Summary
Intra-embryo genome editing by CRISPR/Cas9 enables easy generation of gene-modified animals by non-homologous end joining (NHEJ)-mediated frameshift mutations or homology-directed repair (HDR)-mediated point mutations. However, large modifications, such as gene replacement or gene fusions, are still difficult to introduce in embryos without costly micromanipulators. Moreover, micromanipulation techniques for intra-embryo genome editing have been established in only a small set of animals. To overcome these issues, we developed a method of large-fragment DNA knockin without micromanipulation. In this study, we successfully delivered the knockin donor DNA into zygotes by adeno-associated virus (AAV) without removing the zona pellucida, and we succeeded in both large-DNA fragment knockin and whole exon exchange with electroporation of CRISPR/Cas9 ribonucleoprotein. By this method, we can exchange large DNA fragments conveniently in various animal species without micromanipulation.
Subject Areas: Techniques in Genetics, Genetic Engineering
Graphical Abstract

Highlights
-
•
AAV infects zygotes of various mammals through intact zona pellucida
-
•
AAV vector delivers large knockin cassettes into zygotes without micromanipulation
-
•
Cas9 RNP electroporation and donor AAV enable efficient intra-embryo knockin
Techniques in Genetics; Genetic Engineering
Introduction
Genome editing techniques, especially clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) technology, have significantly simplified the creation of genetically modified cells and animals. CRISPR/Cas9 utilizes programmable nucleases that form a complex with a guide RNA (gRNA). The ribonucleoprotein (RNP) utilizes the RNA to target a specific site in any chromosome and produce a double-stranded break (DSB). The DSB is repaired by non-homologous end joining (NHEJ), homology-directed repair (HDR), or microhomology-mediated end joining (MMEJ). In the NHEJ pathway mediated by Ku70/Ku80, the ends of the DSB are combined with small insertions or deletions. In MMEJ, microhomology near the cleavage site is annealed and repaired with a small defect. Both NHEJ and MMEJ repair mechanisms are imperfect and may cause a frameshift mutation, thereby destroying the function of the gene. In contrast, targeted gene knockins are used to integrate reporter genes downstream of desired promoters, insert gene expression cassettes into safe harbors such as the Rosa26 or AAVS1 loci, or exchange large regions of DNA such as an exon. Although knockins are mainly carried out by HDR-mediated repair mechanisms, MMEJ-mediated target gene integration method (precise integration into target chromosome) and NHEJ-mediated target gene integration (homology-independent targeted integration) have been recently developed (Sakuma et al., 2015, Suzuki et al., 2016). All methods of knockin require delivery of donor DNA into the cell nucleus in addition to the CRISPR/Cas9 complex.
Genetically modified rodents are often developed by first producing embryonic stem (ES) cells with the desired genotype. These cells are selected and injected into embryos, producing chimeric pups. If the injected cells contributed to the germline, the rodents can be mated to produce new offspring with the intended genotype. This process requires multiple generations of animals and can be inefficient. Genome editing performed in zygotes could produce genetically modified animals in the first generation. This would enable site-directed transgenesis even in non-rodent mammals, from which ES cells have not been shown to contribute to germline chimeras.
Genetically modifying zygotes can be achieved by somatic cell nuclear transfer or direct injection of CRISPR/Cas9. This requires advanced training and expensive micromanipulation equipment. Alternatively, RNP-mediated editing in zygotes can be performed by electroporation of the CRISPR/Cas9 complex (Kaneko and Mashimo, 2015); however, large templates of donor DNA cannot be transfected as efficiently (Chen et al., 2016, Hashimoto et al., 2016). Thus, simple gene modifications in zygotes are limited in adaptation to point mutation/repair or small insertions/deletions and require specific gRNAs for each mutated sequence. These small modifications are not sufficient for all applications including disease modeling, as it is difficult to faithfully reproduce disease phenotypes caused by large insertions/deletions. To overcome this issue, technology for replacing a large fragment, such as a whole exon, is necessary. Although genome editing technology has made it easy to generate genetically modified cells, it is still difficult to add or replace large fragments in fertilized embryos.
Results
Trans-Zona Pellucida DNA Delivery by AAV Vector
To stably deliver the donor DNA for gene knockin into the nucleus, a viral vector whose genome is covered with a capsid is suitable. Because adeno-associated viral (AAV) vectors, adenoviral vectors, and lentiviral vectors are commonly used as gene delivery vehicles for experimental and therapeutic applications, the infectivity of these viral vectors on embryos was first confirmed. For AAV, we chose the serotype 6 AAV (AAV6) vector based on its infectivity in mouse and human ES cells as previously reported (Ellis et al., 2013).
In earlier studies, embryos were infected with viral particles by microinjecting the virus into the perivitelline space. Therefore, each viral vector (AAV, adenoviral, and lentiviral) encoding enhanced green fluorescent protein (EGFP) driven by the CAG promoter was microinjected into the perivitelline space of pronuclear-stage mouse embryos, and the expression of EGFP was confirmed under a fluorescence microscope at the morula/blastocyst stage. The infection was confirmed with adenoviral and lentiviral vectors; however, transgene expression was not obvious in embryos with AAV vector (Figures 1I, 1J, S1C–S1F, S2C, and S2D). Removal of the zona pellucida also enabled efficient infection by the lentiviral vector as reported previously (Figures S1G and S1H) (Ikawa et al., 2003).
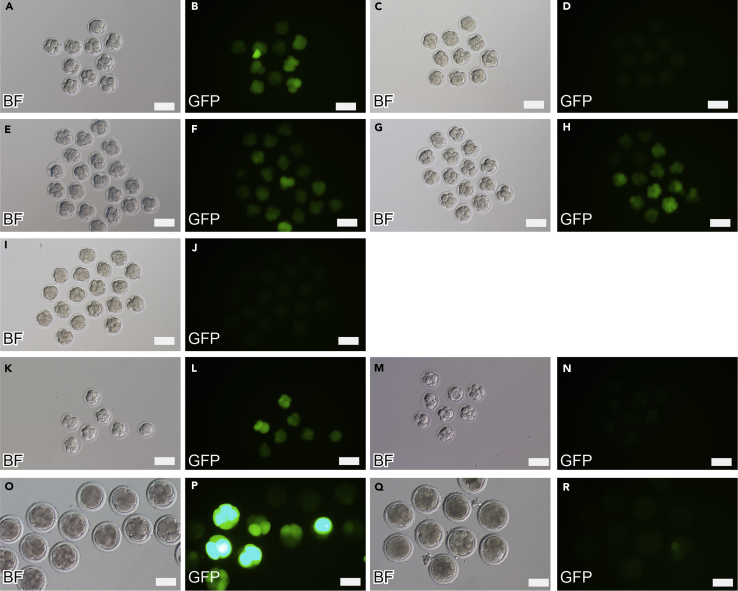
Figure 1.
AAV-Mediated Transduction of Embryos with Zona Pellucida
(A–J) Zygotes of mice were co-cultured with EGFP-expressing scAAV6 for 16–24 hr. The expression of EGFP was analyzed by fluorescence microscopy from morula to blastocyst stage. The fertilized zygotes co-cultured with scAAV6-CAG-EGFP at a concentration of 1 × 105 IU/mL (A and B) showed high EGFP expression compared with that of the mock-treated embryos (C and D). Parthenogenetic oocytes exposed to scAAV6-CAG-EGFP after activation at 1 × 105 IU/mL for 16 hr (E and F) and before activation at 1 × 106 IU/mL for 1 hr (G and H) also showed EGFP expression. Microinjection of AAV at a concentration of 1 × 108 IU/mL to perivitelline space shows no EGFP expression (I and J).
(K–R) Fertilized zygotes of rats (K and L) and cattle (O and P) co-cultured with scAAV6-CAG-EGFP at a concentration of 1 × 106 IU/mL for 16–24 hr also resulted in high EGFP expression compared with that of mock-transduced embryos of rats (M and N) and cattle (Q and R).
Scale bars, 100 μm. See also Figures S1–S4.
As a control, embryos with an intact zona pellucida were co-cultured with the viral vectors, resulting in no infection with lentiviral or adenoviral vectors as expected (Figures S1A, S1B, S2A, and S2B). However, efficient infection was observed with the AAV vector (Figures 1A and 1B). Transgene expression was AAV vector dose-dependent, and high from the 4-cell stage to the morula stage in mice embryos (Figure S3). We also confirmed AAV serotypes other than serotype 6, and AAV6 showed the highest transduction efficiency (Figure S4). Moreover, we examined rat embryos and bovine embryos for AAV transduction. AAV6 transduced both of them efficiently. Since the zona pellucida was thought to be a defense against viral infection, we assumed that this infection was established by the virus passing through the holes created during fertilization. To test this hypothesis, we infected parthenogenetic embryos with the AAV vector, and unexpectedly the infection was established (Figures 1E–1H). These results indicate that the AAV vector can pass through the intact zona pellucida and infect the embryos. This is consistent with the result of AAV microinjection into perivitelline space. We could inject femto-picoliter virus suspension at 1 × 108 IU/mL, which is equivalent to 1 × 1010 vg/mL = 10 vg/pL, into the perivitelline space of each zygote. If zona pellucida is a barrier for virion transmission, all virions might be enclosed in the perivitelline space until zygote infection, and such small amount of virion would be enough for zygote transduction, as in case of other viral vectors. In case of AAV, virion would be diffused from perivitelline space into the culture media, and the multiplicity of infection (MOI) would be insufficient for zygote transduction.
The zona pellucida of mammals is divided into three types depending on the type of constituent glycoprotein. The zona pellucida of mice consists of ZP1, ZP2, and ZP3; the zona pellucidae of humans and rats consist of ZP1, ZP2, ZP3, and ZP4; and the zona pellucida of cattle consists of ZP2, ZP3, and ZP4 (Izquierdo-Rico et al., 2009). We found that the AAV vector can pass through both rat and bovine zona pellucidae to efficiently infect the embryo (Figures 1K–1R). The results indicate that the AAV vector is suitable for delivering donor DNA to the fertilized eggs of any mammalian species without the need for micromanipulation.
Mouse Rosa26 Locus Knockin with AAV Vector
We investigated whether the delivery of donor DNA into embryos by the AAV vector is effective for large-fragment knockin using the CRISPR/Cas9 system. After introducing the Cas9-RNP into pronuclear-stage embryos by electroporation, we infected with a self-complementary AAV (scAAV) vector harboring a 1.8-kb CAG-GFP cassette flanked by two 100-bp Rosa26 homology arms (2 kb in total) (Figures 2A and 2B). At the blastocyst stage, the efficiency of knockin in the Rosa26 locus was 15.5%, and the concentration of scAAV did not affect the efficiency of knockin (Table 1). The birth rate of offspring was 19.3%, of which 6.3% had the cassette inserted into the Rosa26 locus (Table 2). EGFP expression was confirmed by fluorescence stereomicroscopy and flow cytometry of peripheral blood (Figures 2C, 2D, and 2F). Moreover, we found that the cassette was correctly inserted in the Rosa26 locus without indel mutations at the site of junction (Figures 2E and S5A). Germline transmission was confirmed by breeding the knockin founders and wild-type C57BL/6N mice, followed by genotyping of N1 generation offsprings (Figure S7A).
Figure 2.
Large-Fragment Knockin by Donor AAV Transduction and CRISPR/Cas9 RNP Electroporation in Mouse Embryos
(A–F) Knockin by scAAV donor vector. (A) Schematic representation of knockin for Rosa26 locus. Two pronuclear embryos of wild-type mice were electroporated with Cas9 protein and gRNA targeting the Rosa26 locus and cultured in vitro for 16–24 hr with donor AAV6. The embryos that developed normally to the 2-cell stage were collected and transferred to oviducts of surrogate mothers. (B) Donor scAAV vector containing 1,850-bp CAG-EGFP cassette flanked by approximately 100-bp homology arms next to the gRNA target. Magenta arrows, mouse Rosa26 primers (forward and reverse); blue arrow, CAG promoter primer; green arrow, EGFP primer. (C and D) Fluorescence stereomicroscopy of knockin and wild-type neonates. (E) Representative of genotyping of knockin mice. Primers flank the 5′- and 3′-junctional regions. (F) Flow cytometry of peripheral blood myeloid cells.
(G–I) Knockin by ssAAV donor vector. (G) Donor ssAAV vector containing 1,850-bp CAG-EGFP cassette flanked by approximately 1,000-bp homology arms next to the gRNA target. Magenta arrows, mouse Rosa26 primers (forward and reverse); blue arrow, CAG promoter primer; green arrow, EGFP primer. (H) Representative of genotyping of knockin mice by ssAAV. Primers flank the 5′- and 3′-junctional regions. (I) Flow cytometry of peripheral blood myeloid cells.
See also Figures S5, S7A, and S7B.
Table 1.
Knockin Efficiency of In Vitro-Cultured Mouse Blastocyst after Zygote Genome Editing with Donor AAV Transduction
| Vector Type | AAV Concentration | Number of 2PN Zygotes Treated | Number of Zygotes in Blastocyst Stage | Number of Zygotes Genotyped | Number of Knockin Embryosa | Number of Embryos with Partial Insertionb |
|---|---|---|---|---|---|---|
| scAAV6 | 1 × 105 IU/mL | 80 | 53 (66.3) | 47 (88.7) | 7 (14.9) | 4 (8.5) |
| scAAV6 | 1 × 106 IU/mL | 40 | 29 (72.5) | 24 (82.8) | 4 (16.7) | 1 (4.2) |
| ssAAV6 | 1 × 107 vg/mL | 21 | 18 (85.7) | 18 (100) | 2 (11.1) | 2 (11.1) |
| ssAAV6 | 1 × 108 vg/mL | 12 | 7 (58.3) | 7 (100) | 0 (0) | 3 (42.9) |
Embryos with positive genotype at both 5′ and 3′ junctions of the mouse Rosa26 knockin allele.
Embryos with positive genotype at either 5′ or 3′ junction of the mouse Rosa26 knockin allele.
Table 2.
Knockin Efficiency of Mouse Offspring after Zygote Genome Editing with Donor AAV Transduction
| Vector Type | AAV Concentration | Number of 2PN Zygotes Treated | Number of Zygotes in 2-Cell Stage | Number of Zygotes Transferred | Number of Offsprings | Number of Knockin Offspringsa | Number of Offsprings with Partial Insertionb |
|---|---|---|---|---|---|---|---|
| scAAV6 | 1 × 105 IU/mL | 370 | 265 (71.6) | 166 (62.6) | 32 (19.3) | 2 (6.3) | 8 (25) |
| ssAAV6 | 1 × 107 vg/ml | 109 | 93 (85.3) | 84 (90.3) | 29 (34.5) | 4 (13.8) | 1 (3.4) |
Offspring with positive genotype at both 5′ and 3′ junctions of the mouse Rosa26 knockin allele.
Offspring with positive genotype at either 5′ or 3′ junction of the mouse Rosa26 knockin allele.
Furthermore, we performed the same experiment with single-strand AAV (ssAAV) vector harboring a 1.8-kb CAG-GFP cassette flanked by two 1-kb Rosa26 homology arms (3.8 kb in total) (Figure 2G). The birth rate of offspring was 34.5%, and the rate of Rosa26 locus insertion in offspring was 13.8% (Table 2). EGFP expression was confirmed by fluorescence stereomicroscopy and flow cytometry of peripheral blood (Figure 2I). The cassette was correctly inserted in the Rosa26 locus without indel mutations at the site of junction (Figures 2H and S5B). Germline transmission was confirmed by breeding the knockin founders and wild-type C57BL/6N mice, followed by genotyping of N1 generation offsprings (Figure S7B). Some offspring showed positive genotype either at the 5′ or 3′ junction of the mouse Rosa26 knockin allele (Table 2). We could not identify the whole sequences at those integration sites. This might be partly due to non-HDR integration of AAV donor with ITR (Inverted Terminal Repeat), whose secondary structure hampers PCR. However, we could not determine vector-derived sequences, even by PCR with 7-deaza-dGTP, which improves PCR for AAV-ITR (data not shown) (Mroske et al., 2012). We could not rule out intra-vector deletion of concatemeric insertion either (Nowrouzi et al., 2012).
These results indicate that both scAAV and ssAAV vectors can stably deliver a large fragment of donor DNA for CRISPR/Cas9-mediated site-specific knockin without micromanipulation.
Rat Rosa26 Locus Knockin with AAV Vector
We next examined the knockin efficiency in rat embryos. Similar to the experiment described above, the scAAV vector harboring 1.8-kb CAG-GFP cassette flanked by two 100-bp Rosa26 homology arms (2 kb in total) was injected into the rat embryo after electroporation with Cas9-RNP (Figure 3A). The birth rate of offspring was 16.7%, and the rate of Rosa26 locus insertion in offspring was 25.0% (Table 3). We also found that the cassette was correctly inserted in the Rosa26 locus without indel mutations at the site of junction as described above (Figures 3B and S6). The EGFP expression of the rat in which the knockin cassette was correctly inserted was confirmed by fluorescence stereomicroscopy and flow cytometry of peripheral blood (Figures 3C–3E). Germline transmission was confirmed by breeding the knockin founders and wild-type Wistar rats, followed by genotyping of N1 generation offsprings (Figure S7C).
Figure 3.
Large-Fragment Knockin by Donor AAV Transduction and CRISPR/Cas9 RNP Electroporation in Rat Embryos
(A–E) Knockin by scAAV donor vector. (A) Schematic representation of targeting strategy. Donor scAAV vector containing 1,850-bp CAG-EGFP cassette flanked by approximately 100-bp homology arms next to the gRNA target. Magenta arrows, rat Rosa26 primers (forward and reverse); blue arrow, CAG promoter primer; green arrow, EGFP primer; brown arrow, rat Rosa26 primers (forward and reverse) for droplet digital PCR; brown line, rat Rosa26 probe for droplet digital PCR; dark green allow, EGFP primers (forward and reverse) for droplet digital PCR; dark green line, EGFP probe for droplet digital PCR. (B) Representative of genotyping of knockin rat. Primers flanked the 5′- and 3′-junctional regions. (C and D) Fluorescence stereomicroscopy of knockin and wild-type neonates. (E) Flow cytometry of peripheral blood white blood cells. CD45-positive cells in knockin rat show EGFP expression.
(F–G) Knockin by ssAAV donor vector. (F) Donor ssAAV vector containing 1,850-bp CAG-EGFP cassette flanked by approximately 1,000-bp homology arms next to the gRNA target. Magenta arrows, rat Rosa26 primers (forward and reverse); blue arrow, CAG promoter primer; green arrow, EGFP primer; brown arrow, rat Rosa26 primers (forward and reverse) for droplet digital PCR; brown line, rat Rosa26 probe for droplet digital PCR; dark green arrow, EGFP primers (forward and reverse) for droplet digital PCR; dark green line, EGFP probe for droplet digital PCR. (G) Representative of genotyping of knockin rat by ssAAV. Primers flank the 5′- and 3′-junctional regions. (H) Copy number quantification of EGFP and rat Rosa26 allele without integration by droplet digital PCR. Absolute copies were normalized to copies of reference genome targeting endogenous Zeb2 locus and expressed as mean ± SEM (n = 4, technical replicates). Gray column, EGFP copies/genome; open column, copies of uninserted rat Rosa26 allele/genome. (I) Frequency of EGFP-positive cells in peripheral blood CD45+ cells of the knockin rats, assessed by flow cytometry.
See also Figures S6, S7C, and S7D.
Table 3.
Knockin Efficiency of Rat Offspring after Zygote Genome Editing with Donor AAV Transduction
| Vector Type | AAV Concentration | Number of 2PN Zygotes Treated | Number of Zygotes in 2-Cell Stage | Number of Zygotes Transferred | Number of Offsprings | Number of Knockin Offspringsa | Number of Offsprings with Partial Insertionb |
|---|---|---|---|---|---|---|---|
| scAAV6 | 1 × 105 IU/mL | 139 | 120 (86.3) | 120 (100) | 20 (16.7) | 5 (25) | 0 (0) |
| ssAAV6 | 1×107 vg/ml | 60 | 56 (93.3) | 56 (100) | 3 (5.4) | 3 (100) | 0 (0) |
Offspring with positive genotype at both 5′ and 3′ junctions of the rat Rosa26 knockin allele.
Offspring with positive genotype at either 5′ or 3′ junction of the rat Rosa26 knockin allele.
We also performed the same experiment with ssAAV vector harboring a 1.8-kb CAG-GFP cassette flanked by two 1-kb Rosa26 homology arms (3.8 kb in total) (Figure 3F). The birth rate of offspring was 5.4%, and the rate of Rosa26 locus insertion in offspring was 100% (Figure 3G and Table 3). EGFP expression was confirmed by fluorescence stereomicroscopy and flow cytometry of peripheral blood (Figure 3I). The cassette was correctly inserted in the Rosa26 locus without indel mutations at the site of junction (data not shown).
Moreover, droplet digital PCR was used to quantify the number of EGFP inserts and wild-type (un-inserted) Rosa26 alleles per cell (Figure 3H). Three of the five knockin offsprings (#1, #2, and #5) in scAAV group showed inconsistent copy numbers of transgene and uninserted Rosa26 allele. The copy numbers per cell were not integer in those cases. This indicates that they were definitely mosaic, despite none of the three in ssAAV. After germline transmission, offsprings of the knockin founder with 1.3 EGFP copies/cell (#1 in Figure 3H) had integer copies per cell of EGFP from 0 to 2 (Figure S7D). Flow cytometry of white blood cells in peripheral blood showed consistent phenotypes of the mosaic offsprings on droplet digital PCR (Figure 3I).
These results indicate that the trans-zona pellucida donor DNA delivery by AAV vector is effective for large-fragment knockin in the Rosa26 locus even in the rat embryo whose zona pellucida consists of different components than mouse. However, mosaicism of off-target integration was also observed.
Rescue of the Nude Phenotype by Exon Exchange
The nude (Foxn1nu) mutation, which is a single base pair (G) deletion in exon 3, results in a frameshift mutation that induces an athymic and hairless phenotype. To rescue the nude phenotype by exon exchange using a large-fragment knockin technique, we designed gRNAs to target 420 bp upstream (intron 2) and 550 bp downstream (intron 3) of the mutation in exon 3 and generated scAAV harboring functional exon 3 (462 bp) flanked by two homology arms (434 and 400 bp) (Figure 4A). After introducing the Cas9-RNP into pronuclear-stage embryos by electroporation, we infected with the scAAV vector harboring the donor DNA cassette. The birth rate was 20.3%, with exon exchange in 13.8% of the offspring (Table S1). Successful exchange of exon 3 was confirmed by Sanger sequencing and restriction fragment-length polymorphism analysis (Figures 4C and 4D). Furthermore, the restoration of hairless phenotype was observed in all mice with exchanged wild-type exon 3 (Figure 4B). Flow cytometry revealed that the CD3+ T lymphocytes were recovered in the peripheral blood of a mouse with exchanged exon 3: 16.04% ± 3.97%, 0.44% ± 0.22%, 0.72% ± 0.11% in KSN/Slc-Foxn1repaired/nu, KSN/Slc-Foxn1Δexon3/nu, KSN/Slc-Foxn1nu/nu (mean ± SEM, n = 3, 8, 4), respectively. The CD3+ T cells in KSN/Slc-Foxn1repaired/nu showed CD4+CD8 or CD8+CD4 phenotypes as mature T cells in wild-type mice (Figure 4E). These results indicate that an intact exon can be exchanged by CRISPR/Cas9-mediated large-fragment knockin in embryos. In addition, the repaired Foxn1 allele was conserved in the next generation (Figure S7E).
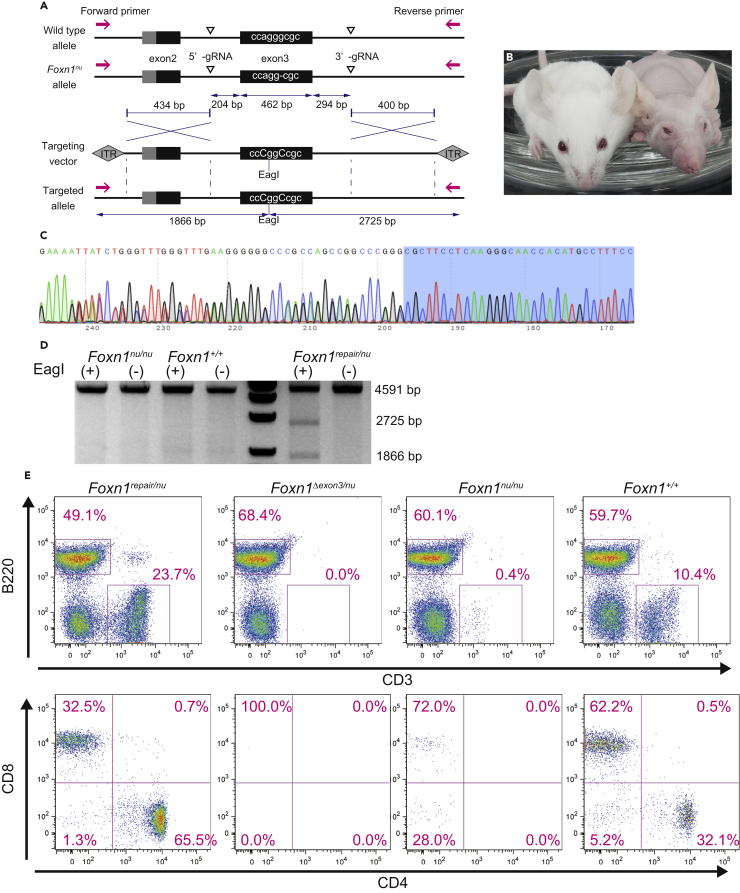
Figure 4.
Correction of Foxn1 Gene in Mouse Embryos by Exon Exchange
(A) Schematic representation of targeting strategy. A 1-bp deletion on exon 3 of Foxn1nu allele results in frameshift mutation. Two gRNAs were designed on introns 2 and 3 flanking exon 3 of Foxn1 gene. Repair donor scAAV vector containing 960-bp gRNA-flanking region with corrected exon 3 sequence and 434-bp 5′ homology arm and 400-bp 3′ homology arm. Magenta arrows, mouse Foxn1 primers (forward and reverse).
(B) Macroscopy of KSN/Slc-Foxn1repaired/nu (left) and KSN/Slc-Foxn1nu/nu (right).
(C) Representative of Sanger sequencing of exon 3 in KSN/Slc-Foxn1repaired/nu. Two sequences were combined from Foxn1nu mutation.
(D) Restriction fragment-length polymorphism analysis of Foxn1repaired/nu. A 4,590- 4,591-bp amplicon of Foxn1-flanking homology arms and exon 3 were digested with EagI. Amplicon derived from Foxn1repaired allele resulted in 1,866- and 2,725-bp products.
(E) Flow cytometry of peripheral blood white blood cells in KSN/Slc-Foxn1repaired/nu, KSN/Slc-Foxn1Δexon3/nu, KSN/Slc-Foxn1nu/nu, and wild-type C57BL/6N mouse. Mature T cells were detected in KSN/Slc-Foxn1repaired/nu, but neither in KSN/Slc-Foxn1Δexon3/nu nor in KSN/Slc-Foxn1nu/nu. Foxn1Δexon3, large deletion around exon 3, resulted from NHEJ between two gRNA targets.
Discussion
In the present study, we successfully performed large-fragment genome editing in embryos via CRISPR/Cas9 and trans-zona pellucida delivery of donor DNA with AAV vectors.
There is a little evidence regarding viral infection in preimplantation embryos with an intact zona pellucida. Bane et al. reported that porcine parvovirus can infect preimplantation porcine embryos with intra-embryonic replication of the viral genome (Bane et al., 1990). While we conducted our study, other groups also demonstrated that the AAV vector can pass through the zona pellucida (Yoon et al., 2018). Previous structural analysis of bovine zona pellucida showed that the average pore size of bovine zona pellucida is about 223 nm, and that 40-nm beads can enter halfway through the zona pellucida (Vanroose et al., 2000). The porcine parvovirus and AAV are relatively small viruses of about 20 nm belonging to the family Parvoviridae. Furthermore, a previous study showed that 20- to –40-nm-sized multi-walled carbon nanotubes can deliver DNA through the zona pellucida (Munk et al., 2016). Therefore, the size of the virus might be a key factor for translocation through zona pellucida, and other viruses with size <20 nm will likely pass through the zona pellucida.
We observed fluorescent reporter gene expression in mouse embryos infected by serotype 6 or serotype 1 AAV, whereas expression was not detected in serotype 2 AAV-infected embryos (Figures S4A, S4D, S4G, and S4J). Yoon et al. performed similar infection experiments in morula-stage mouse embryos and observed efficient gene expression in serotype 2 AAV-infected embryos (Yoon et al., 2018). This might be due to the difference in infection timing, MOI, or fluorescence detection sensitivity. We also detected higher reporter gene expression in rat embryos infected by AAV6 than those infected by AAV1 or AAV2 (Figures S4B, S4E, S4H, and S4K). Interestingly, bovine embryos were transduced with AAV2 as well as AAV1 and AAV6 (Figures S4C, S4F, S4I, and S4L). Our data indicate that serotype 6 AAV might be promising for zygote transduction in a variety of mammals; however, AAV-serotype-specific tropism for zygotes differs among species. Off-target integration and mosaicism were inferred from droplet digital PCR-based transgene copy number analysis. Since the AAV genome can be episomally maintained for a long time, it might be possible for additional insertions to occur after the one-cell stage of embryo development, leading to mosaicism.
Since AAV is nonpathogenic, efficiently infects non-dividing cells, and yields prolonged gene expression, the AAV vector has gained attention in the field of gene therapy. However, there is a concern that AAV might infect germ cells in vivo as it passes through the zona pellucida. At least one previous study has reported that the AAV genome was detected in male germ cells (Erles et al., 2001). Moreover, although Rep is deleted in the AAV vector, integration of the AAV genome into host's chromosomes has been reported (Gil-Farina et al., 2016, Kaeppel et al., 2013). Our data indicate that the AAV vector will likely pass through human zona pellucida and thus warrants consideration while using AAV vectors for gene therapy to avoid genome integration in germ cells.
When generating multiple mutations in one exon for purposes such as disease modeling, we propose that it may be easier to replace an entire exon instead of targeting each mutation individually. This strategy can also be used to repair structural variants, including fusion genes following translocation events or large insertions/deletions, other than just point mutations. Even for gene correction of point mutations, large fragment exchange strategy might be beneficial in some cases. Although point mutation correction by genome editing with single-strand oligodeoxynucleotide would be the best way in many cases, we have to design fine gRNA close to mutations. We can design gRNAs more flexibly around mutation hotspots with large-fragment knockin method, and we would employ the same gRNAs for patients with different point mutation on the same hotspot. The utility of embryonic gene therapy is not limited to humans. Because of inbreeding, genetic diseases are increasing in livestock animals, increasing the demand for livestock gene therapy.
Although several previous studies have succeeded in large-fragment knockin in mouse embryos with similar efficiency (5%–45%) as our AAV-mediated method (6.3%–100%), they delivered CRISPR/Cas9 and donor DNA by pronuclear microinjection (Aida et al., 2015, Miura et al., 2017, Yang et al., 2013, Yoshimi et al., 2016). Recently, Miyasaka et al. succeeded in introducing large fragment into rodent zygotes by electroporation of long single-strand donor DNA without micromanipulation; however, the donor DNA were up to 1.1 kb, including homology arms (Miyasaka et al., 2018). Pronuclear injection is a highly skilled technique and relatively difficult in non-rodent mammals. For example, in bovine or porcine embryos, it is difficult to identify the pronucleus by conventional methods because it is masked by lipid droplets. Our strategy does not require advanced techniques or special equipment such as micromanipulators, and many embryos can be processed simultaneously. Moreover, we could apply the same strategy to non-rodent mammals, in which germline-competent pluripotent stem cells have not been reported, enabling us to establish disease models with mammals that are more similar to those with humans genetically and physiologically. The genome of wild-type AAV is about 4.7 kb, and recombinant AAV vector has a packaging capacity of around 5.2 kb (Grieger and Samulski, 2005, Wu et al., 2010). Since we succeeded in introducing large exogenous fragment by scAAV with 100-bp homology arms and ssAAV with 1-kb homology arms, the donor AAV could contain up to 4.7-kb-length exogenous fragment theoretically. However, ssAAV with longer homology arms showed higher efficiency for precise knockin (Tables 2 and 3). The length of homology arms could affect it, and exogenous cassette would be shortened practically. Micromanipulation is still necessary for the introduction of >5.2-kb fragment. However, inter-AAV genomic homologous recombination could potentially serve longer donor DNA (Bak and Porteus, 2017, Wu et al., 2010).
It seems a good alternative to introduce Cas9 and gRNA by AAV vector. However, we could not expect Cas9 expression in 1-cell stage. Following mice zygote transduction with CAG-EGFP-expressing scAAV6, we could detect very low EGFP fluorescence under microscopy in the 2-cell stage, whereas embryos showed extremely high EGFP fluorescence after the 4-cell stage (Figure S3). Transgene expression from exogenous DNA depends on the embryo's transcription/translation machinery. In mouse zygotes, transcription from zygote genome is repressed in early 1-cell stage, and activated from late 1- to 2-cell stage (Abe et al., 2018, Jukam et al., 2017, Wiekowski et al., 1991). Minor zygotic activation occurred at the late 1-cell stage, whereas major zygotic activation and translation start from the 2-cell stage. These findings on zygotic genome activation explain the time course of EGFP reporter expression. In addition, Cas9 coding sequence is too large for scAAV, which has a capacity limitation of 2.3–2.5 kb. Even for smaller Cas9, including SaCas9, we have to use conventional ssAAV vector. Because transgene expression from ssAAV is much slower than scAAV, Cas9 expression from ssAAV would be insufficient in the 1-cell stage (McCarty et al., 2001, Wang et al., 2003). More importantly, zygotic genome activation starts at a later stage in other mammalian species (e.g., 4-to 8-cell stage in humans). Genome editing by Cas9 RNP electroporation has reduced the risk of mosaicism in rodent zygotes (Chen et al., 2016, Hashimoto et al., 2016, Kaneko and Mashimo, 2015). Cas9 and gRNA introduction by AAV vector might be simpler than Cas9 RNP electroporation, but might cause more mosaic, especially in other animals. The combination of donor DNA introduction by AAV vector and CRISPR/Cas9 genome editing by RNP electroporation could be applied to a multitude of species for both research and therapeutic purposes.
Limitations of the Study
Optimal conditions for embryos co-culture with AAV and Cas9 RNP electroporation were not yet determined except for mice and rats.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Toshiya Nishimura, Dr. Hideki Masaki, Dr. Sanae Hamanaka, and A. Umino for helpful advice. We also thank Y. Yamamoto, J. Iwano, N. Sato, and H. Tsukui for technical support; K. Okada for secretarial support; and M. Watanabe for advice in preparing the manuscript. This work was supported by grants from Leading Advanced Projects for medical innovation, Japan Agency for Medical Research and Development, JSPS KAKENHI Grant Numbers JP18K16276 and JP23500491.
Author Contributions
Conceptualization, N.M., E.M., T.Y., and H.N.; Methodology, N.M., E.M., H.S., F.S., and T.Y.; Investigation, N.M., E.M., H.S., M.K., A.O., and T.Y.; Resources, N.M., E.M., H.S., M.K., A.O., and T.Y.; Writing – Original Draft, N.M. and T.Y.; Writing – Review & Editing, N.M., E.M., F.S., T.Y. and H.N.; Supervision, T.Y. and H.N.; Funding Acquisition, T.Y. and H.N.
Declaration of Interests
The authors declare no competing interests.
Published: November 30, 2018
Footnotes
Supplemental Information includes Transparent Methods, seven figures, and one table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.10.030.
Contributor Information
Tomoyuki Yamaguchi, Email: tomoyama@ims.u-tokyo.ac.jp.
Hiromitsu Nakauchi, Email: nakauchi@stanford.edu.
Supplemental Information
References
- Abe K.-i., Funaya S., Tsukioka D., Kawamura M., Suzuki Y., Suzuki M.G., Schultz R.M., Aoki F. Minor zygotic gene activation is essential for mouse preimplantation development. Proc. Natl. Acad. Sci. U S A. 2018;115:E6780–E6788. doi: 10.1073/pnas.1804309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida T., Chiyo K., Usami T., Ishikubo H., Imahashi R., Wada Y., Tanaka K.F., Sakuma T., Yamamoto T., Tanaka K. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol. 2015;16:87. doi: 10.1186/s13059-015-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak R.O., Porteus M.H. CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep. 2017;20:750–756. doi: 10.1016/j.celrep.2017.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bane D.P., James J.E., Gradil C.M., Molitor T.W. In vitro exposure of preimplantation porcine embryos to porcine parvovirus. Theriogenology. 1990;33:553–561. doi: 10.1016/0093-691x(90)90511-q. [DOI] [PubMed] [Google Scholar]
- Chen S., Lee B., Lee A.Y.-F., Modzelewski A.J., He L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J. Biol. Chem. 2016;291:14457–14467. doi: 10.1074/jbc.M116.733154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.L., Hirsch M.L., Barker J.C., Connelly J.P., Steininger R.J., Porteus M.H. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol. J. 2013;10:74. doi: 10.1186/1743-422X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Rohde V., Thaele M., Roth S., Edler L., Schlehofer J.R. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum. Reprod. 2001;16:2333–2337. doi: 10.1093/humrep/16.11.2333. [DOI] [PubMed] [Google Scholar]
- Gil-Farina I., Fronza R., Kaeppel C., Lopez-Franco E., Ferreira V., D'Avola D., Benito A., Prieto J., Petry H., Gonzalez-Aseguinolaza G. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol. Ther. 2016;24:1100–1105. doi: 10.1038/mt.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C., Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Yamashita Y., Takemoto T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 2016;418:1–9. doi: 10.1016/j.ydbio.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Ikawa M., Tanaka N., Kao W.W.Y., Verma I.M. Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Mol. Ther. 2003;8:666–673. doi: 10.1016/s1525-0016(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Rico M.J., Jiménez-Movilla M., Llop E., Pérez-Oliva A.B., Ballesta J., Gutiérrez-Gallego R., Jiménez-Cervantes C., Avilés M. Hamster zona pellucida is formed by four glycoproteins: ZP1, ZP2, ZP3, and ZP4. J. Proteome Res. 2009;8:926–941. doi: 10.1021/pr800568x. [DOI] [PubMed] [Google Scholar]
- Jukam D., Shariati S.A.M., Skotheim J.M. Zygotic genome activation in vertebrates. Dev. Cell. 2017;42:316–332. doi: 10.1016/j.devcel.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppel C., Beattie S.G., Fronza R., van Logtenstein R., Salmon F., Schmidt S., Wolf S., Nowrouzi A., Glimm H., von Kalle C. A largely random AAV integration profile after LPLD gene therapy. Nat. Med. 2013;19:889–891. doi: 10.1038/nm.3230. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Mashimo T. Simple genome editing of rodent intact embryos by electroporation. PLoS One. 2015;10:e0142755. doi: 10.1371/journal.pone.0142755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D.M., Monahan P.E., Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Miura H., Quadros R.M., Gurumurthy C.B., Ohtsuka M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat. Protoc. 2017;13:195–215. doi: 10.1038/nprot.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y., Uno Y., Yoshimi K., Kunihiro Y., Yoshimura T., Tanaka T., Ishikubo H., Hiraoka Y., Takemoto N., Tanaka T. CLICK: one-step generation of conditional knockout mice. BMC Genomics. 2018;19:318. doi: 10.1186/s12864-018-4713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroske C., Rivera H., Ul-Hasan T., Chatterjee S., Wong K.K. A capillary electrophoresis sequencing method for the identification of mutations in the inverted terminal repeats of adeno-associated virus. Hum. Gene Ther. Methods. 2012;23:128–136. doi: 10.1089/hgtb.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk M., Ladeira L.O., Carvalho B.C., Camargo L.S.A., Raposo N.R.B., Serapião R.V., Quintão C.C.R., Silva S.R., Soares J.S., Jorio A. Efficient delivery of DNA into bovine preimplantation embryos by multiwall carbon nanotubes. Sci. Rep. 2016;6:33588. doi: 10.1038/srep33588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrouzi A., Penaud-Budloo M., Kaeppel C., Appelt U., Le Guiner C., Moullier P., Kalle C.v., Snyder R.O., Schmidt M. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol. Ther. 2012;20:1177–1186. doi: 10.1038/mt.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T., Nakade S., Sakane Y., Suzuki K.-I.T., Yamamoto T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat. Protoc. 2015;11:118–133. doi: 10.1038/nprot.2015.140. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tsunekawa Y., Hernandez-Benitez R., Wu J., Zhu J., Kim E.J., Hatanaka F., Yamamoto M., Araoka T., Li Z. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanroose G., Nauwynck H., Soom A.V., Ysebaert M.-T., Charlier G., Oostveldt P.V., de Kruif A. Structural aspects of the zona pellucida of in vitro-produced bovine embryos: a scanning electron and confocal laser scanning microscopic study. Biol. Reprod. 2000;62:463–469. doi: 10.1095/biolreprod62.2.463. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ma H.I., Li J., Sun L., Zhang J., Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Miranda M., DePamphilis M.L. Regulation of gene expression in preimplantation mouse embryos: effects of the zygotic clock and the first mitosis on promoter and enhancer activities. Dev. Biol. 1991;147:403–414. doi: 10.1016/0012-1606(91)90298-h. [DOI] [PubMed] [Google Scholar]
- Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Shivalila C.S., Cheng A.W., Shi L., Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y., Wang D., Tai P.W.L., Riley J., Gao G., Rivera-Pérez J.A. Streamlined ex vivo and in vivo genome editing in mouse embryos using recombinant adeno-associated viruses. Nat. Commun. 2018;9:412. doi: 10.1038/s41467-017-02706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi K., Kunihiro Y., Kaneko T., Nagahora H., Voigt B., Mashimo T. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat. Commun. 2016;7:10431. doi: 10.1038/ncomms10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.