Abstract
Purpose
Low mitochondrial DNA (mtDNA) content in oocytes and in cumulus cells is an indicator of poor oocyte quality. Moreover, initial evidence showed a correlation between mtDNA content in cumulus cells and mtDNA copy number in peripheral blood cells. On these bases, we deemed of interest investigating the correlation between mtDNA copy number in peripheral blood and natural fecundity.
Methods
This is a nested case–control study drawn from a prospective cohort of pregnant women referred for routine first trimester screening for aneuploidies (from 11 + 0 to 12 + 6 weeks of gestation) between January 2012 and March 2013 at the “Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico” of Milan, Italy. Cases were subfertile women who attempted to become pregnant for 12–24 months. Controls were the two subsequently age-matched women who became pregnant in less than 1 year. MtDNA was quantified using real-time PCR and normalized to nuclear DNA.
Results
One hundred and four subfertile women and 208 controls were selected. The median (IQR) mtDNA copy number was 95 (73–124) and 145 (106–198), respectively (p < 0.001). The area under the ROC curve was 0.73 (95% CI 0.67–0.79) (p < 0.001). The Youden index was 105 mtDNA copy number. The crude OR for subfertility in women with mtDNA copy number below this threshold was 5.72 (95% CI 3.43–9.55). The accuracy of mtDNA copy number assessment in peripheral blood progressively decreased with increasing female age.
Conclusions
Low mtDNA copy number in peripheral blood is associated with an increased risk of subfertility and may represent a biomarker of natural fecundity.
Keywords: Mitochondria, Mitochondrial DNA, Fecundity, Biomarker
Introduction
Mitochondria are key cytoplasmic organelles that play a crucial function in cellular energy metabolism, reactive oxygen species (ROS) generation, cell proliferation, and apoptosis [1, 2]. Not surprisingly, mitochondria play a pivotal role also in both male and female infertility [3].
In particular, they have been claimed to be implicated in the decline of female fertility with age [4]. Murine models suggest that mitochondrial biogenesis during embryonic life concurs in the determination of the initial size of the follicular pool [5]. Moreover, these organelles were shown to regulate follicular atresia and may guide the depletion of the ovarian reserve [4, 6–10]. Noteworthy, the role of mitochondria is not limited to the regulation of the pool of primordial follicles. In fact, by deeper investigating the pathophysiological role of mitochondria in ovarian aging, it clearly emerged a close relation between mitochondrial DNA (mtDNA) and oocytes quality [6]. In particular, the age-related increasing instability of mtDNA results in the accumulation of mtDNA mutations in the oocytes leading to the deterioration of their competence. Interestingly, also the amount of mtDNA per oocyte appears to be a key factor in determining the fertilization outcome. In fact, it is now becoming increasingly apparent that oocyte mtDNA deficiency results in poor oocyte quality and may prevent the oocyte from completing the process of fertilization [11]. Importantly, evidence drawn from animal models and humans converge in suggesting the existence of an oocyte mtDNA copy number threshold below which the fertilization process is more likely to fail. The beneficial effect on fertilization rate of mtDNA supplementation in oocytes exhibiting mtDNA deficiency tends to confirm this hypothesis [11–14].
Mitochondria derived from the oocyte are also the major source of ATP during early embryo development [15]. In fact, from metaphase II oocytes till hatched blastocyst stage, the glycolysis process is limited and the mtDNA count does not change [15, 16].
Unfortunately, mitochondria and mtDNA cannot be directly evaluated without damaging the oocytes. The intriguing observations reported above are of scanty clinical interest in the absence of peripheral biomarkers able to reflect the oocyte mitochondrial function. In this regard, it is noteworthy that oocyte mtDNA content is positively correlated with that of the corresponding cumulus cells [17] and that two recent studies found a significant link between cumulus cell mtDNA content and embryo quality, with higher mtDNA copy numbers being associated with good quality embryos [18, 19]. Furthermore, of utmost relevance is the recent evidence showing a correlation between mtDNA content in cumulus cells and mtDNA content in peripheral blood cells [9, 18].
On these bases, we deemed of interest investigating the correlation between mtDNA copy number in peripheral blood and natural fecundity. To shed light on this issue, we set up a nested case–control study recruiting women at the time of first trimester pregnancy screening for aneuploidies and we compared mtDNA copy number in peripheral blood of women attempting to become pregnant for more than 1 year (subfertile group) with that of age-matched controls who became pregnant in less than 1 year.
Methods
This is a nested case–control study drawn from a prospective cohort of pregnant women referred for routine first trimester screening for aneuploidies (from 11 + 0 to 12 + 6 weeks of gestation) at the “Fondazione IRCCS Ca’ Granda Ospedale Maaggiore Policlinico” between January 2012 and March 2013. All women referred were offered the opportunity to participate in the study. Those who accepted signed an informed consent, provided a blood sample, and were interviewed using a standardized questionnaire. Blood samples were collected in 3 mL EDTA-containing tube and immediately stored at − 20 °C. The study was accepted by the local Institutional Review Board. All recruited patients provided a written informed consent to participate.
Women were defined as subfertile if they had tried to achieve a natural pregnancy for 12–24 months. Controls were the subsequently referred women matched to cases on the basis of age (± 6 months, ratio 2:1). Inclusion criteria for both cases and controls were (i) age > 18 years, (ii) natural conception (women conceiving with the use of controlled ovarian hyper-stimulation with or without assisted reproductive techniques were excluded), and (iii) regular menstrual cycles (24–35 days). Time to pregnancy was the interval between the initiation of unprotected intercourses and conception. If the woman referred transient irregular cycles following hormonal contraceptive discontinuation, the starting point was shifted to the beginning of regular cycles.
Blood samples were all thawed simultaneously. Total genomic DNA was isolated from whole-blood specimens by the Wizard Genomic DNA Purification Kit (Promega). ABI Prism 7900 fast sequence detection system (Applied Biosystems, Foster City, CA) was used for real-time quantitative polymerase chain reaction (PCR) analysis, according to our previously published method [20], using the RNase P gene, as an endogenous control (cat no. 4316844; Applied Biosystems) and AB Mitochondrial Gene 7S, encoding D-loop, as target gene (Hs02596861_s1, Assays-on-Demand Gene Expression Products; Applied Biosystems). RNase P is a single-copy nuclear gene that encodes the RNA moiety for the RNase P enzyme. D-loop is a replication start site of the mtDNA. Real-time quantitative PCR was performed leading to reactions characterized by the point in time during cycling when amplification of a PCR product achieved a fixed level of fluorescence. Target and reference genes were amplified in separate wells in duplicate. Reaction conditions included 10 μL of 2× TaqMan fast universal PCR master mix, 1 μL of primers and probes mixture, 150 ng of template DNA, and nuclease-free water to a 96-well reaction plate. The total reaction volume was 20 μL. The cycling conditions were as follows: 20 s at 95 °C and 40 cycles of 3 s at 95 °C followed by 30 s at 60 °C. Data were analyzed by using the comparative Ct method, where Ct is the cycle number at which fluorescence first exceeds the threshold. The Δ cycle thresholds (ΔCt) values from each sample were obtained by subtracting the values for the reference gene from the sample Ct, thus normalizing to nuclear DNA. For each experimental sample, the 2−ΔCt was calculated and data were graphically indicated as relative quantification. A standard curve for mtDNA quantification was not used. Data were indeed normalized to nuclear DNA and are reported as number of copies per nuclear DNA (mtDNA/nDNA).
The primary end point of the study was the mtDNA copy number assessment in peripheral blood of subfertile and fertile women. Analysis of the data was carried out with the Statistical Package for Social Sciences 18.0 (SPSS Inc., Chicago, IL, USA). Statistically significant differences were determined using Fisher’s exact test, chi-squared test, Student’s t test, or the Mann–Whitney test, as appropriate. The mtDNA copy number in peripheral blood was presented as median (interquartile range-IQR) and compared using the Mann–Whitney test. p values below 0.05 were considered statistically significant. To test the accuracy of mtDNA copy number in peripheral blood in predicting female subfertility, we performed a receiver operator curve (ROC) analysis.
The best threshold to discern between subfertile and fertile women was identified using the Youden index (J) and used to calculate the odds ratio (OR) of being subfertile.
J is a measure for evaluating the biomarker effectiveness. Let Se (c) and Sp (c) be the sensitivity and specificity values associated to a particular cutoff point (c).
J is a function of Se (c) and Sp (c), such that
Overall cutoff point c, cJ, denotes the cutoff point corresponding to J. When the value of J is maximum, cJ is the optimal cutoff point value [21].
The adjusted OR was calculated using a logistic regression analysis that included as covariates baseline characteristics found to differ (p < 0.05) between the study groups. The sample size was calculated based on previous findings of our group [20], setting type I and II errors at the conventional 0.05 and 0.20 and considering clinically relevant a mtDNA copy number in peripheral blood 20% lower in subfertile women. On these bases, the required sample size was 104 cases (and 208 matched controls).
Results
A total of 1200 women were initially eligible to participate. Of these, 1051 accepted and 149 refused for personal reasons. Subfertility was reported in 143 women, of whom 104 fulfilled the selection criteria for being classified as cases. They were matched to 208 fertile controls. The time to pregnancy was 18.3 ± 5.6 and 2.4 ± 2.2 months, respectively (p < 0.001). Baseline characteristics of the two study groups are shown in Table 1. Blood cell counts did not differ between cases and controls, and in none of the included patients, frankly, pathological values were observed. A statistically significant difference was found only for parity.
Table 1.
General characteristics of subfertile women (cases) and controls
| Characteristics | Cases (n = 104) | Controls (n = 208) | p |
|---|---|---|---|
| Woman’s age (years) | 33.4 ± 3.5 | 33.4 ± 3.5 | 0.92 |
| BMI (kg/m2) | 22.1 ± 3.3 | 21.9 ± 3.7 | 0.60 |
| White blood cell (109/L) | 7.91 ± 3.64 | 7.66 ± 3.53 | 0.64 |
| Neutrophil (109/L) | 5.22 ± 3.42 | 4.70 ± 3.10 | 0.31 |
| Lymphocyte (109/L) | 1.80 ± 0.41 | 1.89 ± 0.51 | 0.24 |
| Platelets (109/L) | 205 ± 52 | 211 ± 58 | 0.49 |
| Ethnicity | 0.19 | ||
| Caucasian | 98 (94%) | 203 (98%) | |
| Other | 6 (6%) | 5 (2%) | |
| Marital status | 0.51 | ||
| Married | 77 (74%) | 145 (70%) | |
| Unmarried | 27 (26%) | 63 (30%) | |
| Scholarity | 0.07 | ||
| Elementary–middle school | 14 (14%) | 15 (7%) | |
| High school | 43 (41%) | 74 (36%) | |
| College/university | 47 (45%) | 119 (57%) | |
| Previous pregnancies | 49 (47%) | 117 (56%) | 0.15 |
| Previous live births | 25 (24%) | 83 (40%) | 0.006 |
| Previous ectopic pregnancies | 0 (0%) | 3 (1%) | 0.55 |
| Previous ovarian surgery | 8 (8%) | 6 (3%) | 0.08 |
| Menstrual cycle length (days) | 28.6 ± 2.4 | 28.7 ± 2.2 | 0.55 |
| Recent shortening of the menstrual cycle | 6 (5%) | 7 (3%) | 0.37 |
| Smoking | 16 (15%) | 36 (17%) | 0.11 |
| Alcohol habits | 0.71 | ||
| Never | 71 (68%) | 134 (64%) | |
| Occasional | 20 (19%) | 41 (20%) | |
| Regular | 13 (13%) | 33 (16%) | |
| Twin pregnancy | 1 (1%) | 2 (1%) | 0.78 |
| Neonatal gender | 0.90 | ||
| Male | 56 (54%) | 114 (55%) | |
| Female | 47 (46%) | 92 (45%) |
Data is presented as mean ±SD or n (%) as appropriate
BMI body mass index
aRefers only to singletons
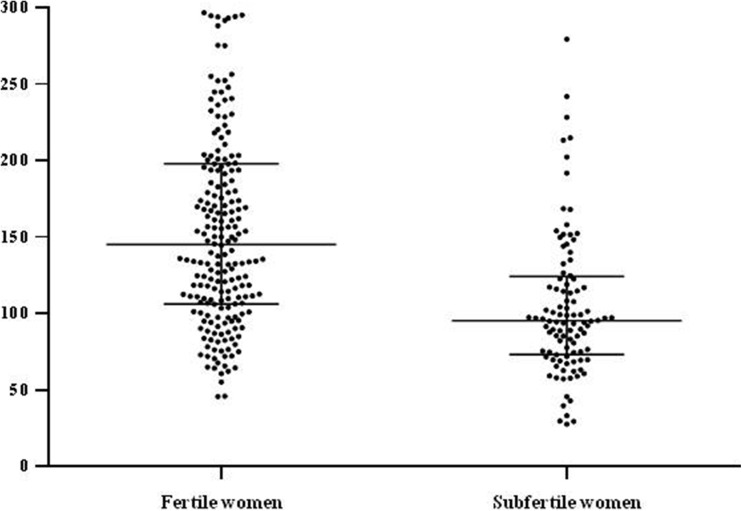
The mtDNA copy number in peripheral blood significantly correlated with age in the whole cohort (Spearman correlation coefficient of − 0.41, p < 0.001). The median (interquartile range-IQR) mtDNA copy number in peripheral blood in subfertile and control women was 95 (73–124) and 145 (106–200), respectively (p < 0.001) (Fig. 1). In both study groups, we failed to observe any significant difference by comparing the median mtDNA copy number in peripheral blood in parous and nulliparous cases ((92 (66–107) and 97 (75–127), respectively (p = 0.54)) and in parous and nulliparous controls ((132 (91–187) and 150 (111–202), respectively (p = 0.35)). Significant differences were confirmed by comparing the median mtDNA copy number in peripheral blood in nulliparous cases and controls (p = 0.01) and in parous cases and controls (p = 0.03).
Fig. 1.
MtDNA copy number in peripheral blood in subfertile (cases) and fertile (controls) pregnant women. Non-parametric Mann–Whitney test was used to determine the difference in mtDNA copy numbers between groups (p < 0.001; error bars represent interquartile range)
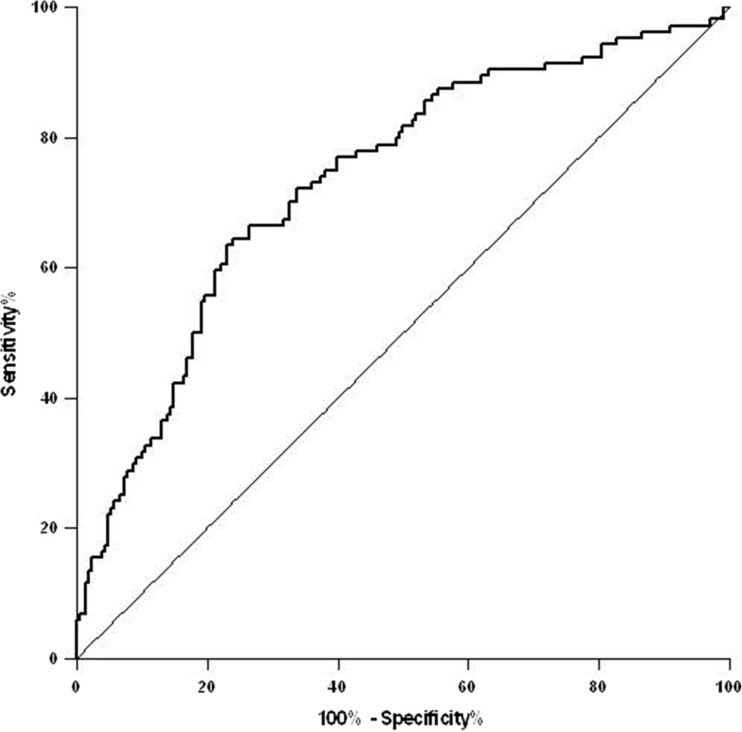
The area under the ROC curve was 0.73 (95% confidence interval (CI) 0.67–0.79) (p < 0.001) (Fig. 2). The Youden index was 105 mtDNA copy number. This cutoff point resulted related to subfertility with a sensitivity of 77% and a specificity of 64%. The crude OR for subfertility in women with mtDNA copy number in peripheral blood below this threshold was 5.72 (95% CI 3.43–9.55). The OR adjusted for parity was 6.49 (95% CI 3.80–11.10). We tested the discrimination ability of mtDNA copy number in peripheral blood in different age groups. Results are shown in Table 2. Accuracy decreased with age.
Fig. 2.
Analysis of the ROC curve demonstrating the predictive value of mtDNA copy number assessment in peripheral blood for subfertility. The area under the ROC curve was 0.73 (95% confidence interval (CI) 0.67–0.79) (p < 0.001)
Table 2.
MtDNA copy number in peripheral blood discrimination potential in different age groups: c-statistics
| Age quartiles | AUC (95% CI) | p | Youden index | Sensitivity (%) | Specificity (%) | OR (95% CI) | Adj. OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Q1 (< 31.42 years) | 0.88 (0.80–0.97) | < 0.001 | 128 | 79 | 84 | 20.05 (5.70–70.58) | 20.80 (5.72–75.64) |
| Q2 (31.42–33.27 years) | 0.73 (0.60–0.86) | 0.001 | 106 | 92 | 50 | 12.00 (3.35–43.04) | 15.02 (3.82–59.11) |
| Q3 (33.28–35.97 years) | 0.65 (0.52–0.78) | 0.024 | 109 | 66 | 64 | 3.49 (1.33–9.21) | 3.68 (1.32–10.25) |
| Q4 (> 35.97 years) | 0.67 (0.55–0.79) | 0.015 | 117 | 45 | 88 | 6.07 (1.62–22.67) | 5.82 (1.51–22.43) |
Q quartile, AUC area under the ROC curve, CI confidence interval, ROC receiver operating characteristics, OR odds ratio for subfertility, Adj. OR odds ratio for subfertility adjusted for parity
Discussion
In this study, we found a significantly lower mtDNA copy number in peripheral blood of subfertile women compared to age-matched fertile controls. Women with a mtDNA/nDNA below 105 had a more than fivefold higher risk of being subfertile. A subgroup analysis based on age showed an increased discriminatory capacity in younger women.
These findings confirm our initial hypothesis. In fact, one may hypothesize that the low mtDNA copy number detected in blood cells may be a peripheral indicator of a low mtDNA content in the oocytes and thus a lower fertilization potential conditioning subfertility [14]. Nevertheless, claiming that the mtDNA copy number in peripheral blood is a marker of poor oocyte quality is speculative. In fact, evidence showing a correlation between mtDNA content of ovarian or follicular cells and mtDNA copy number in peripheral blood are still scanty and the low number of subjects included in studies investigating this issue limits the reliability of the results [9, 18]. Future studies in IVF contexts are needed to disentangle this issue. Of utmost interest here is investigating whether mtDNA copy number in peripheral blood would correlate with the aneuploidy rate of the embryos.
Alternative interpretations may be valid. In this context, an explanation deserving consideration is based on the reported inverse correlation between peripheral mtDNA copy number and the amount of the residual ovarian reserve. In other words, peripheral mtDNA may reflect ovarian reserve rather than oocyte quality. In fact, a study investigating blood cell mtDNA content in patients with premature ovarian aging showed a diminished mtDNA copy number in peripheral blood of women with an impaired ovarian reserve compared to controls [9]. Moreover, dysfunction of the mitochondrial DNA polymerase gamma (POLG), the enzyme that synthesizes mtDNA that causes a generalized mtDNA replication impairment, is associated with phenotypes variably including neurological and muscular defects, diabetes, and primary ovarian insufficiency (POI) [22]. Unfortunately, we did not collect data on ovarian reserve in our study to explore this alternative possibility. A role of impaired ovarian reserve in explaining our results is however unlikely. In fact, only one initial prospective study showed a significantly reduced natural fecundity in women with depleted AMH levels compared with women with higher AMH levels (fecundability ratio 0.38; 95% CI 0.08–0.91) [23]. All the subsequent contributions failed to document an association between AMH levels and effective time to pregnancy in women who conceived naturally [24–27]. Of particular relevance here is the study recently conducted by Steiner et al. [27]. These authors measured a single circulating AMH level (along with levels of follicle-stimulating hormone [FSH], inhibin B, and urinary levels of FSH) for each participant in a time-to-pregnancy study of a community-based sample of women who did not have a history of infertility. They observed that, among women aged 30 to 44 years without a history of infertility who had been trying to conceive for 3 months or less, biomarkers of diminished ovarian reserve (low AMH or high FSH) were not associated with reduced fecundability or a lower cumulative probability of conceiving by 6 or 12 cycles of pregnancy attempt [27].
Finally, it could be hypothesized that oxidative stress per se constitutes the real mediator of female fecundity damage and that the alterations in peripheral blood mtDNA content are only the reflection of its high level. Oxidative phosphorylation, defined as the oxidation of fuel molecules by oxygen and the concomitant transduction of this energy into adenosine triphosphate (ATP), is the most important function performed by mitochondria [28]. A perturbation in this system can disturb mtDNA replication and contribute to mtDNA content decline in peripheral blood [29]. Furthermore, when compared to its nuclear counterpart, mtDNA accumulates damage more extensively when exposed to ROS [30, 31]. Unfortunately, direct evidence showing an association between the amount of ROS and the mtDNA copy number in peripheral blood are lacking. However, several authors observed a reduced peripheral blood mtDNA content in patients suffering from conditions traditionally characterized by an excessive oxidative stress such as metabolic syndrome and coronary heart disease [32–36]. Future studies should consider this aspect and provide the quantification of oxidative stress markers level in peripheral blood together with that of mtDNA in fertile and subfertile women.
Basal characteristics did not differ between study groups with the exception of parity. This result was expected. In fact, it is well known that women who had a previous pregnancy are more susceptible to have another one than nulliparous. We compared mtDNA copy number in peripheral blood in parous and nulliparous women in both study groups, but we failed to observe any significant difference. On the contrary, significant differences were confirmed by comparing the mtDNA amount in peripheral blood in nulliparous cases and controls and in parous cases and controls.
The absence of differences in blood cell counts between the two study groups further supports the validity of our results. In fact, blood cell counts are important covariables influencing mtDNA copy number in peripheral blood. In particular, the relative mtDNA content in peripheral blood largely depends on platelets and white blood cells. Noteworthy, the role of platelets, although it has yet to be fully elucidated, seems to be of considerable importance. Indeed, since they have no nucleus and therefore total DNA does not increase with their total count, one could speculate that the relative amount of mtDNA in peripheral blood directly increases with the increase of platelets concentration. If this hypothesis was confirmed, the platelet count should be always determined in order to correct the estimate of the mtDNA copy number in peripheral blood [37]. Consideration should also be given to peripheral blood monocytes. Their mtDNA content has a large genetic determinant, and not surprisingly, it is postulated to play a role in the pathogenesis of various diseases such as type 2 diabetes [38]. The relative contribution of each peripheral blood cell population in determining the total peripheral blood mtDNA content is not yet precisely known. However, considering the relevance of this aspect, future studies should focus on its determination and on factors able to influence it.
The identification of a biomarker that reflects woman fertility may be of utmost interest. To date, only age was shown to reflect women fecundity. In this regard, it is noteworthy that the ROC analysis of the whole cohort resulted in an AUC value of 0.73 indicating a good performance of mtDNA copy number assessment in discriminating between fertile and subfertile women. The observation that the discriminatory capacity increased in younger age further supports the relevance of our findings. Indeed, natural fecundity steadily decreases with age and, therefore, the arbitrary threshold of 1 year of pregnancy seeking to discern between fertile and subfertile women may be less reliable with increased women’s age [39]. In other words, the proportion of women who have not yet conceived within 1 year just for statistical misfortune (rather than for organic or functional causes) increases with age. Even if the corollary findings of an increased discriminatory capacity in younger age further support the interest of our findings, it is important to underline that it also suggests that age per se remains a critical information. If confirmed in future independent studies, peripheral mtDNA may reveal as a useful biomarker but it is unlikely to fully substitute age. Interestingly, we observed a significant negative correlation between mtDNA and age but the Spearman correlation coefficient indicated a modest association (0.41). This evidence is in line with the results emerging from a recent large population-based observational study [40]. MtDNA is thus unlikely to explain the reduction in fecundity on its own. Further studies specifically designed to address the relative relevance of mtDNA and age on fecundity are required. In this regard, it would be of utmost interest also investigating whether peripheral mtDNA may be improved in subfertile women by modifying life style habits and, if so, whether this would turn into an improved natural fecundity. Noteworthy, for instance, both obesity and diet have been shown to similarly affect mitochondrial function and natural fecundity [41, 42].
Our results might have future clinical application. However, some methodological aspects of mtDNA quantification are still debated [2, 9, 43–47]. Solving this issue is thus of utmost relevance before designing future studies aimed at testing the utility of this biomarker in a clinical setting.
Some limitations of our study deserve to be commented. Firstly, the reliability of mtDNA copy number assessment in peripheral blood during pregnancy may be a matter of concern. Circulating mtDNA was shown to progressively reduce throughout the three trimesters of pregnancy [20], and therefore, confirmation of our findings in a non-pregnant population is necessary. On the other hand, it is noteworthy that this confounder cannot be expected to impact differently in the two study groups. Secondly, we did not perform an infertility diagnostic work-up of the couples and we are thus unable to rule out other potential causes of subfertility. We did not collect a semen sample for analysis. The lack of information about the presence and the severity of male subfertility inevitably weaken the message of the study. Furthermore, women did not undergo examinations aimed at excluding concomitant female factors of subfertility (including tests of ovarian reserve). Also, possible genetic alterations in both members of the couple were not investigated. On the other hand, severe concomitant causes of infertility can be confidently ruled out since all women conceived within 2 years. Thirdly, even if a detailed medical history has been collected, it is not possible to exclude with absolute certainty the presence of underlying pathological conditions that could interfere with the levels of mtDNA in peripheral blood.
Conversely, our study design has some important advantages. The choice of a control group is indeed a major difficulty when studying infertility. Selecting both cases and controls among pregnant women was claimed to overcome most of the possible pitfalls. Time to pregnancy is an epidemiologic metric widely used for the study of factors affecting both male and female human fecundities [47–52].
In conclusion, we observed that mtDNA copy number in peripheral blood is significantly reduced in subfertile women, providing a possible way of predicting which subjects will be predisposed to this condition. Furthermore, we observed a better performance of this test in young women. On this basis, mtDNA copy number assessment in peripheral blood appears to have all the features of the much sought non-invasive biomarker of female fertility. However, further independent studies including in non-pregnant women undergoing high-throughput screening are warranted [53]. In fact, a further mtDNA copy number decrease in peripheral blood of women with long-standing unexplained infertility would support our findings and increase the clinical interest for this biomarker.
Compliance with ethical standards
The study was accepted by the local Institutional Review Board. All recruited patients provided a written informed consent to participate.
Conflict of interest
A.B., D.L., R.R., A.P., L.P., L.F., and E.S., according to Italian laws, filed a patent application for mitochondrial DNA quantification in peripheral blood and for its use as non-invasive biomarker for female subfertility. E.S. handled grants of research from Ferring and Merck-Serono.
Footnotes
Andrea Busnelli and Debora Lattuada should be regarded as joint first authors.
References
- 1.Shen J, Gopalakrishnan V, Lee J, Fang S, Zhao H. Mitochondrial DNA copy number in peripheral blood and melanoma risk. PLoS One. 2015;10:e0131649. doi: 10.1371/journal.pone.0131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Xu S, Xu Y, Liu Y, Li Z, Zhang Y, Jin Y, Xue X, Wang H. Relation of mitochondrial DNA copy number in peripheral blood to postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting. Am J Cardiol. 2017;119:473–477. doi: 10.1016/j.amjcard.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Demain LA, Conway GS, Newman WG. Genetics of mitochondrial dysfunction and infertility. Clin Genet. 2017;91:199–207. doi: 10.1111/cge.12896. [DOI] [PubMed] [Google Scholar]
- 4.May-Panloup P, Boucret L, de la Chao Barca JM, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 5.Aiken CE, Tarry-Adkins JL, Penfold NC, Dearden L, Ozanne SE. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2015;30:1548–1556. doi: 10.1096/fj.15-280800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 7.Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137:4837–4843. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- 8.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, Paffoni A, Mari D, Ragni G, Persani L, the Italian Network for the study of Ovarian Dysfunctions Blood cell mitochondrial DNA content and premature ovarian aging. PLoS One. 2012;7:e42423. doi: 10.1371/journal.pone.0042423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ene AC, Park S, Edelmann W, Taketo T. Caspase 9 is constitutively activated in mouse oocytes and plays a key role in oocyte elimination during meiotic prophase progression. Dev Biol. 2013;377:213–223. doi: 10.1016/j.ydbio.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St John JC, Tsai TS, Cagnone GL. Mitochondrial DNA supplementation as an enhancer of female reproductive capacity. Curr Opin Obstet Gynecol. 2016;28:211–216. doi: 10.1097/GCO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 12.St John JC. Mitochondrial DNA copy number and replication in reprogramming and differentiation. Semin Cell Dev Biol. 2016;52:93–101. doi: 10.1016/j.semcdb.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 15.Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol. 2015;27:175–181. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St John J. The control of mtDNA replication during differentiation and development. Biochim Biophys Acta. 1840;2014:1345–1354. doi: 10.1016/j.bbagen.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Boucret L, de la Chao Barca JM, Moriniere C, Desquiret V, Ferre-L’Hotellier V, Descamps P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30:1653–1664. doi: 10.1093/humrep/dev114. [DOI] [PubMed] [Google Scholar]
- 18.Ogino M, Tsubamoto H, Sakata K, Oohama N, Hayakawa H, Kojima T. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33:367–371. doi: 10.1007/s10815-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desquiret-Dumas V, Clément A, Seegers V, Boucret L, Ferré-L'Hotellier V, Bouet PE, Descamps P, Procaccio V, Reynier P, May-Panloup P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32:607–614. doi: 10.1093/humrep/dew341. [DOI] [PubMed] [Google Scholar]
- 20.Colleoni F, Lattuada D, Garretto A, Massari M, Mandò C, Somigliana E, et al. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol. 2010;203:365.e1–6. doi: 10.1016/j.ajog.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;3762651 [DOI] [PMC free article] [PubMed]
- 22.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 23.Steiner Anne Z., Herring Amy H., Kesner James S., Meadows Juliana W., Stanczyk Frank Z., Hoberman Steven, Baird Donna D. Antimüllerian Hormone as a Predictor of Natural Fecundability in Women Aged 30–42 Years. Obstetrics & Gynecology. 2011;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streuli I, de Mouzon J, Paccolat C, Chapron C, Petignat P, Irion OP, de Ziegler D. AMH concentration is not related to effective time to pregnancy in women who conceive naturally. Reprod BioMed Online. 2014;28:216–224. doi: 10.1016/j.rbmo.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Hagen Casper P., Vestergaard Sonja, Juul Anders, Skakkebæk Niels Erik, Andersson Anna-Maria, Main Katharina M., Hjøllund Niels Henrik, Ernst Erik, Bonde Jens Peter, Anderson Richard A., Jensen Tina Kold. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertility and Sterility. 2012;98(6):1602-1608.e2. doi: 10.1016/j.fertnstert.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Somigliana E, Lattuada D, Colciaghi B, Filippi F, La Vecchia I, Tirelli A, et al. Serum anti-Müllerian hormone in subfertile women. Acta Obstet Gynecol Scan. 2015;94:1307–1312. doi: 10.1111/aogs.12761. [DOI] [PubMed] [Google Scholar]
- 27.Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318:1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiao CP, Hoppel C. Analyzing mitochondrial function in human peripheral blood mononuclear cells. Anal Biochem. 2018;549:12–20. doi: 10.1016/j.ab.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knez J, Marrachelli VG, Cauwenberghs N, Winckelmans E, Zhang Z, Thijs L, Brguljan-Hitij J, Plusquin M, Delles C, Monleon D, Redón J, Staessen JA, Nawrot TS, Kuznetsova T. Peripheral blood mitochondrial DNA content in relation to circulating metabolites and inflammatory markers: a population study. PLoS One. 2017;12:e0181036. doi: 10.1371/journal.pone.0181036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.CIR.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 31.Yakes F, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LP, Cheng K, Ning MA, Li HH, Wang HC, Li F, Chen SY, Qu FL, Guo WY. Association between peripheral blood cells mitochondrial DNA content and severity of coronary heart disease. Atherosclerosis. 2017;261:105–110. doi: 10.1016/j.atherosclerosis.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Ashar FN, Zhang Y, Longchamps RJ, Lane J, Moes A, Grove ML, Mychaleckyj JC, Taylor KD, Coresh J, Rotter JI, Boerwinkle E, Pankratz N, Guallar E, Arking DE. Association of mitochondrial DNA copy number with cardiovascular disease. JAMA Cardiol. 2017;2(11):1247–1255. doi: 10.1001/jamacardio.2017.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, Choi JR, Park IH, Huh JH, Son JW, Kim KW, Park KS, Cha SK, Sohn JH, Jung DH, Koh SB. A prospective study of leucocyte mitochondrial DNA content and deletion in association with the metabolic syndrome. Diabetes Metab. 2017;43(3):280–283. doi: 10.1016/j.diabet.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Révész D, Verhoeven JE, Picard M, Lin J, Sidney S, Epel ES, Penninx BWJH, Puterman E. Associations between cellular aging markers and metabolic syndrome: findings from the CARDIA study. J Clin Endocrinol Metab. 2018;103(1):148–157. doi: 10.1210/jc.2017-01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CH, Su SL, Hsieh MC, Cheng WL, Chang CC, Wu HL, Kuo CL, Lin TT, Liu CS. Depleted leukocyte mitochondrial DNA copy number in metabolic syndrome. J Atheroscler Thromb. 2011;18(10):867–873. doi: 10.5551/jat.8698. [DOI] [PubMed] [Google Scholar]
- 37.Knez J, Winckelmans E, Plusquin M, Thijs L, Cauwenberghs N, Gu Y, Staessen JA, Nawrot TS, Kuznetsova T. Correlates of peripheral blood mitochondrial DNA content in a general population. Am J Epidemiol. 2016;183:138–146. doi: 10.1093/aje/kwv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong J, McLennan SV, Molyneaux L, Min D, Twigg SM, Yue DK. Mitochondrial DNA content in peripheral blood monocytes: relationship with age of diabetes onset and diabetic complications. Diabetologia. 2009;52:1953–1961. doi: 10.1007/s00125-009-1424-6. [DOI] [PubMed] [Google Scholar]
- 39.Somigliana E, Paffoni A, Busnelli A, Filippi F, Pagliardini L, Vigano P, Vercellini P. Age-related infertility and unexplained infertility: an intricate clinical dilemma. Hum Reprod. 2016;31:1390–1396. doi: 10.1093/humrep/dew066. [DOI] [PubMed] [Google Scholar]
- 40.Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toledo FG, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab. 2006;91:3224–3227. doi: 10.1210/jc.2006-0002. [DOI] [PubMed] [Google Scholar]
- 42.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 43.Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, Wells D, Fragouli E. Mitochondrial DNA quantification as a tool for embryo viability assessment: retrospective analysis of data from single euploid blastocyst transfers. Hum Reprod. 2017;32:1282–1292. doi: 10.1093/humrep/dex070. [DOI] [PubMed] [Google Scholar]
- 44.Wells D, Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, Fragouli E. Reply: mitochondrial DNA quantification-the devil in the detail. Hum Reprod. 2017;32:2150–2151. doi: 10.1093/humrep/dex279. [DOI] [PubMed] [Google Scholar]
- 45.Barnes FL, Victor AR, Zouves CG, Viotti M. Mitochondrial DNA quantitation-making sense of contradictory reports. Hum Reprod. 2017;32:2149–2150. doi: 10.1093/humrep/dex278. [DOI] [PubMed] [Google Scholar]
- 46.Wang Tianren, Zhang Man, Jiang Zongliang, Seli Emre. Mitochondrial dysfunction and ovarian aging. American Journal of Reproductive Immunology. 2017;77(5):e12651. doi: 10.1111/aji.12651. [DOI] [PubMed] [Google Scholar]
- 47.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, Kokocinski F, Cohen J, Munne S, Wells D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- 49.Te Velde ER, Eijkemans R, Habbema HD. Variation in couple fecundity and time to pregnancy, an essential concept in human reproduction. Lancet. 2000;355:1928–1929. doi: 10.1016/S0140-6736(00)02320-5. [DOI] [PubMed] [Google Scholar]
- 50.Joffe M. Time trends in biological fertility in Britain. Lancet. 2000;355:1961–1965. doi: 10.1016/S0140-6736(00)02328-X. [DOI] [PubMed] [Google Scholar]
- 51.Vélez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the maternal-infant research on environmental chemicals (MIREC) study. Fertil Steril. 2015;103:1011–1020. doi: 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, Maisog JM, Gore-Langton R, Eisenberg ML, Chen Z. Semen quality and time to pregnancy: the longitudinal investigation of fertility and the environment study. Fertil Steril. 2014;101:453–462. doi: 10.1016/j.fertnstert.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, Jia WH, Jie Y, Chen MS, Chen MX, Fang JH, Zeng C, Zhang Y, Guo RP, Wu Y, Lin G, Zheng L, Zhuang SM. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]