Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) through its graft-versus-tumor (GVT) effects is a curative therapy against many hematological malignancies. However, GVT is linked to harmful graft-versus-host disease (GVHD) after allo-HCT. Both GVT and GVHD require allogeneic T cell responses, which is an energetically costly process that causes oxidative stress. Sirtuin 3 (SIRT3), a mitochondrial histone deacetylase (HDAC), plays an important role in cellular processes through inhibition of reactive oxygen species (ROS). Nonmitochondrial class of HDACs regulate T cell responses, but the role of mitochondrial HDACs, specifically SIRT3, on donor T cell responses after allo-HCT remains unknown. In this study, we report that SIRT3-deficient (SIRT3−/−) donor T cells cause reduced GVHD severity in multiple clinically relevant murine models. The GVHD protective effect of allogeneic SIRT3−/− T cells was associated with a reduction in their activation, reduced CXCR3 expression, and no significant impact on cytokine secretion or cytotoxic functions. Intriguingly, the GVHD protective effect of SIRT3−/− T cells was associated with a reduction in ROS production, which is contrary to the effect of SIRT3 deficiency on ROS production in other cells/tissues and likely a consequence of their deficient activation. Notably, the reduction in GVHD in the gastrointestinal tract was not associated with a substantial reduction in the GVT effect. Collectively, these data reveal that SIRT3 activity promotes allogeneic donor T cell responses and ROS production without altering T cell cytokine or cytolytic functions and identify SIRT3 as a novel target on donor T cells to improve outcomes after allo-HCT.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for many malignant and nonmalignant hematological diseases. Unfortunately, acute graft-versus-host disease (GVHD) is a major life-threatening complication of allo-HCT (1, 2). The pathophysiology of acute GVHD is complex, but it is well established that alloreactive donor T cells play an important role in mediating acute GVHD (3, 4). However, allogeneic T cells are also indispensable for the therapeutic graft-versus-tumor (GVT) effect (5). For this reason, meaningfully separating GVHD from the GVT effect is critical for successful outcomes after allo-HCT. Prophylaxis against GVHD has targeted T cells with calcineurin inhibitors; however, 30–60% of patients continue to develop acute GVHD (6), suggesting that new GVHD prophylaxis and treatment strategies are needed.
To meet the metabolic demands of activation, naive T cells alter their metabolism by shifting from oxidation of free fatty acids to glycolysis and glutaminolysis (7–10). Specifically, alloreactive donor T cells demonstrate increased aerobic glycolysis (11, 12), oxidative phosphorylation (13), and fatty acid metabolism (14, 15), resulting in increased oxidative stress. Reactive oxygen species (ROS) production, and thus the degree of oxidative stress, is controlled in part by sirtuins (SIRTs), which are class III histone deacetylases (HDACs) (16) known to influence a variety of aging related disorders, in part, by controlling mitochondrial functions, including inhibition of ROS production (17, 18). However, little is known regarding the immune functions of most SIRTs, but their importance for T cell function is best illustrated by SIRT1, which influences T cell activation, differentiation, and tolerance (19–27). Interestingly, we and others have previously shown that inhibition of HDACs, other than class III, including the mitochondrial HDACs, mitigates GVHD (28–30). However, the role of mitochondrial HDACs, specifically SIRT3, in regulation of T cells in vitro and in vivo during GVHD remains unknown.
SIRTs are ubiquitously expressed in mammals but exhibit a distinct, predominant subcellular localization, including nuclear (SIRT1, SIRT6, SIRT7), mitochondria (SIRT3, SIRT4, SIRT5), and cytoplasm (SIRT1, SIRT2) (18). SIRT3 promotes generation of energy by regulating the function of mitochondrial proteins involved in oxidative phosphorylation, fatty acid oxidation, the urea cycle, antioxidant responses, and stress responses (31–39). SIRT3 expression is greatest in metabolically active tissues and is increased by metabolic stress and nutrient deprivation (40). Consistent with this, SIRT3-deficient (SIRT3−/−) animals show a 50% reduction of ATP (33). Because of the high metabolic demand of allogeneic T cells and their dependence on mitochondrial metabolism, we hypothesized that SIRT3 would influence their function. In this report, we demonstrate that SIRT3−/− donor T cells in experimental allogeneic models of bone marrow (BM) transplantation (BMT) protect against GVHD without significantly affecting GVT effect, indicating that selective SIRT3 inhibition in donor T cells may provide a novel strategy for improving outcomes after allo-HCT.
Materials and Methods
Mice
Female C57BL/6 (B6, H-2b), B6D2F1 (H-2b/d), and BALB/c (H-2d) mice were purchased from National Cancer Institute (Frederick, MD). SIRT3−/− mice were generated as before (35). All animal studies were approved by the University Committee on Use and Care of Animals of the University of Michigan, based on university laboratory animal medicine guidelines.
BM transplantation
Splenic T cells from donor mice were enriched, whereas BM cells were depleted of T cells by using CD90.2 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). BALB/c and B6D2F1 recipient mice were irradiated with 8 and 11 Gy ([137Cs] source), respectively, on day −1. On day 0, recipient mice received via tail vein injection 5 × 106 T cell–depleted BM (TCDBM) cells from either syngeneic or allogeneic B6 wild-type (WT) mice as well as CD90.2+ splenic T cells from either syngeneic or allogeneic B6 WT or SIRT3−/− mice.
Systemic and histopathological analysis of GVHD
We monitored survival after BMT daily and assessed the degree of clinical GVHD weekly, as described previously (41). Histopathological analysis of GVHD target organs (skin, liver, and gastrointestinal [GI] tract) was performed using a semiquantitative scoring system by a single pathologist (C. Liu), as described previously (42).
MLR
T cells (2 × 105 per well) were magnetically isolated from mice spleen using CD90.2 microbeads (Miltenyi Biotec) and subsequently cultured with irradiated (30 Gy) BALB/c BM-derived dendritic cells (DCs) (BMDCs) at 40:1 (5 × 103 per well) for 72 and 96 h. BMDCs were generated by culturing for 7 d in the presence of 20 ng/ml recombinant GM-CSF (PeproTech, Rocky Hill, NJ). For the exogenous ROS MLRs, T cells were negatively isolated using the PanT Cell Kit (Miltenyi Biotec) after first undergoing RBC lysis (Sigma-Aldrich). The non–T cell fraction from the PanT Cell Kit was collected, irradiated (30 Gy), mixed with the T cell fraction at a ratio of 2:1 (T cells/stimulator cells), and cocultured for 96 h. Incorporation of [3H]thymidine (1 μCi/well) by proliferating T cells during the final 6 h of culture was measured by a TopCount instrument (PerkinElmer, Waltham, MA).
Regulatory T suppression assay
CD4+CD25– and CD4+CD25+ T cells were isolated from the spleen of B6-WT or SIRT3−/− mice using CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec) with MACS by the manufacturer’s protocol. The purity of each type of cells was >90%. CD4+CD25+ T cells were serially diluted from 2 × 104 to 2500 cells per well and incubated with 2 × 104 CD4+CD25– T cells and 2.5× 103 irradiated BALB/c-derived BMDCs for 120 h. Incorporation of [3H]thymidine (1 μCi/well) by proliferating cells was measured during the last 18 h of culture.
FACS analysis
Flow cytometry analysis was performed with fluorescein-labeled mAbs to mouse CD4, CD8a, CD25, H-2Kb, H-2Kd, Foxp3, IFN-γ, CXCR3, KLRG, CD44, CD62L, CD69, CD127, granzyme B, and IL-17A (eBioscience, San Diego, CA), as described previously (42). For intracellular staining (Foxp3, IFN-γ, and IL-17A), permeabilization buffer (eBioscience) was used. IFN-γ and IL-17A production by donor T cells was assessed following in vitro restimulation for 5 h with Cell Stimulation Cocktail (eBioscience), according to the manufacturer’s instructions. To measure mitochondrial mass and matrix oxidant burden, cells, following MLR, were incubated for 15 min at 37°C in PBS supplemented with 25 nM of Mitotracker Green FM and 1 μM of CellRox Deep Red (Invitrogen, Carlsbad, CA), respectively. Fatty acid transport was assessed by staining with BoDipyC1–C12 (Thermo Fisher Scientific, Waltham, MA). Cells were analyzed using a BD Accuri C6 (BD Biosciences, San Jose, CA) or an Attune NxT (Invitrogen) flow cytometer. Annexin V (Biolegend, SanDiego, CA), 7-AAD (Biolegend), and CellRox Deep Red were used to stain tissue culture cells according to manufacturer protocol.
Cell culture
Primary colonic BALB/c epithelial cells were purchased from Cell Biologics and cultured in Epithelial Cell Media (Cell Biologics) on gelatin-coated plates (Cell Biologics) per manufacturer protocol. P815, A20, BCL1, and EL4 cells were obtained from American Type Culture Collection and accommodated to Epithelial Cell Media for at least three passages prior to experimentation. H2O2 (Sigma-Aldrich) was diluted in PBS (Life Technologies, Cincinnati, OH) prior to application.
ELISA
Serum cytokines were measured by ELISA with specific anti-mouse mAbs for IL-2 (BD Biosciences), IFN-γ (BD Biosciences), and IL-17A (Biolegend) according to the manufacturer’s protocol and read at 450 nm by using a microplate reader (Model 3550; Bio-Rad Labs, Hercules, CA). All samples and standards were run in duplicate.
Quantitative RT-PCR for SIRT3 mRNA expression
After isolation of total RNA using TRIZOL reagent (Life Technologies), 2 μg of RNA was reverse transcribed to cDNA using random primers (Invitrogen) and M-MLV reverse transcriptase (Promega, Fitchburg, WI). Quantitative RT-PCRs were performed in triplicate with SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) and then subjected to melting curve analysis using an Eppendorf Realplex2 machine (Eppendorf, Westbury, NY). The primer sequences of murine SIRT3 genes were as follows: 5′-ATCCCGGACTTCAGATCCCC-3′ and 5′-CAACATGAAAAAGGGCTTGGG-3′. SIRT3 expression levels were normalized against the GAPDH control levels using the comparative threshold cycle (ΔΔCT) method, as previously described (43).
51Cr release assay
Splenic T cells (2 × 106/ml) were isolated from both B6-WT and SIRT3−/− animals and cocultured with irradiated allogeneic BALB/c whole splenocytes (5 × 106/ml) for 5 d. Activated T cells were harvested and used as effector cells. Syngeneic MBL-2 and allogeneic P815 target tumor cells were labeled by incubating 2 × 106 cells with 2 MBq of Na251CrO4 (PerkinElmer, Boston, MA) for 2 h at 37°C in a 5% CO2 atmosphere. After washing, 5 × 103 labeled targets were resuspended and added to triplicate wells at varying E:T ratios and then incubated for 4 h. Maximum and minimum release was determined by the addition of Triton-X (MP Biomedicals, Santa Ana, CA) or media alone to targets, respectively. Supernatants were collected onto a LumaPlate (PerkinElmer) after 4 h, and 51Cr activity was determined using an auto gamma counter (Packard, Meridian, CT).
Statistical analysis
The Mann–Whitney U test was used for the statistical analysis of in vitro data and clinical scores. We plotted survival curves using Kaplan–Meier estimates, and the Wilcoxon rank test was used to analyze survival data. A p value <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism (GraphPad Software).
Results
Absence of SIRT3 does not alter T cell development and differentiation at steady state
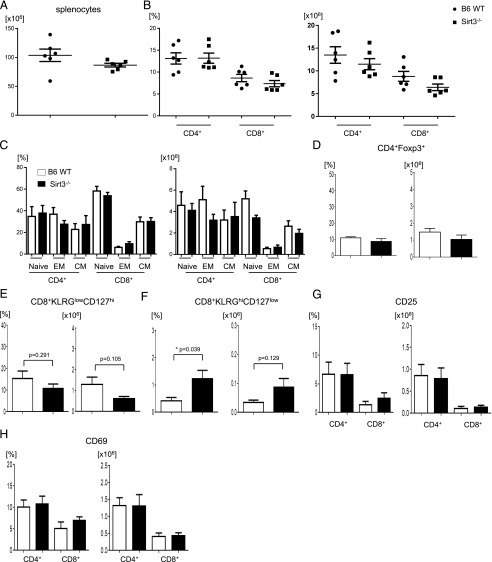
T cells were harvested from 8- to 12-wk-old naive WT and SIRT3-deficient C57BL/6 animals and analyzed for naive, regulatory, and memory T cell subsets. We found that absence of SIRT3 did not have any impact on the numbers or distribution of CD4+ T cells, CD8+ T cells, naive T cells, effector memory T cells, central memory T cells, regulatory T (Treg) cells, or memory precursor effector cells (Fig. 1A–E). There was a higher percentage of short-lived effector T cells (SLECs) in SIRT3-deficient animals; however, there was no difference in absolute numbers of SLECs (Fig. 1F). Absence of SIRT3 expression did not affect the expression of activation markers, such as CD25 and CD69 (Fig. 1G, 1H). These data show that SIRT3 has no large impact on T cell development, differentiation, and activation under resting, in vivo homeostatic conditions.
FIGURE 1.
Phenotypic analysis of T cell subsets and activation markers in naive B6 WT and SIRT3−/− mice. (A–H) Splenocytes were isolated from B6 WT or SIRT3−/− mice (n = 6 mice per group) and analyzed for absolute total splenocyte numbers (A); the percentages and absolute numbers of CD4+ and CD8+ T cells (B); naive (CD44−CD62L+), effector memory (EM) T cell (EM: CD44+CD62−), and central memory (CM) T cell (CM: CD44+CD62L+) subsets (C); Treg cells (CD4+Foxp3+) (D); memory precursor effector cells (CD8+KLRGlowCD127high) (E); terminal differentiated SLECs (CD8+KLRGhiCD127low) (F); and activation marker expression (CD25 and CD69) in CD4+ or CD8+ T cells (G and H). Error bars show the mean ± SEM from three independent experiments. *p < 0.05.
SIRT3 promotes T cell responses in vitro
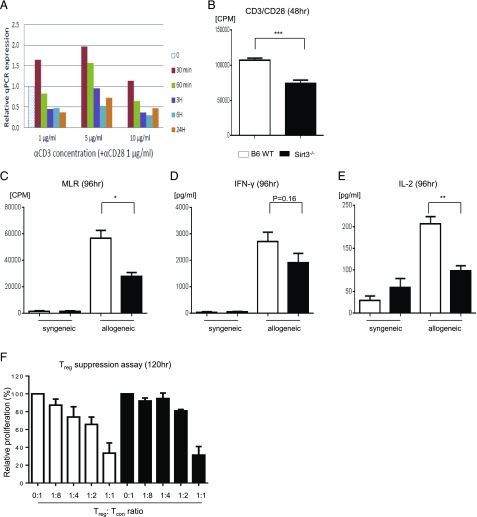
Because SIRT3 expression is known to be induced under conditions that cause increased metabolic demand (40), we first examined whether T cell activation via nonspecific TCR signaling affects SIRT3 expression in splenic T cells from B6 WT mice. We found that nonspecific TCR stimulation with anti-CD3 (1–10 μg/ml) and -CD28 (1 μg/ml) Abs transiently induced Sirt3 mRNA expression after 30 min (Fig. 2A).
FIGURE 2.
Absence of SIRT3 in T cells reduced nonspecific TCR as well as allogeneic responses in vitro. (A) Naive CD90.2+ T cells were isolated from B6 WT spleen with MACS and stimulated with anti-CD3 (1–10 μg/ml)/CD28 (1 μg/ml) Abs. The expression of SIRT3 at various time points after stimulation was evaluated with real-time quantitative PCR. Data presented are representative of one of three independent experiments. (B) Isolated splenic CD90.2+ T cells from either B6 WT or SIRT3−/− mice were incubated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) Abs for 48 h and analyzed for proliferation following [3H]thymidine incorporation during the last 6 h of incubation. (C) In vitro MLR. Isolated splenic CD90.2+ T cells from either B6 WT or SIRT3−/− mice were cultured with BMDCs from syngeneic B6 or allogeneic BALB/c mice for 96 h and analyzed for proliferation following [3H-]thymidine incorporation during the last 16 h of incubation. (D and E) IFN-γ (D) and IL-2 (E) production from allogeneic-stimulated T cells from (C) was measured by ELISA. (F) Treg suppression assay. BMDCs from BALB/c mice were used as stimulators, cocultured with effector T cells (CD4+CD25−) and Treg cells (CD4+CD25+) from either B6-WT or SIRT3−/− mice at different ratios in an MLR, and analyzed for T cell proliferation following [3H]thymidine incorporation during the last 16 h of incubation. Error bars show the mean ± SEM. Three independent experiments were performed for (B–F). *p < 0.05, **p < 0.01, ***p < 0.001.
Next, to specifically test whether SIRT3 functionally regulates in vitro naive T cell nonspecific TCR and/or allogeneic T cell activation, we stimulated WT or SIRT3-deficient (SIRT3−/−) T cells with anti-CD3/CD28 Abs or allogeneic BALB/c BMDCs and measured their proliferation and cytokine production in MLR. We found that proliferation (Fig. 2B, 2C) was reduced in SIRT3−/− T cells in response to both nonspecific TCR and allogeneic stimulation. In addition, the decreased proliferation of allostimulated SIRT3−/− T cells was associated with reduction in IL-2 production and a trend toward less IFN-γ production (Fig. 2D, 2E). We next explored whether SIRT3 affects the functions of Treg cells by comparing the function of SIRT3−/− Treg cells with WT Treg cells in regulating allogeneic naive T cell responses (44, 45) in vitro. We found that SIRT3−/− Treg cells equally suppressed allogeneic WT naive conventional T cell proliferation when compared with WT Treg cells, suggesting that reduced allogeneic T cell response in the absence of SIRT3 is not due to Treg-intrinsic suppression (Fig. 2F).
Absence of SIRT3 in donor T cells mitigates experimental GVHD
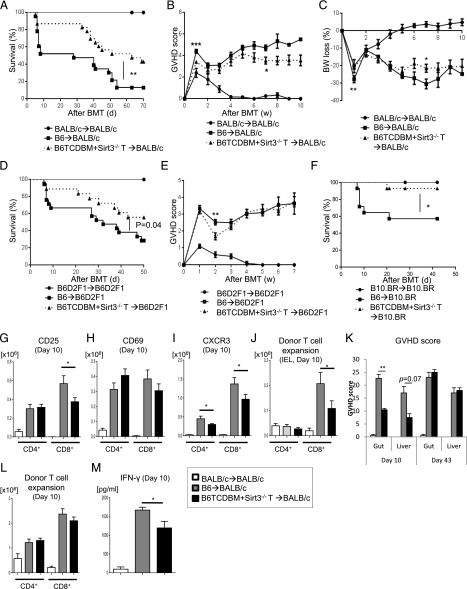
We next determined whether SIRT3 regulates T cell responses in vivo. To do so, we tested in vivo allogeneic T cell function using the well-characterized major histocompatibility Ag–mismatched B6→BALB/c model of allogeneic BMT (allo-BMT). Briefly, BALB/c animals were lethally irradiated and transplanted with either syngeneic BALB/c, allogeneic WT, or SIRT3−/− splenic T cells along with TCDBM from either syngeneic BALB/c or allogeneic B6 WT donors. We found that the absence of SIRT3 in donor T cells ameliorated GVHD mortality and severity (Fig. 3A–C). To eliminate strain-dependent factors, we tested our findings in other BMT models, namely major histocompatibility Ag–mismatched haploidentical B6→B6D2F1 and major histocompatibility Ag–mismatched B6→B10.BR models. In both systems, the allorecipients of SIRT3−/− T cells demonstrated significantly less GVHD mortality than those that received B6 WT T cells (Fig. 3D–F). It is important to note that, although significant, the changes observed in GVHD clinical scores are modest relative to overall survival. This likely reflects the less-sensitive and specific nature of GVHD clinical scoring relative to overall survival and GVHD-specific pathology (see below) and as such remains a limitation. Altogether, these data indicate that SIRT3 augments GVHD in vivo.
FIGURE 3.
Absence of SIRT3 in donor T cells ameliorates GVHD. (A–C) BALB/c mice received 8.5 Gy on day −1 and were transplanted with 0.5 × 106 CD90.2+ splenic T cells from either syngeneic BALB/c or allogeneic major histocompatibility Ag–mismatched B6-WT or SIRT3−/− mice along with 5 × 106 TCDBM cells from either BALB/c or B6 donors (n = 10–23 mice per group). Data are pooled from two independent experiments. (A) Overall survival; p = 0.009 when B6-WT versus SIRT3−/− mice were compared. (B) GVHD clinical score. (C) Percent weight loss. ***p < 0.001, **p < 0.01, *p < 0.05 when B6-WT versus SIRT3−/− mice were compared. (D and E) B6D2F1 mice were used as recipients and received 11 Gy on day −1 and 3 × 106 CD90.2+ T cells along with 5 × 106 TCDBM cells from either syngeneic B6D2F1 or allogeneic B6 donors on day 0. (D) Overall survival (n = 10–28 mice per group). Data are pooled from three independent experiments. (E) Clinical GVHD score. **p < 0.01 when B6-WT versus SIRT3−/− mice were compared. (F) B10.BR mice were used as recipients and received 9.5 Gy on day −1 and 1 × 106 CD90.2+ T cells along with 5 × 106 TCDBM cells from either syngeneic B10.BR or allogeneic B6 donors on day 0. Overall survival is displayed (n = 6–14 mice per group). Data are pooled from two independent experiments. *p < 0.05 when B6-WT versus SIRT3−/− mice were compared. (G–M) Used the B6 into BALB/c GVHD model described in (A–C). (G–I) CD25+(G), CD69+ (H), and CXCR3+ (I) expression in splenic donor T cells at day 10 after allo-BMT (n = 7–8 mice per group, pooled from two experiments). *p < 0.05 when B6-WT versus SIRT3−/− mice were compared. (J) Donor T cell (H-2kb+CD4+CD8+) expansion in intestinal epithelial lymphocytes of the GI tract on day 10 after allo-BMT (n = 7–8 mice per group, pooled from two experiments). *p < 0.05 when B6-WT versus SIRT3−/− mice were compared. (K) The histopathological GVHD grade in GI tract (small and large intestine) and liver on day 10 and day 43 after allo-BMT (n = 8–10 mice per group, pooled from three experiments). **p = 0.038 for GI GVHD and p = 0.07 for liver GVHD; recipients of B6-WT versus SIRT3−/− mice. (L) Donor T cell (H-2kb+CD4+CD8+) expansion in the spleen on day 10 after allo-BMT (n = 7–8 mice per group, pooled from two experiments) (M) Serum IFN-γ levels on day 10 after allo-BMT (n = 8–10 mice per group, pooled from three experiments). *p < 0.05 when B6-WT versus SIRT3−/− mice were compared. Error bars show the mean ± SEM.
In an effort to elucidate why SIRT3 augments GVHD, we analyzed donor T cell characteristics, inflammatory cytokine levels, and GVHD-specific histopathological scores in target organs after allo-BMT in the B6→BALB/c model. We found that decreased GVHD severity 10 d after allo-BMT was associated with decreased expression of the activation marker CD25 primarily on donor CD8+ T cells; however, there was no effect on CD69 expression (Fig. 3G, 3H). Interestingly, the expression of CXCR3 was decreased in allogeneic SIRT3−/− relative to B6 WT T cells (Fig. 3I). CXCR3 is a marker of T cell activation and a chemokine receptor known to be operant in allogeneic effector T cell trafficking to GVHD target organs, especially the gut (46, 47). As would be predicted by decreased CXCR3 expression on allogeneic T cells, we also observed decreased donor CD8 T cell accumulation within intraepithelial lymphocytes (IELs) of the GI tract (Fig. 3J). Furthermore, CD8+ IELs demonstrated decreased expression of CD25 and CXCR3 (Supplemental Fig. 1A, 1B). Corresponding with the decreased donor T cell accumulation in IELs, we also observed a decrease in gut GVHD histopathological score and a trend toward decreased liver histopathological score 10 d after allo-BMT in allogeneic SIRT3−/− donor T cell group relative to WT T cells (Fig. 3K). Interestingly, there was no increased T cell accumulation in the liver or colon between allogeneic B6 WT and SIRT3−/− donor T cell groups (Supplemental Fig. 1C, 1D). Furthermore, donor splenic CD4+, CD8+, and Treg expansion was not altered between allogeneic groups (Fig. 3L, Supplemental Fig. 1E, 1F).
Analysis of cytokines after BMT demonstrated a decrease in serum levels of IFN-γ (Fig. 3M) in the allogeneic SIRT3−/− relative to the B6 WT donor T cell group. However, the decrease in serum levels of IFN-γ appeared to be independent of T cell production as we observed no difference of IFN-γ+ T cells between allogeneic groups (Supplemental Fig. 1G). Similarly, there was no difference in IL-17A or granzyme B–expressing donor T cells between the allogeneic groups (Supplemental Fig. 1H, 1I). Altogether, these data indicate that the absence of SIRT3 on donor T cells attenuates GVHD especially within the GI tract, which may be due to decreased CXCR3-dependent CD8 T cell trafficking to the GI tract, but did not attenuate cytokine and cytotoxic granzyme expression.
SIRT3−/− T cells show reduced ROS production
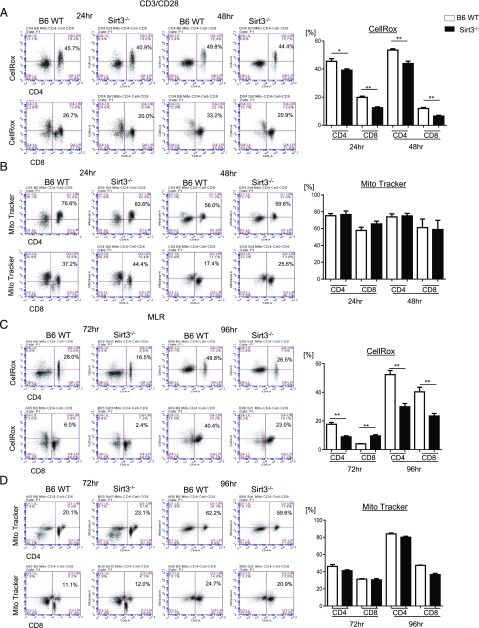
SIRT3 is a major mitochondrial deacetylase and regulates metabolic homeostasis and ROS production via deacetylation of mitochondrial proteins (40, 48). T cells increase ROS production upon allostimulation (13); hence, we wondered whether the decreased GVHD observed with allogeneic donor SIRT3−/− T cells was associated with increased and potentially T cell–harmful oxidative stress. To test this, we examined whether the absence of SIRT3 in T cells alters the levels of mitochondrial ROS in nonspecifically activated and allostimulated T cells in vitro. Surprisingly, we found that SIRT3−/− CD4+ and CD8+ T cells showed reduced production of ROS compared with WT T cells after both nonspecific TCR and allogeneic stimulation (Fig. 4A, 4C). This was dependent on T cell activation because there was no difference in ROS production between WT or SIRT3−/− naive T cells at steady state in vivo (Supplemental Fig. 2A).
FIGURE 4.
SIRT 3 augments ROS production in activated T cells. (A and B) Splenic CD90.2+ T cells were isolated from both B6 WT and SIRT3−/− mice via MACS and stimulated with anti-CD3 (2 μg/ml)/CD28 (1 μg/ml) Abs for 24 or 48 h. ROS levels and mitochondrial accumulation were measured using CellROX staining and Mito Tracker Green Probe, respectively. (A) ROS levels in nonspecific TCR-stimulated CD4+ or CD8+ T cells. Representative FACS profiles (percentage of CD4 or CD8 CellROX-positive cells in upper right quadrant) are shown on the left, and average percentage of CellROX-positive CD8 and CD4 T cells are shown on the right. (B) Mitochondrial accumulation in nonspecific TCR-stimulated CD4+ or CD8+ T cells. Representative FACS profiles are shown on the left (percentage of CD4 or CD8 Mito Tracker–positive cells in upper right quadrant), and average percentages of CellROX-positive CD8 and CD4 T cells are shown on the right. (C and D) Isolated splenic CD90.2+ T cells from either B6 WT or SIRT3−/− mice were cultured with BMDCs from allogeneic BALB/c mice for 72 or 96 h. The cells were stained as above. (C) ROS levels in allogeneic-stimulated CD4+ or CD8+ T cells. Representative FACS profiles are shown on the left, and average percentages are shown on right. (D) Mitochondrial accumulation in allogeneic-stimulated CD4+ or CD8+ T cells. Representative FACS profiles are shown on the left, and average percentages are shown on right. Representative data are from one of three independent experiments, and summarized data were combined from three independent experiments. Error bars show the mean ± SEM. *p < 0.05, **p < 0.01.
Cellular stress, including allogeneic T cell stimulation (13), affects mitochondrial accumulation (49). To assess whether the decrease in ROS production in nonspecifically activated and allostimulated SIRT3−/− T cells was due to a deficiency in mitochondrial content, we stained T cells with MitoTracker Green. Interestingly, there was no difference in mitochondrial abundance in SIRT3−/− T cells relative to WT T cells when stimulated with nonspecific or allogeneic stimulators in vitro (Fig. 4B, 4D) or in naive T cells at steady state in vivo (Supplemental Fig. 2B). SIRT3 is also an important regulator of mitochondrial fatty acid oxidation (36), and allogeneic effector T cells rely on fatty acids for the optimal promotion of murine GVHD (14). However, we observed no dependence on SIRT3 for fatty acid transport in nonspecifically activated T cells in vitro as measured by BoDipyC1–C12 uptake (Supplemental Fig. 3). These data demonstrate that ROS levels are lower in alloantigen or nonspecific TCR-stimulated SIRT3-deficient T cells, which is contrary to SIRT3’s known inhibition of ROS production in other cells (37, 50).
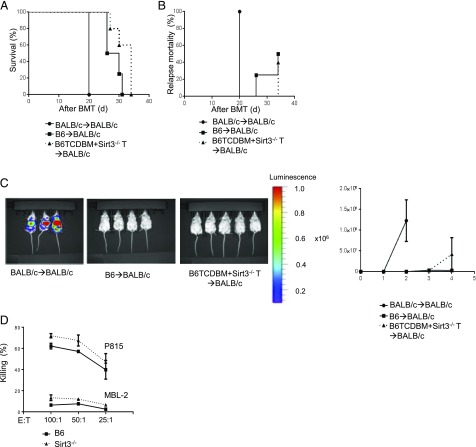
SIRT3−/− T cells retain their GVT effect
GVHD is tightly linked with the GVT effect. Hence, we next explored whether SIRT3−/− donor T cells preserved enough reactivity to sustain GVT activity. To accomplish this, we used the B6→BALB/c model of allo-BMT but added syngeneic mastocytoma P815 tumor cells at the time of BMT. Vitally, the mice that received SIRT3−/− T cells showed equivalent GVT responses as the mice that received WT T cells as measured by overall survival, tumor-related mortality, and tumor growth determined by bioluminescence (Fig. 5A–C). Furthermore, B6 WT and SIRT3−/− T cells showed equivalent killing of allogeneic P815 tumor cells in an in vitro cytotoxic T cell killing assay (Fig. 5D). These data demonstrate that despite SIRT3’s requirement for optimal T cell activation and enhancement of GVHD, it did not substantially affect cytotoxic effects and in vivo GVT responses.
FIGURE 5.
SIRT3−/− T cells retain their GVT effect. BALB/c animals received 8.5 Gy on day −1 and were transplanted with 0.5 × 106 CD90.2+ splenic T cells from either syngeneic BALB/c or allogeneic major histocompatibility Ag–mismatched B6 WT or SIRT3−/− mice along with 5 × 106 TCDBM cells from either BALB/c or B6 donors concurrently with syngeneic 1 × 102 P815 tumor cells at the same time of allo-BMT (n = 4–8 mice per group, data are pooled from two experiments). (A) Overall survival. (B) Tumor related mortality. (C) Tumor growth was monitored using bioluminescence imaging on day 14 after allo-BMT (n = 2–5 mice). Representative data from two independent experiments are shown. (D) 51Cr release assay. Splenic T cells were isolated from either B6 WT or SIRT3−/− mice and cocultured with allogeneic splenocytes from BALB/c for 5 d and used as effector T cells against syngeneic MBL-2 or allogeneic P815 target tumor cells. Target killing was measured by release of 51Cr into the supernatant. One representative experiment from three independent experiments is shown. The error bars represent mean ± SEM.
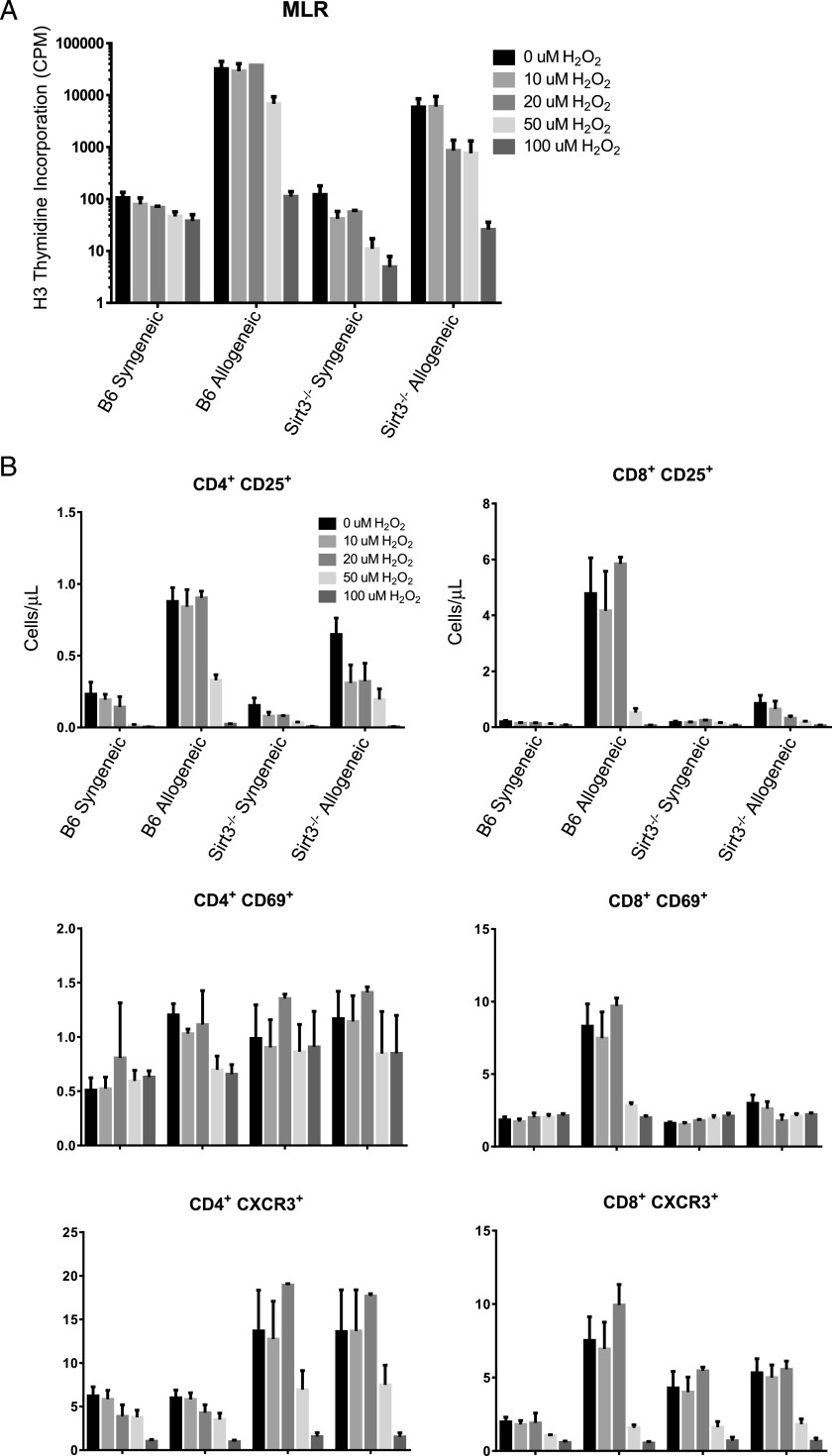
Exogenous ROS does not rescue allogeneic SIRT3−/− T cell proliferation, expression of CD25, CD69, or CXCR3
Mitochondrial-derived ROS are required for T cell activation and proliferation (9, 10, 51). Therefore, we questioned whether the lower levels of ROS in stimulated SIRT3-deficient T cells directly contributed to their deficient proliferation and activation phenotypes or if they were an indirect effect of insufficient T cell activation. To test this, we asked if exogenous ROS in the form of H2O2 could rescue SIRT3−/− T cell proliferation and expression of CD25, CD69, and CXCR3 in an MLR. Varying concentrations of H2O2 spanning the previously reported ranges for its effects on both T cell viability and activation were applied to the MLRs at hour 2 and replenished at hour 48. The lower concentrations of exogenous H2O2 had no effect on WT or SIRT3−/− allogeneic or syngeneic T cell proliferation, whereas the higher concentrations of H2O2 were inhibitory (Fig. 6A). Similarly, exogenous H2O2 failed to increase the absolute number of CD25+, CD69+, or CXCR3+ lymphocytes (Fig. 6B). Altogether, these data suggest that the lower levels of ROS in allogeneic SIRT3−/− T cells were an indirect consequence of blunted T cell activation.
FIGURE 6.
Exogenous ROS does not rescue Sirt3−/− allogeneic T cell proliferation, CD25 expression, CD69 expression, or CXCR3 expression. (A) Negatively selected B6 or Sirt3−/− T cells were cocultured with lethally irradiated (30 Gy) syngeneic (B6) or allogeneic (BALB/c) positively selected nucleated splenocytes for 4 d. Varying concentrations of H2O2 were applied to the MLRs at hour 2 and replenished at hour 48. Proliferation was measured by [3H]thymidine incorporation during the last 6 h. (B) CD25, CD69, and CXCR3 expression in CD4 or CD8 single-positive splenic T cells described in (A) was assessed via flow cytometry after 4 d of coculture. Absolute numbers (cells per microliter) are depicted. Data represent the mean from two independent experiments (n = 4 mice per group), and error bars represent the SEM.
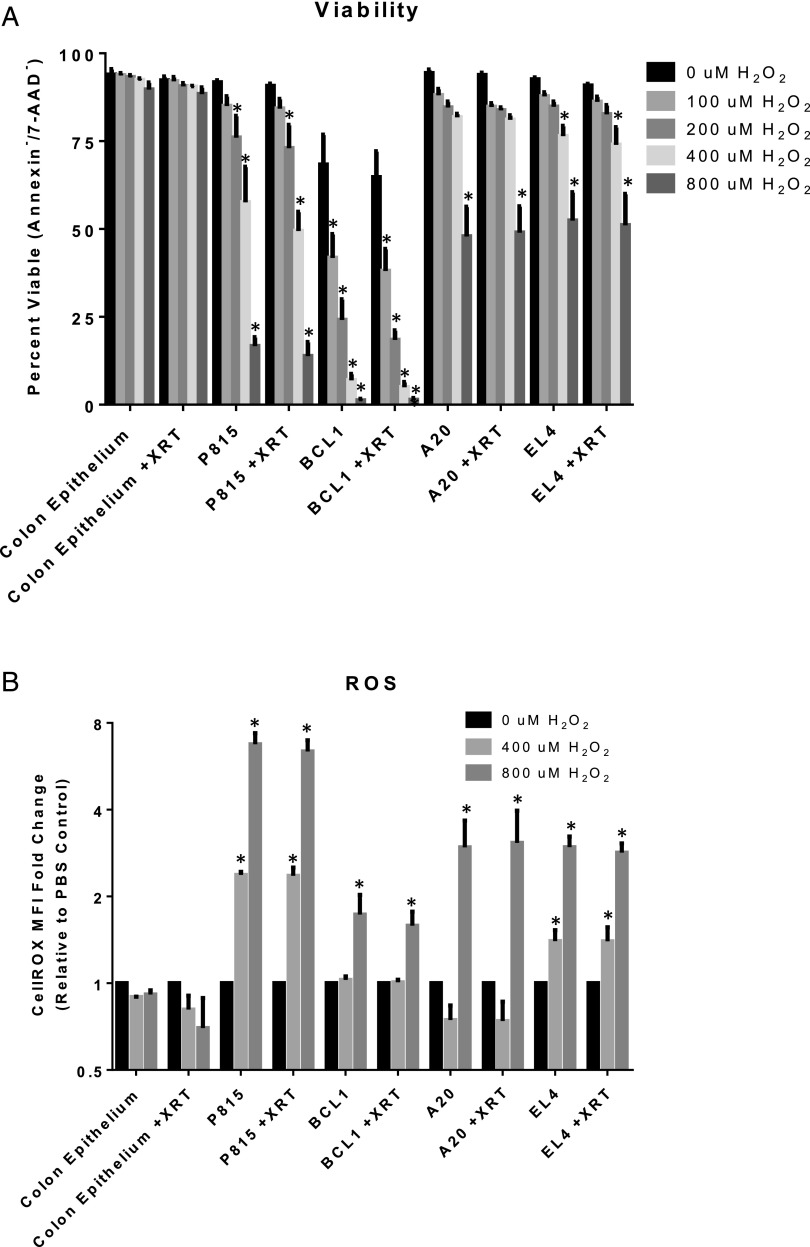
Primary colonic epithelial cells resist exogenous ROS-mediated cell death
Many types of cancer have increased levels of ROS, which often renders them selectively sensitive to exogenous ROS stress (52, 53). Therefore, we wondered if GVHD target tissues were more resistant to exogenous ROS stress than tumor cells lines. If so, this may be one factor contributing to why GVT was preserved and GVHD was attenuated by SIRT3 deficiency in donor T cells whose activation status and ROS levels were blunted. To test this, we used primary colonic epithelial cells as a relevant GVHD target tissue because GI GVHD pathology scores were most affected by loss of SIRT3 in allogeneic T cells (Fig. 3K). As representative tumor cell lines, we chose P815 (mastocytoma), BCL1 (B cell leukemia), A20 (B cell lymphoma), and EL4 (T cell lymphoma) cells and adapted them to the identical media required for primary colonic epithelial cell culture. We then exposed the tumor cell lines and epithelial cells to increasing concentrations of H2O2 and assessed viability by exclusion of annexin V and 7-AAD 20 h later. Interestingly, there was no significant loss of colonic epithelial cell viability even at the highest H2O2 concentration tested (Fig. 7A). In addition, there was no increased sensitivity of the colonic epithelial cells to H2O2 following a noncytotoxic dose of irradiation in an attempt to approximate the conditioning regimen used in our above in vivo experiments. In contrast, all of the tumor cell lines tested showed reduced viability following H2O2 treatment, with the most dramatic loss of viability observed in P815 and BCL1 cells (Fig. 7A). The increased sensitivity of the tumor cell lines to H2O2 was accompanied by a reduced ability to maintain cellular ROS levels following exposure to H2O2, whereas colonic epithelial cells showed no significant changes in cellular ROS levels (Fig. 7B). These data suggest that colonic epithelial cells are better able to maintain cellular ROS homeostasis following an exogenous ROS insult than tumor cell lines, which may contribute to why GVHD was attenuated, yet GVT was preserved by SIRT3−/− allogeneic T cells.
FIGURE 7.
Primary colonic epithelial cells resist exogenous ROS-mediated cell death. (A) Primary BALB/c colonic epithelial cells, P815, BCL1, A20, and EL4 cells were treated with 1 Gy of irradiation or mock irradiated. Two hours later, varying concentrations of H2O2 were applied for 20 h followed by staining for annexin V and 7-AAD and analysis via flow cytometry. Percent viability (Annexin−/7-AAD−) is shown. (B) Cells were treated as described in (A), except 30 min after H2O2 was applied, the cells were stained with 5 μM of CellROX Deep Red and then analyzed via flow cytometry. Fold change from PBS-treated controls is depicted. Data represent the mean from at least three independent experiments, and error bars represent the SEM. *p < 0.05.
Discussion
Allogeneic donor–derived T cells are required for both the beneficial GVT effect and harmful GVHD following allo-HCT (54). Therefore, identifying potential therapeutic strategies that separate the GVT effect from GVHD may improve allo-HCT outcomes and make it widely available. In this study, we found that SIRT3-deficient donor T cells ameliorate GVHD severity and mortality while preserving the GVT effect. Our data suggest that targeting SIRT3 in donor T cells may be a useful strategy for GVHD prevention and treatment.
The influence of SIRTs on T cell functions has been characterized in the context of SIRT1, which regulates T cell activation, proliferation, and tolerance (19–27). Overall, SIRT1 acts to dampen T cell activity, and its absence is associated with increased inflammation and autoimmunity (20, 26). In contrast to the role of SIRT1 in T cells, we demonstrate in this study that SIRT3 augments T cell activation and proliferation but did not substantially affect cytokine production in response to nonspecific TCR signaling or an allogeneic stimulus in vitro. Furthermore, SIRT3 had no impact on ex vivo cytotoxic functions. The activation defect in SIRT3−/− T cells was validated in vivo in response to an allogeneic stimulus, as evidenced by reduced expression of the activation markers CD25 and CXCR3 and lower levels of ROS. Furthermore, this defect in T cell activation correlated with protection from GVHD. The preservation of sufficient GVT might be a consequence of retention of significant cytotoxicity and cytokine secretion functions despite the absence of SIRT3 in T cells. In addition, our data suggest that GVT may also be maintained because of relative differences in susceptibility of tumor cells to residual levels of exogenous ROS. However, the GVT models are subject to limitations, and our results should be interpreted within the context of limitations. Whether SIRT3 is required for nonallogeneic T cell responses in vivo is an intriguing unanswered question.
CXCR3 is an early marker of T cell activation and is also important for the trafficking of mainly CD8+ allogeneic effector T cells to the GI tract (46, 47, 55, 56). In addition, CXCR3 expression in T cells positively correlates with GVHD severity, and inhibiting CXCR3 ameliorates GVHD in an experimental model (47). As would be predicted by its known role for allogeneic T cell trafficking to the GI tract, reduced expression of CXCR3 on allogeneic SIRT3−/− T cells was associated with decreased accumulation of donor T cells in IELs of the GI tract but not in the liver or spleen. The decreased accumulation of SIRT3−/− T cells in IELs of the GI tract also correlated with a decrease of GVHD histopathological damage in the GI tract and to a lesser degree in the liver. All of these observations, including the decreased expression of CD25 in SIRT3−/− T cells, were more pronounced in CD8+ allogeneic T cells, consistent with previous reports showing that CXCR3 mainly augments CD8+ T cell–dependent model of GVHD (46). Nonetheless, to confirm SIRT3 exerts its GVHD-augmenting effect primarily in CD8+ T cells and is dependent on CXCR3 will require further investigation.
SIRT3 is a deacetylase located in the mitochondrial matrix where it regulates cellular metabolism and survival (35). In response to cellular stress, SIRT3 negatively regulates ROS production by enhancing ROS scavenging through the activation of antioxidants (37, 50). Importantly, 90% of cellular ROS are mitochondrial derived, cause mitochondrial damage (57), and mediate allogeneic T cell–dependent GVHD (13, 58). In contrast to these reports showing that SIRT3 negatively regulates ROS production in response to metabolic stress (59), we found that SIRT3 augments ROS production in nonspecific TCR and allostimulated T cells, likely because of incomplete T cell activation resulting in limited ROS production given that exogenous ROS could not complement SIRT3−/− allogeneic T cell activation. It is feasible that SIRT3 contributes to TCR-mediated signaling given that mitochondria are known to translocate to the immunological synapse and that mitochondrial proteins participate in TCR-mediated signaling (9, 60). Importantly, we saw no confounding change in mitochondrial abundance or fatty acid transport in SIRT3-deficient allogeneic T cells as alternative explanations for their decreased levels of ROS. Certainly, SIRT3’s role in TCR-mediated signaling and T cell activation will need further validation and additional studies to elucidate the molecular mechanisms involved.
Our and others’ data suggest that SIRT3 regulates ROS production in a tissue and context-dependent fashion. For instance, SIRT3 in models of systemic sclerosis decreases ROS, resulting in less skin and lung fibrosis, which may also prove relevant for chronic GVHD pathogenesis given its similarities with systemic sclerosis (61). In contrast to this and consistent with our observation of SIRT3 augmenting ROS production, K562 leukemic cells require SIRT3 for optimal ROS production (62). Hence, the regulation of ROS by SIRT3 appears more complicated than uniform induction of anti-oxidant mechanisms.
We and others previously showed that HDAC inhibition mitigates GVHD by potentially modulating IDO expression in DCs (63, 64), reducing activated T cell function (65) through HDAC6 inhibition (66), and/or increasing Treg expansion (67). Because of this, it is tempting to speculate that because SIRT3 possesses deacetylase activity (35), the GVHD-inhibiting effect of allogeneic SIRT3−/− T cells may approximate that of HDAC inhibitors (63, 64). However, the HDAC inhibitors that were used inhibit classes I, II, and IV but do not affect class III deacetylases, such as SIRTs. Thus, the impact of SIRT3 is independent of the effects observed with previous reports of HDAC inhibitors on Treg cells. Furthermore, our in vitro data suggest that SIRT3 had no impact on Treg suppressive functions. However, whether the deacetylase activity of SIRT3 is required for augmentation of T cell activation or GVHD and what the relevant deacetylated protein targets are for these processes will need further investigation.
In conclusion, our data demonstrate several novel features. We demonstrate that SIRT3 positively regulates T cell functions after allo-HCT enough to partially mitigate GVHD but not GVT. The effects are not because of similar effects noted from HDAC inhibitor studies as these drugs do not affect the deacetylase activity of SIRT3. In addition, our data highlight the differing effects of SIRT3 on T cell function with regards to its reported effects in other cells and tissues in its ability to regulate ROS. Our data suggest SIRT3 in donor T cells as a potential novel target for improving outcomes after allo-HCT.
Supplementary Material
This work was supported by National Institutes of Health Grants HL090775, CA173878, HL128046, and CA203542 (to P.R.).
The online version of this article contains supplemental material.
- allo-BMT
- allogeneic BMT
- allo-HCT
- allogeneic hematopoietic cell transplantation
- BM
- bone marrow
- BMDC
- BM-derived DC
- BMT
- BM transplantation
- DC
- dendritic cell
- GI
- gastrointestinal
- GVHD
- graft-versus-host disease
- GVT
- graft-versus-tumor
- HDAC
- histone deacetylase
- IEL
- intraepithelial lymphocyte
- ROS
- reactive oxygen species
- SIRT
- sirtuin
- SLEC
- short-lived effector T cell
- TCDBM
- T cell–depleted BM
- Treg
- regulatory T
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Gooley T. A., Chien J. W., Pergam S. A., Hingorani S., Sorror M. L., Boeckh M., Martin P. J., Sandmaier B. M., Marr K. A., Appelbaum F. R., et al. 2010. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 363: 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R., Blazar B. R. 2017. Acute graft-versus-host disease - biologic process, prevention, and therapy. N. Engl. J. Med. 377: 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korngold R., Sprent J. 1978. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J. Exp. Med. 148: 1687–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlomchik W. D. 2007. Graft-versus-host disease. Nat. Rev. Immunol. 7: 340–352. [DOI] [PubMed] [Google Scholar]
- 5.Storb R., Gyurkocza B., Storer B. E., Sorror M. L., Blume K., Niederwieser D., Chauncey T. R., Pulsipher M. A., Petersen F. B., Sahebi F., et al. 2013. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 31: 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald K. P., Shlomchik W. D., Reddy P. 2013. Biology of graft-versus-host responses: recent insights. Biol. Blood Marrow Transplant. 19: S10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox C. J., Hammerman P. S., Thompson C. B. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 5: 844–852. [DOI] [PubMed] [Google Scholar]
- 8.Verbist K. C., Wang R., Green D. R. 2012. T cell metabolism and the immune response. Semin. Immunol. 24: 399–404. [DOI] [PubMed] [Google Scholar]
- 9.Sena L. A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D. A., Wang C. R., Schumacker P. T., Licht J. D., Perlman H., et al. 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchina D. G., Dostert C., Brenner D. 2018. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol. 39: 489–502. [DOI] [PubMed] [Google Scholar]
- 11.Glick G. D., Rossignol R., Lyssiotis C. A., Wahl D., Lesch C., Sanchez B., Liu X., Hao L. Y., Taylor C., Hurd A., et al. 2014. Anaplerotic metabolism of alloreactive T cells provides a metabolic approach to treat graft-versus-host disease. J. Pharmacol. Exp. Ther. 351: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen H. D., Chatterjee S., Haarberg K. M., Wu Y., Bastian D., Heinrichs J., Fu J., Daenthanasanmak A., Schutt S., Shrestha S., et al. 2016. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J. Clin. Invest. 126: 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatza E., Wahl D. R., Opipari A. W., Sundberg T. B., Reddy P., Liu C., Glick G. D., Ferrara J. L. 2011. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl. Med. 3: 67ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byersdorfer C. A., Tkachev V., Opipari A. W., Goodell S., Swanson J., Sandquist S., Glick G. D., Ferrara J. L. 2013. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood 122: 3230–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raha S., Raud B., Oberdörfer L., Castro C. N., Schreder A., Freitag J., Longerich T., Lochner M., Sparwasser T., Berod L., et al. 2016. Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur. J. Immunol. 46: 2233–2238. [DOI] [PubMed] [Google Scholar]
- 16.Yang X. J., Seto E. 2008. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonnell E., Peterson B. S., Bomze H. M., Hirschey M. D. 2015. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol. Metab. 26: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkel T., Deng C. X., Mostoslavsky R. 2009. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preyat N., Leo O. 2013. Sirtuin deacylases: a molecular link between metabolism and immunity. J. Leukoc. Biol. 93: 669–680. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Lu Y., Zhang Z., Wang J., Yang H., Liu G. 2015. Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology 145: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Lee S. M., Shannon S., Gao B., Chen W., Chen A., Divekar R., McBurney M. W., Braley-Mullen H., Zaghouani H., Fang D. 2009. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 119: 3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuroda S., Yamazaki M., Abe M., Sakimura K., Takayanagi H., Iwai Y. 2011. Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proc. Natl. Acad. Sci. USA 108: 14885–14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong S., Kim S. J., Sandal B., Lee S. M., Gao B., Zhang D. D., Fang D. 2011. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J. Biol. Chem. 286: 16967–16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier U. H., Wang L., Han R., Akimova T., Liu Y., Hancock W. W. 2012. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci. Signal. 5: ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao B., Kong Q., Kemp K., Zhao Y. S., Fang D. 2012. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc. Natl. Acad. Sci. USA 109: 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuprin A., Avin A., Goldfarb Y., Herzig Y., Levi B., Jacob A., Sela A., Katz S., Grossman M., Guyon C., et al. 2015. The deacetylase Sirt1 is an essential regulator of Aire-mediated induction of central immunological tolerance. Nat. Immunol. 16: 737–745. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Bi Y., Chen X., Li C., Li Y., Zhang Z., Wang J., Lu Y., Yu Q., Su H., et al. 2016. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4(+) T cells. Immunity 44: 1337–1349. [DOI] [PubMed] [Google Scholar]
- 28.Choi S., Reddy P. 2011. HDAC inhibition and graft versus host disease. Mol. Med. 17: 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S. W., Braun T., Chang L., Ferrara J. L., Pawarode A., Magenau J. M., Hou G., Beumer J. H., Levine J. E., Goldstein S., et al. 2014. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 15: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S. W., Gatza E., Hou G., Sun Y., Whitfield J., Song Y., Oravecz-Wilson K., Tawara I., Dinarello C. A., Reddy P. 2015. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood 125: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwer B., North B. J., Frye R. A., Ott M., Verdin E. 2002. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 158: 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finley L. W., Carracedo A., Lee J., Souza A., Egia A., Zhang J., Teruya-Feldstein J., Moreira P. I., Cardoso S. M., Clish C. B., et al. 2011. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 19: 416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn B. H., Kim H. S., Song S., Lee I. H., Liu J., Vassilopoulos A., Deng C. X., Finkel T. 2008. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 105: 14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallows W. C., Lee S., Denu J. M. 2006. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. USA 103: 10230–10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombard D. B., Alt F. W., Cheng H. L., Bunkenborg J., Streeper R. S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., et al. 2007. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27: 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., et al. 2010. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu X., Brown K., Hirschey M. D., Verdin E., Chen D. 2010. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12: 662–667. [DOI] [PubMed] [Google Scholar]
- 38.Shimazu T., Hirschey M. D., Hua L., Dittenhafer-Reed K. E., Schwer B., Lombard D. B., Li Y., Bunkenborg J., Alt F. W., Denu J. M., et al. 2010. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 12: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallows W. C., Yu W., Smith B. C., Devries M. K., Ellinger J. J., Someya S., Shortreed M. R., Prolla T., Markley J. L., Smith L. M., et al. 2011. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. [Published erratum appears in 2011 Mol. Cell. 41: 493.] Mol. Cell 41: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman J. C., He W., Verdin E. 2012. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J. Biol. Chem. 287: 42436–42443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooke K. R., Kobzik L., Martin T. R., Brewer J., Delmonte J., Jr., Crawford J. M., Ferrara J. L. 1996. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 88: 3230–3239. [PubMed] [Google Scholar]
- 42.Tawara I., Liu C., Tamaki H., Toubai T., Sun Y., Evers R., Nieves E., Mathewson N., Nunez G., Reddy P. 2013. Influence of donor microbiota on the severity of experimental graft-versus-host-disease. Biol. Blood Marrow Transplant. 19: 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buler M., Aatsinki S. M., Izzi V., Hakkola J. 2012. Metformin reduces hepatic expression of SIRT3, the mitochondrial deacetylase controlling energy metabolism. PLoS One 7: e49863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beier U. H., Angelin A., Akimova T., Wang L., Liu Y., Xiao H., Koike M. A., Hancock S. A., Bhatti T. R., Han R., et al. 2015. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 29: 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edinger M., Hoffmann P., Ermann J., Drago K., Fathman C. G., Strober S., Negrin R. S. 2003. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 46.Duffner U., Lu B., Hildebrandt G. C., Teshima T., Williams D. L., Reddy P., Ordemann R., Clouthier S. G., Lowler K., Liu C., et al. 2003. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp. Hematol. 31: 897–902. [DOI] [PubMed] [Google Scholar]
- 47.He S., Cao Q., Qiu Y., Mi J., Zhang J. Z., Jin M., Ge H., Emerson S. G., Zhang Y., Zhang Y. 2008. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J. Immunol. 181: 7581–7592. [DOI] [PubMed] [Google Scholar]
- 48.Jing E., Emanuelli B., Hirschey M. D., Boucher J., Lee K. Y., Lombard D., Verdin E. M., Kahn C. R. 2011. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 108: 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raimundo N. 2014. Mitochondrial pathology: stress signals from the energy factory. Trends Mol. Med. 20: 282–292. [DOI] [PubMed] [Google Scholar]
- 50.Tao R., Coleman M. C., Pennington J. D., Ozden O., Park S. H., Jiang H., Kim H. S., Flynn C. R., Hill S., Hayes McDonald W., et al. 2010. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 40: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belikov A. V., Schraven B., Simeoni L. 2015. T cells and reactive oxygen species. J. Biomed. Sci. 22: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trachootham D., Alexandre J., Huang P. 2009. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8: 579–591. [DOI] [PubMed] [Google Scholar]
- 53.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P. J., Achanta G., Arlinghaus R. B., Liu J., Huang P. 2006. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10: 241–252. [DOI] [PubMed] [Google Scholar]
- 54.Ferrara J. L., Levine J. E., Reddy P., Holler E. 2009. Graft-versus-host disease. Lancet 373: 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groom J. R., Richmond J., Murooka T. T., Sorensen E. W., Sung J. H., Bankert K., von Andrian U. H., Moon J. J., Mempel T. R., Luster A. D. 2012. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabin R. L., Alston M. A., Sircus J. C., Knollmann-Ritschel B., Moratz C., Ngo D., Farber J. M. 2003. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J. Immunol. 171: 2812–2824. [DOI] [PubMed] [Google Scholar]
- 57.Balaban R. S., Nemoto S., Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120: 483–495. [DOI] [PubMed] [Google Scholar]
- 58.Tkachev V., Goodell S., Opipari A. W., Hao L. Y., Franchi L., Glick G. D., Ferrara J. L., Byersdorfer C. A. 2015. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J. Immunol. 194: 5789–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown K., Xie S., Qiu X., Mohrin M., Shin J., Liu Y., Zhang D., Scadden D. T., Chen D. 2013. SIRT3 reverses aging-associated degeneration. Cell Rep. 3: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quintana A., Schwindling C., Wenning A. S., Becherer U., Rettig J., Schwarz E. C., Hoth M. 2007. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl. Acad. Sci. USA 104: 14418–14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akamata K., Wei J., Bhattacharyya M., Cheresh P., Bonner M. Y., Arbiser J. L., Raparia K., Gupta M. P., Kamp D. W., Varga J. 2016. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7: 69321–69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang Y., Wang J., Xu L., Cao Y., Xu F., Yan L., Nie M., Yuan N., Zhang S., Zhao R., et al. 2016. Autophagy maintains ubiquitination-proteasomal degradation of Sirt3 to limit oxidative stress in K562 leukemia cells. Oncotarget 7: 35692–35702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy P., Maeda Y., Hotary K., Liu C., Reznikov L. L., Dinarello C. A., Ferrara J. L. 2004. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl. Acad. Sci. USA 101: 3921–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reddy P., Sun Y., Toubai T., Duran-Struuck R., Clouthier S. G., Weisiger E., Maeda Y., Tawara I., Krijanovski O., Gatza E., et al. 2008. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Invest. 118: 2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N., Zhao D., Kirschbaum M., Zhang C., Lin C. L., Todorov I., Kandeel F., Forman S., Zeng D. 2008. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc. Natl. Acad. Sci. USA 105: 4796–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuji G., Okiyama N., Villarroel V. A., Katz S. I. 2015. Histone deacetylase 6 inhibition impairs effector CD8 T-cell functions during skin inflammation. J. Allergy Clin. Immunol. 135: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lucas J. L., Mirshahpanah P., Haas-Stapleton E., Asadullah K., Zollner T. M., Numerof R. P. 2009. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell. Immunol. 257: 97–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.