Abstract
Cadmium (Cd) is a common environmental toxicant that has harmful effects on plants, animals, and humans. The present study evaluated the protective effects of Fragaria ananassa methanolic extract (SME) on cadmium chloride (CdCl2)-induced neuronal toxicity in rats. Male albino rats were intraperitoneally (i.p) injected with CdCl2 (6.5 mg/kg) for 5 days with or without the SME (250 mg/kg). We measured the levels of Cd, lipid peroxidation (LPO), nitric oxide, glutathione (GSH), and oxidative enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase, and glutathione reductase (GR) in the whole brain homogenate. Compared with the control group, the Cd-intoxicated group showed a marked increase in the brain levels of Cd, LPO, and nitric oxide and a decrease in the levels of GSH and all tested antioxidant enzymes. Compared with Cd-intoxicated rats, the rats pretreated with SME showed restoration of oxidative balance in the brain tissue. While the expression of brain SOD2, CAT, glutathione peroxidase 1, and GR was down-regulated in the Cd-treated group, the expression of these enzymes was up-regulated in rats pretreated with SME. In addition, administration of SME before CdCl2 increased the Bcl-2 expression, but significantly decreased the expression of Bax. Immunohistochemical analysis showed that compared with Cd-intoxicated rats, rats pretreated with SME showed a decrease in the protein expression of tumor necrosis factor α (TNF-α). Our findings indicate that SME protects the brain tissue from Cd-induced neuronal toxicity by improving the antioxidant system and increasing antiapoptotic and anti-inflammatory activities.
Keywords: apoptosis, brain, cadmium, Fragaria ananassa, oxidative stress
Introduction
Cadmium (Cd) is one of the most toxic heavy metals and a common industrial pollutant present in the environment [1,2]. The main sources of exposure to Cd are smoking tobacco, air pollution, and drinking contaminated water [3]. Additional sources include exposure through fertilizers, sewage, sludge, wastewater, pesticides, metal plating, pigments, plastics, glass, and batteries [4]. Cd is poorly excreted; Cd has a long biological half-life (20 years) and cannot be degraded, and thus accumulates in different organs of the body [5]. The bioaccumulation of Cd in the living system may cause severe damage to the reproductive system, gastrointestinal tract, mucous tissues, and nervous system [6]. Cd inhaled though the nasal mucosa or olfactory pathways enters the central nervous system (CNS) thus causing neurotoxicity [7]. The neurotoxicity of Cd has been observed in cultured rat cortical neurones [8] and rat primary midbrain neurone-glia cultures [9]; Cd disturbs the normal neurochemistry of the animal brain [10,11]. The mechanisms of Cd-induced neurotoxicity have not been clarified thus far. Oxidative stress has been proposed as a possible mechanism for Cd toxicity in a number of tissues such as the kidney [12], liver [2,13], and brain [14]. Cd is indirectly involved in the generation of reactive oxygen species (ROS) via the inactivation of thiol groups in critical molecules, inhibiting antioxidant defenses and DNA repair mechanisms, and disturbing the antioxidant system [15].
The use of herbal plants as health promoters is gaining increasing popularity in the consumer market and in the scientific circle [16,17]. Fruits and vegetables have been used as medicines for a long time because of they are economical and have few side effects on animals and humans [18]. They are important sources of vitamins, minerals, antioxidants, and phytochemicals [19].
Strawberry (Fragaria ananassa) is one of the most consumed fruits in the world. The major active ingredients of this fruit include the vitamin β-carotene and phenolic compounds such as phenolic acids, flavonoids, and anthocyanins [20]. Our group previously demonstrated that the methanolic extract of strawberry contains many active ingredients such as gallic acid, p-coumaric acid, cyanidin, pelargonidin, ellagic acid, and quercetin. Furthermore, the extract has high concentration of total phenolics content ranged from 98.3 to 112.7 mg/g extract, total flavonoids content ranged from 44.2 to 57.1 mg/g extract, and the total anthocyanin content ranged from 12.6 to 25.3 mg/g extract [21]. These bioactive compounds provide health benefits through several mechanisms, including the scavenging of ROS, protecting and regenerating other dietary antioxidants, and chelation of pro-oxidant metals [22]. These active ingredients have various pharmacological activities such as prevention of inflammation, hepatotoxicity, cancer, obesity, and oxidative stress [21,23]. The antioxidant properties of strawberry enable its use in models in which oxidative stress might play a role; therefore, in the present study, we investigated the effect of F. ananassa methanolic extract (SME) on Cd induced-neurotoxicity in rats.
Materials and methods
Chemicals
Cadmium chloride (CdCl2) anhydrous was purchased from Sigma Chem. Co. (St. Louis, MO, U.S.A.). Tris/-HCl was obtained from Fluka Chemie (Buchs, Switzerland). Thiobarbituric acid was obtained from Merck (Darmstadt, Germany), nitroblue tetrazolium and phenazine methosulphate were purchased from Alfa Aesar (Tewksbury, MA, U.S.A.).
TRIzol isolation kit was obtained from Invitrogen (Carlsbad, CA, U.S.A.). RevertAid H minus Reverse Transcriptase was purchased from Thermo Fisher Scientific Inc. The PCR primers were synthesized by Jena Bioscience GmbH (Jena, Germany). All other routine chemicals and solvents were of pure analytical grade.
Fruit material
F. ananassa fresh fruit was purchased from a local market in Cairo, Egypt in April–May 2016. The plant specimen was identified by the Botany Department, Faculty of Science, Helwan University. Fruits were homogenized as 1:10 w/v of 70% methanol aqueous solution. Homogenate was filtered out and left to dry using a vacuum evaporator (IKA, Germany). The residues were dissolved in distilled water and stored in an air-tight bottle at −20°C and was labeled as SME.
GC-MS analysis
The GC-MS analysis of SME was performed with a Thermo Scientific, Trace GC Ultra & ISQ Single Quadruple MS. Helium (99.999%) was used as the carrier gas with a flow rate at 1.7 ml min−1.1 μl injected (1:10) using the splitless injection technique; Agilent 19091S-433UI column (HP-5ms Ultra Inert GC Column, 30 m, 0.25 mm, 0.25 µm, 7-inch cage). Temperatures: injector: 250°C, detector: 280°C, column: 100°C with a temperature gradient of 20°C.min−1, 260°C held for 10 min. The total GC running time is at 20 min. The MS ionization voltage was 70 eV. The MS scan parameters included a mass range of m/z 40–1000 atomic mass units (amu). Identification of compounds was matched using the database of Wiley9, replib, and mainlib Libraries. The name, retention time, and area under peak of the components of the test materials were ascertained.
Animal treatment
To evaluate the potential neuroprotective effects of SME on CdCl2-intoxicated rats, we used 32 adult male Wistar rats, 35–42 days old, with an initial body weight of 160–180 g. The animals were obtained from the Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). The rats were housed in polypropylene cages and were maintained at room temperature (22 IS ± 3°C) on 12-h light/12-h dark cycle throughout the experiment. The rats were supplied water ad libitum and fed with a commercial pelleted rat chew diet of standard quality. They were allowed to adapt to the laboratory conditions for 7 days before starting the experiment. Animal care was in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals 8th edition (NIH Publication No. 85-23, revised 1985) and the study protocol and animal handling procedures were approved by the Ethical Committee of the Faculty of Pharmacy, Helwan University under approval number 0015A-16.
The animals were randomly divided into four equal groups (n=8) as follows:
Control group (Con): the rats were injected daily intraperitoneally (i.p) with normal saline for 5 days.
CdCl2-intoxicated group: the rats were injected i.p with 6.5 mg/kg CdCl2 for 5 days according to the procedure described by Dkhil et al. [13].
SME group: animals were orally administered SME (250 mg/kg) daily for 5 days.
Combined treatment group (SME + CdCl2): animals were pretreated with SME orally, then after 1 h, they were injected i.p with CdCl2 for 5 days.
The animals were killed by sudden decapitation 24 h after the last treatment, their brains were rapidly excised from the skulls, blotted, and chilled. The brain tissue was rapidly wiped dry using filter paper and homogenized in ice-cold medium of 50 mM Tris/HCl (pH 7.4) to give a 10% (w/v). The total protein content of the homogenized brain was then determined using the standard method of Lowry et al. [24].
Cadmium concentration
Cadmium concentration in the brain samples was quantitatively estimated after the end of the experiment using an atomic absorption spectrophotometer at 228.8 nm according to the protocol described by Kubaszewski et al. [25]. The tissue sample was weighed and ashed in a muffle furnace at 500°C, and then digested using nitric acid at 150°C for 2 h. The digested sample was diluted with deionized water to 50 ml. The level of cadmium was expressed as ng/g wet tissue.
Biochemical assay
Estimation of oxidants
The level of malondialdehyde (MDA) was estimated as a lipid peroxidation (LPO) marker in the brain tissue using the method described by Ohkawa et al. [26]. Briefly, a mixture consisting of distilled water, 0.67% thiobarbituric acid, and 0.22% sulphuric acid was added to 500 µl of brain supernatant. The formed mixture was boiled at 95°C for 30 min and then cooled at room temperature and centrifuged for 15 min at 1000 g. Finally, the supernatant was estimated spectrophotometrically 540 nm. The obtained data are expressed as nanomoles MDA per milligram of protein. Nitric oxide (NO) level was measured using the Griess reagent according to Green et al. [27]. Briefly, 100 μl of brain supernatant was mixed for 10 min with Griess reagent at room temperature. The formed reddish purple azo dye was measured spectrophotometrically at 540 nm.
Estimation of antioxidants
Glutathione (GSH) was estimated by the reduction of Elman’s reagent (5,5′-dithiobis (2-nitrobenzoic acid) (DTNB)) with GSH to produce a yellow compound. The reduced chromogen is directly proportional to the GSH concentration, and its absorbance can be measured at 405 nm [28]. Catalase (CAT) activity was determined as described by Aebi [29]. Briefly, 0.1 ml of the brain homogenate was mixed with mixture containing phosphate buffer (50 mM, pH 7.0) and H2O2 (30 mM). The decrease in absorbance was observed for 3 min and the enzyme activity was determined as μM H2O2 decomposed/s/mg protein. The activity of superoxide dismutase (SOD) was determined using the method of Nishikimi et al. [30]. Briefly, 0.05 ml of brain homogenate was added to mixture containing phenazine methosulphate (93 µM), reduced NAD (0.47 mM), nitroblue tetrazolium (0.3 mM), and phosphate bufer (0.1 M; pH 8.5). The increase in absorbance was estimated at 560 nm for 5 min. The obtained results were expressed as units per milligram of protein. Meanwhile, glutathione peroxidase activity was measured using the method of Paglia and Valentine [31]. GPx activity was estimated in terms of the decrease in NADH per min using a reaction coupled with glutathione reductase (GR). The decrease in absorbance at 340 nm was recorded, and GPx activity was expressed as U/mg protein. In addition, GR activity was measured by quantitating GSH-dependent oxidation of NADPH at 340 nm and expressed as U/mg protein.
Quantitative reverse-transcriptase PCR analysis
Total brain tissue RNA was extract from frozen samples using the TRIzol reagent. Approximately 5 μg of total RNA was converted into cDNA. The Power SYBR® Green Master Mix kit was used for real-time PCR analysis. The reaction program was set as follows: 95°C for 30 s followed by 42 cycles at 94°C for 5 s and 58°C for 60 s. The relative differences in gene expression between groups were expressed using cycle time (Ct) values, and the relative differences between groups were expressed as relative increases setting control as one fold. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as a control, and the expression of GAPDH was unchanged by any treatment used. The sense and antisense primers for the selected gene sets used are as follows:
GAPDH (accession number: NM_017008.4; product length: 248): S: 5′-AGTGCCAGCCTCGTCTCATA-3′; AS: 5′-GATGGTGATGGGTTTCCCGT-3′ [32].
SOD2 (accession number: NM_017051.2; product length: 191): S: 5′-AGCTGCACCACAGCAAGCAC-3′; AS: 5′-TCCACCACCCTTAGGGCTCA-3′ [32].
CAT (accession number: NM_012520.2; product length: 362): S: 5′-TCCGGGATCTTTTTAACGCCATTG-3′; AS: 5′-TCGAGCACGGTAGGGACAGTTCAC-3′ [32].
GPx1 (accession number: NM_030826.4; product length: 245): S: 5′-CGGTTTCCCGTGCAATCAGT-3′; AS: 5′-ACACCGGGGACCAAATGATG-3′ [32].
GR (accession number: NM_053906.2; product length: 229): S: 5′-TGCACTTCCCGGTAGGAAAC-3′; AS: 5′-GATCGCAACTGGGGTGAGAA-3′ [32].
Bcl-2 (accession number: NM_016993.1; product length: 228): S: 5′-CTGGTGGACAACATCGCTCTG-3′; AS: 5′-GGTCTGCTGACCTCACTTGTG-3′ [32].
Bax (accession number: NM_017059.2; product length: 217): S: 5′-GGCGAATTGGCGATGAACTG-3′; AS: 5′-ATGGTTCTGATCAGCTCGGG-3′ [32].
Preparation of histological sections
The brains were removed and fixed in 10% neutral formalin for 24 h at room temperature and processed for paraffin sectioning at a thickness of 5 μm. The slides were stained with Hematoxylin and Eosin stain (H&E) according to the previously described method of Bancroft and Gamble [33] for general examination.
Immunohistochemistry analysis
Formaldehyde/PBS-fixed, paraffin-embedded sections (4-μm thick) were mounted on glass slides. Sections were deparaffinized, blocked with methanol containing 0.1% hydrogen peroxide (H2O2) for 10 min to quench the endogenous peroxidase activity. After blocking, the sections were stained with polyclonal rabbit anti-tumor necrosis factor α (TNF-α) antibody at 4°C overnight. Then, the sections were washed with phosphate buffer and incubated with horseradish peroxidase–conjugated goat anti-rabbit antibody at 37°C for 30 min. The antibody-binding sites were visualized by incubation with diaminobenzidine (DAB)-H2O2 at room temperature for 10 min [34]. Images were taken at original magnification of 400× (Nikon Eclipse E200-LED, Tokyo, Japan).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) was used for data analysis. The results were expressed as the mean ± S.E.M. One-way ANOVA followed by Duncan’s test was applied for determining the significance. The acceptable level of significance was established at P<0.05.
Results
GC-MS results
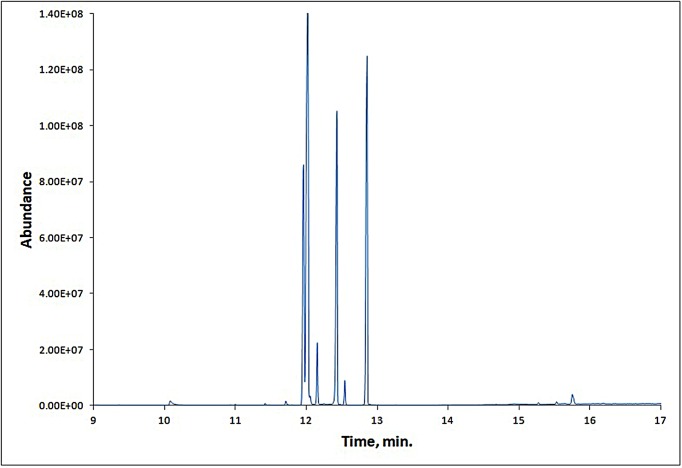
GC-MS chromatogram of the strawberry fruit extract (Figure 1) showed 41 peaks indicating the presence of 41 compounds. On comparison of the mass spectra of the constituents with Wiley9, replib, and mainlib libraries, the 41 compounds were characterized and identified (Supplementary Table S1). The identified compounds indicated the presence of many compounds that are considered as precursors for compounds responsible for strawberry fruits aroma and flavor besides other phytochemicals [35].
Figure 1. GC-MS chromatogram of SME.
Strawberry attenuates cadmium accumulation in brain tissue
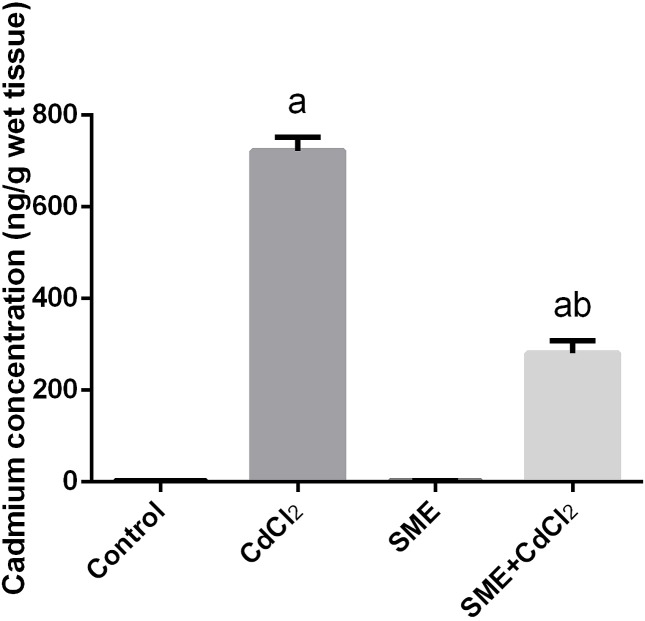
The Cd levels in the whole brain were significantly higher than normal levels (P<0.05) in rats injected with CdCl2 (700 ng/g wet tissue), this increase was significantly inhibited by treatment with SME (Figure 2).
Figure 2. Neuroprotective effects of SME on cadmium accumulation (ng/g wet tissue) in the brain homogenate of rats exposed to CdCl2 for 5 days.
Data are expressed as the mean ± S.E.M. (n=8). aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Strawberry attenuates cadmium alerted oxidant/antioxidant status
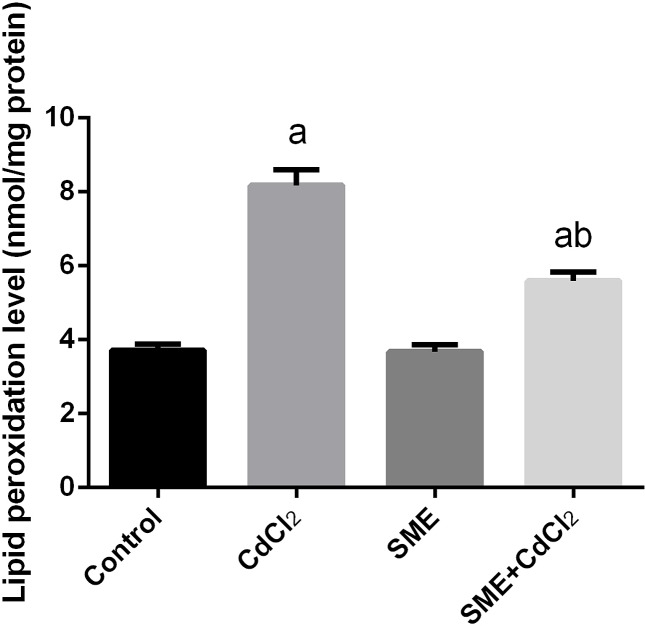
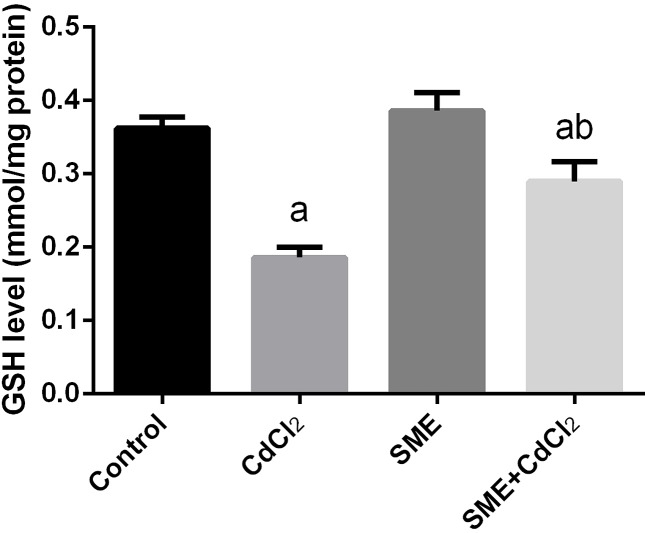
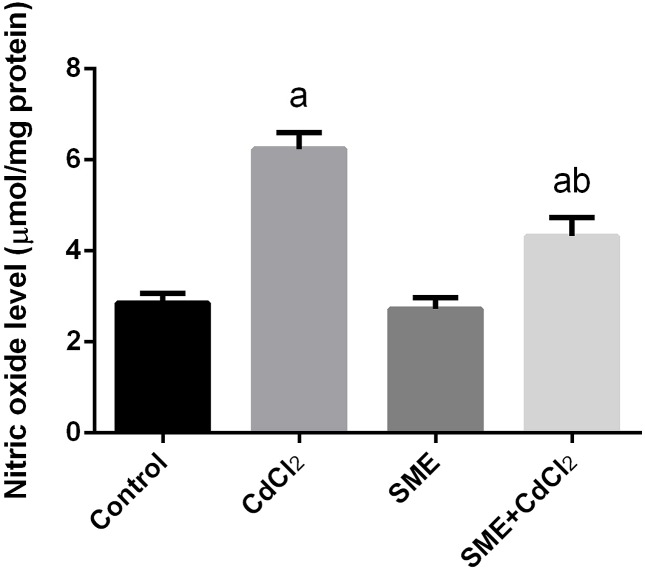
Compared with the control group, the group exposition to CdCl2 injection showed an increase in the levels of LPO and nitric oxide (NO) and a significant decrease in the GSH levels (P<0.05) thus indicating an impairment in the oxidative balance in the brain tissue. The rats treated with a combination of CdCl2 and SME showed restoration of these levels to the control levels (P<0.05) (Figures 3–5).
Figure 3. Ameliorative effects of the pretreatment with SME on the LPO level in rats intoxicated with CdCl2 for 5 days.
Data are expressed as the mean ± S.E.M. (n=8). aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Figure 5. Ameliorative effects of the pretreatment with SME on GSH content in rats intoxicated with CdCl2 for 5 days.
Data are expressed as the mean ± S.E.M. (n=8). aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Figure 4. Ameliorative effects of the pretreatment with SME on the NO level in rats intoxicated with CdCl2 for 5 days.
Data are expressed as the mean ± S.E.M. (n=8). aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
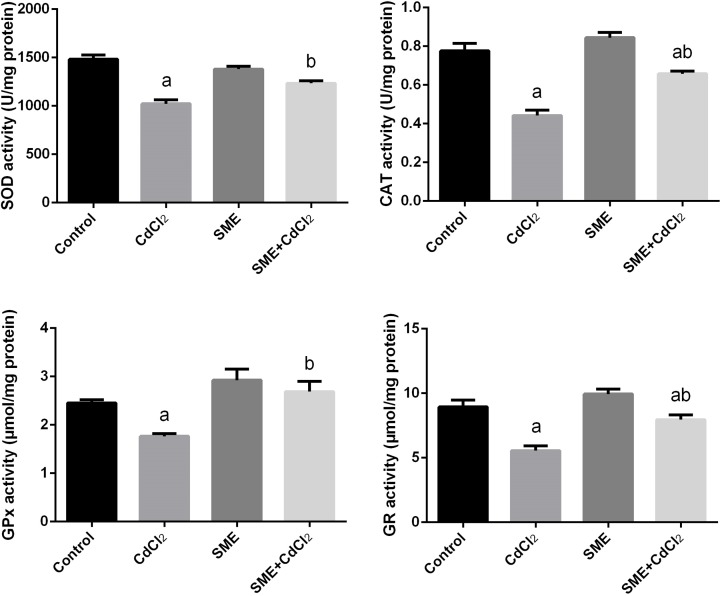
Our study showed changes in the activities of antioxidant enzymes namely SOD, CAT, GPx, and GR in the brain homogenate of the experimental rats (Figure 6). We observed a significant decrease (P<0.05) in the activities of SOD, CAT, GPx, and GR enzymes in CdCl2-intoxicated rats; moreover, compared with CdCl2-intoxicated rats, rats treated with SME showed an increase in the activities of these antioxidant enzymes.
Figure 6. Ameliorative effects of the pre-administration of SME on the antioxidant enzyme activities in the brain tissue of rats exposed to CdCl2 for 5 days.
Data are expressed as the mean ± S.E.M. (n=8). aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Exposition to CdCl2 significantly down-regulated (P<0.05) the mRNA expression of SOD2, CAT, GPx1, and GR with respect to their control values. Rats pretreated with SME showed a significant (P<0.05) increase in the expression of these antioxidant enzymes in the brain homogenate of CdCl2-intoxicated rats (Figure 7).
Figure 7. Ameliorative effects of the pre-administration of SME on the mRNA expression of antioxidant enzymes in the brain tissue of rats exposed to CdCl2 for 5 days.
Data of mRNA levels (mean ± S.E.M. of three assays) were normalized to GAPDH mRNA level and are shown as fold induction (in log2 scale) relative to the mRNA level in the control. aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Strawberry attenuates cadmium-induced apoptosis
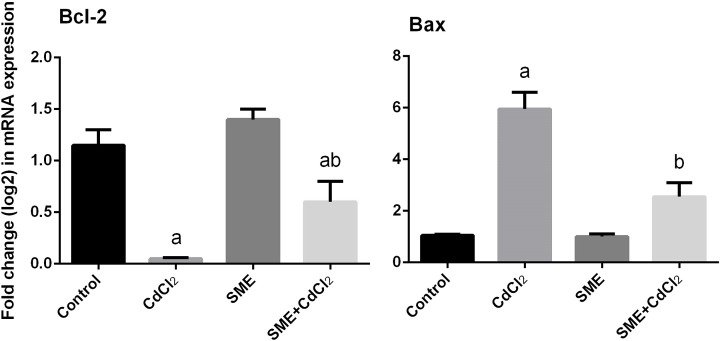
We determined the antiapoptotic activities of SME by examining the expression of Bcl-2 and Bax in the brain tissue. qRT-PCR findings showed that the mRNA expression level of Bcl-2 was down-regulated, whereas the Bax expression was up-regulated in CdCl2-treated rats. In addition, administration of SME before exposition to CdCl2 significantly alleviated the changes (P<0.05) in the levels of these proapoptotic markers (Figure 8).
Figure 8. Mitigation effects of the pre-administration of SME on the mRNA levels of Bcl-2 and Bax in the brain tissue of rats exposed to CdCl2 for 5 days.
Data of mRNA levels (mean ± S.E.M. of three assays) were normalized to GAPDH mRNA level and are shown as fold induction (in log2 scale) relative to the mRNA level in the control. aP<0.05, significant change with respect to control; bP<0.05, significant change with respect to CdCl2 using Duncan’s post-hoc test.
Strawberry attenuates cadmium-induced histopathological alternation
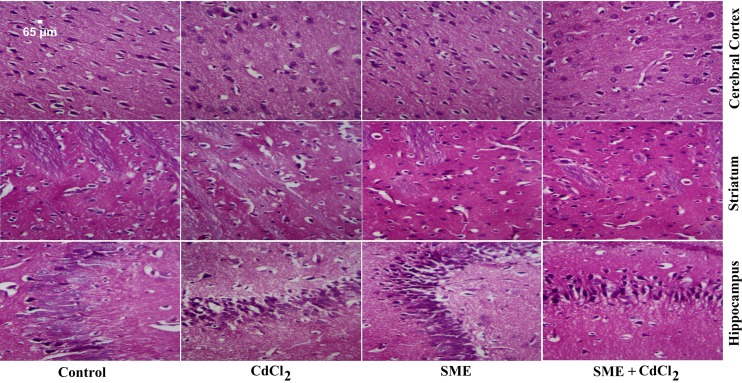
The brains of control rats exhibited their general characteristic shape. The nuclei of these neurones were normal, large, and centrally located (Figure 9). CdCl2-intoxicated rats showed marked damage to different regions of the brain, indicated by the degenerated and pyknotic nuclei in the hippocampus, degenerated, and pyknotic neurones in the striatum, and the presence of small shrunken cells in the cortex. Treatment with SME before administration of CdCl2 had protective effects on neurones, which suggested that the SME can alleviate neural injury induced by CdCl2 injection; however, despite pretreatment with SME, the morphology of some neuronal cells remained altered.
Figure 9. Effect of SME on the histological changes caused by CdCl2 injection in the brain of rats.
Sections of control rats show normal architecture in the different parts of brain. Sections from the CdCl2-intoxicated group show extensive neuronal damage, apoptotic neurones, and degeneration of Purkinje neurones. Sections of the SME-treated group show a normal structure. Sections of brains where SME was pre-administered to CdCl2 show improvement in the neuronal structure (H&E, ×400).
Strawberry attenuates cadmium-induced inflammation
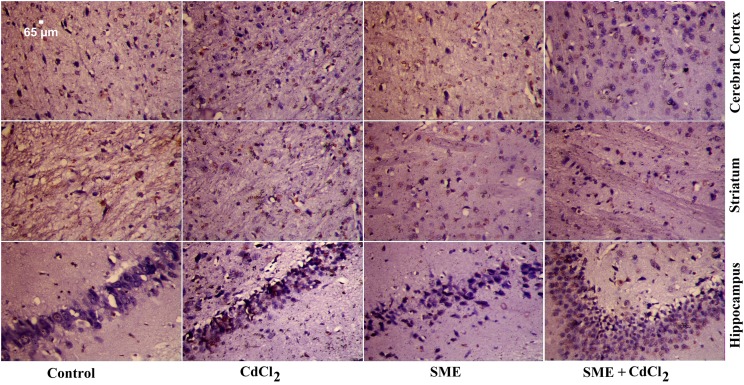
We determined the anti-inflammatory activity of SME by measuring the protein expression of TNF-α. While the protein expression of TNF-α was significantly up-regulated in the CdCl2-intoxicated group (Figure 10), the group pretreated with SME showed a marked down-regulation of TNF-α in the brain homogenate.
Figure 10. Immunohistochemical study of TNF-α in different regions of the brain.
Sections of the cerebral cortex, striatum, and hippocampus were obtained from control, CdCl2, SME, and SME + CdCl2-intoxicated rats. Few TNF-α-positive cells were observed in the control and SME groups. Increased TNF-α immunoreactivity was observed in CdCl2-intoxicated group. Moderate TNF-α immunoreactivity was observed in SME + CdCl2-treated group (×400).
Discussion
To determine the biological activities of constituents present in the fruits and vegetables, the use of the whole extract is better than using individual compounds purified or isolated from the extract [36]. Recent studies have shown protective effects of strawberry extract, which contains high levels of phytochemicals, in different animal and cellular models of oxidative stress-induced diseases [37,38]. This study was performed to investigate the neuroprotective role of SME in Cd-induced neuronal toxicity in the brain tissue of male albino rats.
Cd is a toxic heavy metal that causes harmful effects on the cellular and metabolic systems in humans and animals. Our results showed that rats intoxicated with Cd had high levels of the metal in the brain tissue (approximately 700-fold of the control values) after 5 days of treatment. The accumulation of Cd in the brain tissue may be because of its ability to cross the blood–brain barrier [39]. Once inside, Cd accumulates in different brain tissue and causes cellular dysfunction [7,40]. Oral administration of SME was effective and markedly accelerated the clearance of Cd from the brain tissue after termination of exposure. The protective effects of SME may be attributed to the flavonoids content in the SME which sequester metal ions [41]. Previous reports showed that strawberry and its active constituents can cross BBB and may chelate Cd [42,43].
Cd neurotoxicity is due to the production of ROS, which leads to oxidative stress [44]. To confirm the mechanism underlying Cd neurotoxicity, we measured the levels of LPO, NO, GSH, and the activities of antioxidant enzymes (SOD, CAT, GR, and GPx) in the brain homogenate of rats. Our findings showed that exposure to Cd (6.5 mg/kg body weight) for 5 consecutive days induced neuronal alterations through the depletion of antioxidant defense mechanisms, which disturbs the cellular redox and leads to oxidative stress. This finding was confirmed by an increase in the levels of LPO and NO and a decrease in the GSH levels and the activities of SOD, CAT, GR, and GPx. The decrease in the levels of antioxidant enzymes may be attributed to the accumulation of Cd in the brain tissue which leads to the consumption of the GSH pool [45]. Depletion in the GSH content inactivates the antioxidant enzymes; in addition, Cd binds to the sulphydryl (–SH) group of the oxidative enzymes, which leads to their inhibition [11]. Treatment with SME significantly restored the balance between the production of ROS and the antioxidant system through the inhibition of the LPO, NO production, and an improvement in the oxidant/antioxidant status in the brain homogenate. High levels of flavonoids in the SME act as a chelator for Cd [41].
The effect of SME on neuronal antioxidant enzymes in Cd-intoxicated rats was determined by measuring the transcriptional levels of SOD2, CAT, GPx1, and GR. Our results showed down-regulation of all these genes compared with their control levels, which may be because of production of ROS after Cd exposure. The pre-administration of SME up-regulates the expression of the examined antioxidant genes in the brain tissue. Overexpression of antioxidant genes provides neuroprotection against the consequence of oxidative stress [46].
Heavy metals have been widely associated with severe histopathological alterations in the brain tissue [47]. Histological examination of the brain tissue indicates that Cd intoxication causes abnormal structural changes in the brain tissue, including apoptosis, nuclear vacuolization, and lymphocytic inflammatory changes. Once inside the brain, Cd changes the cortical micro- and macrostructures. In the hippocampus, Cd destroys the neurones and glial cells in the white matter [48].
Cd induces neuropathological and neurochemical alterations in the brain, which causes severe damage, including encephalopathy, peripheral neuropathy, and hemorrhage. Moreover, the exposition to Cd alters the cortical neuroglia and pyramidal and granule cells [49]. In addition, Cd decreases attention, impairs memory and olfactory functions, and hypernociception by affecting the structure of nerve cells and parenchyma [50]. Furthermore, the cells are distorted and shrunken because of damage to the structural and functional biosynthesis of cellular proteins, nucleic acids, certain enzymes, and various neurotransmitters [49].
A previous study has shown that Cd leads to apoptosis by affecting the calcium homeostasis in different cells by suppressing calcium-dependent ATPase or by stimulating the inositol triphosphate pathway or interfering with protein kinase C (PKC), mitogen-activated protein kinase, and phospholipase C [51]. qRT-PCR analysis showed an up-regulation in the expression of Bax (an apoptosis-inducing gene) and down-regulation in the expression of Bcl-2 (an apoptosis-inhibiting gene) in the brain tissue. This finding may be attributed to the ability of Cd to enhance the influx of Ca2+ into the mitochondria, which disrupts the normal metabolism of mitochondria leading to apoptosis and growth arrest in neuronal cells [52,53]. Rats pretreated with SME suppressed apoptosis in the brain tissue, which suggests that SME increases neuronal viability via the inhibition of Bax-induced apoptosis.
Liu et al. [54] showed that the level of serum and hepatic TNF-α increased after exposure of mice to different doses of Cd in the drinking water for 21 days. Moreover, the i.p. injection of Cd (1 mg of Cd/kg) increased the protein expression of TNF-α in the splenic tissue of rats [55]. In addition, the expression of TNF-α in rat testis increased after injection with CdSO4 (3 mg/kg body weight) after 12, 24, and 48 h. Treatment with different strawberry extracts showed anti-inflammatory effects in different experimental models. Oral administration of strawberry leaf extract decreases the serum levels of TNF-α in a rat model of diabetic nephropathy [56]. Oral administration of different strawberry extracts down-regulated the expression of TNF-α in nicotinamide–streptozotocin-induced diabetes in rats [57]. Furthermore, we previously showed that the methanolic extract of strawberry effectively attenuated cadmium-induced inflammation in liver [21], kidney [5], and testis [22] tissues. Our findings showed that compared with Cd-intoxicated rats, rats treated with SME showed down-regulation of TNF-α expression in the brain tissue.
The present study also has limitations, as we administered SME before Cd and to prove the chelation effect of strawberry fruit, we should administer SME post to cadmium and measure Cd concentration in both brain and urine to confirm the ability of SME to clear Cd from the brain and enhance its excretion through the urine. However, many flavonoids such as those in SME can interact with metal and lead to chelation formation.
Overall, our results showed that the SME exerts protective effects against Cd-mediated brain injury through its potent antioxidant, anti-apoptotic, and anti-inflammatory activities, and these effects may be attributed to the high levels of flavonoids and polyphenols in the SME. However, more experiment will be necessary to confirm the biological activities of strawberry on neuronal tissues.
Supporting information
Table S1. Identification of compounds by GC-MS in Fragaria ananassa methanolic extract.
Abbreviations
- CAT
catalase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GR
glutathione reductase
- GSH
glutathione
- i.p
intraperitoneally
- LPO
lipid peroxidation
- MDA
malondialdehyde
- NIH
National Institutes of Health
- ROS
reactive oxygen species
- SME
Fragaria ananassa methanolic extract
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor α
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
All the authors contributed equally to this work.
Funding
This work was supported by the Deanship of Scientific Research at King Saud University through the Research Group Project [grant number RG-1439-034].
References
- 1.Adefegha S.A., Omojokun O.S., Oboh G., Fasakin O. and Ogunsuyi O. (2016) Modulatory effects of ferulic acid on cadmium-induced brain damage. J. Evid. Based Complement. Altern. Med. 21, NP56–NP61 10.1177/2156587215621726 [DOI] [PubMed] [Google Scholar]
- 2.Almeer R.S., Alarifi S., Alkahtani S., Ibrahim S.R., Ali D. and Moneim A. (2018) The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 106, 1490–1498 10.1016/j.biopha.2018.07.089 [DOI] [PubMed] [Google Scholar]
- 3.Satarug S., Swaddiwudhipong W., Ruangyuttikarn W., Nishijo M. and Ruiz P. (2013) Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 121, 531–536 10.1289/ehp.1104769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luparello C., Sirchia R. and Longo A. (2011) Cadmium as a transcriptional modulator in human cells. Crit. Rev. Toxicol. 41, 75–82 10.3109/10408444.2010.529104 [DOI] [PubMed] [Google Scholar]
- 5.Elkhadragy M.F., Al-Olayan E.M., Al-Amiery A.A. and Abdel Moneim A.E. (2018) Protective effects of Fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol. Trace Elem. Res. 181, 378–387 10.1007/s12011-017-1062-7 [DOI] [PubMed] [Google Scholar]
- 6.Gupta V.K., Singh S., Agrawal A., Siddiqi N.J. and Sharma B. (2015) Phytochemicals mediated remediation of neurotoxicity induced by heavy metals. Biochem. Res. Int. 2015, 534769 10.1155/2015/534769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Omairi N.E., Radwan O.K., Alzahrani Y.A. and Kassab R.B. (2018) Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab. Brain Dis. 33, 1121–1130 10.1007/s11011-018-0222-6 [DOI] [PubMed] [Google Scholar]
- 8.Lopez E., Figueroa S., Oset-Gasque M.J. and Gonzalez M.P. (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 138, 901–911 10.1038/sj.bjp.0705111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z., Yang S., Qian S.Y., Hong J.S., Kadiiska M.B., Tennant R.W.. et al. (2007) Cadmium-induced toxicity in rat primary mid-brain neuroglia cultures: role of oxidative stress from microglia. Toxicol. Sci. 98, 488–494 10.1093/toxsci/kfm106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez-Armenta M. and Rios C. (2007) Cadmium neurotoxicity. Environ. Toxicol. Pharmacol. 23, 350–358 10.1016/j.etap.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Renugadevi J. and Prabu S.M. (2009) Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 256, 128–134 10.1016/j.tox.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 12.Chater S., Douki T., Garrel C., Favier A., Sakly M. and Abdelmelek H. (2008) Cadmium-induced oxidative stress and DNA damage in kidney of pregnant female rats. C. R. Biol. 331, 426–432 10.1016/j.crvi.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 13.Dkhil M.A., Al-Quraishy S., Diab M.M., Othman M.S., Aref A.M. and Abdel Moneim A.E. (2014) The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem. Toxicol. 74, 98–106 10.1016/j.fct.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 14.Abdel Moneim A.E., Bauomy A.A., Diab M.M., Shata M.T., Al-Olayan E.M. and El-Khadragy M.F. (2014) The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol. Trace Elem. Res. 160, 392–399 10.1007/s12011-014-0066-9 [DOI] [PubMed] [Google Scholar]
- 15.Shagirtha K., Bashir N. and MiltonPrabu S. (2017) Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol. Ind. Health 33, 454–468 10.1177/0748233716665301 [DOI] [PubMed] [Google Scholar]
- 16.Upadhyay R.K. (2011) Plant natural products: their pharmaceutical potential against diseases and drug resistant microbial pathogens. J. Pharm. 4, 1179–1185 [Google Scholar]
- 17.Hashemi S. and Davoodi H. (2012) Herbal plants as new immuno-stimulator in poultry industry: a review. Asian J. Anim. Vet. Adv. 7, 105–116 10.3923/ajava.2012.105.116 [DOI] [Google Scholar]
- 18.Bak M.J., Das Gupta S., Wahler J. and Suh N. (2016) Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Biol. 40–41, 170–191 10.1016/j.semcancer.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goff S.A. and Klee H.J. (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311, 815–819 10.1126/science.1112614 [DOI] [PubMed] [Google Scholar]
- 20.Oszmiański J. and Wojdyło A. (2009) Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur. Food Res. Technol. 228, 623–631 10.1007/s00217-008-0971-2 [DOI] [Google Scholar]
- 21.Elkhadragy M.F. and Abdel Moneim A.E. (2017) Protective effect of Fragaria ananassa methanolic extract on cadmium chloride (CdCl2)-induced hepatotoxicity in rats. Toxicol. Mech. Methods, 27, 335–345, 10.1080/15376516.20171285973 [DOI] [PubMed] [Google Scholar]
- 22.Elmallah MIY, Elkhadragy M.F., Al-Olayan E.M. and Abdel Moneim A.E. (2017) Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 18, 10.3390/ijms18050957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamed S.S., Al-Yhya N.A., El-Khadragy M.F., Al-Olayan E.M., Alajmi R.A., Hassan Z.K.. et al. (2016) The protective properties of the strawberry (Fragaria ananassa) against carbon tetrachloride-induced hepatotoxicity in rats mediated by anti-apoptotic and upregulation of antioxidant genes expression effects. Front. Physiol. 7, 325 10.3389/fphys.2016.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 25.Kubaszewski L., Ziola-Frankowska A., Frankowski M., Nowakowski A., Czabak-Garbacz R., Kaczmarczyk J.. et al. (2014) Atomic absorption spectrometry analysis of trace elements in degenerated intervertebral disc tissue. Med. Sci. Monit. 20, 2157–2164 10.12659/MSM.890654 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Ohkawa H., Ohishi N. and Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 27.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S. and Tannenbaum S.R. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138 10.1016/0003-2697(82)90118-X [DOI] [PubMed] [Google Scholar]
- 28.Ellman G.L. (1959) Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. (1984) Catalase in vitro. Methods Enzymol. 105, 121–126 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 30.Nishikimi M., Appaji N. and Yagi K. (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854 10.1016/S0006-291X(72)80218-3 [DOI] [PubMed] [Google Scholar]
- 31.Paglia D.E. and Valentine W.N. (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169 [PubMed] [Google Scholar]
- 32.Almeer R.S. and Abdel Moneim A.E. (2018) Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxid. Med. Cell. Longev. 2018, 8487248 10.1155/2018/8487248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bancroft J. and Gamble M. (eds). (2008) Bancroft’s Theory and Practice of Histological Techniques, 6th edn, Elsevier, London: Churchill Livingstone [Google Scholar]
- 34.Sternberger L.A. (1979) Immunocytochemistry, 2nd edn, Wiley Medical Publication, Wiley, New York [Google Scholar]
- 35.Zhang J., Wang X., Yu O., Tang J., Gu X., Wan X.. et al. (2011) Metabolic profiling of strawberry (Fragaria x ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 62, 1103–1118 10.1093/jxb/erq343 [DOI] [PubMed] [Google Scholar]
- 36.Somasagara R.R., Hegde M., Chiruvella K.K., Musini A., Choudhary B. and Raghavan S.C. (2012) Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE 7, e47021 10.1371/journal.pone.0047021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amatori S., Mazzoni L., Alvarez-Suarez J.M., Giampieri F., Gasparrini M., Forbes-Hernandez T.Y.. et al. (2016) Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 6, 30917 10.1038/srep30917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spagnuolo C., Flores G., Russo G.L. and Ruiz Del Castillo M.L. (2016) A phenolic extract obtained from methyl jasmonate-treated strawberries enhances apoptosis in a human cervical cancer cell line. Nutr. Cancer 68, 1140–1150 10.1080/01635581.2016.1208831 [DOI] [PubMed] [Google Scholar]
- 39.Shukla G.S. and Chandra S.V. (1987) Concurrent exposure to lead, manganese, and cadmium and their distribution to various brain regions, liver, kidney, and testis of growing rats. Arch. Environ. Contam. Toxicol. 16, 303–310 10.1007/BF01054947 [DOI] [PubMed] [Google Scholar]
- 40.Sinha M., Manna P. and Sil P.C. (2008) Cadmium-induced neurological disorders: prophylactic role of taurine. J. Appl. Toxicol. 28, 974–986 [DOI] [PubMed] [Google Scholar]
- 41.Malesev D. and Kunti V. (2007) Investigation of metal–flavonoid chelates and the determination of flavonoids via metal–flavonoid complexing reactions. J. Serb. Chem. Soc. 72, 921–939 10.2298/JSC0710921M [DOI] [Google Scholar]
- 42.Campos-Bedolla P., Walter F.R., Veszelka S. and Deli M.A. (2014) Role of the blood–brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 45, 610–638 10.1016/j.arcmed.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 43.Faria A., Mateus N. and Calhau C. (2012) Flavonoid transport across blood-brain barrier: Implication for their direct neuroprotective actions. Nutr. Aging 1, 89–97 [Google Scholar]
- 44.Chen L., Xu B., Liu L., Luo Y., Zhou H., Chen W.. et al. (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 50, 624–632 10.1016/j.freeradbiomed.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onyema O.O., Farombi E.O., Emerole G.O., Ukoha A.I. and Onyeze G.O. (2006) Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J. Biochem. Biophys. 43, 20–24 [PubMed] [Google Scholar]
- 46.Sheldon R.A., Jiang X., Francisco C., Christen S., Vexler Z.S., Tauber M.G.. et al. (2004) Manipulation of antioxidant pathways in neonatal murine brain. Pediatr. Res. 56, 656–662 10.1203/01.PDR.0000139413.27864.50 [DOI] [PubMed] [Google Scholar]
- 47.Al-Quraishy S., Dkhil M.A., Ibrahim S.R. and Abdel Moneim A.E. (2016) Neuroprotective potential of Indigofera oblongifolia leaf methanolic extract against lead acetate-induced neurotoxicity. Neural. Regen. Res. 11, 1797–1803 10.4103/1673-5374.194749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Tarras A.E.-S., Attia H.F., Soliman M.M., El Awady M.A. and Amin A.A. (2016) Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 29, 398–407 10.1177/0394632016651447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afifi O.K. and Embaby A.S. (2016) Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. J. Microsc. Ultrastruct. 4, 36–45 10.1016/j.jmau.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B. and Du Y. (2013) Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013, 12 10.1155/2013/898034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son Y.O., Lee J.C., Hitron J.A., Pan J., Zhang Z. and Shi X. (2010) Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicol. Sci. 113, 127–137 10.1093/toxsci/kfp259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu B., Chen S., Luo Y., Chen Z., Liu L., Zhou H.. et al. (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 6, e19052 10.1371/journal.pone.0019052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Y., Jiang C.Y., Xu H., Sun Y., Hu F.F., Bian J.C.. et al. (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS ONE 8, e64330 10.1371/journal.pone.0064330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L., Tao R., Huang J., He X., Qu L., Jin Y.. et al. (2015) Hepatic oxidative stress and inflammatory responses with cadmium exposure in male mice. Environ. Toxicol. Pharmacol. 39, 229–236 10.1016/j.etap.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 55.Demenesku J., Mirkov I., Ninkov M., Popov Aleksandrov A., Zolotarevski L., Kataranovski D.. et al. (2014) Acute cadmium administration to rats exerts both immunosuppressive and proinflammatory effects in spleen. Toxicology 326, 96–108 10.1016/j.tox.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim D.S. and Abd El-Maksoud M.A. (2015) Effect of strawberry (Fragaria x ananassa) leaf extract on diabetic nephropathy in rats. Int. J. Exp. Pathol. 96, 87–93 10.1111/iep.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandave P., Khadke S., Karandikar M., Pandit V., Ranjekar P., Kuvalekar A.. et al. (2017) Antidiabetic, lipid normalizing, and nephroprotective actions of the strawberry: a potent supplementary fruit. Int. J. Mol. Sci. 18, 10.3390/ijms18010124 [DOI] [PMC free article] [PubMed] [Google Scholar]