Abstract
Background
We determined the age-related changes in atrioventricular junction (AVJ) velocities and displacements by feature tracking cardiovascular magnetic resonance (FT-CMR) in a healthy community-based population. We also investigated the importance of age-matching for the identification of altered AVJ dynamics.
Methods
FT-CMR was performed in 230 controls (18–78 years) and in two patient groups each consisting of 40 subjects (group 1: 23–55 years, group 2: 56–80 years). AVJ dynamic parameters, including systolic velocity Sm, early diastolic velocity Em, late diastolic velocity Am, maximal systolic excursion MAPSE and the new parameter sweep surface area velocity SSAV were measured.
Results
Increasing age in the control group was significantly associated with reductions in Sm, Em, MAPSE (r = −0.40, −0.76, −0.34, all P < 0.001) and an increase in Am (r = 0.45, P < 0.001). For patient group 1, the selection of an age-unmatched control group (56–76 years) underestimated the number of patients with abnormal AVJ dynamics during systole and early diastole (38% vs. 70% for Sm; 20% vs. 60% for Em; 35% vs. 50% for MAPSE). In contrast, for patient group 2, the number of patients with systolic and early diastolic AVJ dynamic abnormalities was overestimated (88% vs. 63% for Sm; 90% vs. 68% for Em; 73% vs. 58% for MAPSE) when compared with age-unmatched controls (24–55 years). Fifty-percent (20/40) of the sub-group of patients with normal left ventricular ejection fraction exhibited abnormal systolic Sm or MAPSE measurements.
Conclusions
Significant correlations exist between age and AVJ dynamics. Age matching is important for evaluating AVJ long-axis function.
Keywords: Cardiovascular magnetic resonance, Reference values, Age-matching, Feature tracking, Atrioventricular junction dynamics
Highlights
-
•
4D atrioventricular junction (AVJ) dynamics by feature tracking CMR
-
•
Provide age- and gender-specific normal ranges of AVJ dynamics
-
•
AVJ dynamics significantly associated with age and gender
-
•
Age matching is necessary for assessing severity of AVJ dynamic dysfunction.
1. Introduction
Cardiovascular magnetic resonance (CMR) imaging has emerged as a new way of quantifying long-axis function of the atrioventricular junction (AVJ) with good reproducibility [[1], [2], [3], [4], [5], [6], [7]]. Advantages of CMR over conventional tissue Doppler imaging (TDI) include absence of angle dependency [8], and higher robustness to mask (similar to sample volume in TDI) size and location [9,10]. The CMR-derived parameters of annular systolic velocity (Sm), early diastolic velocity (Em), late diastolic velocity (Am), and mitral annular plane systolic excursion (MAPSE) have great potential for routine use in clinical CMR for assessing left ventricular (LV) function. Feasibility and effectiveness have been demonstrated in diagnosing heart failure with preserved ejection fraction (HFpEF), myocardial infarction (MI), hypertrophic cardiomyopathy (HCM), and pulmonary hypertension (PH) [4,5]. The diagnostic importance of these parameters motivates efforts to obtain age- and gender-stratified reference values in healthy individuals from large, population-based studies. The necessity of age-matching for the detection of abnormal AVJ dynamics also needs investigation.
In achieving study objectives we 1) used feature tracking (FT)-CMR to derive the regional and global AVJ velocities and displacements in a population of 230 subjects without known cardiovascular disease, diabetes and hypertension; 2) further derived the new dynamic parameters, viz., sweep surface area velocities (SSAV) following FT-CMR; 3) assessed the age- and gender-related changes in the AVJ dynamic measurements; and 4) investigated the importance of age-matching for the identification of altered AVJ dynamics for LV function assessment.
2. Methods
2.1. Study population
In 2011–2016, 424 subjects aged 18–87 years old free of clinically recognized cardiovascular diseases were enrolled at the National Heart Centre Singapore and Beijing Anzhen Hospital China. All subjects either 1) did not report physician-diagnosed cardiovascular disease; or 2) underwent electrocardiography and echocardiography to define the absence of cardiovascular disease. Of the 424 participants, 420 had CMRs of which 396 were interpretable for AVJ measures. We then excluded those with cardiovascular risk factors, i.e., hypertension and diabetes mellitus; eventually 230 subjects (age 18–78 years, 111 male and 119 female) were selected in this study. Hypertension was defined by current use of anti-hypertensive drugs or physician-diagnosed hypertension. Diabetes mellitus was defined by current use of anti-diabetic agents or physician-diagnosed diabetes mellitus by blood test. The screening of participants was conducted by a trained nurse using a standard protocol that included medical history, family history, cardiovascular risk factors and drug history and final assessment was confirmed by cardiologist. To investigate the influence of age matching, measurements in 40 young to mid-aged patients (group 1, 30 male, mean 45 ± 8 years, range = 23–55 years, n = 10 with HCM, 10 with MI, 10 with heart failure with reduced ejection fraction (HFrEF), 5 with heart failure with mid-ranged ejection fraction (HFmrEF), and 5 with HFpEF) and 40 elderly patients (group 2, 26 male, mean 66 ± 6 years, range = 56–80 years, n = 10 with HCM, 10 with MI, 10 with HFrEF, 5 with HFmrEF, and 5 with HFpEF) were compared to those in an appropriate age-matched control group and an unmatched control group (control age group 56–76 years for young to mid-aged patients and control age group 24–55 years for elderly patients). Inclusion criteria for HF patients required the presence of signs or symptoms of congestive HF based on modified Framingham criteria [11]. HCM and MI patients were recruited from specialized cardiomyopathy clinics. The SingHealth Centralized Institutional Review Board and the institutional review board of Beijing Anzhen Hospital approved the study protocol. Informed consent was obtained from each participant. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. CMR acquisition
Imaging was performed using a 3 T magnetic resonance imaging system (Ingenia, Philips Healthcare, Netherlands). End-expiratory breath hold balanced steady-state free precession cine images were acquired in multi-planar long-axis views (2-, 3-, and 4-chamber views). Typical parameters were as follows: repetition time (TR)/echo time (TE), 3/1 ms; matrix, 240 × 240; flip angle, 45°; field of view, 300 × 300 mm2; pixel bandwidth, 1776 Hz; pixel spacing, 1.25 × 1.25 mm; slice thickness, 8 mm; frame rate, 30/40 per cardiac cycle.
2.3. CMR data analysis
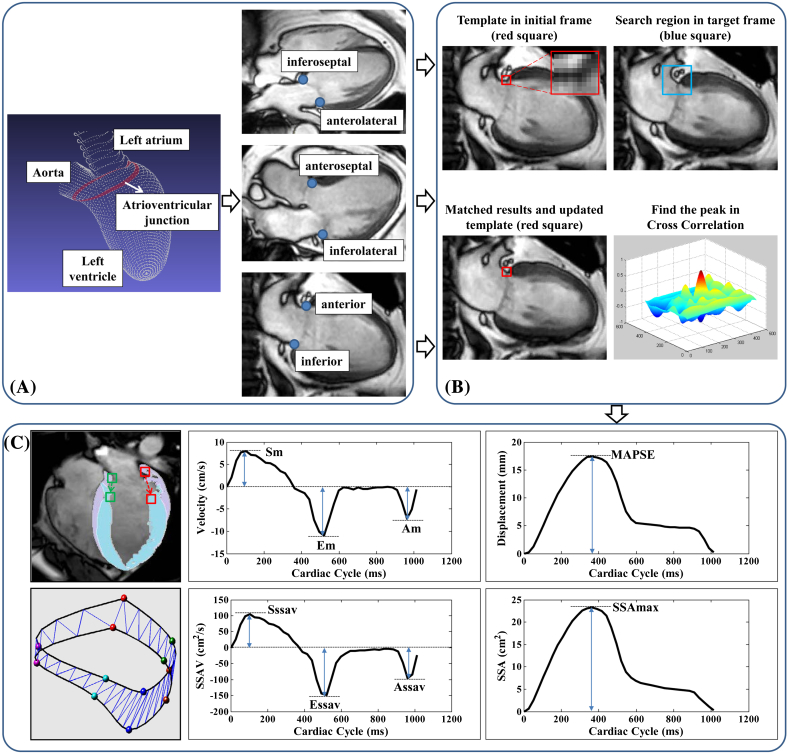
Custom software, developed in the MATLAB environment (MathWorks Inc., MA, USA), was used to perform the semi-automatic tracking of AVJ (Fig. 1(A)) motion in 2-, 3-, and 4-chamber long-axis CMR views (inferoseptal and anterolateral AVJs obtained from 4-chamber view, anteroseptal and inferolateral AVJs obtained from 3-chamber view, and anterior and inferior AVJs obtained from 2-chamber view are shown in Fig. 1(A)) throughout the whole cardiac cycle. The tracking system used the method of template matching (Fig. 1(B)), which is an algorithm for searching and finding the location of a template image within a larger image (called the search region). More detailed descriptions of the semi-automated tracking method can be found in previous studies [4,5].
Fig. 1.
(A) Atrioventricular junction (AVJ) denoted by the red line and the 6 AVJ points (inferoseptal and anterolateral in 4-chamber view; anteroseptal and inferolateral in 3-chamber view; anterior and inferior in 2-chamber view). (B) Semi-automatic tracking of AVJ points using template matching. (C) AVJ dynamic outputs including peak velocities and displacement Sm, Em, Am and MAPSE and sweep surface area based measurements Sssav, Essav, Assav and SSAmax. Details see text.
Following the AVJ tracking, dynamic measurements including Sm, peak systolic velocity; Em, peak early diastolic velocity; Am, peak late diastolic velocity during atrial contraction; and MAPSE, mitral annular plane systolic excursion (Fig. 1(C)) were derived for each of the six AVJ points in CMR 2-, 3- and 4-chamber views.
The semi-automatic tracking system provided spatial coordinates of 6 points located along the AVJ as a function of CMR frame time. The 2D coordinates of AVJ motion trajectories obtained in 2-, 3-, and 4-chamber CMR views were transformed into a 3D coordinate system. A spline curve interpolation was performed to reconstruct the 3D AVJ geometry, using a fully automated smoothing procedure based on a penalized least squares method [12]. Two CMR-based indices, sweep surface area (SSA) and sweep surface area velocity (SSAV), were adopted to further quantitatively characterize 3D AVJ motion [5,7]. Following the AVJ reconstruction by a curve at each CMR frame, the SSA (area bounded between the corresponding curves) was computed as the surface area swept out by the AVJ at successive CMR frames by Delaunay triangulation. The rate of AVJ motion was then quantified using SSAV by taking the first order time derivative of SSA. Parameters extracted from the resulting SSAV and SSA curves were: peak systolic SSAV (Sssav), peak early diastolic SSAV (Essav), peak late diastolic SSAV (Assav), and maximal SSA (SSAmax) in systole (Fig. 1(C)).
2.4. Statistical analysis
Data was analyzed using SPSS (version 17.0, Chicago, IL, USA) and SAS (version 9.3, Cary, NC, USA). Continuous variables were tested for normality by the Shapiro-Wilk test and expressed as mean ± standard deviation (SD). The AVJ dynamics were analyzed using a two-way analysis of variance model with factors Age Category (<30, 30–39, 40–49, 50–59, 60–69, and ≥70 years), Gender (Male/Female) and Age × Gender interaction. F-tests were performed to test for significant differences among factor levels, and a contrast was used to test for a linear trend across age categories. Statistical significance was declared at P < 0.05.
Reproducibility analysis was conducted on 30 randomly selected subjects using the Bland-Altman method and the coefficient of variation (CV). The CV was calculated as the overall residual standard deviation divided by the overall mean of the observations.
3. Results
3.1. Baseline characteristics of control population
Baseline demographics for the entire cohort of 230 (male/female 111/119, age 18–78 years) healthy individuals are shown in Table 1. Mean ± SD age was 51.8 ± 18.2 years among men and 48.6 ± 17.4 among women. Men had significantly higher LV end-diastolic volume (EDV), LV end-systolic volume (ESV), LV stroke volume (SV) and LV mass index than women (all P < 0.05). All control participants were divided according to age group (<30, 30–39, 40–49, 50–59, 60–69, and ≥70 years).
Table 1.
Baseline characteristics of the control population.
| Total (n = 230) | Men (n = 111) | Women (n = 119) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 50.1 ± 17.8 | 51.8 ± 18.2 | 48.6 ± 17.4 | 0.179 |
| Height (cm) | 163.7 ± 8.5 | 169.5 ± 6.9 | 158.3 ± 5.9 | <0.0001 |
| Weight (kg) | 63.0 ± 12.4 | 68.8 ± 12.6 | 57.6 ± 9.5 | <0.0001 |
| BSA (m2) | 1.68 ± 0.20 | 1.79 ± 0.19 | 1.57 ± 0.15 | <0.0001 |
| Heart rate (bmp) | 74.8 ± 12.2 | 75.7 ± 12.6 | 74.0 ± 11.9 | 0.286 |
| Sinus rhythm (n) | 230 | 111 | 119 | NS |
| Left ventricular volumes | ||||
| LVEDV index (ml/m2) | 67.1 ± 11.6 | 69.6 ± 12.2 | 64.8 ± 10.6 | 0.002 |
| LVESV index (ml/m2) | 25.5 ± 6.9 | 26.4 ± 7.0 | 24.6 ± 6.7 | 0.049 |
| LVSV index (ml/m2) | 41.7 ± 7.7 | 43.2 ± 7.9 | 40.4 ± 7.2 | 0.005 |
| LVEF (%) | 62.4 ± 6.5 | 62.3 ± 6.3 | 62.5 ± 6.6 | 0.858 |
| LV mass index (g/m2) | 43.5 ± 10.2 | 48.9 ± 10.1 | 38.4 ± 7.3 | <0.0001 |
Data are presented as mean ± SD. P value is for t-test between genders. BSA: body surface area; LV: left ventricular; EDV: end-diastolic volume; ESV: end-systolic volume; SV: stroke volume; EF: ejection fraction; NS: non-significant.
3.2. Feasibility and reproducibility
Out of 1380 (6 points from each of the 230 control subjects) AVJ points potentially available for the study population, 1356 points (98.3%) were adequately tracked in the semi-automatic manner. Manual adjustments were necessary for the remaining 24 points due to image artifacts. Supplementary Table S1 shows the intra- and inter-observer variability for global values and values for each point separately. Intra-observer CV was 1.2% to 4.0% for Sm, Em, Am and MAPSE averaged for the 6 AVJ points and 1.0% to 10.2% for each point separately. The corresponding inter-observer CV was 1.9% to 5.0% and 1.4% to 10.9%, respectively. Intra- and inter-observer CVs for Sssav, Essav, Assav and SSAmax were 2.2% to 3.7% and 3.8% to 5.4%. Regional and global AVJ dynamic measurements in all gender and age are shown in Supplementary Table S2. The global Sm, Em, Am, Em/Am and MAPSE were 8.0 ± 1.6 cm/s, 9.5 ± 3.1 cm/s, 7.7 ± 2.0 cm/s, 1.4 ± 0.7, and 14.8 ± 2.2 mm, respectively.
3.3. Normal controls: influence of age and gender on AVJ dynamics
Comprehensive results of the statistical analysis on the global AVJ dynamics stratified into gender and age groups (<30, 30–39, 40–49, 50–59, 60–69, and ≥70 years) are given in Table 2. Sm velocity (P < 0.0001), Em velocity (P < 0.0001), Em/Am velocity ratio (P < 0.0001) and MAPSE (P < 0.0001) decreased significantly with age, while Am velocity increased with age (P < 0.0001). Peak systolic velocity Sm (Male vs. Female, least squares mean 8.4 vs. 7.7 cm/s, P < 0.0001) and late diastolic velocity Am (Male vs. Female, 8.1 vs. 7.3 cm/s, P = 0.002) were significantly higher in males than in females. Peak early diastolic velocity Em (Male vs. Female, 9.4 vs. 9.6 cm/s, P = 0.524) and maximal displacement MAPSE (Male vs. Female, 14.6 vs. 15.0 cm/s, P = 0.110) were similar among males and females. There was significant gender difference in Em/Am ratio (Male vs. Female, 1.3 vs. 1.5, P = 0.002) with greater values in females than in males. Only Sm velocity demonstrated a significant Age × Gender interaction (P = 0.045). For sweep surface area based measurements, Sssav (P < 0.0001), Essav (P < 0.0001) and SSAmax (P < 0.0001) were inversely related to age, while positive relationship to age existed for Assav (P = 0.001). Gender differences were found for Sssav (P < 0.001), Essav (P = 0.006) and Assav (P < 0.0001) with higher values in males. Relationships between regional Sm, Em, Am, MAPSE at all 6 AVJ points and age group are shown in Supplementary Fig. S1, which demonstrated regional heterogeneity of AVJ motion in all age groups. The relationship plots separated in males and females are given in Supplementary Figs. S2 and S3. Supplementary Figs. S4 and S5 present the 5th, 25th, 50th, 75th, and 95th percentile plots for all AVJ dynamic measurements versus age.
Table 2.
Summary of statistical analysis on AVJ dynamics.
| Variable | Gender | Age category (years) |
P values |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <30 (n = 43, M/F 21/22) |
30–39 (n = 30, M/F 13/17) |
40–49 (n = 44, M/F 18/26) |
50–59 (n = 27, M/F 11/16) |
60–69 (n = 37, M/F 19/18) |
≥70 (n = 49, M/F 29/20) |
LS mean, gender | ANOVA main effect: Age Gender Interaction (age × gender) Linear trend: age |
||
| Sm (cm/s) | M | 8.9 (1.1) | 10.2 (1.5) | 8.7 (1.7) | 7.3 (1.3) | 7.9 (1.8) | 7.3 (1.5) | 8.4 |
A: <0.0001 G: <0.0001 I: 0.045 |
| F | 8.6 (1.3) | 8.4 (1.4) | 7.7 (1.3) | 7.2 (1.1) | 7.2 (1.1) | 7.0 (1.2) | 7.7 | ||
| LS mean, age | 8.8 | 9.3 | 8.2 | 7.3 | 7.6 | 7.2 |
LT: <0.0001 Slope = −0.04 (P < 0.0001) |
||
| Em (cm/s) | M | 12.7 (1.8) | 11.9 (2.6) | 8.9 (2.4) | 8.6 (2.3) | 8.0 (2.0) | 6.5 (1.4) | 9.4 |
A: <0.0001 G: 0.524 I: 0.173 |
| F | 12.8 (2.6) | 12.2 (2.6) | 10.6 (2.4) | 8.7 (1.9) | 7.4 (1.7) | 6.3 (1.5) | 9.6 | ||
| LS mean, age | 12.7 | 12.0 | 9.8 | 8.6 | 7.7 | 6.4 |
LT: <0.0001 Slope = −0.13 (P < 0.0001) |
||
| Am (cm/s) | M | 6.8 (1.4) | 7.9 (2.3) | 8.3 (1.9) | 7.8 (2.0) | 8.8 (2.2) | 8.8 (2.0) | 8.1 |
A: <0.0001 G: 0.002 I: 0.370 |
| F | 5.9 (1.3) | 6.5 (1.1) | 7.2 (1.5) | 7.3 (1.3) | 8.1 (2.2) | 9.0 (1.7) | 7.3 | ||
| LS mean, age | 6.3 | 7.2 | 7.7 | 7.6 | 8.5 | 8.9 |
LT: <0.0001 Slope = 0.05 (P < 0.0001) |
||
| Em/Am | M | 2.0 (0.4) | 1.7 (0.6) | 1.2 (0.4) | 1.2 (0.4) | 0.9 (0.3) | 0.8 (0.3) | 1.3 |
A: <0.0001 G: 0.002 I: 0.075 |
| F | 2.3 (0.6) | 2.0 (0.5) | 1.6 (0.5) | 1.3 (0.4) | 1.0 (0.3) | 0.7 (0.2) | 1.5 | ||
| LS mean, age | 2.2 | 1.8 | 1.4 | 1.2 | 1.0 | 0.8 |
LT: <0.0001 Slope = −0.03 (P < 0.0001) |
||
| MAPSE (mm) | M | 15.2 (2.1) | 15.4 (2.5) | 14.8 (2.8) | 14.6 (2.6) | 14.2 (1.6) | 13.7 (1.8) | 14.6 |
A: <0.0001 G: 0.110 I: 0.689 |
| F | 15.8 (1.9) | 16.4 (2.6) | 15.3 (2.3) | 14.3 (1.8) | 14.3 (2.2) | 14.1 (1.4) | 15.0 | ||
| LS mean, age | 15.5 | 15.9 | 15.1 | 14.4 | 14.2 | 13.9 |
LT: <0.0001 Slope = −0.05 (P < 0.0001) |
||
| Sssav (cm2/s) | M | 141 (20) | 169 (23) | 136 (30) | 115 (12) | 108 (29) | 102 (29) | 129 |
A: <0.0001 G: <0.001 I: 0.048 |
| F | 116 (16) | 129 (27) | 112 (18) | 107 (15) | 94 (17) | 90 (20) | 108 | ||
| LS mean, age | 129 | 149 | 124 | 111 | 101 | 96 |
LT: <0.0001 Slope = −0.66 (P < 0.0001) |
||
| Essav (cm2/s) | M | 202 (36) | 195 (51) | 151 (41) | 146 (29) | 120 (38) | 95 (32) | 151 |
A: <0.0001 G: 0.006 I: 0.289 |
| F | 164 (35) | 193 (44) | 149 (40) | 129 (29) | 102 (28) | 86 (27) | 137 | ||
| LS mean, age | 183 | 194 | 150 | 137 | 111 | 91 |
LT: <0.0001 Slope = −2.09 (P < 0.0001) |
||
| Assav (cm2/s) | M | 111 (25) | 128 (32) | 133 (30) | 108 (41) | 123 (31) | 125 (31) | 121 |
A: 0.001 G: <0.0001 I: 0.056 |
| F | 83 (14) | 90 (29) | 108 (30) | 107 (24) | 112 (28) | 127 (35) | 104 | ||
| LS mean, age | 97 | 109 | 120 | 107 | 118 | 126 |
LT: <0.001 Slope = 0.48 (P < 0.001) |
||
| SSAmax (cm2) | M | 27.9 (5.5) | 27.0 (5.2) | 25.2 (5.0) | 24.8 (2.6) | 23.7 (4.0) | 22.3 (4.8) | 25.1 |
A: <0.0001 G: 0.075 I: 0.105 |
| F | 23.5 (3.3) | 28.3 (5.3) | 24.8 (4.1) | 22.8 (3.7) | 22.3 (4.1) | 21.8 (3.3) | 23.9 | ||
| LS mean, age | 25.5 | 27.7 | 25.0 | 23.8 | 23.0 | 22.1 |
LT: <0.001 Slope = −0.10 (P < 0.0001) |
||
Data are presented as mean (SD). Sm: peak systolic annular velocity; Em: peak early diastolic annular velocity; Am: peak late diastolic annular velocity; MAPSE: mitral annular plane systolic excursion; Sssav: peak systolic sweep surface area velocity; Essav: peak early diastolic sweep surface area velocity; Assav: peak late diastolic sweep surface area velocity; SSAmax: maximum sweep surface area; ANOVA: analysis of variance; M: male; F: female; LS: least squares; A: age; G: gender; I: interaction; LT: linear trend. Bold data indicates the significance of P value.
3.4. Patients: impact of age matching
AVJ dynamics of the subjects in the patient group are summarized in Supplementary Table S3. Elderly patients had significantly smaller Sm, Em and MAPSE than young to mid-aged patients. The values of (Mean − 2 × SD) from the global AVJ dynamic measurements in the age-matched and unmatched normal controls were used as the cutoff points for abnormality. Notably, the evaluation of abnormal AVJ function based on unmatched control groups significantly impacted the results (Table 3). Specifically for young to mid-aged patients, the selection of an older control group (56–76 years) underestimated the number of patients with abnormal AVJ dynamics during systole and early diastole (38% vs. 70% for Sm; 20% vs. 60% for Em; 35% vs. 50% for MAPSE). In contrast, for elderly patients, the number of patients with systolic and early diastolic AVJ dynamic abnormalities was overestimated (88% vs. 63% for Sm; 90% vs. 68% for Em; 73% vs. 58% for MAPSE) when compared with an age-unmatched controls group (24–55 years).
Table 3.
Number of patients diagnosed with abnormal AVJ dynamics with age-matched and age-unmatched normal controls.
| Variable | Patient group 1: 23–55 years (n = 40) |
Patient group 2: 56–80 years (n = 40) |
||
|---|---|---|---|---|
| Matched | Unmatched | Matched | Unmatched | |
| Sm* | 28/40 (70%) | 15/40 (38%) | 25/40 (63%) | 35/40 (88%) |
| Em* | 24/40 (60%) | 8/40 (20%) | 27/40 (68%) | 36/40 (90%) |
| Am* | 18/40 (45%) | 27/40 (68%) | 27/40 (68%) | 16/40 (40%) |
| MAPSE* | 20/40 (50%) | 14/40 (35%) | 23/40 (58%) | 29/40 (73%) |
Data presented are “number of patients diagnosed with abnormal AVJ dynamics/total number of patients (percentage)”. AVJ: atrioventricular junction; Sm: peak systolic annular velocity; Em: peak early diastolic annular velocity; Am: peak late diastolic annular velocity; MAPSE: mitral annular plane systolic excursion. *Global values obtained by taking average of 6-point measurements. Bold values indicate underestimation of abnormal AVJ dynamics in the patient groups when compared with age-unmatched controls. Italic values indicate overestimation of abnormal AVJ dynamics in the patient groups when compared with age-unmatched controls.
3.5. Systolic parameters
Velocity Sm and displacement MAPSE reflect the LV systolic function. Among the 80 patients included in the current study, 40 had normal LVEF (≥50%). By using the value of (Mean − 2 × SD) from the global Sm and MAPSE in the age- and gender-matched normal controls as the cutoff points for abnormality, it was found that, 97.5% (39 out of 40) of the sub-group of patients with reduced LVEF (<50%, n = 40) had abnormal Sm or MAPSE. In addition, 50% (20 out of 40) of the sub-group of patients with normal LVEF exhibited abnormal systolic Sm or MAPSE measurements.
4. Discussion
In this study, we investigated age-related changes in FT-CMR-derived AVJ dynamics in a cohort of 230 healthy subjects. Increasing age was associated with significant reductions in Sm, Em, MAPSE and an increase in Am. These associations with respect to age had a significant effect on the quantification and grading of abnormal AVJ function in the patient groups. Hence, age matching is important when evaluating the severity of AVJ long-axis dysfunction.
4.1. Normal values of AVJ dynamics
Several studies have attempted to derive normative values for TDI mitral annular velocities [[13], [14], [15]]. This is the first study to report CMR-derived AVJ dynamic reference values, which are necessary to accelerate the technique and measurements to become clinical routine in complex cardiac disease diagnosis. There were differences between FT-CMR normal ranges presented herein and those reported using TDI method [[13], [14], [15]]. In this respect, CMR-based method evaluates the peak myocardial motion using feature tracking technique with typical mask size of 8 × 8 mm, while PW TDI measures peak myocardial velocity based on Doppler theory with typical sample volume size of 5 mm. CMR is also acquired at lower frame rates than PW TDI. Hence, FT-CMR dynamic measurements reported herein were lower than PW TDI values. In fact, despite the existence of systematic bias, our earlier study has demonstrated a strong correlation between FT-CMR- and TDI-derived AVJ velocities [4]. For MAPSE measurements, one prior study reported normal value of 12 ± 2 mm (average of inferoseptal, anterior, anterolateral, and inferolateral) derived from M-mode of echocardiography [16]. Our CMR-based reference MAPSE range (14.8 ± 2.2 mm) was larger than echo-based values, mainly due to the different calculation methodologies where the former (CMR) represents the total distance of excursion moved by the annulus along its arc-shaped trajectory but the latter (echo) measures the point-to-point longitudinal distance between ED and ES. Systematic bias is generally expected between modalities [17]. Thus, physicians applying or interpreting the technique should be aware that each modality has its own set of reference values and they may not be interchangeable.
In our data, CVs of intra- and inter-observer reproducibility were reduced for Sm, Em, Am and MAPSE when averaging measurements of all 6 AVJ points. One prior study [18] has demonstrated 25% higher variability by single walls echocardiographic TDI than the 4-wall average. Hence, our results concur with previous findings that averaging yields more robust measurements [13].
4.2. Influence of gender and age on AVJ dynamics
Our results showed a decline in cardiac function with aging as measured by AVJ velocities and displacement. The systolic velocity Sm and displacement MAPSE were inversely related to age. The general decrease in Em velocity and increase in Am velocity with aging resulted in a significant decrease in the Em/Am ratio. The influence of age on the AVJ longitudinal function was most evident in Em, which is in agreement with the findings of previous studies [15]. Prior study of conventional echocardiographic Doppler measurements has reported gender differences that Sm and Am were higher in men than in women, and Em was higher in women [13]. In our study, we also observed significantly larger Sm and Am in male subjects. The Em, however, did not show significant difference between men and women. The broad age range and division of the study population into 6 age groups allowed us to reveal the progressive decline in AVJ dynamics with age. Therefore, we believe our more granular data can provide more accurate age- and gender-dependent assessment of LV systolic and diastolic function with FT-CMR used as the method of investigation.
4.3. Heterogeneous AVJ motion
In accordance with previous findings [13,15], the segmental heterogeneity was ascertained by our regional dynamic measurements. It has been hypothesized that the highest annular velocities in anterolateral, inferolateral and inferior segments could be explained by the most longitudinal orientation of myocardial fibers in these regions [15]. In fact, the global Sm velocity averaged from the 6-basal segments has been demonstrated to be a more sensitive index of early systolic dysfunction than is LVEF [19]. The early diastolic velocity Em is widely used to assess LV diastolic function as it is linked to long-axis myocardial relaxation [20]. In current study, the magnitude of Em velocity was found to vary according to the location and the highest Em was observed at the anterolateral site in each of the age groups, which was in accordance with prior findings [15]. By using pulsed mode TDI, Wang et al. [21] demonstrated that mitral annular velocity averaged from four sites – inferoseptal, anterolateral, inferior, and anterior – in early diastole gives the most predictive power for cardiac mortality.
4.4. Importance of age matching
In view of the significant association between AVJ function and age, it is essential to establish age-dependent reference ranges to accurately interpret AVJ measurements in patient samples. The importance of age-specific normal values for LV and right ventricular (RV) function, including ventricular mass, volumes and ejection fraction have been well demonstrated in prior studies for the determination of normality, or the severity of abnormality [22,23]. In another study [24], Ooij et al. investigated the effect of age-mismatched normal controls on the identification of abnormal aortic hemodynamics in patients with aortic valve disease. Our data in present study also suggested that in clinical practice, age-specific normal references should be used in order to correctly identify or exclude abnormality of AVJ function.
4.5. Systolic function assessment
The LVEF is an important determinant of the severity of systolic heart dysfunction. In this study, significant positive correlation was found between LVEF and the CMR-derived peak systolic velocity Sm and displacement MAPSE in the controls and patients, which was similar to the previously presented results [4]. It has been demonstrated in earlier studies that patients with valvular heart diseases, hypertensive heart diseases, and HCM tend to have impaired systolic function despite preserved LVEF [25,26]. In the current study, we also found decreased AVJ motion values in HCM and HFpEF patients even though their LVEF values were normal. Hence, LVEF alone does not always reliably reflect the severity of systolic dysfunction in all heart diseases and the AVJ dynamics offer incremental values in the ventricular function assessment.
4.6. Sweep surface area velocity
Our AVJ motion evaluation was further extended based on CMR-based SSAV and SSA, which reflect radial, longitudinal and circumferential changes of the atrioventricular annulus. One prior study [3] has demonstrated the clinical potential of 3D mitral annular sweep volume in diastolic function assessment. It was shown in that study that patients with diastolic dysfunction had significantly lower peak volume sweep rates in early diastole (5.3 ± 1.4 vs. 7.7 ± 1.7 s−1), higher peak volume sweep rates in late diastole (6.6 ± 2.0 vs. 4.7 ± 1.4 s−1), and lower ratio of the two (0.9 ± 0.4 vs. 1.8 ± 0.7). In current clinical practice, echocardiography and CMR has parity in assessment of ventricular systolic function, but CMR lags echocardiography for evaluation of diastolic function. Our CMR-derived sweep surface area velocity is a step toward achieving this parity. We observed significantly reduced early diastolic SSAV (Essav: 71.1 ± 25.9 vs. 139.3 ± 51.9 cm2/s) and late diastolic SSAV (Assav: 55.2 ± 29.5 vs. 113.2 ± 32.2 cm2/s) in HF patients compared to controls. Further studies are needed to demonstrate the capability of SSAV parameters in assessing ventricular diastolic dysfunction in a large cohort of patients.
4.7. Limitations
There were some limitations in present study. First, the associations between age and CMR parameters were cross-sectional but not longitudinal, since the CMR examinations were not performed repeatedly on the same subjects over time. Second, the temporal resolution of CMR is markedly lower compared to echocardiographic techniques. The practical implication is that CMR may systematically underestimate peak velocities compared to TDI with a higher temporal resolution, although the level of underestimation does not appear to be clinically relevant [4]. Lastly, we used 6 points in the routine long-axis views for AVJ reconstruction. By using the 3 routine slices, our prior study reported mean differences of −0.7 ± 0.6 cm2 and −5.8 ± 4.7 mm for mitral annular 3D area and perimeter, respectively, in comparison to reference values obtained from a series of radially rotational long-axis cine CMR [10]. This study showed the importance of mitral annular plane motion in cardiac mechanics. Left atrial (LA) function quantified by tracking the distance from mitral annulus to a user defined point at the base of LA [27,28] warrants further investigations in a large group of normal controls to determine the age-related changes in LA phasic function.
5. Conclusions
We demonstrated in 230 normal controls with large age span that significant correlations exist between age and the AVJ dynamics. Therefore, age matching is important for evaluation of the severity of AVJ long-axis dysfunction.
Grants
This study received funding support from the National Medical Research Council of Singapore (NMRC/TA/0031/2015; NMRC/OFIRG/0018/2016), Hong Leong Foundation and Edwards Lifesciences. The funder had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgements
We appreciate the support and medical editing assistance from the Duke-NUS/SingHealth Academic Medicine Research Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2018.11.001.
Contributor Information
Xiaohai Ma, Email: maxi8238@yahoo.com.
Liang Zhong, Email: zhong.liang@nhcs.com.sg.
Appendix A. Supplementary data
Supplementary material
References
- 1.Maffessanti F., Gripari P., Pontone G. Three-dimensional dynamic assessment of tricuspid and mitral annuli using cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging. 2013;14:986–995. doi: 10.1093/ehjci/jet004. [DOI] [PubMed] [Google Scholar]
- 2.Saba S.G., Chung S., Bhagavatula S. A novel and practical cardiovascular magnetic resonance method to quantify mitral annular excursion and recoil applied to hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014;16:35. doi: 10.1186/1532-429X-16-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu V., Chyou J.Y., Chung S., Bhagavatula S., Axel L. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J. Cardiovasc. Magn. Reson. 2014;16:71. doi: 10.1186/s12968-014-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng S., Zhao X.D., Huang F.Q. Automated quantitative assessment of cardiovascular magnetic resonance-derived atrioventricular junction velocities. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1923–H1935. doi: 10.1152/ajpheart.00284.2015. [DOI] [PubMed] [Google Scholar]
- 5.Leng S., Jiang M., Zhao X.D. Three-dimensional tricuspid annular motion analysis from cardiac magnetic resonance feature-tracking. Ann. Biomed. Eng. 2016;44:3522–3538. doi: 10.1007/s10439-016-1695-2. [DOI] [PubMed] [Google Scholar]
- 6.Leng S., Zhao X., Tan R.S., Zhong L. Novel method for atrioventricular motion assessment from three-dimensional cine magnetic resonance imaging. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015;2015:319–322. doi: 10.1109/EMBC.2015.7318364. [DOI] [PubMed] [Google Scholar]
- 7.Xiao P.D., Zhao X.D., Leng S., Tan R.S., Wong P., Zhong L. A software tool for heart AVJ motion tracking using cine cardiovascular magnetic resonance images. IEEE J. Transl. Eng. Health Med. 2017;5 doi: 10.1109/JTEHM.2017.2738623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho C.Y., Solomon S.D. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396–e398. doi: 10.1161/CIRCULATIONAHA.105.579268. [DOI] [PubMed] [Google Scholar]
- 9.Hill J.C., Palma R.A. Doppler tissue imaging for the assessment of left ventricular diastolic function: a systematic approach for the sonographer. J. Am. Soc. Echocardiogr. 2005;18:80–88. doi: 10.1016/j.echo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Leng S., Zhang S., Jiang M. Imaging 4D morphology and dynamics of mitral annulus in humans using cardiac cine MR feature tracking. Sci. Rep. 2018;8:81. doi: 10.1038/s41598-017-18354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho K.K., Anderson K.M., Kannel W.B., Grossman W., Levy D. Survival after the onset of congestive heart failure in Framingham Heart study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 12.Garcia D. Robust smoothing of gridded data in one and higher dimensions with missing values. Comput. Stat. Data Anal. 2010;54:1167–1178. doi: 10.1016/j.csda.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalen H., Thorstensen A., Vatten L.J., Aase S.A., Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ. Cardiovasc. Imaging. 2010;3:614–622. doi: 10.1161/CIRCIMAGING.109.926022. [DOI] [PubMed] [Google Scholar]
- 14.Chahal N.S., Lim T.K., Jain P., Chambers J.C., Kooner J.S., Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur. J. Echocardiogr. 2010;11:51–56. doi: 10.1093/ejechocard/jep164. [DOI] [PubMed] [Google Scholar]
- 15.Nikitin N.P., Witte K.K., Thackray S.D., de Silva R., Clark A.L., Cleland J.G. Longitudinal ventricular function: normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color Doppler tissue imaging. J. Am. Soc. Echocardiogr. 2003;16:906–921. doi: 10.1016/S0894-7317(03)00279-7. [DOI] [PubMed] [Google Scholar]
- 16.Simonson J.S., Schiller N.B. Descent of the base of the left ventricle: an echocardiographic index of left ventricular function. J. Am. Soc. Echocardiogr. 1989;2:25–35. doi: 10.1016/s0894-7317(89)80026-4. [DOI] [PubMed] [Google Scholar]
- 17.Wood P.W., Choy J.B., Nanda N.C., Becher H. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography. 2014;31:87–100. doi: 10.1111/echo.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Støylen A., Skjaerpe T. Systolic long axis function of the left ventricle. Global and regional information. Scand. Cardiovasc. J. 2003;37:253–258. doi: 10.1080/14017430310015000. [DOI] [PubMed] [Google Scholar]
- 19.Yu C.M., Lin H., Yang H., Kong S.L., Zhang Q., Lee S.W. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 20.Okada K., Mikami T., Kaga S. Early diastolic mitral annular velocity at the interventricular septal annulus correctly reflects left ventricular longitudinal myocardial relaxation. Eur. J. Echocardiogr. 2011;12:917–923. doi: 10.1093/ejechocard/jer154. [DOI] [PubMed] [Google Scholar]
- 21.Wang M., Yip G.W., Wang A.Y. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J. Am. Coll. Cardiol. 2003;41:820–826. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 22.Maceira A.M., Prasad S.K., Khan M., Pennell D.J. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 23.Maceira A.M., Prasad S.K., Khan M., Pennell D.J. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur. Heart J. 2006;27:2879–2888. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- 24.Van Ooij P., Garcia J., Potters W.V. Age-related changes in aortic 3D blood flow velocities and wall shear stress: implications for the identification of altered hemodynamics in patients with aortic valve disease. J. Magn. Reson. Imaging. 2016;43:1239–1249. doi: 10.1002/jmri.25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K., Akashi Y.J., Mizukoshi K. Relationship between left ventricular ejection fraction and mitral annular displacement derived by speckle tracking echocardiography in patients with different heart diseases. J. Cardiol. 2012;60:55–60. doi: 10.1016/j.jjcc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Young A.A., Kramer C.M., Ferrari V.A., Axel L., Reichek N. Three-dimensional left ventricular deformation in hypertrophic cardiomyopathy. Circulation. 1994;90:854–867. doi: 10.1161/01.cir.90.2.854. [DOI] [PubMed] [Google Scholar]
- 27.Leng S., Tan R.S., Zhao X.D., Allen J.C., Koh A.S., Zhong L. Validation of a rapid semi[HYPHEN]automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2018;20:71. doi: 10.1186/s12968-018-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh A.S., Gao F., Leng S. Dissecting clinical and metabolomics associations of left atrial phasic function by cardiac magnetic resonance feature tracking. Sci. Rep. 2018;8:8138. doi: 10.1038/s41598-018-26456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material