Abstract
Background
Most current cell‐based regenerative therapies are based on the indirect induction of the affected tissues repair. Xenogeneic cell‐based treatment with expanded human placenta stromal cells, predominantly from fetal origin (PLX‐RAD cells), were shown to mitigate significantly acute radiation syndrome (ARS) following high dose irradiation in mice, with expedited regain of weight loss and haematopoietic function. The current mechanistic study explores the indirect effect of the secretome of PLX‐RAD cells in the rescue of the irradiated mice.
Methods
The mitigation of the ARS was investigated following two intramuscularly (IM) injected 2 × 106 PLX‐RAD cells, 1 and 5 days following 7.7 Gy irradiation. The mice survival rate and their blood or bone marrow (BM) cell counts were followed up and correlated with multiplex immunoassay of a panel of related human proteins of PLX‐RAD derived secretome, as well as endogenous secretion of related mouse proteins. PLX‐RAD secretome was also tested in vitro for its effect on the induction of the migration of BM progenitors.
Results
A 7.7 Gy whole body mice irradiation resulted in ~25% survival by 21 days. Treatment with two IM injections of 2 × 106 PLX‐RAD cells on days 1 and 5 after irradiation mitigated highly significantly the subsequent lethal ARS, with survival rate increase to nearly 100% and fast regain of the initial weight loss (P < 0,0001). This was associated with a significant faster haematopoiesis recovery from day 9 onwards (P < 0.01). Nine out of the 65 human proteins tested were highly significantly elevated in the mouse circulation, peaking on days 6–9 after irradiation, relative to negligible levels in non‐irradiated PLX‐RAD injected mice (P < 0.01). The highly elevated proteins included human G‐CSF, GRO, MCP‐1, IL‐6 and lL‐8, reaching >500 pg/mL, while MCP‐3, ENA, Eotaxin and fractalkine levels ranged between ~60–160pg/mL. The detected radiation‐induced PLX‐RAD secretome correlated well with the timing of the fast haematopoiesis regeneration. The radiation‐induced PLX‐RAD secretome seemed to reinforce the delayed high levels secretion of related mouse endogenous cytokines, including GCSF, KC, MCP‐1 and IL‐6. Additional supportive in vitro studies also confirmed the ability of cultured PLX‐RAD secretome to induce accelerated migration of BM progenitors.
Conclusions
A well‐regulated and orchestrated secretion of major pro‐regenerative BM supporting secretome in high dose irradiated mice, treated with xenogeneic IM injected PLX‐RAD cells, can explain the observed mitigation of ARS. This seemed to coincide with faster haematopoiesis regeneration, regain of severe weight loss and the increased survival rate. The ARS‐related stress signals activating the IM injected PLX‐RAD cells for the remote secretion of the relevant human proteins deserve further investigation.
Keywords: Placental stromal cells, X‐ray irradiation, Acute radiation syndrome, Cytokines, Secretome, Intramuscular injection
1. Introduction
Acute radiation syndrome (ARS) following exposure to high dose ionizing radiation is associated with multiple severe systemic multi‐organ failures. The damages depend on the modality, the source, the dose and the rate of the radiation exposure.1, 2, 3, 4, 5, 6 Even low doses of whole body irradiation can severely damage the radiosensitive haematopoietic system,7 while moderate high dose may lead to other systemic effects such as cachexia and muscle damages.8 Early effects of ARS are manifested by haematopoietic and gastrointestinal failure, which are followed by the delayed effects of acute radiation exposure, characterized by time and dose‐dependent multi‐organ injury. A common possible solution to treat the bone marrow (BM) failure is the transplantation of haematopoietic stem cell (HSC). However, individually matched BM transplantation (BMT) is not always available and may not be practical in events associated with multiple casualties with no accurate estimation of the doses of the radiation exposure.1, 9, 10, 11, 12, 13 It also does not address damages to other organs. Other proposed approaches are based on the treatment with pertinent growth factors, such as erythropoietin (EPO), granulocyte and granulocyte‐macrophage colony stimulating factors (G‐CSF and GM‐CSF, respectively).14, 15, 16, 17, 18, 19 Some of these factors have already been approved as pro‐regenerative emergency and investigative new drug to treat BM failure following aggressive radiotherapy or chemotherapy1, 18, 20 Several other drugs and growth factors, as well as anti‐inflammatory cytokines and chemokines and prostaglandins, have also been proposed for mitigation of ARS, based on various mechanisms, such as their anti‐oxidative and anti‐apoptotic activity.21, 22, 23, 24
Early studies employing BM or other tissue derived mesenchymal stromal/stem cells (MSC) proposed that the implanted cells take an active role in replacing the depleted cells in damaged tissues by their differentiation to various relevant mesenchymal cell types and their subsequent integration in the damaged tissues.25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Nevertheless, to date, it is becoming more commonly accepted that the main mechanism of pro‐regenerative action of such cells is via their paracrine/endocrine effects.33, 35, 36, 37, 38, 39 Cell therapies based on BM derived MSC (BM‐MSC) were also proposed as an option for enhancing the recovery from ARS.25, 35, 37, 38, 40, 41, 42, 43 A wide variety of cell types have been investigated for their potency in this respect.25, 40, 44, 45, 46, 47, 48, 49, 50, 51, 52 Nevertheless, some studies questioned the pro‐regenerative effect of these cells on the haematopoietic system.53 To date, the best explanation for the role of BM‐MSC in mitigation of radiation effects seems to be their induced systemic support of the affected BM by the secretion of relevant pro‐regenerative and haematopoiesis promoting factors.28, 37, 38
The human placenta is an easily accessible source for isolation of rapidly proliferating human stromal cells.54 Placental stromal cell populations were initially investigated as an easily available alternative to BM‐MSC for stem‐cell based tissue repair. As to the trophic effect of these cells, some studies suggested that they may have advantages over stromal cells from other tissues for enhancing early development of the haematopoietic system.55, 56
It was previously reported that intravenously injected cells, particularly stromal cells, such as BM‐MSCs, are predominantly trapped in the lung immediately following their administration.36, 57 In contrast, intramuscular (IM) injection allows the delivery of higher numbers of cells, which remain within the area of the injection site for at least 1–3 weeks with no apparent adverse effects.26 Therefore, the apparently complication‐free IM cell delivery was adopted for the current study.
PLacenta‐eXpanded (PLX) cells are adherent human‐derived placental cells with typical mesenchymal cell phenotype, manufactured for cell therapy in highly regulated standards. The cell product PLX, consisting of placental stromal cells (PSC) from pure maternal tissues, was initially developed and tested pre‐clinically and clinically as an allogeneic cell therapy for treating different chronic conditions for peripheral artery disease (e.g. critical limb ischemia), neurological disorders, pulmonary hypertension, muscle injury and preeclampsia.26, 40, 58, 59, 60, 61, 62 While conducting a preliminary study on mitigation of radiation effects, cells consisting predominantly of expanded mesenchymal stromal cells of the newborn tissues of the placenta (which we termed PLX‐RAD) were found to be most effective in mitigating ARS. Following IM injections with PLX‐RAD cells to 7.7 Gy pre‐irradiated mice, close to 100% survival was recorded, relative to ~30% survival of the irradiated untreated controls.26
The current continuation study was set in order to comprehend the mode‐of‐action of PLX‐RAD‐based cell therapy by monitoring the secreted human and murine protein profile in the plasma of the high dose irradiated mice at different time points. This mechanistic animal study, supported by in vitro data, proposes a mechanism of action of the PLX‐RAD cells as a well‐controlled highly effective cell therapy for lethal ARS which could be implied for other similar cell‐based therapies.
2. Methods and martials
2.1. Animals
C3H/HeNHsd male mice, 8–10 weeks old, were purchased from Harlan/Envigo‐RMS Israel Ltd (ISO 9001:200) The mice were kept in specific pathogen free conditions at Hadassah Hebrew University animal colony or at Harlan (Envigo) Israel el, Ltd. They were acclimated for at least 5 days before the initiation of the experiments. BALB‐C mice for BM extraction (ethics approval # IL‐14‐04‐120) were purchased from Harlan/Envigo‐RMS Israel. The animal model experiments were approved with Ethical Animal Welfare Certificates #GB06/68708 of the Institutional Animal Welfare Committee of the Hebrew University of Jerusalem #MD‐12‐13296‐4 (with modified approved versions/amendments MD‐16‐14727‐4 and MD 11‐12877‐4).
The personnel involved in the animal part of the study were supervised personally by the Institutional responsible veterinary staff on the humane handling of mice in this specific high‐risk protocol associated with expected severe life‐threatening heavy irradiation effects. They were instructed how to monitor the animals discomfort at all stages of the study and assure their minimal suffering.
2.2. Mice irradiation and follow‐up
All the irradiated mice were subjected to total body irradiation (TBI) of 7.7 Gy on day 0 (1 day prior to the first IM injection of cells or vehicle control solution). The mice were irradiated by a clinical 6–18 MeV LINAC (Varian, Medical Systems, CA, USA), in a sterilized box with height restriction for homogenous dose distribution. A 1 cm plastic dose build‐up layer was used to assure uniform, accurate and homogenous dose exposure as calibrated in the actual experimental setup by high sensitivity ionizing chambers.
All the irradiated mice were weighed daily in all working days in the week and in weekends in case of stress associated with their pre‐irradiation. They were inspected twice daily upon the early appearance of any signs of stress or sharp weight loss. In the cages housing mice suffering from severe weight loss (>20%), wetted food was supplied. Mice which suffered from dehydration were injected IP with 05‐1 mL of saline. In spite of the close tight follow‐up of the mouse condition, in about 20–25%, the deadly radiation induced pancytopenia occurred by fast deterioration of their health condition between the routine follow‐ups. If severe signs of stress occurred, including decreased mobility, heavy breathing, curving back, sleepiness or decreased response to stimulation, all hinting for irreversible deterioration of their health condition, the mice were immediately humanely euthanized and counted as non‐surviving at that time point. As previously reported, 2 × 106 PLX‐RAD cells injected IM on day 1 and 5 following 7.7 Gy irradiation, mitigate very significantly the expected lethal radiation‐induced ARS, as reflected by major haematopoiesis failure and weight loss.26
2.3. Experimental setup
The experimental design is presented in Figure 1A. For cytokine analyses, the tested mice were divided into three main treatment protocols. The main experimental group was irradiated and injected IM with 2 × 106 PLX‐RAD cells in the vehicle solution (Plasma‐lyte, Baxter, USA) 1 and 5 days after irradiation. In parallel, the control irradiated mice were injected only with the vehicle solution. A control group of non‐irradiated mice was treated with PLX‐RAD cells 1 and 5 days after the initiation of the experiment. Another naïve control group was not treated or irradiated. At different time points, between days 2 and 23 of the experiment, four to five mice were sacrificed from each of the experimental groups, and their blood was collected for further analyses. In vitro experiments were set in parallel to study the pro‐migratory effects of factors in conditioned medium (CM) secreted by PLX‐RAD cells in culture conditions as described in Figure 1B.
Figure 1.
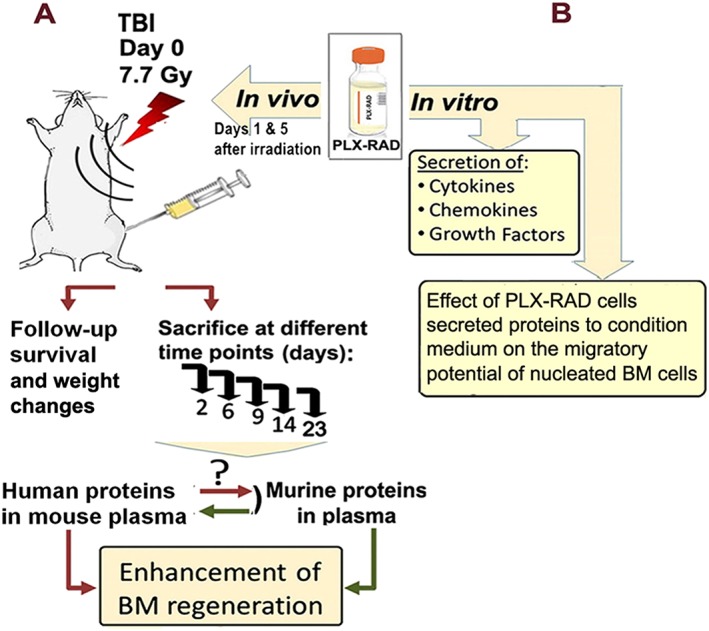
Experimental overview flow of the animal study and in vitro experiments for evaluation of the mechanism of action of PLX‐RAD cells in mitigation of ARS. The mice were irradiated by 7.7 Gy, and blood was collected at selected time points after irradiation and PLX‐RAD treatment for analysis of human and mouse proteins secretion in the plasma (A). For the studies on PLX‐RAD secretome activity in vitro, CM from cultured PLX‐RAD cells was collected and tested for the relevant proteins of interest. Then, the conditioned medium was further tested for its effect on the migration of harvested nucleated mouse BM cells by the trans‐well migration assay (B).
ARS induced mice death occurred within a period of 20 days after their irradiation. The survivors in the different experimental groups at this stage were already in the process of recovery from BM‐related ARS. The follow‐up that was terminated on day 23 still allowed to assay the residual radiation‐induced damages to the haematopoietic system, as reflected by peripheral blood cell counts, before further recovery of the survivors could blur these differences. The updated survival data of the irradiated mice presented in the current report includes previously reported survival data26 with additional mice which were added in this study to assay their secretome for the full period of to 23 days.
The mice to be sacrificed were deeply anaesthetized with ketamine/xylazine, and blood was collected directly from the right ventricle of the heart for complete blood count. Then, these mice were euthanized. BM cells were harvested from both the femurs and tibias of the sacrificed mice as follows: the extremities of the bones were cut off, and the BM plugs were flushed out of the bone cavities with PBS + 2% FCS. The extracted BM cells were suspended in PBS + 2% FCS, and the RBC were lysed by a lysis buffer (Biological Industries, Israel), then the nucleated cells were counted by hematocytometer.
2.4. PLX‐RAD cells and their administration
PLX‐RAD adherent PSCs (also termed PLX‐R18) were isolated from full term placentae of Caesarean section of normal healthy male births in GMP conditions by Pluristem Ltd. (Haifa, Israel). The cells were digested from placental tissue, initially expanded in large tissue culture flasks and then transferred to bioreactors for further controlled 3D‐expansion on non‐woven fibre made carriers. After harvesting, cells were cryopreserved in liquid nitrogen. PLX‐RAD cells consisted of >70% fetal (X/Y) cells.58, 61 The extended surface marker profile of the PLX‐RAD cells is shown in Figure 2. By FACS analysis, these cells were negative to haematopoietic end endothelial markers, expressing surface marker profile identical to mesenchymal stromal cells from other sources. Those include also CD146 and CD166 which are shared with other cell types including pericytes, which are proposed to be the source of mesenchymal stromal cells63, 64, 65 (Figure 2). The PLX‐RAD cells in all the treated groups were injected on days 1 and 4 after the mice irradiation. Four to five mice were sacrificed in each of the arms tested at different time points of 0, 2, 6, 9, 14 and 23 days following irradiation for the cytokine assay in the blood plasma. For their delivery, the cryopreserved PLX‐RAD cells were thawed, washed and re‐suspended in PlasmaLyte‐A solution as the vehicle for their injection (Baxter, Deerfield, IL, USA).
Figure 2.

Surface markers profile of the PLX‐RAD placenta derived stromal cells. FACS histograms of the PLX‐RAD cells surface markers similar to those expressed by stromal cells with no expression of typical haematopoietic and endothelial cells markers. The cells are positive to CD146 and 166 which are also shared with other cell types like pericytes.
PlasmaLyte‐A with no cells was used as a vehicle control. In each mouse, a total 2 × 106 viable cell/0.1 mL (50 μL/limb) were injected slowly to the right and left lateral and cranial thigh muscles 1 and 4 days post‐irradiation using fine 27G needles as previously described.26 No discomfort of the animals following this IM cell administration was recorded in any of the experiments.
2.5. Collection of conditioned medium from PLX‐RAD cells for in vitro assays
PLX‐RAD cells were thawed and suspended in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma Aldrich), supplemented with 10% fetal bovine serum + 2 mM L‐Glutamine, and cultured in six well‐plate (each well with 0.5 × 106 cells/4 mL DMEM) for 24 h in humidified CO2 incubator (5% CO2, at 37°C). Twenty four hours later, the DMEM was replaced with fresh and neat DMEM for the cytokine assay or alternatively with RPMI 1640 medium +2 mM L‐Glutamine (Biological Industries, Beit Haemek, Israel) supplemented with 0.5% Human Serum Albumin for BM migration assay. Then, PLX‐RAD cells were incubated in the fresh media for additional 24 h. Finally, the CM was centrifuged by 4500 g at 4 °C for 1 min and collected, and cell debris discarded.
2.6. Human and murine protein concentrations in murine plasma or CM of cultured PLX‐RAD cells
The experimental model of the current study is based on the use of multiplex immunoassay for the measurement of the plasma levels of an array of proteins of interest at different time points. A minimal cross‐reactivity was detected between the mouse and human proteins, allowing the separate follow‐up of the human proteins secreted by the IM injected PLX‐RAD cells and the endogenously derived murine proteins.
For the irradiated mice secretome experiments for each time point of interest for each arm of the experiment 4–5 mice were sacrificed and their blood collected. For the time point of day 23, 10 mice of the vehicle control were included for each arm since over 14 days a significant mortality from ARS was recorded, but the four surviving mice in this group could be tested in this time point.
Plasma samples were obtained from the sacrificed mice on day 0, 2, 6, 9, 14 and 23 after irradiation. For in vitro secretome analyses, CM of cultured PLX‐RAD cells was used.
The cytokine levels in the peripheral blood were analysed by Bio‐Plex protein assays of BIO‐RAD (Hercules, CA, USA) with BIO‐RAD software data analysis. The assays were read by Luminex 100 system (Perkin Elmer, Waltham, MA, USA). The levels of human factors were assessed by 39‐Plex platform for the detection and analysis of Eotaxin, Flt‐3L, Fractalkine, G‐CSF, GRO, IL‐6, IL‐8, MCP‐1, MCP‐3, TGFα, IFNγ, IL‐10, MDC, IL‐13, IL‐17A, IL‐1α, IL‐2, MIP‐1β, VEGF, EGF, GM‐CSF, IFNα, IL‐12p40, IL‐12p70, IL‐15, IL‐1RA, sIL‐2RA, IL‐9, IL‐1ß, IL‐3, IL‐4, IL‐5, IL‐7, IP‐10, MIP‐1a, TNFα, TNFβ, sol CD40‐ligand and FGF‐2. Additional human proteins were tested with a 23‐plex platform, designed for analysis of ENA‐78, Eotaxin‐2, BCA‐1, MCP‐4, TARC, Eotaxin‐3, LIF, IL‐33, IL23, I‐309, IL‐16, 6CKine, TPO, SCF, TSLP, TRAIL, CTACK, IL‐28A, IL‐20, MCP‐2, MIP‐1d, IL‐21 and SDF‐1α,β. An additional multiplex platform was set for analysis of PDGF‐AA, PDGF‐BB and RANTES. All antibodies were tested to be prominently specific to detect human proteins with <10% cross‐reactivity with mouse proteins. The levels of murine cytokines were analysed by using murine 15‐plex platform for the following proteins: G‐CSF, GM‐CSF, IFNγ, IL‐1β, IL‐6, IL‐10, IL‐12p70, KC, MCP‐1, MIP‐1α, MIP‐2, IL‐2, IL‐4, IL‐17 and TNFα. All Luminex assays were performed as per the manufacturer's instructions and protocols.
2.7. Collection and process of BM cells for in vitro migration assays
BM was collected from the bones of Balb/C mice. The BM cells were harvested from the long bones as described above. The isolated harvested cells were centrifuged for 10 min at 300 g and re‐suspended in RPMI 1640 medium (Biological Industries, Beit Haemek, Israel), supplemented with 0.5% HSA to be used for BM migration.
2.8. BM cell migration assay in vitro
For the BM cell migration assay, 106 nucleated mouse BM cells were seeded in duplicates per each experimental group on the upper insert of Transwell 24 well plate with 5 μm pores polyester permeable membrane (Corning, purchased from Sigma Aldrich). Subsequently, 0.5 mL of either PLX‐RAD derived CM or fresh RPMI medium with 0.5% HSA (which served as a negative control) were added to the lower chambers of the Transwell plate. A day later, the upper inserts were gently removed, and the migrated cells were collected from the lower chambers and counted by CyQuant NF assay (Life Technologies Corporation, Carlsbad, CA, USA).
2.9. Statistical analyses
Statistical analysis of the data for comparison between the different groups of interest was done by Student's t‐tests of two‐sample assuming equal variances. The significance of the difference between the survival curves was analysed by a Log‐Rank test of the Kaplan–Meier survival curves for both the survival duration and for the endpoint survival rate following different treatments.
3. Results
3.1. Follow‐up with time after irradiation of human PLX‐RAD and murine endogenous proteins
The treatment with 2 × 106 PLX‐RAD cells injected IM on day 1 and 5 after 7.7 Gy irradiation mitigated very significantly the lethal radiation‐induced ARS symptoms of weight loss and haematopoiesis failure.26 The survival of the control irradiated vehicle treated mice by day 23 was only ~ 20% so that higher number of mice had to be used (55) to allow enough survivors to monitor at the end of the follow‐up of survival. In the group of PLX‐RAD treated mice with a survival rate of almost 100% at the end of the follow‐up, 31 mice were sufficient for the followed up.
The multiplex immunoassay measurement of the levels of plasma proteins of interest at different time points allowed monitoring the kinetics the specific human secretome in the different arms tested. The detailed kinetics of the levels of human proteins of interest in the plasma of the PLX‐RAD treated mice and the controls are shown in Figure 3B–J. Only nine out of the 63 human proteins tested showed significantly elevated levels in the mouse circulation. Among these human proteins secreted by the PLX‐RAD cells, G‐CSF, GRO (CXCL1), MCP‐1 (CCL2), IL‐6 and lL‐8 (CXCL8) reached high peak levels of >500 pg/mL 6 to 9 days after irradiation and 2 to 5 days after the two cell injections, respectively. The timing of the proteins secretion seems to correspond to the induction of regeneration of the haematopoietic system. The PLX‐RAD secretome contained growth factors which induce HSC proliferation while participating in their differentiation (such as GCSF), or as inducers of cell migration from the BM into the circulation (i.e. GRO). Additional chemokines which relate to the recruitment of new blood cells to the circulation, such as the https://en.wikipedia.org/wiki/Monocyte and https://en.wikipedia.org/wiki/Macrophage attractant and function regulator MCP‐3 (CCL7), the neutrophil activating protein ENA78 (CXCL5), the eosinophil chemotactic protein Eotaxin (CCL11) and https://en.wikipedia.org/wiki/T_cell and https://en.wikipedia.org/wiki/Monocyte chemoattractant Fractalkine (CX3CL1) were elevated. All these proteins showed similar kinetics with peak proteins concentrations in the mouse circulation in the range of 50–200 pg/mL.
Figure 3.
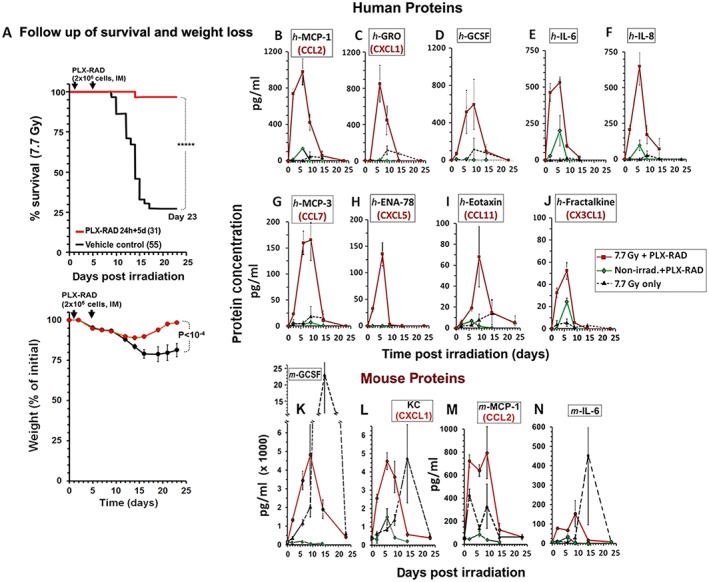
Kinetics of plasma protein levels in irradiated and non‐irradiated mice treated with PLX‐RAD. (A) The time points in which plasma samples were taken for analyses of protein secretion are posed relative to the survival data of the mice following 7.7 Gy irradiation with or w/o cell treatment (***** P < 0.00001). The survival and weight follow‐up, based on a previous report,26 are given in order to illustrate the correlation between the protein secretion kinetics and the fate of the mice at every time point tested. (B) Secreted human proteins in the mouse plasma, as analysed by Luminex multi‐protein panels. The cross‐reactivity of the antibodies to human and mouse homologous proteins was <10%. Out of 63 human proteins tested in the mouse plasma the 9 that were significantly elevated are presented in panels B–J. Red lines represent the secretion profile of PLX‐RAD treated by 7.7 Gy irradiated mice. The green lines represent data from non‐irradiated mice treated IM with PLX‐RAD cells. Broken black lines represent the untreated irradiated mice. For all the presented proteins, the difference in the peak concentration in PLX‐RAD treated groups between irradiated and non‐irradiated mice was significant (P < 0.01). The four murine proteins which were significantly elevated out of a panel of the 15 tested are shown in panels K‐N (n = 4 for most of the time points examined), the broken grey lines represent the levels of these proteins in the non‐cell‐treated mice (P < 0.01).
Remarkably, when the PLX‐RAD cells were injected IM in the same manner in naïve non‐irradiated mice, only negligible levels of the PLX‐RAD secreted human proteins were detected in the plasma (with very limited short‐term elevations of IL‐6, MCP‐1 and Fractalkine). This suggests that the systemic stress signals related to ARS stimulate the IM injected PLX‐RAD cells in the acute phase in which they were needed to trigger the controlled transient co‐secretion of these proteins to the circulation in a manner in the acute phase in which they were most needed (Figure 3B–J).
As expected, following high‐dose irradiation, the mice also secreted their own haematopoiesis supporting growth factors and chemokines, including G‐CSF, KC (a homologue of the human chemokine GRO), MCP‐1 (CCL2) and IL‐6 (Figure 3K–N). These proteins were elevated with somehow earlier kinetics in the irradiated mice treated with PLX‐RAD cells reaching peak levels between days 6 and 9 after irradiation. This hints for the possible contribution of the human PLX‐RAD secretome to the earlier critical time point of the induction of intrinsic secretion of the murine proteins. In the irradiated non PLX‐RAD treated mice, the secreted murine protein levels peaked later, between days 9 and 14 with higher levels of mouse derived G‐CSF and IL‐6 in the critical phase of pancytopenia.
The above data suggest that PLX‐RAD derived human secretome, combined with the intrinsically secreted mouse proteins, may join force to support an accelerated earlier proliferation of the BM progenitors. This seems to explain the mitigation of the ARS and recovery of the seriously BM depleted PLX‐RAD treated mice from lethal stage of pancytopenia, which was recorded typically from days 9–12 onward in untreated mice (as shown in Figure 3A). Only negligible residual levels of the elevated human proteins were present in the mouse circulation of the PLX‐RAD treated mice from day 14 onwards, while the slope of the decline of most inherent murine proteins seemed to be further expanded to later days. Therefore, the murine derived proteins levels were still elevated on day 14 in the few surviving non PLX‐RAD treated mice. At this time point, the most affected weaker mice have already died, assuming that the secretome records in this group on the later time points, from ~day 14 and 23, represent the data of only the few stronger surviving mice. As to the irradiated PLX‐RAD treated group, all human proteins secreted by the PLX‐RAD and the endogenous mice secretome resumed to their normal low levels in the blood plasma by day 23, in a stage by which the surviving mice were already in an advanced process of BM recovery (Figure 3B–J).
To demonstrate the synchronized kinetics of the elevated secretion of the human haematopoiesis‐related proteins, their secretion kinetics profiles were normalized to their peak levels and superimposed, as presented in Figure 4A. This illustrates the impressive overlap of the pharmacokinetics of the elevated secretome, peaking on days 6–9 following irradiation. To correlate the timing of the cytokine secretion in relation to their protective effects, the common pharmacokinetics profile of all secreted PLX‐RAD derived proteins is presented in Figure 4B as a shaded filled peak. By superimposing this secretion kinetics profile on the outline of the survival and weight follow‐up curves, it is apparent that the peak PLX‐RAD derived secretome just precedes the eventual recovery of the PLX‐RAD treated mice, resulting in their enhanced survival from day 14 onward. This effect is best demonstrated when this secretion peak is superimposed on the records of the cell counts of main blood lineages. Up to ~ 9 days after irradiation, the number of nucleated cells of the BM, as well as RBC, WBC and platelets counts in peripheral blood dropped sharply to reach critically low levels with similar initial kinetics for untreated and the PLX‐RAD treated mice. Then, supported by the high‐level secretion of the proteins by PLX‐RAD cells to the circulation, a sharp faster recovery is seen in all these blood related cell compartments to rescue the mice form lethal ARS (Figure 4C).
Figure 4.
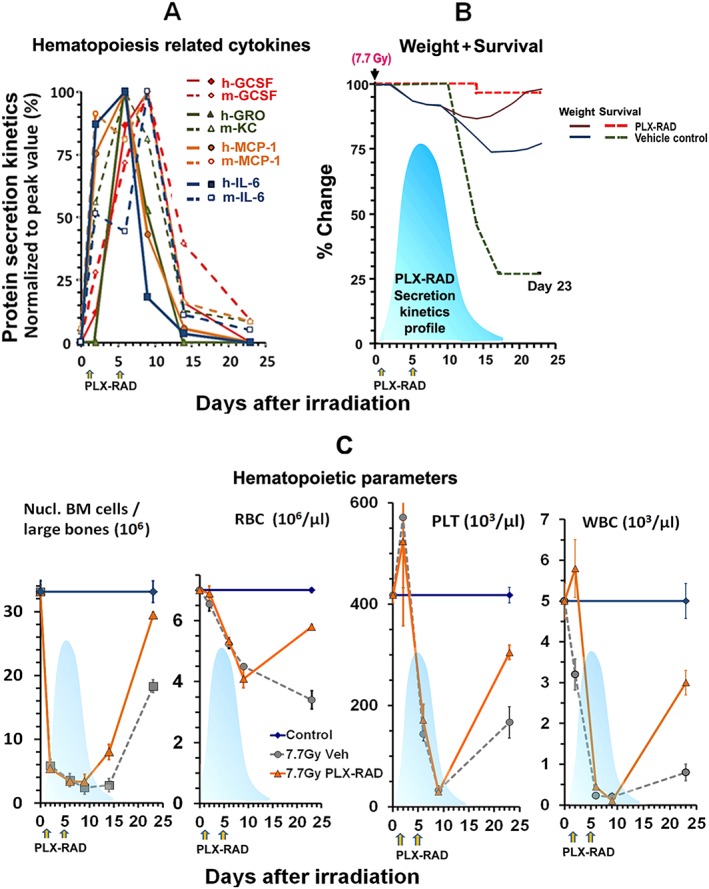
Correlation of the combined kinetics of main PLX‐RAD secreted and murine analogues of haematopoiesis related cytokines in 7.7 Gy irradiated mice. (A) The superimposed kinetics of human secretome associated with induction haematopoiesis in PLX‐RAD treated mice as compared to their murine homologues in the mouse circulation, based on the data from Figure 3 B–E,K–N. The human and murine proteins in PLX‐RAD treated mice were normalized to their peak level and superimposed to show the orchestrated kinetics of their secretion. The human proteins are presented as solid and the mouse as broken lines, indicating a common secretion peak on days 6 and 9 after irradiation. (B) The basic outline of the survival trend and the weight of the PLX‐RAD treated and control non‐cell treated mice at the points tested for proteins. The sequence of events of the elevated survival of treated surviving vs. untreated mice and their weight regain occur shortly after the peak of PLX‐RAD and murine derived protein secretion. The light blue shaded area represents the kinetics of orchestrated PLX‐RAD secreted proteins, preceding and possibly supporting the fast recovery of the cell treated mice at the critical point (**** P < 0.0001). (C) A follow‐up of whole BM counts, RBC, WBC and platelet counts with time following irradiation and PLX‐RAD cell treatment (days 1 and 5). The shaded secretion profile of the combined kinetics of the major elevated cytokine secretion as seen in (A) is superimposed as a shadow on each of the parameters tested, showing the timing of the effect of the secretome relative to the preceding enhanced regeneration of the different haematopoietic components which seems to be directly related to the increased survival rate of the treated mice, as shown in (B). Data points of panel C are taken from preliminary published data.26
3.2. In vitro effects of PLX‐RAD cell secretion of haematopoiesis‐supporting growth factors and chemokines
To further explore the secretion of the relevant haematopoiesis inducing factors by PLX‐RAD cells, their CM were analysed by a Multiplex assay (Figure 5A). The results provide in vitro support for the ability of PLX‐RAD cells to produce and secrete these factors.26 Even with no major apparent stress triggered signals, the cells secreted basal levels of factors associated with haematopoietic cell mobilization, which in culture conditions could accumulate in the CM in detectable levels as seen in Figure 5. The CM collected from cultured PLX‐RAD cells was shown to induce in vitro the migration of harvested BM‐residing nucleated cells (Figure 5B). Of interest is the observation that the effect of the PLX‐RAD CM was ~three‐fold more pronounced than the chosen positive control—SDF‐1, which is considered as a highly active pro‐migratory factor.
Figure 5.
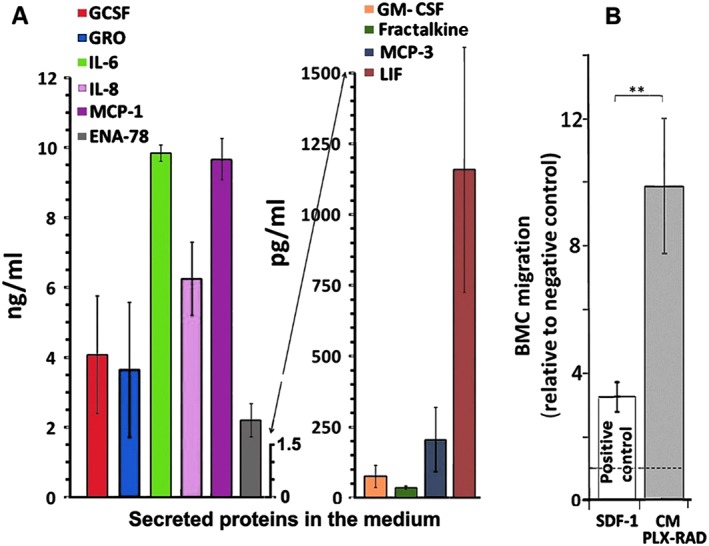
Cytokine levels in the CM of PLX‐RAD and their effect in vitro on the migration potential of isolated BM cells. (A) High levels of selected human proteins are expressed in the CM of cultured PLX‐RAD cells (n = 3, average of three different placentae). (B) The CM of the PLX‐RAD cells showed a significant ability to induce in vitro migration of BM cells in trans‐well plate preloaded with mouse whole BM cells on the upper insert. SDF‐1 supplemented RPMI medium was used as positive control and neat RPMI medium as a negative control. The fold change relative to negative control is represented as mean ± SE in 14 independent experiments.
4. Discussion
As we demonstrated in earlier report, cell therapy by IM injections of human‐derived PLX‐RAD cells on days 1 and 5 after high dose of 7.7 Gy TBI dramatically mitigated lethal ARS due to BM failure. This improved the survival rate of the irradiated mice from ~28% to ~98% associated with a complete recovery from a sharp weight loss by the end of the experiment on day 23, when the experiments were terminated.26 The selected highly active PLX‐RAD cells with high proportion of the neonate PSCs were shown to be much more active for treating ARS than the cell product PLX‐PAD cells consisting predominantly of expanded PSCs of maternal origin.26
The choice of the IM route administration of PLX‐RAD cells was adopted based on previous successful results showing a lack of apparent adverse effects of the IM injected PLX‐RAD cells. The IM injected cells were found to be restricted within the injection site in the highly vascular muscle. In contrast, in systemic cell intravenous delivery, the cells are immediately trapped in the lungs, as previously reported with other stromal cells.26, 36, 57
The increased survival of the high dose irradiated mice treated with PLX‐RAD was found to be associated with highly significant accelerated recovery of BM. This resulted in the fast increase of peripheral blood cells counts within the critical life‐threatening period of radiation induced pancytopenia, relative to much lower regeneration in non‐treated irradiated controls. The main goals of the current study were to explore the mechanism of action of the IM injected PLX‐RAD cells in the mitigation of the severe haematopoietic ARS.
In irradiated mice treated with PLX‐RAD cells, nine out of a panel of 63 tested human proteins were significantly elevated in the mice circulation, coinciding with the development of severe pancytopenia and sharp weight loss. These included major haematopoiesis related cytokines, such as G‐CSF, GRO, MCP‐1, IL‐6 and IL‐8, whose concentrations peaked on days 6–9 of the experiment. Other cytokines, mostly associated with WBC recruitment and migration, included MCP‐3 (CCL7), ENA (CXCL5), Eotaxin (CCL11) and Fractalkine (CX3CL1), were also significantly elevated in a similar kinetics.
The kinetics and peak secretion of the elevated PLX‐RAD derived human proteins in the mouse circulation following 7.7 Gy TBI seemed to correspond well with the recovery time from ARS of the cell injected mice (Figure 4), resulting in threefold higher survival rate relative to the untreated irradiated controls. This suggests that the IM injected PLX‐RAD cells respond from their remote injection site in the highly vascularized muscle to the systemic stress signals in the circulation. The radiation induced stress signals may be associated with the massive cell death and the failing of the haematopoietic system and systemic hypoxia due to reduction in the circulating RBC. This response is associated with almost simultaneous secretion of a wide panel of relevant proteins of interest. Moreover, the relatively extended presence of the injected low immunogenic xenogeneic PLX‐RAD cells in the muscle before they are cleared, as previously demonstrated,26 may provide a dynamic secretion of high levels of these factors, when needed, over a period of up to several weeks. The stress is maximal when the number of both BM and different blood lineage cells reach their lowest levels, ~4–6 days after irradiation. Therefore, the peak secretome concentrations released by the cells in response to the irradiation are reached for most proteins tested only ~6–9 days after the exposure and not immediately after the cells delivery. These findings on the need of activate the cells to increase the cytokines and growth factors secretion are supported by our findings that only negligible levels of this secretome were detected when PLX‐RAD cells were injected to non‐irradiated mice (Figure 3).
G‐CSF, both from human and murine source, was one of the cytokines which were secreted at highest levels. It plays a major role in the regulation and induction of proliferation of leukocyte progenitors, as well as in activation of HSC mobilization. Therefore, growth factor‐based therapies, and specifically those based on G‐CSF, were proposed to treat BM failure following nuclear disasters and/or high dose irradiation.13, 66, 67, 68, 69, 70 Of note is that most non cell‐treated irradiated mice survived ARS developed high levels of endogenously secreted murine G‐CSF in a delayed phase relative to its secretion by the PLX‐RAD treated mice. These findings suggest that the earlier controlled secretion of G‐CSF by the PLX‐RAD cells helped supporting the recovery of the high dose irradiated mice. Moreover, it is likely that G‐CSF alone is not sufficient to ideally induce the haematopoiesis regeneration in the mice with depleted haematopoietic system.
Among other highly expressed factors of note GRO (CXCL1) or its KC homologue in mice. These factors are inducers of proliferation and migration of progenitor cells71, 72, 73 and promote arteriogenesis through enhanced monocyte recruitment into neo‐vascularized tissues.74
Both IL‐6 and IL‐8 have a significant role in haematopoiesis. They were secreted in high levels by the PLX‐RAD cells, both in the CM of the in vitro models and into the circulation of the irradiated PLX‐RAD treated mice. The IL‐8 secretion could be associated with radio‐protective activity by inducing rapid mobilization of HSCs with the capacity for long‐term myelolymphoid repopulation.75 The administration of IL‐6 secreting stromal cells in combination with syngeneic BM transplant was shown to accelerate the recovery of peripheral blood counts and HSC in mouse BM following high dose irradiation.76 The activities of IL‐6 as an acute‐phase induced cytokine include the triggering haematopoiesis by inducing multi‐lineage HSC stimulation and acceleration of the regeneration of WBC, platelets and RBC in high dose irradiated mice.77 This could also aid the BM reconstitution after syngeneic BMT, leading to the extension of IL‐6 therapy from irradiated rodents and primates to the clinical practice.78
Whereas murine IL‐6 was not detectable in the few surviving irradiated control mice before the end of the second week post‐irradiation, the mice treated with PLX‐RAD cells expressed both human and murine IL‐6 levels as early as day 2 after irradiation. This early secretion of both IL‐6 and IL‐8 by PLX‐RAD cells in response to ARS‐related stress signals was associated with significantly lower levels of murine IL‐6 secretion within the first week. This may also explain the higher survival rate of the PLX‐RAD treated mice relative to irradiated vehicle treated controls.
It should be noted that highly relevant haematopoiesis inducing factor EPO was not included in the panel of assayed proteins since it is highly conserved in different species and the cross‐reactivity in immunoassays limits the ability to discriminate between the mouse or human protein.79, 80 Nevertheless, it was previously shown that IL‐6 induced by hypoxic or haematopoietic stress may control the subsequent secretion of EPO to enhance haematopoiesis stimulation in the BM.81, 82 Therefore, the high IL‐6 secretion, both by the PLX‐RAD and the mouse, may suggest a possible subsequent recruitment of EPO in the molecular cascade that leads to the faster regeneration of the BM and the haematopoietic system.
Based on our findings, we propose that the accelerated recovery of the BM in heavily irradiated animals treated with PLX‐RAD cells was aided by the earlier secretion of PLX‐RAD derived chemokines and growth factors. Those might have further stimulated endogenous secretion of relevant mice proteins to help overcome the ARS and severe pancytopenia by boosting earlier proliferation of the BM progenitor cells and faster repopulation of the cell depleted BM.
It should be noted that the critical radiation induced pancytopenia does not develop immediately. The possibility to delay the treatment with first PLX‐RAD cells injection to 24 h or more following radiation exposure is based on the findings that the PLX‐RAD cells express their elevated secretome only upon the development of major stress, more than 2 days following irradiation. This may be a critical advantage of a relevant life‐saving treatment for high dose irradiated individuals, providing the grace time window needed for global mobilization of deep frozen PLX‐RAD cells to any remote disaster site around the globe.
The finding that PLX‐RAD cells in culture secrete some of their secretome proteins to the CM may contradict our in vivo findings where systemic stress regulated this secretome elevation. This can be resolved considering that in vitro these cells may continuously secrete basal low levels of these proteins which accumulate. But in vivo, in the animal experiments, these baseline protein secretion levels are probably cleared fast and dissipate in the mouse circulation and are not accumulated to reach detectable levels. However, following the development of severe ARS, the endogenous stress signals probably boost the secretion of high levels of these cytokines in the critical time points with transient accumulation to reach higher detectable concentrations in the blood plasma. Then, upon the fast mice recovery, the secretion of these human proteins by the injected PLX‐RAD cells is immediately reduced below detectable levels.
Our findings, as summarized in Figure 6, suggest a possible complex mechanism for the dramatic recovery from lethal radiation‐induced pancytopenia by IM PLX‐RAD cells delivery with an early involvement of pro‐regenerative factors of the haematopoietic niche and inducers of HSC proliferation. Moreover, the early increase in human IL‐6 and IL‐8 levels in the mouse circulation may also activate intrinsic secretion of pro‐regenerative murine factors, resulting in earlier regeneration of the compromised BM.83, 84, 85
Figure 6.

Schematic summary of the proposed mechanistic effect of the PLX‐RAD secretome on mitigation of ARS by PLX‐RAD cells. IM injected PLX‐RAD cells in irradiated mice seem to respond to the systemic radiation‐induced stress. This triggers a fast secretion of high levels of the relevant proteins by the PLX‐RAD cells. The induced PLX‐RAD derived human secretome is accompanied by enhanced secretion of murine counterparts. The human cytokine peak profiles seem to precede the recovery of damaged haematopoiesis by a short interval, and they occur just prior to the anticipated radiation‐related mortality of the untreated control group. The proteins known to be directly associated with haematopoiesis regeneration are highlighted in red, but other proteins could also be indirectly involved in processes such as cell migration from the haematopoietic niches into the circulation.
The data on the pro‐regenerative secretome of the PLX‐RAD cells in response to stress may have implications for other wide range of regenerative therapies. Muscle regeneration by stem/progenitor cell recruitment, proliferation and survival are supported by the inflammatory cells which are affected by the PLX‐RAD cell secretome in response to ARS.
We can hypothesize that the mechanism of PLX‐RAD effects may be related to their possible physiological role in the placenta, where they respond to messages carried by the embryo circulation, potentially serving as both sensors and responders to its stress signals and as secretors of pro‐regenerative proteins. In the current study which is focused on short‐term follow‐up of acute effects of less than 8 Gy irradiation, no muscle damage was expected. But in other degenerative processes, the haematopoietic system is recruited by the PLX‐RAD secretome, boosting different haematopoietic lineages including eosinophils, macrophages and T cells. In such circumstances, the induced secretome of potent PLX‐RAD cells may participate in the regeneration of tissues such as damaged muscles,86, 87, 88 an issue which deserves further investigation.
In summary, we provide detailed mechanistic insights into simple well‐regulated allogeneic/xenogeneic PLX‐RAD cell‐based treatments. The mode of action of these cells in mitigation of ARS following high dose irradiation is based on the induction of a faster regeneration of highly damaged or depleted BM, thus reversing subsequent life‐threatening pancytopenia and severe weight loss.
4.1. Disclosure of potential conflicts of interest
H.D.V. and P.R. serve as immunological and clinical consultants for Pluristem. The experimental work of R.G. and his group was partially sponsored by Pluristem Therapeutics. R.O., L.P. and Z.A. were involved in the study as employees of Pluristem Therapeutics which supplied all the GMP grade cells used in the current study.
The studies performed by the Laboratory of RG at Hadassah were partially sponsored by Pluristem Therapeutics which supplied the different cells used for this study.
4.2. Vertebrate animals ethical approvals
In the animal experiments, all methods were carried out in accordance with relevant guidelines and regulations, and experimental protocols were approved by the following institutional committees as follows: All experiments with 3H/HeNHsd mice at Hadassah‐Hebrew University Medical Center were approved by the Institutional Animal Welfare Committee as covered by the Ethical Animal Welfare Certificates #GB06/68708 #MD‐12‐13296‐4 (NIH approval number OPRRA01‐5011). The limited use of Balb‐c mice for BM extraction in experimental protocols performed by Harlan for Pluristem Therapeutics was approved by Harlan (Envigo) Laboratories' Ethical Committee, certificate no. IL‐14‐04‐120.
4.3. Original figures
All figures in this manuscript (1–6) are original and were drawn by the authors of this manuscript. Where indicated, survival experiments in which some of the data were previously published were combined with additional new data to be presented in the current manuscript as a reference to the kinetics of the relevant proteins secretion. The authors organized and drew the data presented in the different figures.
Authorship Contributions
L.P.: contribution in animal and in‐vitro experiments, cytokine assays, data analysis, manuscript writing; L.A./C.C.: cytokine assays, conceptual contribution and manuscript editing; Z.A.: conceptual ideas; R.O.: conceptual contribution, development and characterization of PLX‐RAD cells, study supervision; M.B./E.Z.: technical assistance; P.R./C.C./D.J.W.: conceptual and technical assistance; L.A.: performing the cytokine experiments; L.L./E.V.: technical contribution to the animal experiments and data analysis preparation; H.D.V.: Co‐PI, design of cytokine/protein expression studies, conceptual contribution and assisting manuscript writing; R.G.: Co‐PI, conceptual contribution and earlier animal studies on the discovery of the potential of PLX‐RAD cells activity, experimental design, technical help and study supervision, data analysis, manuscript writing.
Acknowledgements
The project was partially supported by a research grant of the Israeli Science Foundation (ISF# 1639/14) to R.G. It was also sponsored by the German Federal Ministry of Education and Research (BMBF) to the BCRT (HDV) and by the Israel Innovation Authority (I/A) to Pluristem Therapeutics.
We thank Elena Gaberman, Ilana Stav, Astar Hailu and Boaz Adani, from Hadassah, as well as Noa Sher, Tal Prezma and Shiran Rekhes from Pluristem for their valuable technical contribution in earlier or parallel projects that emerged in part from the current study. We greatly appreciate the helpful consultation along the different stages of this project with Prof. Eli Keshet and Dr Myriam Grunewald from the Hebrew University, Jerusalem. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.89
Pinzur, L. , Akyuez, L. , Levdansky, L. , Blumenfeld, M. , Volinsky, E. , Aberman, Z. , Reinke, P. , Ofir, R. , Volk, H.‐D. , and Gorodetsky, R. (2018) Rescue from lethal acute radiation syndrome (ARS) with severe weight loss by secretome of intramuscularly injected human placental stromal cells. Journal of Cachexia, Sarcopenia and Muscle, 9: 1079–1092. 10.1002/jcsm.12342.
References
- 1. Donnelly EH, Nemhauser JB, Smith JM, Kazzi ZN, Farfán EB, Chang AS, et al Acute radiation syndrome: assessment and management. South Med J 2010;103:541–546. [DOI] [PubMed] [Google Scholar]
- 2. Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, et al Radiation‐associated kidney injury. Int J Radiat Oncol Biol Phys 2010;76:S108–S115. [DOI] [PubMed] [Google Scholar]
- 3. Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing‐radiation lethality. Health Phys 2003;84:565–575. [DOI] [PubMed] [Google Scholar]
- 4. Van Dyk J. Magna‐field irradiation: physical considerations. Int J Radiat Oncol Biol Phys 1983;9:1913–1918. [DOI] [PubMed] [Google Scholar]
- 5. Meineke V, Fliedner TM. Radiation‐induced multi‐organ involvement and failure: challenges for radiation accident medical management and future research. BJR Suppl 2005;27:196–200. [Google Scholar]
- 6. Vacca P, Montaldo E, Vitale C, Croxatto D, Moretta L, Mingari MC. MSC and innate immune cell interactions: a lesson from human decidua. Immunol Lett 2015;168:170–174. [DOI] [PubMed] [Google Scholar]
- 7. Port M, Herodin F, Valente M, Drouet M, Lamkowski A, Majewski M, et al First generation gene expression signature for early prediction of late occurring hematological acute radiation syndrome in baboons. Radiat Res 2016;186:39–54. [DOI] [PubMed] [Google Scholar]
- 8. Cui W, Bennett AW, Zhang P, Barrow KR, Kearney SR, Hankey KG, et al A non‐human primate model of radiation‐induced cachexia. Sci Rep 2016;6:23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant 2010;45:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanda J. Effect of HLA mismatch on acute graft‐versus‐host disease. Int J Hematol 2013;98:300–308. [DOI] [PubMed] [Google Scholar]
- 11. Buckley RH. 27. Transplantation immunology: organ and bone marrow. J Allergy Clin Immunol 2003;111:S733–S744. [DOI] [PubMed] [Google Scholar]
- 12. Gregori S, Amodio G, Quattrone F, Panina‐Bordignon P. HLA‐G orchestrates the early interaction of human trophoblasts with the maternal niche. Front Immunol 2015;6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams JP, McBride WH. After the bomb drops: a new look at radiation‐induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol 2011;87:851–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barria E, Mikels A, Haas M. Maintenance and self‐renewal of long‐term reconstituting hematopoietic stem cells supported by amniotic fluid. Stem Cells Dev 2004;13:548–562. [DOI] [PubMed] [Google Scholar]
- 15. MacVittie TJ, Farese AM, Jackson W 3rd. Defining the full therapeutic potential of recombinant growth factors in the post radiation‐accident environment: the effect of supportive care plus administration of G‐CSF. Health Phys 2005;89:546–555. [DOI] [PubMed] [Google Scholar]
- 16. Liu W, Morschauser A, Zhang X, Lu X, Gleason J, He S, et al Human placenta‐derived adherent cells induce tolerogenic immune responses. Clin Transl Immunology 2014;3:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moroni M, Ngudiankama BF, Christensen C, Olsen CH, Owens R, Lombardini ED, et al The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G‐colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total‐body gamma‐irradiation. Int J Radiat Oncol Biol Phys 2013;86:986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, et al Comparison of autologous cell therapy and granulocyte‐colony stimulating factor (G‐CSF) injection vs. G‐CSF injection alone for the treatment of acute radiation syndrome in a non‐human primate model. Int J Radiat Oncol Biol Phys 2005;63:911–920. [DOI] [PubMed] [Google Scholar]
- 19. Hofer M, Pospisil M, Komurkova D, Hoferova Z. Granulocyte colony‐stimulating factor in the treatment of acute radiation syndrome: a concise review. Molecules 2014;19:4770–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quesenberry PJ, Stewart FM, Becker P, D'Hondt L, Frimberger A, Lambert JF, et al Stem cell engraftment strategies. Annals of the New York Academy of Sciences 2001;938:54–61; discussion 61–52. [DOI] [PubMed] [Google Scholar]
- 21. Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, et al Gamma‐tocotrienol protects hematopoietic stem and progenitor cells in mice after total‐body irradiation. Radiat Res 2010;173:738–747. [DOI] [PubMed] [Google Scholar]
- 22. Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic‐Haberle I, Thrasher BA, Gauter‐Fleckenstein B, et al Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res 2007;41:1273–1282. [DOI] [PubMed] [Google Scholar]
- 23. Basile LA, Ellefson D, Gluzman‐Poltorak Z, Junes‐Gill K, Mar V, Mendonca S, et al HemaMax, a recombinant human interleukin‐12, is a potent mitigator of acute radiation injury in mice and non‐human primates. PloS one 2012;7:e30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gratwohl A, John L, Baldomero H, Roth J, Tichelli A, Nissen C, et al FLT‐3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood 1998;92:765–769. [PubMed] [Google Scholar]
- 25. Lange C, Brunswig‐Spickenheier B, Cappallo‐Obermann H, Eggert K, Gehling UM, Rudolph C, et al Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PloS one 2012;6:e14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, et al Mitigation of lethal radiation syndrome in mice by intramuscular injection of 3D cultured adherent human placental stromal cells. PloS one 2013;8:e66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 2007;82:241–243. [DOI] [PubMed] [Google Scholar]
- 28. Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, Fan L, et al Mesenchymal stromal cell‐conditioned medium prevents radiation‐induced small intestine injury in mice. Cytotherapy 2011;14:267–273. [DOI] [PubMed] [Google Scholar]
- 29. Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, Enzmann V, et al Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res 2012;83:267–275. [DOI] [PubMed] [Google Scholar]
- 30. Chung JK, Park TK, Ohn YH, Park SK, Hong DS. Modulation of retinal wound healing by systemically administered bone marrow‐derived mesenchymal stem cells. Korean J Ophthalmol 2011;25:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acar N, Soylu H, Edizer I, Ozbey O, Er H, Akkoyunlu G, et al Expression of nuclear factor erythroid 2‐related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6) proteins in healthy and pathologic placentas of human and rat. Acta Histochem 2014;116:1289–1300. [DOI] [PubMed] [Google Scholar]
- 32. Sugino N, Miura Y, Yao H, Iwasa M, Fujishiro A, Fujii S, et al Early osteoinductive human bone marrow mesenchymal stromal/stem cells support an enhanced hematopoietic cell expansion with altered chemotaxis‐ and adhesion‐related gene expression profiles. Biochem Biophys Res Commun 2016;469:823–829. [DOI] [PubMed] [Google Scholar]
- 33. Li O, Tormin A, Sundberg B, Hyllner J, Le Blanc K, Scheding S. Human embryonic stem cell‐derived mesenchymal stroma cells (hES‐MSCs) engraft in vivo and support hematopoiesis without suppressing immune function: implications for off‐the shelf ES‐MSC therapies. PloS one 2013;8:e55319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng K, Wu W, Yang S, Huang L, Chen J, Gong C, et al Treatment of radiation‐induced acute intestinal injury with bone marrow‐derived mesenchymal stem cells. Exp Ther Med 2016;11:2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation‐induced gastrointestinal syndrome in mice. PloS one 2011;6:e24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell 2009;5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thierry D, Bertho JM, Chapel A, Gourmelon P. Cell therapy for the treatment of accidental radiation overexposure. BJR Suppl 2005;27:175–179. [Google Scholar]
- 38. Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol 2010;83:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang C, Dai W, Chen H, Wu B. Application of human bone marrow‐derived mesenchymal stem cells in the treatment of radiation‐induced gastrointestinal syndrome. Sci China Life Sci 2014;57:1177–1182. [DOI] [PubMed] [Google Scholar]
- 40. Prather WR, Toren A, Meiron M. Placental‐derived and expanded mesenchymal stromal cells (PLX‐I) to enhance the engraftment of hematopoietic stem cells derived from umbilical cord blood. Expert Opin Biol Ther 2008;8:1241–1250. [DOI] [PubMed] [Google Scholar]
- 41. Yang X, Balakrishnan I, Torok‐Storb B, Pillai MM. Marrow stromal cell infusion rescues hematopoiesis in lethally irradiated mice despite rapid clearance after infusion. Adv Hematol 2012;2012:142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, et al Pre‐activation of mesenchymal stem cells with TNF‐alpha, IL‐1beta and nitric oxide enhances its paracrine effects on radiation‐induced intestinal injury. Sci Rep 2015;5:8718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Guo M, Dong Z, Qiao J, Yu C, Sun Q, Hu K, et al Severe acute radiation syndrome: treatment of a lethally 60Co‐source irradiated accident victim in China with HLA‐mismatched peripheral blood stem cell transplantation and mesenchymal stem cells. J Radiat Res 2014;55:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng JC, Schultheiss TE, Wong JY. Impact of drug therapy, radiation dose, and dose rate on renal toxicity following bone marrow transplantation. Int J Radiat Oncol Biol Phys 2008;71:1436–1443. [DOI] [PubMed] [Google Scholar]
- 45. Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90) Y microspheres: the experience of the National Tumor Institute of Milan. Q J Nucl Med Mol Imaging 2011;55:168–197. [PubMed] [Google Scholar]
- 46. Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev 2011. [DOI] [PubMed] [Google Scholar]
- 47. Bifari F, Lisi V, Mimiola E, Pasini A, Krampera M. Immune modulation by mesenchymal stem cells. Transfus Med Hemother 2008;35:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 49. Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 2006;8:559–561. [DOI] [PubMed] [Google Scholar]
- 50. Sioud M, Mobergslien A, Boudabous A, Floisand Y. Mesenchymal stem cell‐mediated T cell suppression occurs through secreted galectins. Int J Oncol 2011;38:385–390. [DOI] [PubMed] [Google Scholar]
- 51. Freier CP, Kuhn C, Rapp M, Endres S, Mayr D, Friese K, et al Expression of CCL22 and infiltration by regulatory T cells are increased in the decidua of human miscarriage placentas. Am J Reprod Immunol 2015;74:216–227. [DOI] [PubMed] [Google Scholar]
- 52. Xie MW, Gorodetsky R, Micewicz ED, Mackenzie NC, Gaberman E, Levdansky L, et al Marrow‐derived stromal cell delivery on fibrin microbeads can correct radiation‐induced wound‐healing deficits. J Invest Dermatol 2013;133:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Blanc K. Hematopoiesis does not always get support from MSC. Cytotherapy 2009;11:674–675. [DOI] [PubMed] [Google Scholar]
- 54. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al Concise review: isolation and characterization of cells from human term placenta: outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008;26:300–311. [DOI] [PubMed] [Google Scholar]
- 55. Talwadekar MD, Kale VP, Limaye LS. Placenta‐derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord‐derived counterparts‐a paired sample study. Sci Rep 2015;5:15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alvarez‐Silva M, Belo‐Diabangouaya P, Salaun J, Dieterlen‐Lievre F. Mouse placenta is a major hematopoietic organ. Development 2003;130:5437–5444. [DOI] [PubMed] [Google Scholar]
- 57. Schmuck EG, Koch JM, Centanni JM, Hacker TA, Braun RK, Eldridge M, et al Biodistribution and clearance of human mesenchymal stem cells by quantitative three‐dimensional cryo‐imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl Med 2016;5:1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prather WR, Toren A, Meiron M, Ofir R, Tschope C, Horwitz EM. The role of placental‐derived adherent stromal cell (PLX‐PAD) in the treatment of critical limb ischemia. Cytotherapy 2009;11:427–434. [DOI] [PubMed] [Google Scholar]
- 59. Ahmed TA, Hincke MT. Mesenchymal stem cell‐based tissue engineering strategies for repair of articular cartilage. Histol Histopathol 2014;29:669–689. [DOI] [PubMed] [Google Scholar]
- 60. Roy R, Brodarac A, Kukucka M, Kurtz A, Becher PM, Jülke K, et al Cardioprotection by placenta‐derived stromal cells in a murine myocardial infarction model. J Surg Res 2013;185:70–83. [DOI] [PubMed] [Google Scholar]
- 61. Ramot Y, Meiron M, Toren A, Steiner M, Nyska A. Safety and biodistribution profile of placental‐derived mesenchymal stromal cells (PLX‐PAD) following intramuscular delivery. Toxicol Pathol 2009;37:606–616. [DOI] [PubMed] [Google Scholar]
- 62. Chatterjee P, Chiasson VL, Pinzur L, Raveh S, Abraham E, Jones KA, et al Human placenta‐derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin Sci 2016;130:513–523. [DOI] [PubMed] [Google Scholar]
- 63. Caplan AI. New MSC: MSCs as pericytes are sentinels and gatekeepers. J Orthop Res 2017;35:1151–1159. [DOI] [PubMed] [Google Scholar]
- 64. da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008;26:2287–2299. [DOI] [PubMed] [Google Scholar]
- 65. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 66. Singh VK, Christensen J, Fatanmi OO, Gille D, Ducey EJ, Wise SY, et al Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res 2012;177:781–791. [DOI] [PubMed] [Google Scholar]
- 67. Suryavanshi S, Sharma D, Checker R, Thoh M, Gota V, Sandur SK, et al Amelioration of radiation‐induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic Biol Med 2015;85:56–70. [DOI] [PubMed] [Google Scholar]
- 68. Kerrigan DP, Castillo A, Foucar K, Townsend K, Neidhart J. Peripheral blood morphologic changes after high‐dose antineoplastic chemotherapy and recombinant human granulocyte colony‐stimulating factor administration. Am J Clin Pathol 1989;92:280–285. [DOI] [PubMed] [Google Scholar]
- 69. Williams JP, Brown SL, Georges GE, Hauer‐Jensen M, Hill RP, Huser AK, et al Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cohen DM, Bhalla SC, Anaissie EJ, Hester JP, Savary CA, Rex JH. Effects of in vitro and in vivo cytokine treatment, leucapheresis and irradiation on the function of human neutrophils: implications for white blood cell transfusion therapy. Clin Lab Haematol 1997;19:39–47. [DOI] [PubMed] [Google Scholar]
- 71. Shen CM, Zhu BF, Deng YJ, Ye SH, Yan JW, Yang G, et al Allele polymorphism and haplotype diversity of HLA‐A, ‐B and ‐DRB1 loci in sequence‐based typing for Chinese Uyghur ethnic group. PloS one 2010;5:e13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bizzetto R, Bonfim C, Rocha V, Socié G, Locatelli F, Chan K, et al Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica 2011;96:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moser B, Clark‐Lewis I, Zwahlen R, Baggiolini M. Neutrophil‐activating properties of the melanoma growth‐stimulatory activity. J Exp Med 1990;171:1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vries MH, Wagenaar A, Verbruggen SE, Molin DG, Dijkgraaf I, Hackeng TH, et al CXCL1 promotes arteriogenesis through enhanced monocyte recruitment into the peri‐collateral space. Angiogenesis 2015;18:163–171. [DOI] [PubMed] [Google Scholar]
- 75. Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin‐8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long‐term myelolymphoid repopulating ability. Blood 1995;85:2269–2275. [PubMed] [Google Scholar]
- 76. Jacobs P, Hailey D, Turner R, MacLean N. Allogeneic stem cell transplantation. An economic comparison of bone marrow, peripheral blood, and cord blood technologies. Int J Technol Assess Health Care 2000;16:874–884. [DOI] [PubMed] [Google Scholar]
- 77. Hagedorn EJ, Durand EM, Fast EM, Zon LI. Getting more for your marrow: boosting hematopoietic stem cell numbers with PGE2. Exp Cell Res 2014;329:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Givon T, Revel M, Slavin S. Potential use of interleukin‐6 in bone marrow transplantation: effects of recombinant human interleukin‐6 after syngeneic and semiallogeneic bone marrow transplantation in mice. Blood 1994;83:1690–1697. [PubMed] [Google Scholar]
- 79. Wen D, Boissel JP, Tracy TE, Gruninger RH, Mulcahy LS, Czelusniak J, et al Erythropoietin structure‐function relationships: high degree of sequence homology among mammals. Blood 1993;82:1507–1516. [PubMed] [Google Scholar]
- 80. Wen D, Boissel JP, Showers M, Ruch BC, Bunn HF. Erythropoietin structure‐function relationships. Identification of functionally important domains. J Biol Chem 1994;269:22839–22846. [PubMed] [Google Scholar]
- 81. Klausen T. The feed‐back regulation of erythropoietin production in healthy humans. Dan Med Bull 1998;45:345–353. [PubMed] [Google Scholar]
- 82. Ranjbaran M, Kadkhodaee M, Seifi B. Renal tissue pro‐inflammatory gene expression is reduced by erythropoietin in rats subjected to hemorrhagic shock. Journal of nephropathology 2017;6:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sohn SK, Moon JH. Favorable effects of low‐dose anti‐thymocyte globulin in a partially‐mismatched HLA group in an unrelated allogeneic stem cell transplantation setting. Annals of transplantation: quarterly of the Polish Transplantation Society 2015;20:7–15. [DOI] [PubMed] [Google Scholar]
- 84. Jaing TH, Huang IA, Chen SH, Yang CP, Liang DC, Hung IJ. Cord blood transplantation in children with relapsed or refractory severe aplastic anemia. J Pediatr Hematol Oncol 2011;33:18–21. [DOI] [PubMed] [Google Scholar]
- 85. Chai L, Ling K, He X, Yang R. Expression of ATF4 and VEGF in chorionic villus tissue in early spontaneous abortion. Eur J Obstet Gynecol Reprod Biol 2013;170:434–438. [DOI] [PubMed] [Google Scholar]
- 86. Shapira I, Fainstein N, Tsirlin M, Stav I, Volinsky E, Moresi C, et al Placental stromal cell therapy for experimental autoimmune encephalomyelitis: the role of route of cell delivery. Stem Cells Transl Med 2017;6:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Quarta M, Rando TA. Mimicking the niche: cytokines expand muscle stem cells. Cell Res 2015;25:761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fu X, Xiao J, Wei Y, Li S, Liu Y, Yin J, et al Combination of inflammation‐related cytokines promotes long‐term muscle stem cell expansion. Cell Res 2015;25:1082–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


