1 (magA), the serotype K1 polymerase gene in Klebsiella pneumoniae cps gene cluster. J Infect Dis. 2010;201:1268–1269. doi: 10.1086/652183. [DOI] [PubMed] [Google Scholar]
- 33.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, et al. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother. 2014;58:5379–5385. doi: 10.1128/AAC.02523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Wu H, Shen D. Clinical and molecular analysis of Klebsiella pneumoniae causing liver abscess in China. J Mol Microbiol Biotechnol. 2016;26:245–251. doi: 10.1159/000444367. [DOI] [PubMed] [Google Scholar]
- 35.Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82:2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wand ME, McCowen JW, Nugent PG, Sutton JM. Complex interactions of Klebsiella pneumoniae with the host immune system in a Galleria mellonella infection model. J Med Microbiol. 2013;62:1790–1798. doi: 10.1099/jmm.0.063032-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and microbiological characteristics of the two reference strains