Abstract
Background
Cancer cachexia (CC) is a multifactorial syndrome, often irreversible, that affects patients with cancer influenced, in part, by the inflammatory condition. Peritumoural adipose tissue produces adipokines and angiogenic, apoptotic, and growth factors; given the possible crosstalk between the peritumoural adipose tissue and tumour, these may play an important role in cancer biology and carcinogenesis.
Methods
The aim of this study was to evaluate the factors produced by peritumoural adipose tissue in a cohort of 16 colorectal cancer patients with either weight‐stable cancer (WSC; n = 7) or CC (n = 9). The study was approved by the Ethics Research Committee (972.914). Samples of peritumoural adipose tissue were analysed for concentrations of TNF‐α, IL‐1β, STAT‐1, STAT‐3, RANTES, IL‐1Ra, IP‐10, IL‐15, MCP‐1, IFN‐α, GCSF, FADD, and TGF‐β. The cytokines and proteins were measured using Multiplex. Correlations between the proteins and cytokines were evaluated.
Results
TNF‐α, STAT‐1, and FADD, a factor involved in apoptosis, were significantly higher in CC group than in the WSC group. In the peritumoural adipose tissue of the CC group, RANTES showed a significant positive correlation with IL‐1Ra and IP‐10 and a negative correlation with IFN‐α; and GCSF showed significant negative correlations with IL‐1Ra, IP‐10, IL‐15, and MCP‐1 and a positive correlation with IFN‐α. In the peritumoural adipose tissue of the WSC group, no significant correlations were detected between RANTES, GCSF, IL‐3, FADD, and STAT‐1 and the cytokines/chemokines analysed.
Conclusions
These results indicated that inflammatory and tumorigenic pathways were altered in peritumoural adipose tissue in CC. Furthermore, inflammatory cytokines were correlated with growth factors in the peritumoural adipose tissue of cachectic patients, suggesting that inflammatory cytokines modulated the proliferative environment closely linked to the tumour.
Keywords: Peritumoural adipose tissue, Cancer cachexia, Cytokines, Apoptosis
Introduction
Cancer is one of the leading causes of death in the world, and colorectal cancer is the third most prevalent type of cancer.1 Around half of cancer sufferers experience cachexia, a multifactorial syndrome characterized by involuntary weight loss. Although the relationship between cancer and cachexia has been extensively discussed, the mechanisms responsible for this relationship have not been totally elucidated.
Cachexia involves the loss of skeletal muscle and adipose tissue, depending partially of the grade of systemic inflammation. The muscle loss can have a dramatic impact on the patient's quality of life. In addition, cachectic patients exhibit several other symptoms, including anorexia, anaemia, fatigue, early satiety, weakness, altered blood biochemistry parameters, and increased levels of inflammatory factors in various organs and tissues. The inflammatory changes are of extreme importance for a better understanding of the clinical picture of cancer cachexia (CC).2
The reduction in adipose tissue represents a sustained energy imbalance. Decreased body fat and body mass index (BMI) are associated with CC in both experimental animal models3 and in humans.4 Adipose tissue acts as an energy store and an endocrine organ that secretes a variety of multifunctional molecules known as adipokines.5 These cytokines regulate physiological functions systemically through endocrine‐like activity.6 They include adiponectin, leptin, and numerous cytokines, such as tumour necrosis factor alpha (TNF‐α), interleukin 6 (IL‐6), interleukin 8 (IL‐8), interleukin 10 (IL‐10), vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein development 1 (MCP‐1). Many of these cytokines have been implicated in the development and progression of tumours.7 Our research group has previously demonstrated a relationship between changes in cytokines and the growth factor profile and wasting in CC patients.8, 9, 10 Circulating levels of pro‐inflammatory adipokines have been shown to correlate with the total mass of visceral adipose tissue.7 Some of these adipokines, such as IL‐6 and TNF‐α, exert potent pro‐inflammatory actions and have been reported to be strong promoters of tumour initiation and progression.11
In addition to inflammatory markers, adipose tissue cells also produce angiogenic factors, such as VEGF12 and insulin‐like growth factor type I (IGF‐1), which contribute to the growth of solid tumours.13 Of all the adipose tissues examined, visceral adipose tissues express the highest level of VEGF.12 Furthermore, resident and infiltrated macrophages in adipose tissue appear to be the major producers of pro‐inflammatory cytokines, and their number correlates positively with BMI.14
Several factors are expressed in the adipose tissue, especially under inflammatory conditions, including RANTES, MCP‐1, GCSF, IL‐3, TNF‐α, and IP‐10. These are associated with the promotion of recruitment by the immune system of macrophages, monocytes, lymphocytes, and T cells, among others, and they may promote changes to the apoptosis pathway. This association is well illustrated by the interaction between TNF‐α and FADD and the negative regulation of GCSF in the apoptosis process.15, 16, 17
Recent studies have shown that the interaction of cancer with adipocytes can promote cellular reprogramming, resulting in cancer‐associated adipocytes or dedifferentiation into fibroblast‐like cells. These fibroblasts may contribute to the growth of tumour cells and to cell array changes and the immune response, promoting an increase in the production of cytokines and growth factors, which results in increased angiogenesis and metastasis.18, 19, 20 In an experimental model using mice, Wagner et al.21 demonstrated that cell tumours implanted distant from adipose tissue depots developed less quickly than those implanted in a subcutaneous adipose tissue depot, suggesting that crosstalk between adipose tissue and tumour cells is important for tumour development.
A previous study suggested that peritumoural adipose tissue may play an important role in inflammatory and angiogenic processes, secreting several factors that affect tumour biology and promote the progression of the tumour, resulting in poor clinical prognosis.14 However, few studies have investigated the role peritumoural adipose tissue in patients with colorectal cancer.20 The purpose of the present study, therefore, was to evaluate the pro‐inflammatory and pro‐tumorigenic properties of peritumoural adipose tissue in the presence of systemic inflammation in a cohort of colorectal cancer patients with and without cachexia. To our knowledge, this is the first study to analyse whether there are differences on the peritumoral adipose tissue inflammatory condition between cachetic and weight stable colorectal cancer patient, which could be involved on presence of cachexia.
Material and methods
Patients and sample collection
This study was approved by the Ethics Committee of the Institute of Biomedical Sciences and by the Human Ethics Committee of the University of São Paulo Hospital (CEP‐ICB/USP 1117/13, CEP‐HU/USP 752/07, and 1117/13, CAAE 0031.0.198.019‐07) and was conducted in accordance with the principles of the Declaration of Helsinki (2013). All subjects provided written informed consent prior to participation in the study. The study included 16 colorectal cancer patients who were enrolled in the study at the time of diagnosis and had not received anticancer or continuous anti‐inflammatory treatment and who were willing to participate. The exclusion criteria were liver failure, renal failure, AIDS, inflammatory diseases of the bowel, and autoimmune disorders. The characteristics of the subjects are summarized in Table 1.
Table 1.
General characteristic of patients
| N | WSC | CC | P |
|---|---|---|---|
| 7 | 9 | ||
| Gender | F (2)/M (5) | F (4)/M (5) | |
| Age (years) | 61.57 ± 5.70 | 65.56 ± 5.07 | 0.5839a |
| BMI (kg/m2) | 25.73 [18.17–26.40] | 24.53 [18.70–30.88] | 0.3968b |
| Weight loss (kg) | 0.00 [0.00–0.00] | 6.00 [00.00–15.00] | 0.0063b |
| Weight loss (%) | 0.00 [0.00–0.00] | 9.00 [0.00–20.60] | 0.0031b |
| CRP (mg/L) | 6.84 ± 1.99 | 8.72 ± 1.81 | 0.4968a |
| Albumin (g/dL) | 4.23 ± 0.52 | 3.27 ± 0.59 | 0.2326a |
| CRP/Albumin | 1.58 [0.46–7.54] | 3.53 [0.33–10.84] | 0.2991b |
| Haemoglobin (g/dL) | 13.57 ± 0.85 | 11.40 ± 0.43 | 0.0287b |
| IL‐6 (pg/mL) | 0.13 [0.13–2.45] | 12.10 [4.88–45.49] | 0.0042b |
| Tumour stage (n) | |||
| I–II | 2 | 3 | |
| III–IV | 5 | 6 | |
Student's t‐test.
Mann–Whitney test.
The subjects were subdivided into two groups: those with CC group (n = 9) and those with weight‐stable cancer (WSC group, n = 7). Patients were considered cachectic based on criteria from the international consensus,22 as follows: weight loss >5% in the past 6 months or any degree of weight loss >2% in the last 6 months plus BMI <20 kg/m2. The WSC group subjects had undergone no important weight change during the previous year and had a BMI <25 kg/m2. The primary tumour location for all subjects in both groups was the intestine. All the subjects underwent surgery to resect the tumour; the peritumoural adipose tissue was dissected from the tumour and kept at −80°C for later analyses.
Clinical parameter assessment
The subject's height and weight were measured. After an overnight fast, within the venous access procedure for anaesthesia during the surgery, 10 mL of blood was collected for the analysis of plasma C‐reactive protein (CRP), albumin, haemoglobin, and IL‐6. The plasma samples were then immediately frozen at −80°C until analysis. TNM tumour staging was determined post‐operatively according to the guidelines of the Union for International Cancer Control.
Multiplex analysis of protein content
Approximately 100–200 mg of the peritumoural adipose tissue from each sample was homogenized in 300 μL of ice‐cold extraction protein buffer (10 mM Tris base, 0.01 mM EDTA, 0.1 mM sodium chloride and 1% Triton X‐100) to which a protease inhibitor cocktail was added (1 tablet/50 mL extraction buffer; Roche Diagnostics). The homogenate was then centrifuged at 18 000 g for 40 min at 4°C and the fatty layer discarded. Aliquots of the supernatant were stored at −80°C. Samples of peritumoural adipose tissue were incubated with a mixture of Magplex microspheres and covered with the specific antibodies. After incubation for 1 h followed by incubation for 30 min with streptavidin labelled with phycoerythrin, target antigens bound to the microspheres were detected with a mixture of biotinylated capture antibodies. The microspheres were then analysed using a MAGPIX® instrument (Life Technologies, Grand Island, NY, USA). Each cytokine value was corrected to the total protein concentration. MILLIPLEX® Analyst 5.1 software was used, which integrated the data acquisition and analysis.
Protein expression of ANGPTL‐4
ANGPTL‐4 was assessed quantitatively using ELISA. The results are expressed as pg/mg of protein.
Statistical analysis
Data are expressed as mean ± standard error, when the data showed normal distribution or median [interquartile range] when not normal distributed. The distributions for all samples were tested for whether they were Gaussian using either the D'Agostino–Pearson omnibus test or the Shapiro–Wilk test. Student's t‐test or the Mann–Whitney test with multiple comparisons was employed for the parametric and non‐parametric data, respectively. The significance level was set at P ≤ 0.05. Pearson or Spearman correlation coefficients were calculated to assess the relationships between variables. Graphpad Prism 5.0 was used for the analysis.
Results
General characteristics of the subjects
Table 1 presents the general characteristics of the subjects. Age and BMI were similar between the two groups. The weight loss during the 6 months before participation in the study, as reported by the subjects at the recruitment interview, was used to divide the groups in CC and WSC, in line with the criteria of Evans et al.22 used to define the groups. Haemoglobin levels were lower in the CC group than in the WSC group and IL‐6 levels were higher.
Peritumoural adipose tissue protein expression analysis
The concentrations in peritumoural adipose tissue of the various pro‐inflammatory and anti‐inflammatory cytokines/chemokines, tumour growth factors, signal proteins related to pro‐inflammatory pathways, and cachexia‐related factors are shown in Tables 2 and 3. There were no significant differences between the two subject groups for the majority of the proteins analysed, but TNF‐α, STAT‐1, and FADD (an apoptotic factor) were significantly higher in the CC group than in the WSC group. The protein concentration of IP‐10, a chemokine secreted by interferon‐stimulated cells, was not significantly different but showed a tendency to be higher in the CC group (p = 0.07).
Table 2.
Peritumoral white adipose tissue cytokines and protein content (pg/mg of protein) in weight‐stable cancer patient (WSC) and in cachectic cancer patient (CC)
| Protein | WSC | CC | P |
|---|---|---|---|
| IL‐6 | 6.85 [4.13–21.44] | 8.71 [1.17–24.74] | 0.75b |
| IL‐10 | 0.49 [0.27–0.79] | 0.17 [0.05–0.78] | 0.17b |
| IL‐15 | 1.16 ± 0.17 | 1.30 ± 0.19 | 0.57a |
| MCP‐1 | 106.30 [25.37–633.20] | 90.97 [35.74–307.40] | 0.69b |
| TNF‐α | 0.57 ± 0.11 | 1.04 ± 0.19 | 0.05a |
| IL‐7 | 2.58 [2.45–4.57] | 3.61 [1.90–12.95] | 0.39b |
| IFN‐ALPHA | 12.46 [5.98–86.04] | 15.59 [8.97–21.02] | 0.61b |
| IFN‐GAMMA | 0.83 [0.08–2.44] | 0.97 [0.62–1.75] | 0.75b |
| IL‐1α | 1.15 [0.43–2.05] | 0.87 [0.12–2.06] | 0.53b |
| IL‐1Rα | 8.41 ± 2.49 | 15.10 ± 3.29 | 0.12a |
| IL‐2 | 0.36 ± 0.07 | 0.36 ± 0.03 | 0.98a |
| IL‐8 | 14.59 [6.64–29.96] | 16.78 [15.41–55.35] | 0.14b |
| IL‐17α | 0.42 ± 0.06 | 0.40 ± 0.13 | 0.88a |
| IP‐10 | 12.93 [6.19–19.62] | 54.17 [7.65–178.9] | 0.07b |
| ANGPTL‐4 | 10029.79 ± 1168.79 | 8949.79 ± 1136.50 | 0.49a |
| MIP‐1α | 11.44 [5.30–24.47] | 14.20 [4.32–40.28] | 0.85b |
| MIP‐1β | 9.33 ± 2.01 | 8.53 ± 1.13 | 0.71a |
| TNF‐β | 0.82 ± 0.21 | 0.87 ± 0.11 | 0.79a |
| IL‐12p40 | 5.48 ± 0.73 | 3.78 ± 0.41 | 0.04a |
| Il‐12p70 | 1.48 ± 0.22 | 1.20 ± 0.11 | 0.22a |
| IL‐13 | 6.24 ± 1.59 | 5.82 ± 0.78 | 0.79a |
| VEGF | 14.80 ± 3.27 | 14.91 ± 1.17 | 0.97a |
| IL‐3 | 0.42 [0.18–0.88] | 0.62 [0.05–1.06] | 0.70b |
| IL‐5 | 0.16 ± 0.04 | 0.17 ± 0.02 | 0.81a |
| EOTAXIN | 3.33 ± 0.51 | 3.20 ± 0.47 | 0.85a |
| GM‐CSF | 2.76 [1.28–5.23] | 1.04 [0.32–2.84] | 0.13b |
| RANTES | 415.43 ± 129.53 | 516.88 ± 113.65 | 0.54a |
| G‐CSF | 8.02 [5.44–44.54] | 6.52 [3.78–64.69] | 0.78b |
| IL‐10/TNF‐α | 0.63 [0.41–1.46] | 0.10 [0.03–1.10] | 0.07b |
Data expressed as median [first quartile; third quartile] or as mean ± SEM; n = 5–9 per group.
Student's t‐test.
Mann–Whitney test.
Table 3.
Peritumoral white adipose tissue growth factors and protein content (pg/mg of protein or MFI, medium fluorescente intensity) in weight‐stable cancer patient (WSC) and in cachectic cancer patient (CC)
| Protein | WSC | CC | P |
|---|---|---|---|
| TGF‐β1 (pg/mg) | 138.78 ± 27.70 | 142.50 ± 21.75 | 0.911a |
| TGF‐β2 (pg/mg) | 7.68 ± 1.71 | 9.64 ± 2.01 | 0.4391a |
| TGF‐β3 (pg/mg) | 2.27 [0.28–4.25] | 1.72 [1.13–9.10] | 0.7308b |
| NFkB (MFI) | 69.00 [47.00–85.00] | 84.00 [51.00–107.00] | 0.2677b |
| TNF‐R1 (MFI) | 153.00 [115.00–247.00] | 215.00 [150.00–333.00] | 0.202b |
| c‐MYC (MFI) | 107.50 [76.00–140.50] | 130.00 [83.00–146.00] | 0.3524b |
| IKK (MFI) | 67.00 [56.00–102.00] | 84.00 [50.50–118.00] | 0.5303b |
| IkB (MFI) | 194.00 [116.00–290.50] | 249.00 [146.50–466.00] | 0.3434b |
| Src (MFI) | 71.00 [53.00–79.00] | 68.00 [66.00–70.00] | 0.7551b |
| ERK/MAPK 1–2 (MFI) | 483.25 [328.00–996.00] | 565.25 [346.00–638.00] | 1.00b |
| STAT‐1 (MFI) | 1361.00 [216.00–2087.00] | 2654.00 [1598.00–4865.00] | 0.0303b |
| STAT‐3 (MFI) | 141.00 [66.00–181.50] | 138.00 [111.00–175.00] | 0.8763b |
| FADD (MFI) | 122.00 [103.00–137.00] | 181.00 [145.00–200.00] | 0.0139b |
Data expressed as median [first quartile; third quartile] or as mean ± SEM; n = 5–9 per group. MFI, median fluorescente intensity.
Student's t‐test.
Mann–Whitney test.
Correlation analysis
Correlation analysis (Spearman or Pearson, for non‐parametric and parametric data, respectively, as stated in Figure 1A–1E) was applied to the relationships between cytokines/chemokines and RANTES, GCSF (both growth tumour factors) and VEGF (an angiogenic factor) in the peritumoural adipose tissue.
Figure 1.
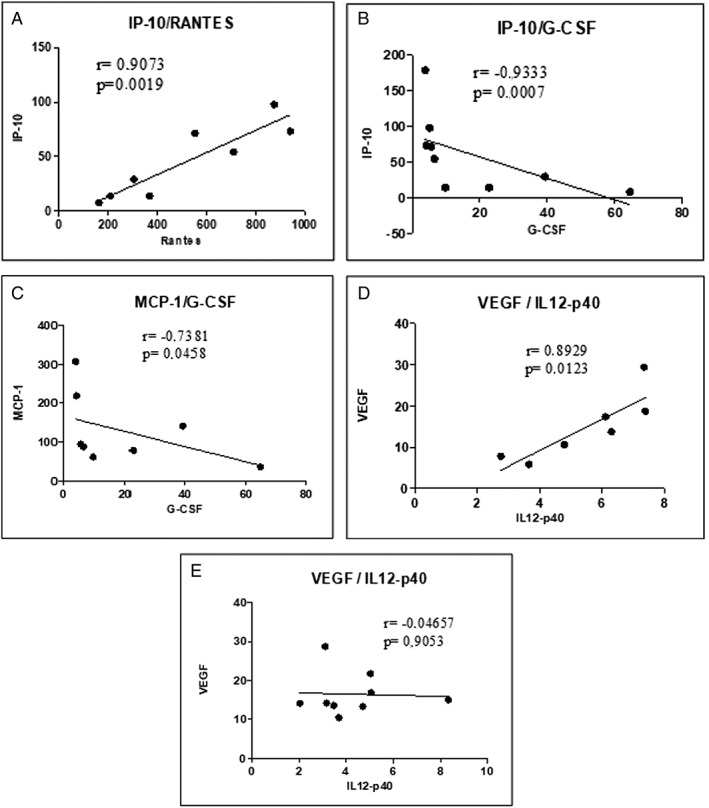
Correlation analysis in peritumoutal adipose tissue; n = 8–9. (A) Correlation between RANTES and IP‐10 in peritumoural adipose tissue of cancer with cachexia patients. (B) Correlation between GCSF and IP‐10 in peritumoural adipose tissue of cancer with cachexia patients. (C) Correlation between GCSF and MCP‐1 in peritumoural adipose tissue of cancer with cachexia patients. (D) Correlation between IL12p40 and VEGF in peritumoural adipose tissue of weight‐stable cancer patients. (E) Correlation between IL12p40 and VEGF in peritumoural adipose tissue of cancer with cachexia patients.
In the CC group, RANTES showed a positive significant correlation with IP‐10 (P < 0.05) (Figure 1A) and a negative correlation with IFN‐α (Table S1). GCSF showed significant negative correlations (all P < 0.05) with IL‐1Ra (TS1), IP‐10 (Figure 1B), IL‐15 (Table S1), and MCP‐1 (Figure 1C) and a positive correlation with IFN‐α (P = 0.01) (Table S1).
A positive correlation between VEGF and IL12p40 was observed for the WSC group but not for the CC group (Figure 1D and 1E). In the CC group, IL‐3 showed significant positive correlations with IL‐2, IL‐17α, and IL‐1α (Table S1). No correlation was found between FADD and STAT‐1 and the cytokines and chemokines analysed (TS1).
In the WSC group, no significant correlations were detected between RANTES, GCSF, IL‐3, FADD, and STAT‐1 and the cytokines/chemokines analysed (Table S2).
Discussion
In this study, we evaluated many proteins and factors in peritumoural adipose tissue from patients with colorectal cancer with either cachexia or stable body weight. Overall, the results were similar between the two groups, but TNF‐α, FADD, and STAT‐1 were higher and IL‐1β and IL‐12p40 lower in the patients with cachexia, indicating more pronounced pro‐inflammatory conditions in the peritumoural adipose tissue of the cachectic patients than in the stable weight patients. This difference may have contributed to the development of cachexia and more aggressive tumour progression.
The CC group patients all experienced significant weight loss over the previous 6 months. In addition, they showed a reduction in the level of haemoglobin and increased levels of IL‐6 and CRP, with the CRP level higher than the normal reference value. These conditions confirmed that all these patients presented with cachexia, in accordance with the criteria proposed by Evans et al.22
Systemic inflammation is a common condition in CC. Circulating pro‐inflammatory mediators, such as IL‐6 and TNF‐α, and acute‐phase proteins, such as CRP, are upregulated in cachectic patients and this correlates positively with weight loss, resulting in a poor prognosis. In addition to the tumour, white adipose tissue contributes to the inflammatory state in cachexia by actively secreting several pro‐inflammatory mediators.23
Previously, our group24 reported that tumours from cachectic and stable weight cancer patients with the same diagnosis showed different secretory profiles of inflammatory factors and different proportions of macrophage phenotypes, with lower levels of M2 macrophages in the tumours of the cachectic patients. Based on these findings, we proposed an association between tumour‐originating factors and adipose tissue inflammatory changes, as a positive correlation was found between cytokines derived from the tumour and subcutaneous adipose tissue and inflammatory factors.
In the present study, the major differences in cytokine levels between the groups were that the IL‐6 serum concentration and TNF‐α content in peritumoural adipose tissue were higher in the CC group patients than in the WSC group patients. In contrast, de Matos‐Neto et al.24 did not detect a difference in the TNF‐α protein content of subcutaneous adipose tissue and tumours between cachexia and stable weight patients. Taking together the result of the present study and reported by de Matos‐Neto et al.,24 it is possible suggesting that the tumoural micro‐environment was not altered by the increase of TNF‐α content in the peritumoural adipose tissue or that this change was not sufficient to modify the tumour micro‐environment. Conversely, it could be suggested that the cytokines produced by peritumoural adipose tissue may contribute to the development of cachexia in patients with tumours. IL‐6 and TNF‐α are considered to be the major mediators of a network of interactive signals. TNF‐α is a pleiotropic cytokine implicated as a local and systemic factor involved in the development of CC, and IL‐6 has been shown to be associated with cancer progression.25
TNF‐α, like other pro‐inflammatory factors such as IFN‐γ and TLR, can stimulate the M1 phenotype and therefore suppress M2 macrophages.26 In addition, TNF‐α negatively regulates the expression of M2 macrophages in vivo and in vitro,27 indicating that the downstream signals from type I TNFR, such as NF‐kB, act in a gene‐specific way.
Macrophage migration and polarization in the tumour micro‐environment has been implicated in increasing pro‐inflammatory conditions and tumour development.28 Several factors may contribute to macrophage migration. In the present study, we evaluated levels in peritumoural adipose tissue of IP‐10 and RANTES, which are chemiotaxis factors. We observed a non‐significant increase in IP‐10 in the CC group compared to the WSC group (P = 0.07), with a positive correlation between IP‐10 and RANTES, which could contribute to a pro‐inflammatory tumour environment in cancer cachectic patients. In addition, there were negative correlations between IP‐10 and GCSF, an antiapoptotic, antiangiogenic, and anti‐inflammatory factor,29, 30 and between MCP‐1 and GCSF, in the peritumoural adipose tissue of the CC group patients but not the WSC group patients. It has also been found that LPS‐challenged macrophages secrete IL‐1β, IL‐6, and TNF‐α, increasing the proliferation and migration of colon cancer cells. This suggests that these inflammatory mediators could contribute to the development and metastasis of colon cancer.31
Our results considered together with those of previous studies could suggest that the TNF‐α produced by peritumoural adipose tissue may have acted as a paracrine factor that contributed to an increase in the malignancy of the cancer in the patients of the present study, suggesting the existence of crosstalk between peritumoural adipose tissue and tumour cells.
In addition to this local effect, TNF‐α exerts systemic effects on cachexia associated with cancer, contributing via activation of the ubiquitin proteasome pathway to the decreased synthesis rates and increased protein degradation rates (protein turnover) responsible for the loss of skeletal muscle mass. TNF‐α also changes the metabolism of lipids by promoting an increase in lipolysis, reducing lipoprotein lipase activity and suppressing the expression of peroxisome proliferator‐activated receptor gamma (PPARγ), which is responsible for the differentiation and function of adipocytes. These findings indicate the important role of TNF‐α in the loss of adipose tissue and in the development of CC.32
IL‐6 and TNF‐α are important multifunctional cytokines involved in tumour growth and metastasis. A retrospective clinical study of lung cancer patients found a possible link between metastasis and cachexia that was associated with the inherent tumour characteristics.33
Many tumour suppressor genes oncogenes have been reported to be implicated in the development of colorectal cancer. Protein interactions could contribute to tumour development. The role of FADD in the mechanism of tumour cell apoptosis is dependent on the tumour type; it is pro‐apoptotic for certain types of cancer, and anti‐apoptotic for others.34 Marikar et al.35 demonstrated that the interaction of pFADD with metallothionein 2A (MT2A, identified as a potential biomarker in colorectal cancer)36 was correlated with the suppression of apoptosis and the induction of cell proliferation. That study also verified that both MT RNAi and FADD RNAi significantly inhibited the growth of Colo 205 solid tumours.
In the present study, we detected a significant increase in FADD protein content in peritumoural adipose tissue in the CC group; this may interact with other tumour proteins, such as MT2, resulting in an increase in tumour cell proliferation and malignancy in CC patients compared to those with a stable weight.
Angiogenesis is an important factor favouring tumour development.37 We therefore analysed the content levels of VEGF, a proangiogenic factor, and IL12p40, an anti‐angiogenic factor,38 in peritumoural adipose tissue. IL12p40 was lower in the CC group than in the WSC group, but there was no difference between the groups in VEGF content. A significant positive correlation between VEGF and IL12p40 was observed only in the WSC group, suggesting the angiogenic factors in peritumoural adipose tissue are disrupted in CC patients.
STAT‐1 has traditionally been considered to be associated with a good prognosis. Recently, however, an increase in this protein has been shown to be associated with the progression of cancer. STAT‐1 can promote tumour growth through the evasion and suppression of tumour immune surveillance and an increase in invasion or metastasis, and this can lead to resistance to radiotherapy and chemotherapy.39 Mice with ectopic overexpression of STAT‐1 exhibited mobilization of myeloid‐derived suppressor cells, resulting in a greater aggressiveness of the tumour accompanied by an increased level of TNF‐α and tumour growth; conversely, gene knockdown of STAT‐1 reversed this process.40 In a review, Meissl et al.39 revealed discrepancies in the literature regarding the role of STAT‐1; these could be explained by heterogeneity of the tumours, such as differences in tumour type and/or stage. Our analysis of the protein concentration of STAT‐1 in peritumoural adipose tissue in the present study showed an increase in the CC group compared with the WSC group. The elevated concentration of this protein may be associated with an increase in pro‐inflammatory factors such as TNF‐α, which may increase tumour malignancy in these patients.
This study had several limitations. The subjects' previous body weight was self‐reported and so inaccuracies were possible. The number of subjects in each group was low because of the difficulty of obtaining sampled of peritumoural adipose tissue during surgery. Because of the intrinsic variation in human tissue samples, some of the analyses were not performed for all the subjects initially enrolled, as some samples fell outside the detection range of the assays. The relatively small sample sizes could result in type 2 statistical error, although the statistical differences observed did not interfere with this error. However, as far as we know, this is the first study which points out differences on the peritumoral adipose tissue inflammatory condition between cachetic and weight stable colorectal cancer patient, which could be involved on presence of cachexia.
Conclusion
The findings of this study showed that increased inflammatory processes in patients with CC promoted changes in the peritumoural adipose tissue. These changes may contribute to tumour development by modifying either angiogenic factors or factors involved in apoptosis. Changes of the content of these proteins in the peritumoural adipose tissue of patients with CC may lead to a deterioration in prognosis, resulting in reduced quality of life and further complications, which in turn could increase the mortality rate. However, the effects of these pro‐inflammatory factors and those involved with angiogenesis and apoptosis remain to be fully elucidated. Their roles are dependent on the type and staging of the tumour, and further studies are needed to shed light on these factors in cachexia associated with cancer.
Supporting information
Table S1. Correlation between protein expression of inflammatory factors in peritumoural adipose tissue from CC group. n = 6‐9.
Table S2. Correlation between protein expression of inflammatory factors in peritumoural adipose tissue from WSC group. n= 5‐7.
Acknowledgements
This work was supported mainly by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2012/50079‐0), Conselho Nacional de Desenvolvimento Científico, Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). L.M.O., C.M.O.N., and M.S. are recipients of CNPq fellowships. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.41 The authors declare that they have no conflict of interest.
Neto, N. I. P. , Murari, A. S. P. , Oyama, L. M. , Otoch, J. P. , Alcântara, P. S. M. , Tokeshi, F. , Figuerêdo, R. G. , Alves, M. J. , Lima, J. D. C. C. , Matos‐Neto, E. M. , Seelaender, M. , and Oller do Nascimento, C. M. (2018) Peritumoural adipose tissue pro‐inflammatory cytokines are associated with tumoural growth factors in cancer cachexia patients. Journal of Cachexia, Sarcopenia and Muscle, 9: 1101–1108. 10.1002/jcsm.12345.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 2. Tsoli M, Swarbrick MM, Robertson GR. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin Cell Dev Biol 2016;54:68–81. [DOI] [PubMed] [Google Scholar]
- 3. Lee WM, Lu S, Medline A, Archer MC. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N‐methyl‐N‐nitrosourea. Cancer Lett 2001;162:155–160. [DOI] [PubMed] [Google Scholar]
- 4. Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control 1996;7:253–263. [DOI] [PubMed] [Google Scholar]
- 5. Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc 2005;64:163–169. [DOI] [PubMed] [Google Scholar]
- 6. Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 2005;184:285–293. [DOI] [PubMed] [Google Scholar]
- 7. Lysaght J, van der Stok EP, Allott EH, Casey R, Donohoe CL, Howard JM, et al. Pro‐inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett 2011;312:62–72. [DOI] [PubMed] [Google Scholar]
- 8. Batista ML Jr, Henriques FS, Neves RX, Olivan MR, Matos‐Neto EM, Alcantara PS, et al. Cachexia‐associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2016;7:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batista ML Jr, Neves RX, Peres SB, Yamashita AS, Shida CS, Farmer SR, et al. Heterogeneous time‐dependent response of adipose tissue during the development of cancer cachexia. J Endocrinol 2012;215:363–373. [DOI] [PubMed] [Google Scholar]
- 10. Batista ML Jr, Olivan M, Alcantara PS, Sandoval R, Peres SB, Neves RX, et al. Adipose tissue‐derived factors as potential biomarkers in cachectic cancer patients. Cytokine 2013;61:532–539. [DOI] [PubMed] [Google Scholar]
- 11. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 2011;70:i104–i108. [DOI] [PubMed] [Google Scholar]
- 12. Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum‐mediated angiogenesis. J Surg Res 1997;67:147–154. [DOI] [PubMed] [Google Scholar]
- 13. Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 2011;32:550–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amor S, Iglesias‐de la Cruz MC, Ferrero E, Garcia‐Villar O, Barrios V, Fernandez N, et al. Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. Int J Colorectal Dis 2016;31:365–375. [DOI] [PubMed] [Google Scholar]
- 15. Zhong W, Tong Y, Li Y, Yuan J, Hu S, Hu T, et al. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF‐kappaB signaling by paracrine CCL5. Oncotarget 2017;8:73693–73704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 2017;113:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duckett CS. Apoptosis and NF‐kappa B: the FADD connection. J Clin Invest 2002;109:579–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whipple CA. Tumor talk: understanding the conversation between the tumor and its microenvironment. Cancer Cell Microenviron 2015;2:e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V, et al. Adipocyte‐derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 2013;73:5657–5668. [DOI] [PubMed] [Google Scholar]
- 20. Zoico E, Rizzatti V, Darra E, Budui SL, Franceschetti G, Vinante F, et al. Morphological and functional changes in the peritumoral adipose tissue of colorectal cancer patients. Obesity (Silver Spring) 2017;25:S87–S94. [DOI] [PubMed] [Google Scholar]
- 21. Wagner M, Bjerkvig R, Wiig H, Melero‐Martin JM, Lin RZ, Klagsbrun M, et al. Inflamed tumor‐associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis 2012;15:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 23. Camargo RG, Riccardi DM, Ribeiro HQ, Carnevali LC Jr, de Matos‐Neto EM, Enjiu L, et al. NF‐kappaBp65 and expression of its pro‐inflammatory target genes are upregulated in the subcutaneous adipose tissue of cachectic cancer patients. Nutrients 2015;7:4465–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Matos‐Neto EM, Lima JD, de Pereira WO, Figueredo RG, Riccardi DM, Radloff K, et al. Systemic inflammation in cachexia—Is tumor cytokine expression profile the culprit? Front Immunol 2015;6:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL‐6 and TNF‐alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 2004;90:2312–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1‐m2 polarization balance. Front Immunol 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep 2015;12:1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho U, Kim B, Kim S, Han Y, Song YS. Pro‐inflammatory M1 macrophage enhances metastatic potential of ovarian cancer cells through NF‐kappaB activation. Mol Carcinog 2018;57:235–242. [DOI] [PubMed] [Google Scholar]
- 29. Tsai RK, Chang CH, Sheu MM, Huang ZL. Anti‐apoptotic effects of human granulocyte colony‐stimulating factor (G‐CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT‐dependent. Exp Eye Res 2010;90:537–545. [DOI] [PubMed] [Google Scholar]
- 30. Malashchenko VV, Meniailo ME, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, et al. Direct anti‐inflammatory effects of granulocyte colony‐stimulating factor (G‐CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell Immunol 2018;325:23–32. [DOI] [PubMed] [Google Scholar]
- 31. Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology 2010;215:242–249. [DOI] [PubMed] [Google Scholar]
- 32. Patel HJ, Patel BM. TNF‐alpha and cancer cachexia: molecular insights and clinical implications. Life Sci 2017;170:56–63. [DOI] [PubMed] [Google Scholar]
- 33. Shiono M, Huang K, Downey RJ, Consul N, Villanueva N, Beck K, et al. An analysis of the relationship between metastases and cachexia in lung cancer patients. Cancer Med 2016;5:2641–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang R, Liu Y, Hammache K, He L, Zhu B, Cheng W, et al. The role of FADD in pancreatic cancer cell proliferation and drug resistance. Oncol Lett 2017;13:1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marikar FM, Jin G, Sheng W, Ma D, Hua Z. Metallothionein 2A an interactive protein linking phosphorylated FADD to NF‐kappaB pathway leads to colorectal cancer formation. Chin Clin Oncol 2016;5:76. [DOI] [PubMed] [Google Scholar]
- 36. Liang GY, Lu SX, Xu G, Liu XD, Li J, Zhang DS. Expression of metallothionein and Nrf2 pathway genes in lung cancer and cancer‐surrounding tissues. World J Surg Oncol 2013;11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dewangan J, Kaushik S, Rath SK, Balapure AK. Centchroman regulates breast cancer angiogenesis via inhibition of HIF‐1alpha/VEGFR2 signalling axis. Life Sci 2018;193:9–19. [DOI] [PubMed] [Google Scholar]
- 38. Ngiow SF, Teng MW, Smyth MJ. A balance of interleukin‐12 and ‐23 in cancer. Trends Immunol 2013;34:548–555. [DOI] [PubMed] [Google Scholar]
- 39. Meissl K, Macho‐Maschler S, Muller M, Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine 2017;89:12–20. [DOI] [PubMed] [Google Scholar]
- 40. Hix LM, Karavitis J, Khan MW, Shi YH, Khazaie K, Zhang M. Tumor STAT1 transcription factor activity enhances breast tumor growth and immune suppression mediated by myeloid‐derived suppressor cells. J Biol Chem 2013;288:11676–11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation between protein expression of inflammatory factors in peritumoural adipose tissue from CC group. n = 6‐9.
Table S2. Correlation between protein expression of inflammatory factors in peritumoural adipose tissue from WSC group. n= 5‐7.


