Abstract
Introduction
Heart failure is associated with recurrent hospitalizations and high mortality. Guideline directed medical treatment (GDMT), including beta blockers (BBs), angiotensin converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs) and aldosterone antagonists (AAs) has shown to improve outcomes. Current guidelines recommend the use of these medication classes at maximally tolerated dosages. Despite the evidence, < 25% of patients with heart failure with reduced left ventricular ejection fraction (HFrEF) are on the appropriate medical regimen titrated to the target doses. As such, we sought to assess the utility of a focused GDMT clinic to reduce this gap.
Methods
We conducted a retrospective chart review through existing patient data in a single center teaching hospital of patients referred to a focused GDMT clinic primarily staffed with heart failure trained nurse specialists, physician assistants and cardiologists. Management guidelines were developed with protocols for the initiation and uptitration of all therapeutic agents considered as GDMT.
Our primary objective was to determine whether enrollment into a dedicated nursing led guideline directed medical therapy clinic would increase the proportion of patients with heart failure with reduced ejection fraction on appropriate medications as well as medication dosages in patients, the percentage of patients on the following medications and percentage at target doses: Renin-Angiotensin-Aldosterone System Blockers, Evidence Based Beta Blockers, and Aldosterone Antagonists. Our secondary objective was to determine if there was any clinical benefit on objective measures including renal function, hospital admissions, mortality and implantable defibrillator shocks.
Results
Between October 2015 and March 2017, 63 patients were identified by requisition forms, in which 61 were able to be identified based on legibility of identifying information. Mean duration of follow up was 264.44 ± 162.68 days over 7 ± 3.94 days. Mean ejection fraction was 21.8 ± 7.3%. New onset cardiomyopathies (diagnosed within 30 days) compiled 21% of the patient population while those with demonstrated cardiomyopathies (> 90 days) compiled 48% of the patient population. Patients with NYHA class III heart failure compiled 65% of the patient population.
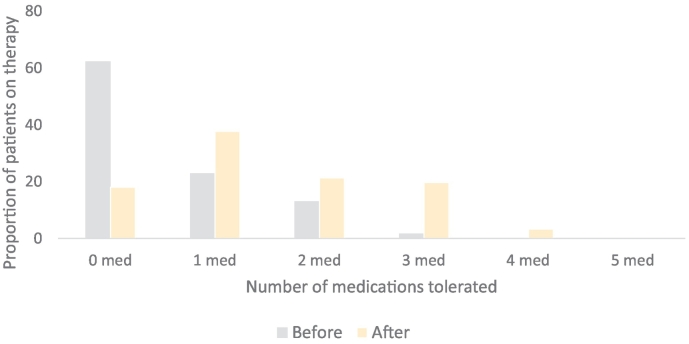
There was a statistically significant increase in the mean number of GDMT at any dose (2.31 ± 0.76 to 2.74 ± 0.66; p < 0.001) and mean number of GDMT at target doses (0.54 ± 0.79 to 1.52 ± 1.1; p < 0.001). Percentage of the population that were on no target doses at initial visit was 62% which was reduced to 18% after intervention.
Clinical improvement was reflected in significant improvement in ejection fraction from 21.8 ± 7.8% to 36.2 ± 14.3% (p < 0.001). Increases in sodium and chloride were statistically small but significant. There a significant reduction in heart failure hospitalizations in comparison to a year prior to after the initial encounter in the clinic (p < 0.001).
Conclusion
This pilot study showed that a nurse directed GDMT titration program successfully increased the number of GDMT that patients were able to tolerate in a timely fashion, all the while enhancing ejection fraction, sodium and chloride levels, with a reduction in rehospitalization rates.
1. Background
Heart failure is associated with recurrent hospitalizations and high mortality. For patients with heart failure with reduced ejection fraction (HFrEF), defined as an ejection fraction less than or equal to 40%, guideline directed medical therapy (GDMT) has been shown to improve morbidity and mortality. The American College of Cardiology (ACC), American Heart Association's(AHA), and Heart Failure Society of America (HFSA) joint guidelines recommend that patients with HFrEF be treated with maximally tolerated doses of appropriate neurohormonal blockers [1,2]. Despite overwhelmingly positive evidence [3,4,5,6,7,8,9,10], <25% of patients with HFrEF are on the appropriate target doses of medical therapy [11].
In the outpatient setting, optimization of GDMT is primarily performed by physicians (cardiologists or primary care providers) typically resulting in delayed optimization due to relatively infrequent visits and other challenges such as lab, blood pressure monitoring, and minor symptoms [12,13,14]. Thus, clinical inertia can be a significant barrier to optimizing vital therapy for patients with HFrEF. Several studies have investigated the utility of nurse led titration of GDMT demonstrating increased utilization rates and an improved proportion of patients on target doses [15,16,17,18]. However, the majority of these have either been conducted outside the US and/or are very small in scale.
As such, we sought to assess the utility of a focused, nurse driven, GDMT clinic to reduce this gap in a typical clinical system of care in the United States.
2. Methods
2.1. Study design
This was a retrospective cohort study conducted at a single tertiary care center between October 2015 and December 2017. Our primary objective was to determine whether enrollment into a dedicated nursing led GDMT clinic would increase adherence to target doses of GDMT prescribed by the referring physician. Renin-Angiotensin-Aldosterone System Blockers, Evidence Based Beta Blockers, and Aldosterone Antagonists were all evaluated. Our secondary objective was to determine if there was any clinical benefit on objective measures including renal function, hospital admissions, mortality and implantable defibrillator shocks.
Primary objective comparisons were made between the time of the initial visit and final visit at the GDMT clinic. Secondary objective comparisons were made within 1 year prior to their initial visit to 1 year after their final visit.
2.2. Guideline directed medical therapy clinic
The GDMT clinic was established at St Francis Hospital and Medical Center in Hartford, Connecticut in late 2015. This clinic is staffed with heart failure trained nurse specialists and physician assistants. A heart failure cardiologist provides oversight. Management guidelines were developed with protocols for the initiation and up-titration of all therapeutic agents recommended by the ACC/AHA/HFSA guideline statement [1,2]. All patients who were referred for GDMT titration with an ejection fraction of 40% or less were evaluated in this study between October 2015 and March 2016. Follow up data was obtained up to November 2017.
Inclusion for the initiation of angiotensin receptor/neprilysin inhibitors (ARNI), angiotensin converting enzyme inhibitor (ACE-I), or angiotensin receptor blocker (ARB) necessitated blood work within 3 days of initiation, a systolic blood pressure >90 mm Hg, no evidence of moderate or severe hepatic dysfunction according to the Child-Pugh classification, not taking Aliskerin, consent for frequent blood draws and follow ups, and a negative pregnancy test if appropriate.
If the referring practitioner ordered ARNI initiation and/or titration, and if the patient presented on an ACE-I, this was held for at least 36 h prior to ARNI initiation. After initiation of the ARNI, ARB or ACE-I, blood work was obtained per protocol within 7 days. Follow up was established within 2 weeks and, given hemodynamic and renal stability, the ARNI, ARB or ACE-I was further up-titrated to the next progressive dose as tolerated (Fig. S3). Target doses of a particular medication was based on ACC/AHA/HFSA guidelines (Fig. S1) [1,2].
Inclusion for aldosterone antagonist (AA) therapy initiation included a serum creatinine of <2.0 mg/dL in women and 2.5 mg/dL in men, baseline serum potassium <5 mEq/L, systolic blood pressure >90 mm Hg, and consent for frequent blood draws and follow up. Follow up was typically established within 2 weeks and if stable, the aldosterone antagonist was up-titrated if necessary. Routine lab follow up was performed per protocol and was based on protocols utilized in the major clinical trials in this area (Fig. S2) [9,10].
Beta blocker (BB) therapy was also managed per protocol. The specific evidence based BB was used as directed by the ordering physician. Protocol for medication titration is shown (Fig. S3).
Patients who met inclusion criteria were followed every two weeks to assess eligibility for medication uptitration, and repeat laboratory assessments. Patients not able to tolerate further medication uptitration.
2.3. Data collection
All patients who were referred to the GDMT clinic between October 2015 and March 2016 were evaluated using a retrospective chart review. All clinical data were entered into the electronic medical record at the time of each patient visit. Patient care documentation between October 2015 and November 2017 were reviewed. Patient data was de-identified for data collection and stored in a password protected, encrypted database. A separate password protected, encrypted file, stored in a different location, acted to link subject numbers to medical record numbers.
2.4. Data analysis
Each variable was summarized by mean and standard deviation or frequencies and percentages at each time point. The before-and-after comparisons were conducted by Wilcoxon signed-rank test for continuous variables and by Mc-Nemar's test for categorical variables. A p-value smaller than 0.05 was considered statistically significant. All the statistical analyses were performed in R 3.4.3.
3. Results
3.1. Patient characteristics
Between October 2015 and March 2017, 61 patients were referred to the GDMT Clinic. Of those patients, all but 2 maintained adequate follow up. Patient characteristics are outlined in Table 1. The mean duration of follow up was 264.44 ± 162.68 days. At baseline, the mean ejection fraction was 21.8 ± 7.3%. New onset cardiomyopathies (diagnosed within 30 days) represented 21% of the patient population while those with chronic heart failure (>90 days) comprised 48% of the patient population. Of patients with chronic heart failure, 53% had been on no target doses of GDMT. Most patients were NYHA class II (33%) or III (65%) at time of referral.
Table 1.
Patient characteristics.
| Population size (N) | 61 |
| Age (yrs, mean ± SD) | 58.7 ± 14.9 |
| Gender | |
| Male | 39 (64) |
| Female | 22 (36) |
| Ethnicity | |
| Caucasian | 28 (46) |
| African American | 20 (33) |
| Hispanic | 13 (21) |
| NYHA Class | |
| I | 1 (2) |
| II | 20 (33) |
| III | 40 (65) |
| Mean ejection fraction (%, mean ± SD) | 21.8 ± 7.3 |
| Duration of heart failure diagnosis | |
| < 30 days | 13 (21) |
| 31–60 days | 14 (23) |
| 61–90 days | 4 (7) |
| 91–180 days | 7 (11) |
| 181–360 days | 7 (11) |
| 361–720 days | 7 (11) |
| > 720 days | 9 (15) |
| Non ischemic cardiomyopathy | 41 (67) |
| Ischemic cardiomyopathy | 20 (33) |
| Diabetes | 19 (31) |
| Hypertension | 46 (75) |
| Hyperlipidemia | 37 (61) |
| Smoker | 35 (57) |
| Coronary artery disease | 29 (48) |
| History of percutaneous intervention | 12 (20) |
| History of coronary bypass surgery | 8 (13) |
| Anemia; hemoglobin <12 mg/dL | 9 (15) |
| Atrial fibrillation/flutter | 20 (33) |
| Chronic kidney disease | 15 (25) |
| COPD/asthma | 7 (11) |
| Implantable defibrillator | 16 (26) |
| Cardiac resynchronization therapy | 15 (25) |
| Cerebrovascular accident | 5 (8) |
| Peripheral vascular disease | 6 (10) |
| Depression | 10 (16) |
3.2. Treatment
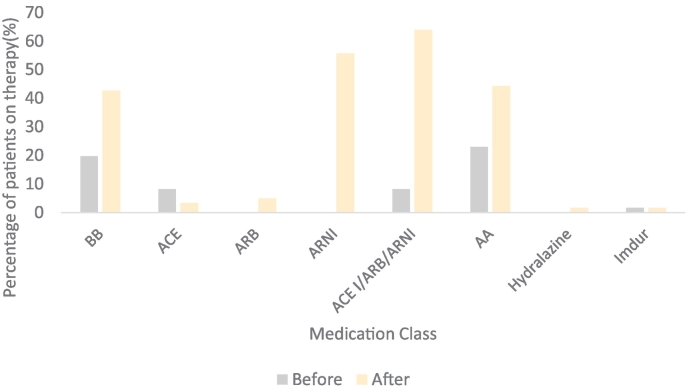
There was a statistically significant increase in the mean number of GDMT agents used by patients at any dose (2.31 ± 0.76 to 2.74 ± 0.66; p:<0.001) and mean number of GDMT agents used at target doses (0.54 ± 0.79 to 1.52 ± 1.1; p: <0.001) (Table 2 and Fig. 1). There was a significant increase in utilization of ARNI and AA with a concurrent decrease in utilization of ACE-I (Fig. 2). Reasons for failure to up-titrate GDMT are outlined in Table S1 in the supplemental content.
Table 2.
Clinical findings and treatment: initial visit versus most recent follow-up.
| Initial visit | Follow up | P value | ||
|---|---|---|---|---|
| GDMT | Beta blocker | 58 (95) | 61 (100) | 0.248 |
| ACE I | 34 (55) | 9 (15) | <0.001 | |
| ARB | 11 (18) | 4 (7) | 0.070 | |
| ARNI | 8 (13) | 47 (77) | <0.001 | |
| ACE I/ARB/ARNI | 53 (86) | 60 (98) | 0.046 | |
| Aldactone | 34 (56) | 38 (63) | 0.009 | |
| Imdur | 2 (3) | 2 (3) | 1.000 | |
| Hydralazine | 2 (3) | 4 (7) | 0.480 | |
| Total number of GDMT | 2.31 ± 0.76 | 2.74 ± 0.66 | <0.001 | |
| Target GDMT doses | 0.54 ± 0.79 | 1.52 ± 1.10 | <0.001 | |
| NYHA class | I | 2 | 4 | 0.528 |
| II | 23 | 27 | ||
| III | 34 | 26 | ||
| IV | 2 | 1 | ||
| Ejection fraction (%) | 21.8 ± 7.8 | 36.2 ± 14.3 | <0.001 | |
| Hemodynamics | Heart rate | 75 ± 12 | 76 ± 11 | 0.965 |
| Systolic BP | 117 ± 15 | 115 ± 14 | 0.179 | |
| Diastolic BP | 70 ± 10 | 68 ± 10 | 0.388 | |
| Biochemistry | Sodium | 137 ± 2.6 | 138 ± 1.8 | 0.032 |
| Chloride | 101.97 ± 3.95 | 102.84 ± 3.30 | 0.011 | |
| Potassium | 4.2 ± 0.4 | 4.3 ± 0.3 | 0.378 | |
| Creatinine | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.937 | |
| Hospitalizations (1 year before and after initial Encounter) | Heart failure | 26 | 8 | <0.001 |
| Renal failure | 0 | 3 | 0.902 | |
| ICD shock | 2 | 2 | 0.178 | |
| Death | 0 | 3 | NA |
Bold lettering indicates significant p values.
Fig. 1.
Proportion of patients tolerating medical therapy at maximal doses before and after nursing directed up titration.
Fig. 2.
Proportion of patients tolerating medical therapy by class at maximal dosage before and after nursing directed up titration.
3.3. Clinical hemodynamic and biochemical changes
Clinical improvement was reflected in significant improvement in ejection fraction (p: <0.001), sodium and chloride.
3.4. Mortality, hospitalizations and ICD therapies
Comparing the year prior to the subsequent time period, there was a significant reduction in heart failure hospitalizations (p < 0.001).
There were a total of 3 deaths during the study. One patient passed away from complications associated with ischemic colitis. Two patients proceeded to stage D heart failure and were transitioned into hospice.
There was no significant difference in the number of ICD shocks before and after the intervention. Among patients with ICDs, there was no difference in mortality, or hospitalizations.
4. Discussion
The goal of this study was to evaluate a simple strategy designed to augment therapy in patients that have HFrEF in the transition and chronic phases of their disease. We were able to demonstrate that a nurse directed GDMT titration program effectively improved adherence to ACC/AHA/HFSA mandated target GDMT in a selected group of patients with HFrEF. In conjunction, we observed an improvement in ejection fraction, sodium and chloride levels, along with a favorable re-hospitalization rate. We were able to recognize patients under-treated for their cardiomyopathy, in addition to patients with advanced diseases states who were appropriately referred for advanced heart failure consultation.
Heart Failure (HF) care, whether in the acute setting, during transitions, or in the chronic phase, is a major focus of efforts to reduce health care costs in many health care systems. Since the passage of the Affordable Care Act (ACA) in 2012, and the Hospital Readmission Reduction Program (HRRP), there has been a concerted effort in the Quality Improvement (QI) arena focused on reduction of 30-day and 90-day HF readmissions. From 2008 through 2014, among the three top diagnoses targeted by the HRRP including acute myocardial infarction, HF, and pneumonia, HF had the highest number of hospitalizations, at almost 3 million within the Medicare fee-for-service population, over 65 years of age [19]. HF also had the highest rate of 30-day readmissions at 23.5% in the same time period [19].
Unfortunately, there continues to be an underutilization of appropriate GDMT in patients with HF with systolic dysfunction despite well-validated guidelines. This pattern appears to be systemic even among general cardiologists. Throughout the United Kingdom, Netherlands, and Sweden, specialist HF clinics have been widely implemented yet clinical evidence of effectiveness of these types of clinics to enhance GDMT has been sparse [20].
Specialty trained nurse driven protocols for the up-titration and maintenance of beta blocker dosing in the heart failure population has shown superior performance when compared to usual care [15]. These successes are not limited to beta blockers, as similar success has been seen with angiotensin-converting enzyme inhibitors and angiotensin receptor blocking agents [16,17,18]. There is a suggestion within the literature that nurse directed titration of neuro-hormonal blocking agents in patients with heart failure with reduced ejection fraction results in fewer hospital admissions and improved mortality [16].
A patient with HFrEF requires close follow up for titration of medications as hemodynamics, electrolytes and symptoms permit. Although the benefits of GDMT have been studied extensively, there remains a significant gap in its usage on a population level.
5. Limitations of this study
This is a retrospective, observational study and thus provides only descriptive data. The demographic and risk factors of the patient population are fairly typical, although our population was relatively young with a preponderance of males. There was also a high incidence of non-ischemic cardiomyopathy in our cohort. The overall sample size was small.
Conclusion.
This study showed that a nurse directed GDMT titration program successfully increased the number of GDMT that patients were able to tolerate in a timely fashion, all the while enhancing ejection fraction, sodium and chloride levels, with a reduction in rehospitalization rates.
This model of care delivery for these high-risk patients should prove to be an effective method for improvement in adherence to guideline directed therapies which, in turn, may result in improvement in clinical outcomes, reduction in hospitalization, and an overall decrease in healthcare cost. We suggest systems of care consider this strategy to improve all care for patients with heart failure.
6. Clinical perpectives
6.1. Clinical competency in patient care
The heart failure patient is often sub optimally treated with target doses of guideline directed medical therapy (GDMT), but through a focused nurse led GDMT clinic, this deficiency can be reduced and thus improve patient care.
6.2. Clinical competency in systems-based practice
The GDMT clinic utilizes a stream lined protocol for medication titration, which allows patients to be timely optimized on appropriate therapy.
6.3. Translational outlook
Further evaluation of the efficacy of a GDMT clinic must be investigated in a prospective randomized fashion.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2018.10.003.
Appendix A. Supplementary data
Supplementary material
References
- 1.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., Johnson M.R., Kasper E.K., Levy W.C., Masoudi F.A., McBride P.E., McMurray J.J.V., Mitchell J.E., Peterson P.N., Riegel B., Sam F., Stevenson L.W., Tang W.W.H., Tsai E.J., Wilkoff B.L. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2013;62(16) doi: 10.1016/j.jacc.2013.05.019. 2013 Oct 15. (e147-239) [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Colvin M.M., Drazner M.H., Filippatos G.S., Fonarow G.C., Givertz M.M., Hollenberg S.M., Lindenfeld J., Masoudi F.A., McBride P.E., Peterson P.N., Stevenson L.W., Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 3.CIBIS-II Writers The cardiac insufficiency Bisoprolol study II (CIBIS-II): a randomized trial. Lancet. 1999;353(9145):9–13. [PubMed] [Google Scholar]
- 4.Cohn J.N., Togoni G., on behalf of Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 5.MERIT-HF Study Group Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERITHF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 6.Packer M., Bristow M.R., Cohn J.N., Colucci W.S., Fowler M.B., Gilbert E.M. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 7.Packer M., Fowler M.B., Roecker E.B., Coats A.J., Katus H.A., Krum H. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 8.Granger C.B., McMurray J.J., Yusuf S., Held P., Michelson E.L., Olofsson B., Ostergren J. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. 2003;362(9386):772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B., Zannad F., Remme W.J., Cody R., Castaigne A., Perez A., Palensky J., Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 10.Zannad F., McMurray J.J., Krum H., van Veldhuisen D.J., Swedberg K., Shi H., Vincent J. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 11.Komajda M., Anker S.D., Cowie M.R., Filippatos G.S., Mengelle B., Ponikowski P., Tavazzi L. Physicians' adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from QUALIFY global survey. Eur. J. Heart Fail. 2016;18(5):514–522. doi: 10.1002/ejhf.510. [DOI] [PubMed] [Google Scholar]
- 12.Phillips S.M., Marton R.L., Tofler G.H. Barriers to diagnosing and managing heart failure in primary care. Med. J. Aust. 2004;181(2):78–81. doi: 10.5694/j.1326-5377.2004.tb06178.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt A.S., Devore A.D., Dewald T.A., Swedberg K., Mentz R.J. Achieving a maximally tolerated beta-blocker dose in heart failure patients: is there room for improvement? J. Am. Coll. Cardiol. 2017;69(20):2542–2550. doi: 10.1016/j.jacc.2017.03.563. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow G.C., Ziaeian B. Gaps in adherence to guideline-directed medical therapy before defibrillator implantation. J. Am. Coll. Cardiol. 2016;67(9):1070–1073. doi: 10.1016/j.jacc.2015.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driscoll A., Srivastava P., Toia D., Gibcus J., Hare D.L. A nurse-led up-titration clinic improves chronic heart failure optimization of beta-adrenergic receptor blocking therapy—a randomized controlled trial. BMC Res Notes. 2014;7 doi: 10.1186/1756-0500-7-668. (668-0500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driscoll A., Currey J., Tonkin A.M. Nurse-led titration of angiotensin-converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2016;1(7):842–843. doi: 10.1001/jamacardio.2016.2332. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll A., Currey J., Tonkin A., Krum H. Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction. Cochrane Database Syst. Rev. 2015;12 doi: 10.1002/14651858.CD009889.pub2. (CD009889. doi(12):CD009889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain A., Mills P., Nunn L.M., Butler J., Luddington L., Ross V. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur. J. Heart Fail. 2005;7:405–410. doi: 10.1016/j.ejheart.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Ansari M., Shlipak M.G., Heidenreich P.A. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003;107(22):2799–2804. doi: 10.1161/01.CIR.0000070952.08969.5B. [DOI] [PubMed] [Google Scholar]
- 20.Jaarsma T., Strömberg A., De Geest S., Fridlund B., Heikkila J., Martensson J., Moons P., Scholte Op Reimer W., Smith K., Stewart S., Thompson D.R. Heart failure management programmes in Europe. Eur. J. Cardiovasc. Nurs. 2006;5:197–205. doi: 10.1016/j.ejcnurse.2006.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material