Abstract
Three peptaibols, trichorzins HA II (1), HA V (2), and HA VI (3), were isolated from okara fermented with Trichoderma harzianum HK-61 as anti-plant viral agents. Their structures were confirmed by spectroscopic and chemical methods. At micro molar concentrations, the trichorzins inhibited infections by Cucumber mosaic virus in the cowpea plant Vigna sesquipedalis.
Keywords: Cucumber mosaic virus, Trichoderma harzianum, anti-plant viral activity, peptaibols, trichorzins
Introduction
Viruses are among the pathogens most agriculturally detrimental to crops. Plant viral diseases cause serious economic losses in agriculture by reducing yield and quality. Because of the simple form of viruses, which consist of a segment of DNA or RNA encoding the genes required for their own multiplication in hosts and coat proteins, the chemical control of their diseases remains difficult or impossible.1,2) Therefore, the discovery of compounds that can inhibit plant viral infections continues to be a priority.
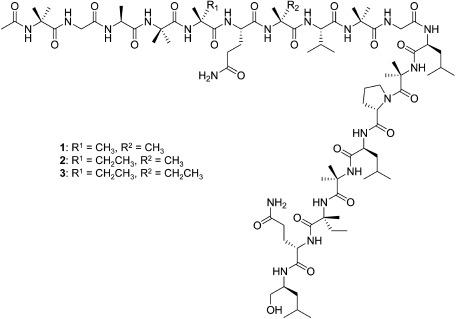
In previous studies, we yielded several fungal strains from soil samples collected in Japan that produced unique natural products. For example, Penicillium simplicissimum ATCC 90288 produced insecticidal indole alkaloids, okaramines,3–8) and the unidentified ascomycete OK-128 produced paralytic cyclic peptides, PF1171s.9,10) In this study, we have screened the fermentation extracts of fungal strains to find anti-plant viral compounds. Here, we report the isolation, identification, and anti-plant viral activity of three peptaibols—trichorzins HA II (1), HA V (2), and HA VI (3) (Fig. 1)—produced by Trichoderma harzianum HK-61.
Fig. 1. Structures of trichorzins HA II (1), HA V (2), and HA VI (3).
Materials and Methods
1. Fermentation
T. harzianum HK-61 was isolated from a soil sample collected in Sakai (Japan) in the usual manner. Identification of this strain was carried out at the CBS Fungal Biodiversity Centre (The Netherlands). A loopful of spores from a slant culture of the strain was inoculated into 30 g of okara in a Petri dish 9 cm in diameter, and cultivation was carried out at 25°C for 14 days.
2. Extraction and isolation
The okara (2 kg) that had been fermented with strain HK-61 was soaked in MeOH for 2 days. Evaporation of the MeOH gave an aqueous concentrate, which was extracted with EtOAc. The EtOAc extract was concentrated and subsequently chromatographed on Wakogel C-200 (Wako Pure Chemical) by eluting with n-hexane and an increasing ratio of EtOAc to afford active eluates (5.0 g; 60 and 80% EtOAc). The active eluates were further chromatographed on Chromatorex ODS (Fuji Silysia Chemical) by eluting with H2O and an increasing ratio of MeOH to afford an active eluate (1.5 g; 100% MeOH). The active fraction was subjected to preparative HPLC [column, Inertsil ODS-3 10×250 mm (GL Sciences); solvent, 75% MeOH in 0.05% aq. TFA; flow rate, 4 mL/min, 50 times] to yield 1 (90 mg), 2 (320 mg), and 3 (150 mg).
3. Spectroscopic analysis of trichorzin HA V (2) and its partial acid hydrolysates
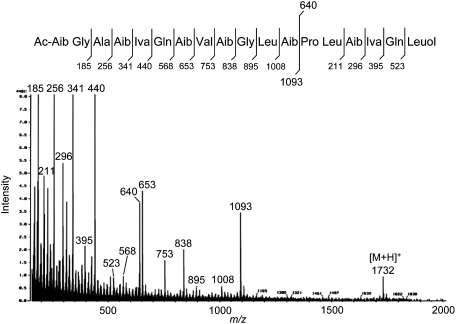
NMR experiments were carried out in DMSO-d6 using a JNM AL-400 NMR spectrometer (JEOL). Chemical shifts were referenced to the solvent peak (δH 2.49, δC 39.7) as an internal standard. FAB-MS was recorded on a JMS-700 (JEOL) using glycerol as the matrix. The 1H- and 13C-NMR signals of 2 could not be assigned due to the severe overlapping of signals. FAB-MS data for 2 are shown in Fig. 2. Compound 2 was partially hydrolyzed in 6 M HCl for 90 min at 90°C to afford five fragment peptides, 4–8. The structures of the peptides were determined by 1D-NMR (1H, 13C) and 2D-NMR (1H–1H COSY, HMQC, HMBC). Spectroscopic data for 4: 1H-NMR (400 MHz) δ: 0.71 (3H, dd, J=7.3, 7.7 Hz, Iva-γ), 0.83 (3H, d, J=6.7 Hz, Leu-δ), 0.90 (3H, d, J=6.7 Hz, Leu-δ′), 1.34 (3H, s, Aib-β), 1.35 (3H, s, Aib-β′), 1.37 (3H, s, Iva-β′), 1.49 (2H, m, Leu-β), 1.63 (1H, m, Leu-γ), 1.74 (1H, m, Iva-β), 1.84 (2H, m, Pro-γ), 1.89 (1H, m, Pro-β), 2.01 (1H, m, Iva-β), 2.28 (1H, m, Pro-β), 3.26 (2H, br, Pro-δ), 4.20 (1H, br, Pro-α), 4.36 (1H, m, Leu-α), 7.13 (1H, s, Iva-NH), 8.16 (1H, s, Aib-NH), 8.54 (1H, d, J=7.9 Hz, Leu-NH), 9.20 (1H, br, Pro-NH). 13C-NMR (100 MHz) δ: 7.9 (Iva-γ), 21.3 (Iva-δ), 21.9 (Iva-δ′), 22.8 (Iva-β′), 23.1 (Pro-γ), 24.0 (Leu-γ), 24.5 (Aib-β), 24.8 (Aib-β′), 28.5 (Iva-β), 29.4 (Pro-β), 40.5 (Leu-β), 45.7 (Pro-δ), 51.6 (Leu-α), 56.2 (Aib-α), 58.7 (Pro-α), 59.0 (Iva-α), 167.8 (Pro-C=O), 171.0 (Leu-C=O), 172.5 (Aib-C=O), 175.1 (Iva-C=O). FAB-MS: m/z 413 [M+H]+ (C20H37N4O5). Spectroscopic data for 5: 1H-NMR (400 MHz) δ: 0.74 (3H, t, J=7.5 Hz, Iva-γ), 1.36 (3H, d, J=8.5 Hz, Ala-β), 1.38 (3H, s, Aib-β′), 1.39 (3H, s, Iva-β′), 1.44 (3H, s, Aib-β), 1.76 (1H, m, Iva-β), 2.03 (1H, m, Iva-β), 3.84 (1H, br, Ala-α), 7.15 (1H, s, Iva-NH), 8.00 (2H, br, Ala-NH2), 8.35 (1H, s, Aib-NH). 13C-NMR (100 MHz) δ: 7.9 (Iva-γ), 17.0 (Ala-β), 21.8 (Iva-β′), 24.0 (Aib-β), 25.3 (Aib-β′), 28.6 (Iva-β), 48.2 (Ala-α), 56.5 (Aib-α), 59.1 (Iva-α), 168.6 (Ala-C=O), 171.7 (Aib-C=O), 174.9 (Iva-C=O). FAB-MS: m/z 274 [M+H]+ (C12H24N3O4). Spectroscopic data for 6: 1H-NMR (400 MHz) δ: 0.90 (3H, d, J=6.1 Hz, Leu-δ), 0.91 (3H, d, J=6.1 Hz, Leu-δ′), 1.38 (3H, s, Aib-β), 1.42 (3H, s, Aib-β′), 1.51 (1H, m, Leu-β), 1.59 (1H, m, Leu-β), 1.70 (1H, m, Leu-γ), 3.72 (1H, br, Leu-α), 8.02 (2H, br, Leu-NH2), 8.49 (1H, s, Aib-NH). 13C-NMR (100 MHz) δ: 21.7 (Leu-δ), 22.4 (Leu-δ′), 23.2 (Leu-γ), 24.4 (Aib-β), 24.7 (Aib-β′), 40.0 (Leu-β), 50.6 (Leu-α), 55.2 (Aib-α), 167.7 (Leu-C=O), 174.3 (Aib-C=O). FAB-MS: m/z 217 [M+H]+ (C10H21N2O3). Spectroscopic data for 7: 1H-NMR (400 MHz) δ: 0.82 (3H, d, J=6.8 Hz, Val-γ), 0.89 (3H, d, J=6.8 Hz, Val-γ′), 1.35 (3H, s, Aib2-β′), 1.36 (3H, s, Aib2-β), 1.41 (3H, s, Aib1-β), 1.43 (3H, s, Aib1-β′), 1.97 (2H, m, Glu-β), 2.00 (1H, m, Val-β), 2.36 (2H, t, J=7.8 Hz, Glu-γ), 3.81 (1H, br, Glu-α), 4.13 (1H, dd, J=7.3, 8.5 Hz, Val-α), 7.09 (1H, d, J=8.8 Hz, Val-NH), 7.95 (1H, s, Aib2-NH), 8.03 (2H, br, Glu-NH2), 8.37 (1H, s, Aib1-NH). 13C-NMR (100 MHz) δ: 18.0 (Val-γ), 18.9 (Val-γ′), 24.4 (Aib1-β), 24.6 (Aib2-β), 24.7 (Aib2-β′), 25.0 (Aib1-β′), 26.1 (Glu-β), 28.9 (Glu-γ), 30.5 (Val-β), 51.7 (Glu-α), 54.7 (Aib2-α), 56.6 (Aib1-α), 57.5 (Val-α), 167.3 (Glu-C=O), 169.7 (Val-C=O), 172.3 (Aib1-C=O), 172.8 (Glu-δ (C=O)), 174.8 (Aib2-C=O). FAB-MS: m/z 417 [M+H]+ (C18H34N5O6). Spectroscopic data for 8: 1H-NMR (400 MHz) δ: 0.74 (3H, t, J=7.6 Hz, Iva-γ), 0.82 (6H, d, J=6.7 Hz, Val-γ,γ′), 1.26 (3H, s, Iva-β′), 1.38–1.44 (15H, Aib1-β′, Aib2-β,β′, Aib3-β,β′), 1.40 (3H, s, Aib1-β), 1.43 (3H, d, J=7.0 Hz, Ala-β), 1.75 (1H, m, Iva-β), 2.03 (3H, Iva-β, Glu-β), 2.19 (1H, m, Val-β), 2.33 (2H, t, J=7.5 Hz, Glu-γ), 3.58 (1H, dd, J=5.8, 18.0 Hz, Gly-α), 3.76 (1H, dd, J=5.8, 18.0 Hz, Gly-α), 3.86 (1H, br, Ala-α), 3.96 (1H, m, Glu-α), 4.07 (1H, dd, J=5.5, 8.9 Hz, Val-α), 6.93 (1H, d, J=8.9 Hz, Val-NH), 7.47 (1H, t, J=5.8 Hz, Gly-NH), 7.49 (1H, s, Aib3-NH), 7.66 (1H, d, J=6.1 Hz, Glu-NH), 7.79 (1H, s, Aib2-NH), 7.85 (1H, s, Iva-NH), 7.98 (2H, br, Ala-NH2), 8.75 (1H, s, Aib1-NH). 13C-NMR (100 MHz) δ: 7.3 (Iva-γ), 16.5 (Ala-β), 17.3 (Val-γ), 19.0 (Val-γ′), 22.4 (Iva-β′), 23.6–25.6 (Aib1-β′, Aib2-β,β′, Aib3-β,β′), 25.7 (Glu-β), 25.9 (Aib1-β), 26.5 (Iva-β), 28.9 (Val-β), 30.3 (Glu-γ), 40.7 (Gly-α), 48.2 (Ala-α), 54.5 (Glu-α), 55.9 (Aib3-α), 56.1 (Aib2-α), 56.3 (Aib1-α), 57.9 (Val-α), 59.0 (Iva-α), 169.4 (Ala-C=O), 170.0 (Val-C=O), 170.7 (Gly-C=O), 172.0 (Glu-C=O), 173.6 (Aib-C=O, Glu-δ (C=O)), 174.0 (Aib2-C=O, Aib3-C=O), 175.8 (Iva-C=O). FAB-MS: m/z 729 [M+H]+ (C32H57N8O11).
Fig. 2. FAB mass spectrum and proposed fragmentation of trichorzin HA V (2).
4. Spectroscopic analysis of trichorzins HA II (1) and HA VI (3)
FAB-MS for 1 (m/z, %): 1718 [M+H]+ (C80H141N20O21, 8), 1079 (12), 994 (4), 823 (10), 738 (10), 640 (24), 639 (18), 553 (40), 426 (56), 395 (13), 341 (100), 296 (37), 256 (100), 211 (29), 185 (100), 128 (71). FAB-MS for 3 (m/z, %): 1746 [M+H]+ (C82H145N20O21, 23), 1107 (48), 1022 (9), 852 (28), 766 (24), 667 (54), 640 (51), 568 (14), 524 (18), 440 (100), 395 (30), 341 (100), 296 (83), 256 (100), 211 (73), 185 (100), 128 (100). Several of the m/z values were not the same as those reported previously.11) This is because Hlimi et al. described the FAB-MS data for these compounds as the nominal mass (C=12, H=1, N=14, O=16).
5. Marfey’s analysis
Peptaibol (2 mg) was hydrolyzed in 6 M HCl at 100°C for 20 hr. After cooling to room temperature, the sample was dried under vacuum. The residue was dissolved in 1 M NaHCO3 (500 µL) and reacted with Marfey’s reagent (5-fluoro-2,4-dinitrophenyl-L-alanine amide, 120 µL of 10 mM acetone solution) and allowed to react at 40°C for 2 hr. After cooling, the samples were quenched with 2 M HCl and dried under vacuum. The solid residue was dissolved in 50% aq. MeCN and analyzed by HPLC (column, Inertsil ODS-3 4.6×250 mm; solvent, linear gradient from 30 to 70% MeCN in 0.05% aq. TFA; flow rate, 0.8 mL/min; detection, 340 nm) over 60 min. The retention times for Marfey’s derivatives of 2 were: L-Glu, 14.8 min; Gly, 16.2 min; L-Ala, 19.0 min; L-Pro, 19.5 min; Aib, 24.8 min; L-Val, 28.3 min; D-Iva, 31.5 min; L-Leuol, 32.8 min; L-Leu, 36.2 min. The retention times for Marfey’s derivatives of 1 were: L-Glu, 14.2 min; Gly, 15.5 min; L-Ala, 18.2 min; L-Pro, 18.6 min; Aib, 24.2 min; L-Val, 27.6 min; D-Iva, 30.7 min; L-Leuol, 32.8 min; L-Leu, 35.9 min. The retention times for Marfey’s derivatives of 3 were: L-Glu, 14.3 min; Gly, 15.7 min; L-Ala, 18.4 min; L-Pro, 18.9 min; Aib, 24.4 min; L-Val, 27.7 min; D-Iva, 30.8 min; L-Leuol, 32.8 min; L-Leu, 35.9 min.
6. Bioassay
An isolate of CMV propagated in tobacco (N. tabacum cv. Xanthi) was purified as described.12) A local lesion host for CMV, cowpea (V. sesquipedalis cv. Kurodane-sanjaku), was grown in a growth chamber at 25°C. The inhibitory effects of the fermentation extracts against CMV infection of cowpea plants were examined using a local lesion assay as reported previously.13) The activity of purified compounds was investigated using hydroponic treatment. All assays were performed in three replicates.
Results and Discussion
Anti-plant viral activity was examined by using a bioassay of Cucumber mosaic virus (CMV) and its local lesion host, cowpea (Vigna sesquipedalis cv. Kurodane-sanjaku), where its activity was evaluated by the inhibition of local lesions resulting from CMV infection.13) MeOH extracts of okara (the insoluble residue of whole soybean) fermented with fungal strains were used for the bioassay. As a result of screening 200 strains, we found that T. harzianum HK-61 produces antiviral compounds against CMV; therefore, we isolated the active compounds. The MeOH extract of okara fermented with HK-61 was concentrated in vacuo, and the resulting aqueous concentrate was extracted with EtOAc. The EtOAc extract demonstrated inhibition of the CMV infection; however, the aqueous layer did not. Bioassay-guided purification of the extract by repeated column chromatography over silica gel and ODS resulted in the isolation of compounds 1–3. NMR experiments suggested that these compounds were peptides. This was also supported by the positive ninhydrin reaction of their hydrolysates.
The FAB-MS of 2, the most active compound, gave a protonated molecule [M+H]+ at m/z 1732 (Fig. 2). The 1H- and 13C-NMR spectra of 2 exhibited signals for 21 amide protons and 20 carbonyl carbons, suggesting that this peptide was composed of nearly 20 amino acid residues (data not shown). However, the severe overlapping of NMR signals hampered further analysis of the structure. Thus, we carried out the partial acid hydrolysis of 2 and obtained five fragment peptides, 4–8. The structure of fragment 4 was determined to be Pro-Leu-Aib (2-aminoisobutyric acid)-Iva (isovaline) by FAB-MS and NMR (1H, 13C, 1H–1H COSY, HMQC, HMBC) experiments. Similarly, fragments 5–8 were deduced to be Ala-Aib-Iva, Leu-Aib, Glu-Aib-Val-Aib, and Ala-Aib-Iva-Glu-Aib-Val-Aib-Gly, respectively. Marfey’s amino acid analysis of the total acid hydrolysate of 2 provided the following amino acids; Aib, L-Ala, L-Glu/Gln, L-Gly, D-Iva, L-Leu, L-Leuol (leucinol), L-Pro, and L-Val.14) Taking into consideration these results, we reanalyzed the FAB-MS data for 2 and assigned the respective fragment ions derived from the cleavage of amide bonds (Fig. 2). Thus, we concluded that compound 2 was trichorzin HA V (Fig. 1), which was previously isolated from T. harzianum M-903602 as an antibacterial compound.11) Similarly, compounds 1 and 3 were identified as trichorzins HA II and HA VI (Fig. 1), respectively.
Finally, the antiviral activity of the purified trichorzins was examined. The compounds were added to hydroponic cultures of cowpea plants, and CMV was inoculated into the leaves. Compound 2 showed the strongest effects, 80.5% (5 μM) and 90.6% (10 μM) inhibition, against CMV (Table 1). Compounds 1 and 3 exhibited 42.6% and 68.5% inhibition at a concentration of 10 μM. This is the first report of the anti-plant viral activity of trichorzins.
Table 1. Inhibitory activity of trichorzins against the CMV infection.
| Compound | Concentration (μM) | Inhibition (%)a) |
|---|---|---|
| Trichorzin HA II (1) | 5 | 33.5±5.3 |
| 10 | 42.6±2.7 | |
| Trichorzin HA V (2) | 5 | 80.5±4.6 |
| 10 | 90.6±4.2 | |
| Trichorzin HA VI (3) | 5 | 16.5±2.8 |
| 10 | 68.5±5.7 |
a) Data shown are the mean±SE for three replicates.
Peptaibols are characterized by an N-terminal acylated amino acid residue and a C-terminal amino alcohol on a lipophilic amino acid chain that includes many α,α-dialkylated amino acids, such as Aib and Iva.15) Numerous peptaibols have been isolated from Trichoderma spp. and several other fungi mainly as antimicrobial substances. Previously, Yeo and co-workers isolated two peptaibols, peptaivirins A and B, from the unidentified fungus KGT142 as antiviral agents against infection by Tobacco mosaic virus (TMV) in the tobacco plant Nicotiana tabacum cv. Xanthi-nc.16) The 18mer peptaibols TvBI and TvBII from T. virens Gv29-8 elicited defense responses in the cucumber plant Cucumis sativus that resulted in resistance against several bacteria.17) Furthermore, trichokonins isolated from T. pseudokoningii SMF2 were revealed to induce defense responses and systemic resistance in N. tabacum var. Samsun NN against TMV infections.18) These results suggest that trichorzins may also induce defense responses in cowpea plants and cause resistance to CMV. Trichorzins’ mechanism of action against CMV in cowpea plants would be an interesting future topic of study.
References
- 1).C. Ritzenthaler: Curr. Opin. Biotechnol. 16, 118–122 (2005). [DOI] [PubMed] [Google Scholar]
- 2).M. Zaitlin and P. Palukaitis: Annu. Rev. Phytopathol. 38, 117–143 (2000). [DOI] [PubMed] [Google Scholar]
- 3).H. Hayashi, K. Takiuchi, S. Murao and M. Arai: Agric. Biol. Chem. 53, 461–469 (1989). [Google Scholar]
- 4).H. Hayashi, T. Fujiwara, S. Murao and M. Arai: Agric. Biol. Chem. 53, 3143–3145 (1991). [Google Scholar]
- 5).H. Hayashi, Y. Asabu, S. Murao and M. Arai: Biosci. Biotechnol. Biochem. 59, 246–250 (1995). [Google Scholar]
- 6).H. Hayashi and A. Sakaguchi: Biosci. Biotechnol. Biochem. 62, 804–806 (1998). [DOI] [PubMed] [Google Scholar]
- 7).Y. Shiono, K. Akiyama and H. Hayashi: Biosci. Biotechnol. Biochem. 64, 103–110 (2000). [DOI] [PubMed] [Google Scholar]
- 8).N. Kato, S. Furutani, J. Otaka, A. Noguchi, K. Kinugasa, K. Kai, H. Hayashi, M. Ihara, S. Takahashi, K. Matsuda and H. Osada: ACS Chem. Biol. 13, 561–566 (2018). [DOI] [PubMed] [Google Scholar]
- 9).K. Kai, H. Yoshikawa, Y. H. Kuo, K. Akiyama and H. Hayashi: Biosci. Biotechnol. Biochem. 74, 1309–1311 (2010). [DOI] [PubMed] [Google Scholar]
- 10).Y. H. Kuo, K. Kai, K. Akiyama and H. Hayashi: Tetrahedron Lett. 53, 429–431 (2012). [Google Scholar]
- 11).S. Hlimi, S. Rebuffat, C. Goulard, S. Duchamp and B. Bodo: J. Antibiot. (Tokyo) 48, 1254–1261 (1995). [DOI] [PubMed] [Google Scholar]
- 12).Y. Takanami: Virology 109, 120–126 (1981). [DOI] [PubMed] [Google Scholar]
- 13).M. Fujiwara, T. Kanamori, S. T. Ohki and T. Osaki: J. Gen. Plant Pathol. 67, 152–158 (2001). [Google Scholar]
- 14).P. Marfey: Carlsberg Res. Commun. 49, 591–596 (1984). [Google Scholar]
- 15).C. P. Kubicek, M. Komoń-Zelazowska, E. Sándor and I. S. Druzhinina: Chem. Biodivers. 4, 1068–1082 (2007). [DOI] [PubMed] [Google Scholar]
- 16).B. S. Yun, I. D. Yoo, Y. H. Kim, Y. S. Kim, S. J. Lee, K. S. Kim and W. H. Yeo: Tetrahedron Lett. 41, 1429–1431 (2000). [Google Scholar]
- 17).A. Viterbo, A. Wiest, Y. Brotman, I. Chet and C. Kenerley: Mol. Plant Pathol. 8, 737–746 (2007). [DOI] [PubMed] [Google Scholar]
- 18).Y. Luo, D. D. Zhang, X. W. Dong, P. B. Zhao, L. L. Chen, X. Y. Song, X. J. Wang, X. L. Chen, M. Shi and Y. Z. Zhang: FEMS Microbiol. Lett. 313, 120–126 (2010). [DOI] [PubMed] [Google Scholar]