Abstract
Purpose
To detect which factors influence decision-making among pregnant FMR1 premutation carriers regarding the preferred mode of genetic diagnosis: IVF-PGT-M (in vitro fertilization with preimplantation genetic testing for monogenic gene diseases), or CVS (chorionic villus sampling), or AC (amniocentesis) after spontaneous conception.
Methods
In Israel FMR1 premutation preconception genetic screening is offered, free of charge, to every woman in her reproductive years. FMR1 premutation carriers with ≥ 70 CGG repeats, or a history of FXS offspring, are offered IVF-PGT-M. This is a historical cohort study including all pregnant FMR1 premutation carriers who underwent prenatal diagnosis between the years 2011 and 2016 at a tertiary medical center. Data were collected from electronic charts and through phone interviews.
Results
One hundred seventy-five women with high-risk pregnancies who were offered IVF-PGT-M were evaluated. In 37 pregnancies (21%), the women decided to undergo IVF-PGT-M. Using the generalized estimating equations (GEE) statistical method including seven parameters, we found that previous termination of pregnancy due to FXS and advanced woman’s age were significantly associated with making the decision to undergo IVF-PGT-M. Previously failed IVF was the most significant parameter in a woman’s decision not to undergo IVF-PGT-M.
Conclusion
The most dominant factor affecting the decision of FMR1 premutation carriers to choose spontaneous conception with prenatal diagnosis versus IVF-PGT-M is a previous experience of failed IVF treatments. Women whose IVF treatments failed in the past tended to try to conceive naturally and later, during the course of the pregnancy, perform CVS or AC. Conversely, women who previously experienced a termination of pregnancy (TOP) due to an affected fetus, and older women, preferred to undergo IVF-PGT-M procedures.
Keywords: FMR1 premutation, IVF-PGT-M, CVS, AC
Introduction
Fragile X syndrome (FXS) is the most common inherited form of severe mental retardation, with a reported incidence of 1 in 4000 males and 1 in 8000 females [1]. The molecular genetic defect underlying FXS is the expansion of a repeated trinucleotide segment of DNA (CGG) in the fragile X mental retardation-1 (FMR1) gene, located on the X chromosome. In the general population, the normal range of CGG repeats is 5–44 [2]. A full fragile X mutation is defined as above 200 CGG repeats, and will result in FXS in all males, and in a variable number of females due to X inactivation [3, 4]. Premutation is defined as the presence of 55 to 200 CGG repeats. FMR1 premutation carriers present a spectrum of health disorders, the most prevalent being Fragile X-associated tremor-ataxia syndrome (FXTAS), affecting both males and females in the fifth or sixth decade of life, and Fragile X-associated primary ovarian insufficiency (FXPOI). In addition, FMR1 premutation carriers are reported to have an increased rate of various psychological, endocrine, autoimmune, and metabolic disorders [5]. Since the premutation CGG segment is unstable and may expand, women carrying a premutation allele are at risk of transmitting a full mutation to their offspring, resulting in a FXS-affected child with some phenotypic variation by gender of the offspring [6]. Previous studies have shown a strong and positive correlation between the number of CGG repeats in the mother and the risk of expansion to full mutation in her offspring [7]. In Israel, all women in their reproductive years who wish to conceive are offered preconception genetic screening free of charge, including FMR1 premutation screening. For preconception genetic screening, a FMR1 premutation carrier is defined as showing between 58 and 199 CGG repeats, since below this range the risk of expansion to a full mutation in one generation is questionable. The recently reported incidence of carriers (58–199 CGG repeats) among genetically screened Israeli women without a family history of mental retardation or developmental abnormalities is 1 out of 256 women [8].
Pregnant carriers are advised to perform prenatal diagnosis by either chorionic villus sampling (CVS) or amniocentesis (AC), free of charge. Alternatively, in vitro fertilization, using preimplantation genetic testing for monogenic gene diseases (IVF-PGT-M), is offered, also free of charge, to couples at high risk for expansion to full mutation. High risk is defined as showing 70 or more CGG repeats or having had a previous pregnancy involving FXS. Hence, women at high risk have the opportunity to choose between conceiving spontaneously and performing genetic testing during their pregnancy or conceiving through IVF using PGT-M for genetic diagnosis, both free of charge. Each approach has its advantages and disadvantages complicating the decision-making process. Spontaneous conception carries a risk of bearing an affected child and the need to decide whether to perform a termination of pregnancy. Termination of pregnancy involves medical, emotional, and ethical issues especially in the case of a full mutation carrier when the final phenotype is uncertain. On the other hand, IVF using PGT-M avoids the need for a termination of pregnancy and offers the opportunity to transfer only non-carrier embryos. However, this procedure arouses emotional difficulties and is not the obvious choice for a fertile couple, especially considering the higher prevalence of ovarian dysfunction and reduced ovarian response (up to 20%) in premutation carriers compared to non-carrier women [1, 9–14]. The option of using donor oocytes is also discussed. However; this procedure is not free of charge in Israel.
Thus, the aim of our current study was to evaluate factors that influence decision-making among FMR1 premutation and full mutation women, regarding the preferred mode of genetic diagnosis: IVF-PGT-M vs. CVS or AC after spontaneous conception.
Methods
We conducted a historical cohort study. This study was approved by the Institutional Ethical Review Board of the Sheba Medical Center, Israel. A total of 216 pregnant fragile X premutation and full mutation carriers, who underwent prenatal diagnosis for FMR1 premutation status at our center between the years 2011 and 2016, were interviewed by phone questionnaire. FMR1 premutation carriers using donor oocytes were excluded. The women’s data were obtained from their electronic medical charts. Additional data including obstetrical history, time of genetic screening, the reason for performing preconception genetic screening, as well as the decision regarding the mode of conception, were collected through phone interviews. Sixty-one women were not available following at least 3 calling attempts, and 13 refused to participate, resulting in a total of 142 women and 461 pregnancies. In 286 pregnancies, the woman was unaware of her FMR1 premutation status prior to conceiving, and these women were therefore excluded. Pregnancies in women with less than 70 CGG repeats and without a history of a previous affected pregnancy were also excluded.
Lastly, our study group included 175 pregnancies in which IVF-PGT-M was offered because of known fragile X carrier status prior to conceiving and a high risk for expansion to full mutation, as was defined earlier. The primary outcome was the choice of genetic diagnosis: undergoing IVF-PGT-M or conceiving spontaneously and performing CVS or AC. We employed a generalized estimating equations (GEE) statistical method to assess the contribution of different variables to the woman’s decision on whether to conceive through IVF-PGT-M or not. Statistical analysis was performed using the following seven parameters as inputs: number of CGG repeats, maternal age, gravidity, history of an affected child, history of termination of pregnancy (TOP) due to FXS, number of TOPs due to FXS, infertility, and previous failure to conceive through IVF-PGT-M treatment. The decision on whether to perform the IVF-PGT-M procedure or not was considered as the output parameter. The GEE statistical method, with a model assuming an unstructured variance-covariance matrix, was used to evaluate the effect of each input parameter on that decision’s outcome. Characteristics of the FMR1 premutation carriers between the two groups were compared using t test and chi-square test as appropriate. All statistical tests consider p values below 5% as being statistically significant.
Descriptive statistics were performed using ®JMP Statistical Discovery software, version 14.0.0 from ®SAS Institute Inc., Cary, NC. GEE modeling was done with R, version 3.5.0, using Gee and Geepack packages, under the Free Software Foundation’s GNU General Public License.
IVF and PGT-M treatment
The treating physician decided which controlled ovarian stimulation protocols were used. In all protocols, gonadotropins were administered in variable doses, depending on patient age and ovarian response in previous cycles, if available. Gonadotropin doses were further adjusted according to serum estradiol (E2) levels and vaginal ultrasound measurements of follicular diameter were obtained every 2 or 3 days. Ultrasound-guided transvaginal route follicular aspiration was done 34–36 h after human chorionic gonadotropin (hCG) injection. Intracytoplasmic sperm injection (ICSI) was performed only if indicated because of impaired sperm parameters [15]. Embryo biopsy was performed on day 3 of development. A multiplex nested polymerase chain reaction protocol was used, with simultaneous amplification of multiple flanking informative polymorphic markers, at least one on each side of the FMR1 gene sequence. In this protocol, it is technically impossible to amplify the number of CGG repeats in the FMR1 gene of the blastomere by PCR, and the diagnosis is based on identification of the wild type maternal allele by linkage. [16]. PGT-A (preimplantation genetic testing for aneuploidy) was not performed. In Israel, all women who conceive using IVF-PGT-M are recommended to perform AC for ratification of the genetic result.
Results
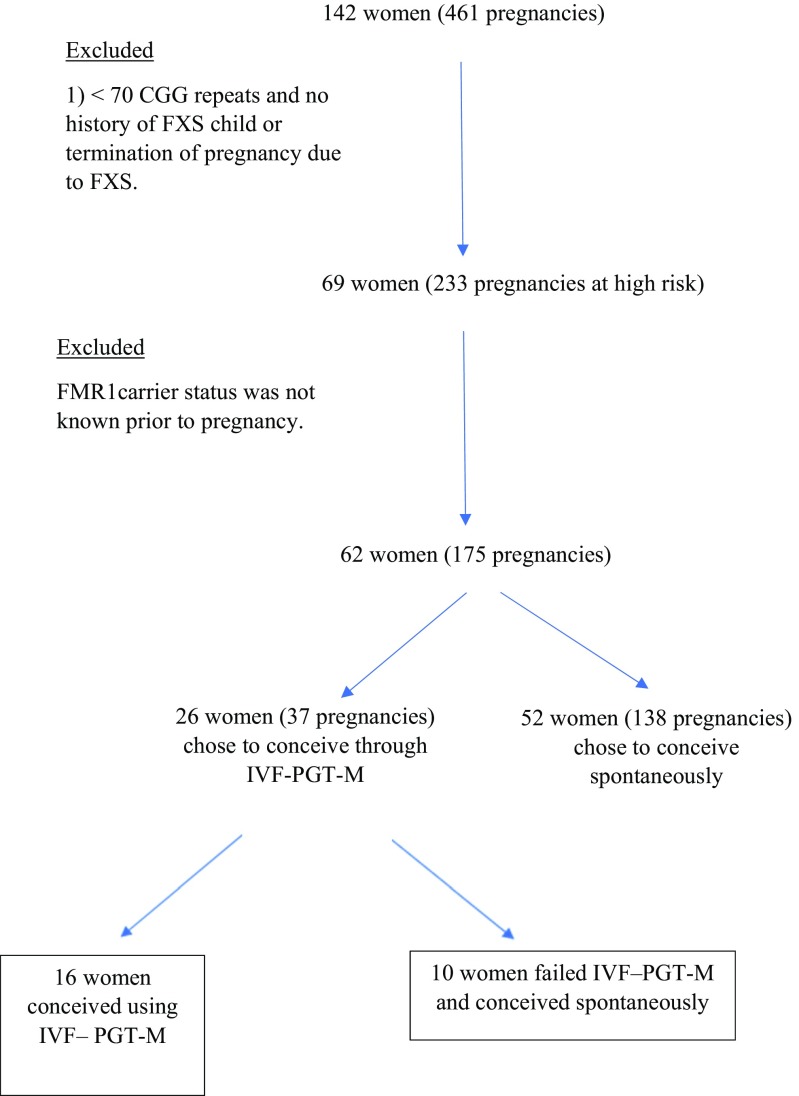
Of the 142 pregnant fragile X carriers recruited, 75 women had 58–69 CGG repeats (approximately 53%), while 67 women (approximately 47%) had CGG repeats above 69, including 10 women with full mutation. Two women had CGG repeats below 70, but they had a history of a termination of pregnancy due to FXS. Hence, a total of 69 women having 233 pregnancies were defined as being at high risk for expansion to full mutation and were offered IVF using PGT-M. Fifty-eight pregnancies were excluded because their genetic status was unknown prior to the current pregnancy. Therefore, our final analysis included 62 women with 175 pregnancies at high risk for expansion to full mutation, recorded from 2011 until 2016.
In 37 pregnancies (21%), the women decided to undergo IVF-PGT-M, and in 138 pregnancies (79%) the women chose not to undergo PGT-M (Fig. 1). The characteristics of the study groups are presented in Table 1. Gravidity, maternal BMI, the incidence of other genetic diseases, and infertility did not differ significantly between the study groups.
Fig. 1.
Pregnant fragile X premutation and full mutation carriers who underwent prenatal genetic diagnosis from 2011 to 2016 at the Genetic Institute, Chaim Sheba Medical Center, Israel
Table 1.
Characteristics of pregnant FMR1 premutation carriers undergoing CVS or AC due to high risk# for an affected child
| Characteristics | Chose IVF-PGT-M 26 women (in 37 pregnancies) |
Chose to conceive spontaneously 52 women (in 138 pregnancies) |
p value |
|---|---|---|---|
| Maternal age at pregnancy [mean (SD) years] | 32.9 (±3.9) | 31.4 (±3.7) | 0.03 |
| Maternal BMI [kg/m2 (SD)] | 21.3 (±3.4) | 21.7 (±3.9) | ns |
| Other genetic disease [%] | 21.6 | 15.9 | ns |
| Infertility [%] | 8.1 | 5.1 | ns |
| Nullipara [%] | 17.1 | 22.1 | ns |
#Having 70 or more CGG repeats or having a previous pregnancy affected with FXS
CVS chorionic villus sampling, AC amniocentesis
The statistical analysis using the GEE model indicates the following factors as having a statistically significant effect on women’s decisions to perform IVF-PGT-M (Table 2). Positive estimates of all parameters, except the one related to previously failed IVF, suggest a preference of PGT-M over CVS or AC: women who had experienced previous failure of IVF preferred not to go through additional IVF-PGT-M treatments (p value = 0.0008). We encountered only a single instance of a woman choosing IVF, despite a previously failed IVF procedure. Women who had undergone TOP due to FXS in the past were in favor of the IVF procedure (p value = 0.01), as were older women, who preferred IVF (p value = 0.03).
Table 2.
Summary results of the generalized estimating equations (GEE) model
| Parameter | Estimates | p values |
|---|---|---|
| Intercept | −14.45 | 0.0335 |
| Number of CGG Repeats | 0.01 | 0.3955 |
| Age | 0.16 | 0.0327 |
| Number of Gravidities | −0.33 | 0.1846 |
| TOP due to FXS | −2.26 | 0.0125 |
| Number of TOP due to FXS | 0.35 | 0.6109 |
| Infertility | 0.25 | 0.8780 |
| Previous failed IVF | 4.60 | 0.0008 |
TOP termination of pregnancy
Twenty-six women chose to perform IVF-PGT-M treatment. Four of them had a full mutation. Ten women (33%) failed to conceive following the treatment, but later conceived spontaneously. Nine of the 22 women with premutation status (41%) were diagnosed with poor ovarian response (POR) according to the Bologna criteria [17]. In 55% of the cycles, a GnRH antagonist protocol was used, in 35% a long agonist protocol, and in 10% a flare-up short agonist protocol was used.
Of note, no obstetrical complications related to the prenatal diagnostic procedure (AC or CVS) were described and no misdiagnosis occurred in the IVF-PGT-M pregnancies.
Discussion
To the best of our knowledge, this is the first study using a generalized estimating equations (GEE) model to assess decision-making in pregnant FMR1 premutation women carriers regarding the genetic evaluation of their fetus. Recent rapid developments in medical genetics, and the emerging discoveries of genes associated with or cause diseases, raise issues regarding the way individuals negotiate decision-making when they face a significant risk for transmitting a genetic disease which might have physical and cognitive implications for their offspring [18–20]. Genetic risk is uncertain because it is stated in probabilities, which leave room for multiple interpretations. Even in FXS, in which penetrance and prognosis are well known, a variety of personal experiences, familial demands, cultural forces and ethical perceptions shape decision-making, hence predicting choices is complex [18, 21].
In the present study, we assessed the decision-making process among pregnant FMR1 premutation carriers at high risk for having an offspring with a full mutation, regarding their decision on whether to underdo IVF-PGT-M or CVS or AC. As mentioned earlier, in Israel, genetic screening is recommended and offered free of charge to every woman who wishes to conceive. If the woman is found to be a carrier for a recessive genetic disease, the male partner is also screened for the same disease. If both parents are found to be carriers for the same genetic disease, they receive genetic consultation and are offered IVF-PGT-M free of charge. In the case of FMR1 premutation women carriers, those defined at “high risk” are offered the opportunity to perform IVF-PGT-M. This unique combination enables us to examine decision-making that is independent of financial considerations.
Our results show that the most dominant factor affecting the decision of whether to choose IVF-PGT-M or CVS or AC is a previous experience of failed IVF treatment (p value = 0.001). Women who failed IVF tend to try to conceive naturally, and later during the pregnancy perform CVS or AC. The other two significant factors are: history of TOP due to FXS, and the women’s age (p values are 0.01 and 0.03, respectively). Women who had experienced a previous TOP due to an affected fetus, and women of advanced age, preferred IVF-PGT-M. Other factors were not statistically significant. Few previous studies have evaluated decision-making among fragile X premutation carriers. Raspberry et al. examined how women negotiate reproductive desires in families with at least one child with FXS. The majority (77%) decided not to have additional biological children after they were diagnosed as carriers [18]. In another study conducted by Xuncia et al, which also evaluated fragile X carriers with at least one affected child, 40.5% of the women said that they would choose to perform prenatal diagnosis in a subsequent pregnancy, 42.9% renounced having additional offspring, 9.5% would choose oocyte donation, and 7.1% PGT-M [22]. However, both these studies did not examine reproductive decisions in fragile X carrier women with no children, or with no children with FXS. The main principles of the genetic consultation are not reported, so there was no standardization or uniformity. Moreover, in those studies the financial costs of assisted reproductive techniques, especially IVF-PGT-M and oocyte donation, definitely played an important role in the decision-making of FMR1 carriers.
It has been previously shown that the main determinant of successful PGT-M cycles in FMR1 premutation carriers is ovarian dysfunction. When embryo transfer is possible, the results are comparable to PGT-M for other monogenic diseases [23]. This emphasizes the importance of early detection of the FMR1 premutation carrier state in order to start IVF-PGT-M as soon as possible before the deterioration in ovarian reserve occurs.
Twenty-six women chose to perform IVF-PGT-M treatment. Four of them had a full mutation. Ten women (33%) failed to conceive following the treatment, but later conceived spontaneously. Not surprisingly, 41% of FMR1 premutation carriers who underwent IVF had poor ovarian response (POR) according to the Bologna criteria [17]. This is in accordance with previous reports [24] and results in high doses of gonadotropins used, low peak estradiol levels, and low numbers of retrieved oocytes during IVF cycles [25]. A possible treatment option is to perform several cycles using the modified natural protocol [26] in order to collect several frozen embryos and later thaw them and perform PGT-M prior to embryo transfer. One of the pregnancies reported here followed this treatment.
One limitation of our study is that it included only pregnant FMR1 carriers who decided to perform prenatal genetic screening. Therefore, carriers who did not conceive, and those who conceived but did not perform prenatal genetic screening or used donor oocyte, are not included and our conclusions might not be applicable to them.
Conclusion
According to our findings, the most dominant factor affecting the decision of pregnant fragile X carriers of whether to choose to undergo IVF-PGT-M or conceive spontaneously followed by CVS or AC is a previous experience of failed IVF. FMR1 premutation carriers who failed IVF treatment tended to try to conceive naturally and perform CVS or AC later during the pregnancy. On the other hand, carriers who had previously experienced a TOP due to an affected fetus, and those of advanced maternal age, preferred to choose IVF-PGT-M.
Acknowledgments
This work was supported by The Azrieli Foundation Canada-Israel.
References
- 1.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Nolin SL, Glicksman A, Ding X, et al. Fragile X analysis of 1112 natal samples from 1991 to 2010. Prenat Diagn. 2011;31(10):925 931–9252011. doi: 10.1002/pd.2815. [DOI] [PubMed] [Google Scholar]
- 3.Tsafrir A, Altarescu G, Margalioth E, Brooks B, Renbaum P, Levy-Lahad E, Rabinowitz R, Varshaver I, Eldar-Geva T. PGT-M for fragile X syndrome: ovarian function is the main determinant of success. Hum Reprod. 2010;25(10):2629–2636. doi: 10.1093/humrep/deq203. [DOI] [PubMed] [Google Scholar]
- 4.Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet. 2000;9:901–908. doi: 10.1093/hmg/9.6.901. [DOI] [PubMed] [Google Scholar]
- 5.Hoyos LR, Thakur M. Fragile X premutation in women: recognizing the health challenges beyond primary ovarian insufficiency. J Assist Reprod Genet. 2017;34(3):315–323. doi: 10.1007/s10815-016-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visootsak J, Warren ST, Anido A, Graham JM. Fragile X syndrome: an update for the primary pediatrician. Clin Pediatr. 2005;44:371–381. doi: 10.1177/000992280504400501. [DOI] [PubMed] [Google Scholar]
- 7.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 8.Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G. Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn. 2007;27(11):991–994. doi: 10.1002/pd.1815. [DOI] [PubMed] [Google Scholar]
- 9.Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spath MA, Feuth TB, Allen EG, Smits APT, Yntema HG, van Kessel AG, Braat DDM, Sherman SL, Thomas CMG. Intra-individual stability over time of standardized anti-Mullerian hormone in FMR1 premutation carriers. Hum Reprod. 2011;26:2185–2191. doi: 10.1093/humrep/der146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- 12.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 13.Martin JR, Arici A. Fragile X and reproduction. Curr Opin Obstet Gynecol. 2008;20:216–220. doi: 10.1097/GCO.0b013e3282fe7254. [DOI] [PubMed] [Google Scholar]
- 14.Fernández Raquel M., Peciña Ana, Lozano-Arana Maria Dolores, Sánchez Beatriz, García-Lozano Juan Carlos, Borrego Salud, Antiñolo Guillermo. Clinical and Technical Overview of Preimplantation Genetic Diagnosis for Fragile X Syndrome: Experience at the University Hospital Virgen del Rocio in Spain. BioMed Research International. 2015;2015:1–6. doi: 10.1155/2015/965839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman B, Aizer A, Brengauz M, Dotan K, Levron J, Schiff E, Orvieto R. Pre-implantation genetic diagnosis – should we use ICSI for all? J Assist Reprod Genet. 2017;34(9):1179–1183. doi: 10.1007/s10815-017-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M M, Naiman T, Yosef DB, Carmon A, Mey-Raz N, Amit A, Vagman I, Yaron Y. Preimplantation genetic diagnosis for fragile X syndrome using multiplex nested PCR. Reprod BioMed Online. 2007;14(4):515–521. doi: 10.1016/S1472-6483(10)60901-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation to in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 18.Raspberry KA, Skinner D. Negotiating desires and options: how mothers who carry the fragile X gene experience reproductive decisions. Soc Sci Med. 2011;72(6):992–998. doi: 10.1016/j.socscimed.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr A, Cunningham-Burley S. On ambivalence and risk: reflexive modernity and the new human genetics. Sociology. 2000;34(2):283–304. doi: 10.1177/S0038038500000183. [DOI] [Google Scholar]
- 20.Lemke T. Disposition and determinism genetic diagnostics in risk society. Sociol Rev. 2004;52(4):550–566. doi: 10.1111/j.1467-954X.2004.00495.x. [DOI] [Google Scholar]
- 21.Belanger D. Indispensable sons: negotiating reproductive desires in rural Vietnam. Gend Place Cult. 2006;13(3):251–265. doi: 10.1080/09663690600701012. [DOI] [Google Scholar]
- 22.Xuncia M, Badenas C, Dominguez M, Rodriguez-Revenga L, Madrigal I, Jimenez L, Soler A, Borrel A, Sanchez A, Mila M. Fragile X syndrome prenatal diagnosis: prenatal attitudes and reproductive responses. Reprod BioMed Online. 2010;21(4):560–565. doi: 10.1016/j.rbmo.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Tsafrir A, Altarescu G, Margalioth E, Brooks B, Renbaum P, Levy-Lahad E, Rabinowitz R, Varshaver I, Eldar-Geva T. PGD for fragile X syndrome: ovarian function is the main determinant of success. Hum Reprod. 2010;25(10):2629–2636. doi: 10.1093/humrep/deq203. [DOI] [PubMed] [Google Scholar]
- 24.Man L, Lekovich J, Rosenwaks Z, Gerhardt J. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci. 2017;12(10):290. doi: 10.3389/fnmol.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elizur SE, Lebovitz O, Derech-Haim S, Dratviman-Storobinsky O, Feldman B, Dor J, Orvieto R, Cohen Y. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One. 2014;9(8):e105121. doi: 10.1371/journal.pone.0105121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elizur SE, Aslan D, Shulman A, Weisz B, Bider D, Dor J. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J Assist Reprod Genet. 2005;22(2):75–79. doi: 10.1007/s10815-005-1496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]