Abstract
Breast cancer is a very heterogeneous disease. The intrinsic molecular subtypes can explain the intertumoral heterogeneity and the cancer stem cell (CSC) hypothesis can explain the intratumoral heterogeneity of this kind of tumor. CD44+/CD24- phenotype and ALDH1 expression are the major CSC markers described in invasive breast cancer. In the present study, 144 samples of invasive breast carcinoma, no special type were distributed in 15 tissue microarrays (TMA) and then evaluated for expression of the CD44+/CD24- phenotype and ALDH1 to understand the importance of these CSC markers and the clinical aspects of breast cancer. The samples were classified into four molecular subtypes according to clinicopathological criteria: Luminal A, Luminal B, HER2, and Basal-like. A statistical association was found between the molecular subtypes and the CSC markers, with HER2 the most frequent subtype for both markers. ALDH1 was also associated with other poor prognostic variables, such as a high histological grade and larger tumors, but it was not associated with the patients’ prognosis in this sample nor was the CD44+/CD24- phenotype in a multivariate analysis. There are still many controversies about the role of these markers in breast cancer molecular subtypes. The identification of these populations of cells, through immunohistochemical markers, can help to better understand the CSC theory in clinical practice and, in the near future, contribute to developing new target therapies.
Introduction
The “cancer stem cell” (CSC) hypothesis considers cancer to originate in a small subset of cells which have the abilities of self-renewal and differentiation into other cancer cells, stimulating tumor formation, growth and spread.1,2 These groups of cells are also thought to be involved in the resistance mechanism to different cancer treatments, such as chemotherapy and radiotherapy. 3 The complete eradication of these cells is essential in cancer treatment and, to achieve this, development of new-targeted therapies to CSC is necessary.2 One stem cell feature is that they are quiescent while chemotherapeutic agents, on the other hand, act on proliferating cells, explaining the chemoresistance of these cells.4,5 Furthermore, the CSC has high levels of ATP binding cassette (ABC) transporter proteins, such as ABCG2, which actively expels the drugs from the cells, protecting the cell during the chemotherapy action.6
The first description of the CSC existence was in acute myeloid leukemia 3 (AML) by Lapidot et al.7 In breast cancer they were described by Al-Hajj in 2003, with the cell membrane markers CD44 and CD24.8 The authors demonstrated that cells expressing CD44 (CD44+), but with low or absent expression of CD24 (CD24-/low) were able to form tumors in immunodeficient mice with as few as 100 cells.8 In 2007, Ginestier et al., using in vitro and in vivo experimental systems found that cells with increased aldehyde dehydrogenase activity (ALDH) have stem cell properties, both in normal and in cancer mammary epithelial cells.9 ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes.10 CD44+/CD24- phenotype and ALDH1 are the principal markers of CSC in breast cancer until now. Very few studies have evaluated both markers at the same time, and although both are important biomarkers in representing CSC, the overlap between CD44+/CD24-/low phenotype and ALDH1 expression in primary tumors is very low at approximately 1%.9 However, cells with both phenotypes seem to be more tumorigenic than other cells, being able to generate tumors from as few as 20 cells.9
The clinical impact and prognostic value of these markers is still uncertain. Indeed, it is probable that each CSC population will have distinct clinical values in different subgroups of breast cancer.11,12 In this study, we aimed to investigate the association between the breast CSC markers, CD44+/CD24- and ALDH1, and the breast cancer molecular subtypes, disease-free survival and overall survival.
Materials and Methods
Patients and tumors
One hundred and sixty-four tumor samples were included from patients submitted to surgery at Nossa Senhora das Graças Hospital (HNSG) Breast Unit, Curitiba, Brazil, from January 1998 to July 2011. All patients had undergone surgery (mastectomy or breast conserving surgery with sentinel node or axillary dissection) and had an invasive breast carcinoma, no special type diagnosis. Cases submitted to neoadjuvant chemotherapy were excluded, as were the cases that had no sufficient paraffin-embedded material for a new analysis. The patients’ ages were analyzed as a continuous variable by arithmetic mean and standard deviation. The histological grade was classified according to the Nottingham- Bloom-Richardson grading system.13 Tumor size was reported according to TNM (8th edition, 2017) classification of the American Joint Committee on Cancer.14 Lymph node status was considered positive or negative, according to the presence or absence of tumor cells in at least one lymph node. Disease-free survival was considered as the period (months) between the surgery and the diagnosis of a disease recurrence at any site. Overall survival was considered as the time (months) between the first operation and the date of death.
This study was approved by the Ethics Committee of the Pontifical Catholic University of Paraná (PUCPR, Registration number: 5365).
Tissue microarray
Tumor samples were distributed in 15 TMAs using 4 mm tissue cores at the Experimental Pathology Laboratory of Pontifical Catholic University of Paraná (PUCPR). From each donor block was extracted one or two cylinders 4 mm in diameter and deposited in the receiver blocks, previously prepared. In each TMA, a sample of normal breast tissue was included as an internal control. Thereafter, 4 μm tissue sections from the TMA blocks were transferred to electrically charged Star Frost® (Braunschweig, Germany) slides and incubated with primary antibodies [anti-CD44 (anti-human mouse monoclonal, clone DF1485, dilution 1/40, Novocastra, Newcastle, UK), anti-CD44v6 (antihuman mouse monoclonal, clone VFF-7, dilution 1/100, Novocastra, Newcastle, UK), anti-CD24 (anti-human rabbit polyclonal, dilution 1/200, Abbiotec, San Diego, CA, USA), and anti-ALDH1 (anti-human rabbit monoclonal, clone E-P1932y, dilution 1/100, Epitomics, Cambridge, Massachusetts, USA)] for 12 h in a humidified chamber at 2−8°C. An Advance Dako (Caripenteria, CA, USA) secondary antibody was incubated with the slides for 30 min at 2−8°C. The reactions were developed using a DAB chromogen-substrate solution (Dako). Harris hematoxylin was used for counterstaining. Positive and negative (incubated without primary antibody) controls were run in parallel with all reactions.
Immunohistochemistry
The estrogen receptor(ER) and progesterone receptor (PgR) were evaluated at the time of diagnosis using the Streptavidin- Biotin-Peroxidase method. In this study the ER and PgR expressions were considered positive when nuclear expression was detected in at least 1% of tumor cells.15 HER2 was analyzed at the time of diagnosis according to the American Society of Clinical Oncology (ASCO) / College of American Pathologists (CAP) protocols, when the HER2 scored 2+, the result was determined by fluorescence in situ hybridization (FISH).16 The basal markers: cytokeratin 5/6 (CK5/6), cytokeratin 14 (CK14), cytokeratin 17 (CK17), c-kit, and epidermal growth factor receptor (EGFR) were carried out in an earlier study of our group.17 They were considered positive when detected in at least 1% of tumor cells.
Immunohistochemistry was considered positive in CD44, CD44v6, and CD24 if it was expressed in more than 5% of tumor cells, as previously reported.12 CD44 and CD44v6 showed mainly membranous staining, and CD24 was mostly cytoplasmic. The CD44+/CD24- phenotype was established when a positive expression of CD44 or CD44v6 and a negative expression of CD24 was found. ALDH1 was considered positive when it showed cytoplasmic expression in more than 1% of tumor cells.9,11 The slides were examined by two investigators (IR and APMS) simultaneously in a multi-observer microscope, without knowledge of the corresponding clinicopathological data.
Molecular subtypes
The molecular subtypes were classified based on clinicopathological criteria as described in Table 1.18
Table 1.
Molecular subtype definition according to clinicopathological criteria.
| Molecular subtype | Clinicopathological definition |
|---|---|
| Luminal A | • ER and/or PR positive |
| • HER2 negative | |
| • Ki-67 low (<14%)* | |
| Luminal B | Luminal B (HER2 negative): |
| • ER and/or PR positive | |
| • HER2 negative | |
| • Ki-67 high (≥14%)* | |
| Luminal B (HER2 positive): | |
| • ER and/or PR positive | |
| • Any Ki-67 | |
| • HER2 over-expressed or amplified | |
| HER2 overexpression | • ER and PR negative |
| • HER2 overexpressed or amplified | |
| “Basal-like” | • ER and PR negative |
| • HER2 negative | |
| • At least one basal marker positive: CK 5/6, CK 14, CK 17, c-kit, EGFR |
*The Ki-67 cut-off labeling index was established based on the Cheang et al. study,42 through comparison to gene array data (PAM50).
Statistical analysis
Statistical analysis was performed using Stata/SE v.14.1. Age was described by mean, standard deviation and range. For categorical variables frequencies and percentages were presented. Associations between two categorical variables were evaluated by Fisher’s exact test, a Chisquare test or a logistic regression model followed by a Wald test. Analysis of age was performed using Student’s t-test. The Fine and Gray model (based on the hazard of the sub distribution) was used to estimate cumulative incidence functions for death caused by disease (other causes of death as competing risks) and for recurrence of cancer (all causes of death as competing risks). A P value <0.05 was considered significant.
Results
A total of 144 cases were included in this cohort. The mean age of the patients was 56.4±13.5 years (27-88 years). Most patients were post-menopausal (97 patients: 67.4%). Seventy-three (50.7%) cases were grade II, 47 (32.6%) were grade III, and 24 (16.7%) were grade I. Tumor size was classified as T1 in 50% (72 cases), and T2 in 44,4% (64). Only eight cases (5.6%) exhibited a locally advanced disease (T3-T4). The lymph node status was negative in eighty patients (55.6%) and positive in sixty-four patients (44.4%). In terms of molecular subtypes, the most common subtype of this cohort was Luminal B, with sixty-two patients (43.1%), 27 (18.8%) cases were Luminal A, 24 (16.7%) were HER2, and 31 (21.5%) were Basal-like.
The expression of CD44, CD44V6, CD24, and ALDH1 was analyzed and the immunostaining of these markers is represented in Figure 1. The CD44+/CD24- phenotype was expressed in eighteen patients (12.9%). Twenty patients (14.5%) expressed the ALDH1. Only five cases (3.7%) expressed both stem cell markers simultaneously. The correlation between the CSC markers and the clinicopathological parameters are described in Table 2. There was a significant correlation between the ALDH1 expression and the variables histological grade, and tumor size. The grade three tumors and the locally advanced tumors (T3 and T4) more frequently expressed the ALDH1. Regarding CD44 expression, the variant isoform CD44v6 was much more sensitive than the standard isoform CD44, with 119 positive cases (85.6%) for CD44v6 versus 78 (46.1%) for CD44. CD24 was positive in one hundred and thirteen cases (80.7%). The correlation between the expression of each of CD24, CD44 and CD44v6 and the clinicopathological parameters (age, menopausal status, histological grade, tumor size, and lymph node status) was not significant.
Figure 1.
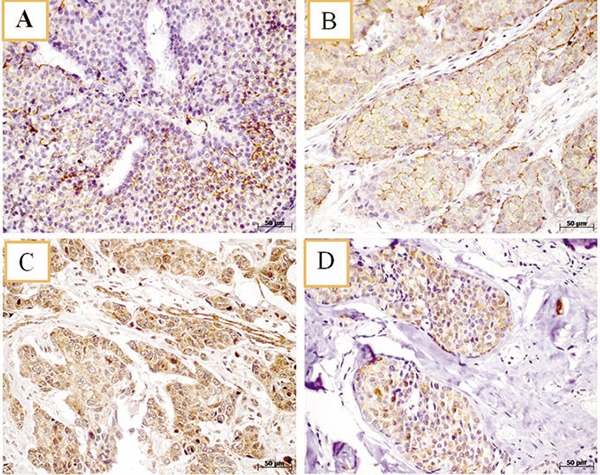
Immunohistochemistry analysis of CD44, CD44v6, CD24, and ALDH1 expression in tumor samples. Representative light photomicrographs showing positive staining of breast cancer tissue samples for CD44 (A); CD44v6 (B); CD24 (C) and, ALDH1 (D). Magnification: 40x; Scale bar: 50m
Table 2.
Correlation between stem cell markers and clinicopathological parameters.
| Stem cell markers expression | Category | CD44+/CD24- | ALDH1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Negative | Positive | P* | n | Negative | Positive | P* | ||
| Age | Mean | 122 | 56.4 | 55.2 | 0,726 | 118 | 56.3 | 58.5 | 0.503 |
| SD | 18 | 13.3 | 15.2 | 20 | 13.7 | 14 | |||
| Menopausal status | Pre | 45 | 38 (84.4) | 7 (15.6) | 44 | 38 (86.4) | 6 (13.6) | ||
| Post | 95 | 84 (88.4) | 11 (11.6) | 0.591 | 94 | 80 (85.1) | 14 (14.9) | 1 | |
| Histological grade | I | 22 | 19 (86.4) | 3 (13.6) | 23 | 22 (95.7) | 1 (4.3) | ||
| II | 72 | 67 (93.1) | 5 (6.9) | 70 | 62 (88.6) | 8 (8.4) | |||
| III | 46 | 36 (78.3) | 10 (21.7) | 0.064 | 45 | 34 (75.6) | 11 (24.4) | 0.049 | |
| pT | T1 | 62 | 55 (88.7) | 7 (11.3) | 60 | 57 (95.0) | 3 (5.0) | ||
| T2 | 70 | 62 (88.6) | 8 (11.4) | 70 | 56 (80.0) | 14 (20.0) | |||
| T3-T4 | 8 | 5 (62.5) | 3 (37.5) | 0.100 | 8 | 5 (62.5) | 3 (37.5) | 0.009 | |
| Lymph node status | Negative | 78 | 69 (88.5) | 9 (11.5) | 75 | 68 (90.7) | 7 (9.3) | ||
| Positive | 62 | 53 (85.5) | 9 (14.5) | 0.621 | 63 | 50 (79.4) | 13 (20.6) | 0.088 | |
Results expressed as frequency (%); n, number of patients
*Fisher’s exact test or Chi-square test, Student’s t-test for independent samples, P<0.05.
Stem cells markers and molecular subtypes
The CSC markers were related to the molecular subtypes, with statistical significance in both markers (Table 3). The HER2 subtype was associated with a greater likelihood of CD44+/CD24- positivity compared to Luminal A (P=0.039), Luminal B (P=0.008), and Basal-like (P=0.039). The HER2 subtype was also associated with a greater probability of ALDH1 positivity compared to Luminal A (P=0.037), and Luminal B (P=0.038), but not in comparison to Basal-like (P=0.477).
Table 3.
Association between stem cells markers and molecular subtypes.
| Molecular subtypes | CD44+/CD24– | ALDH1 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Negative | Positive | P* | n | Negative | Positive | P* | |
| Luminal A | 26 | 24 (92.3) | 2 (7.7) | 26 | 25 (96.2) | 1 (3.8) | ||
| Luminal B | 59 | 54 (91.5) | 5 (8.5) | 59 | 53 (89.8) | 6 (10.2) | ||
| HER2 | 24 | 16 (66.7) | 8 (33.3) | 24 | 17 (70.8) | 7 (29.2) | ||
| Basal-like | 31 | 28 (90.3) | 3 (9.7) | 0.032° | 29 | 23(79.31) | 6 (20.69) | 0.039# |
Results expressed as frequency (%); n, number of patients
*Logistic Regression Model and Wald test, P<0.05; °Luminal A vs HER2 (P=0.039); Luminal B x HER2 (P=0.008); Basal-like (P=0.039); other comparisons (P>0.05)
#Luminal A vs HER2 (P=0.037); Luminal B x HER2 (P=0.038); other comparisons (P>0.05).
Stem cells markers and survival
A greater risk of relapse (P=0.011) and a worse outcome (P=0.019), were observed in the group that expressed the CD44+/CD24- phenotype, but there was not a significant difference in the death risk (P=0.785). The ALDH1 expression did not show a statistically significant correlation with relapse, death, or outcome, as described in Table 4.
Table 4.
Association of stem cells markers and prognosis.
| Prognosis | Category | CD44+/CD24- | ALDH1 | ||||
|---|---|---|---|---|---|---|---|
| Negative (n=122) | Positive (n=18) | P* | Negative (n=118) | Positive (n=20) | P* | ||
| Relapse | No | 92 (75.4) | 8 (44.4) | 83 (70.3) | 14(70) | ||
| Yes | 30 (24.6) | 10 (55.6) | 0.011 | 35 (29.7) | 6(30) | 1 | |
| Death | No | 86 (70.5) | 12 (66.7) | 83 (70.4) | 14(70) | ||
| Yes | 36 (29.5) | 6 (33.3) | 0.785 | 35 (29.6) | 6(30) | 1 | |
| Outcome | Live | 79 (64.8) | 8 (44.5) | 72(61) | 13(65) | ||
| Cancer death | 23 (18.9) | 6 (33.3) | 24 (20.4) | 5(25) | |||
| Death other causes | 13 (10.6) | 0 (0) | 11 (9.3) | 1(5) | |||
| Live with relapse | 7 (5.7) | 4 (22.2) | 0.019 | 11 (9.3) | 1(5) | 0.812 | |
Results expressed as frequency (%); n, number of patients
*Fisher’s exact test or Chi-square test, P<0.05.
In this study, there were 42 cases of relapse (29.2%), 30 (20.8%) cancer death and 13 (9%) of death by other causes, in a mean time of 91 months (8-245). The death by other causes is a competitive risk to the event of relapse, or cancer death, because if the patients had lived long enough they could have presented our interest event. In order to establish the real prognostic value of our CSC markers, all the variables were analyzed using the Fine and Gray model for overall survival and disease-free survival. In the overall survival model, there was not a significant association between the CSC markers (CD44+/CD24-, ALDH1) and overall survival (Table 5, Figure 2). In the disease- free survival model, there was a significant association between the CD24 negativity [P=0.005, SHR 0.39 (0.20-0.76)], the CD44+/CD24- phenotype [P=0.002, SHR 3.07 (1.51-6.22)] and a greater risk of relapse, in the univariate analyses, but in the multivariate analysis with the Fine and Gray model, only the histological grade and lymph node status remained as independent prognostic factors for recurrence (Table 6, Figure 2).
Table 5.
Univariate and multivariate analyses of stem cells markers and other predictors of overall survival.
| Univariate analyses | Multivariate analyses | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Variable | P* | SHR | CI 95% | P* | SHR | CI 95% |
| Age | 0.001 | 1.04 | 1.02-1.07 | 0.177 | 1.03 | 0.99-1.08 | |
| Molecular subtype | Luminal A | ||||||
| Luminal B | 0.175 | 2.35 | 0.68-8.04 | 0.119 | 2.78 | 0.15-5.68 | |
| Basal-like | 0.884 | 0.89 | 0.18-4.46 | 0.923 | 0.91 | 0.15-5.68 | |
| HER2 | 0.023 | 4.55 | 1.24-16.70 | 0.049 | 4.45 | 1.01-19.68 | |
| Menopause | Pre | ||||||
| Post | 0.009 | 4.82 | 1.49-15.58 | 0.190 | 2.86 | 0.59-13.75 | |
| Lymph node | Negative | ||||||
| Positive | 0.040 | 2.13 | 1.04-4.39 | 0.012 | 2.79 | 1.25-6.22 | |
| CD44+/CD24– | Negative | ||||||
| Positive | 0.098 | 2.12 | 0.87-5.16 | 0.274 | 1.63 | 0.68-3.89 | |
| ALDH1 | Negative | ||||||
| Positive | 0.622 | 1.27 | 0.49-3.31 | 0.984 | 0.99 | 0.40-2.43 | |
*Fine & Gray subdistribution hazards model and Wald test, P<0.05.
Figure 2.
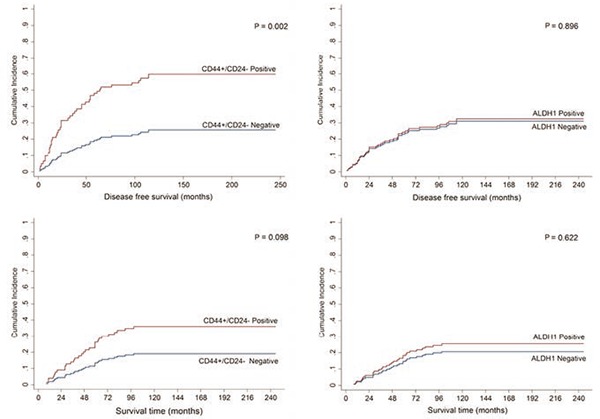
Cumulative incidence curves for recurrence and cancer death by CD44+/CD24- and ALDH1 (positive and negative).
Table 6.
Univariate and multivariate analyses of stem cells markers and other predictors of disease-free survival.
| Univariate analyses | Multivariate analyses | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Variable | P* | SHR | CI 95% | P* | SHR | CI 95% |
| Age | 0.051 | 1.02 | 1.00-1.05 | 0.002 | 1.05 | 1.02-1.08 | |
| Molecular subtype | Luminal A | ||||||
| Luminal B | 0.108 | 2.37 | 0.83-6.81 | 0.168 | 2.48 | 0.68-9.05 | |
| Basal-like | 0.293 | 1.87 | 0.58-6.03 | 0.458 | 1.63 | 0.45-5.99 | |
| HER2 | 0.010 | 4.31 | 1.42-13.11 | 0.119 | 2.86 | 0.76-10.71 | |
| Histological grade | I | ||||||
| II | 0.086 | 2.71 | 0.87-8.49 | 0.065 | 3.52 | 0.92-13.47 | |
| III | 0.013 | 4.31 | 1.35-13.70 | 0.023 | 5.97 | 1.27-27.95 | |
| Lymph node | Negative | ||||||
| Positive | 0.011 | 2.21 | 1.20-4.08 | 0.032 | 2.21 | 1.07-4.57 | |
| CD24 | Negative | ||||||
| Positive | 0.005 | 0.39 | 0.20-0.76 | 0.833 | 0.85 | 0.19-3.80 | |
| CD44+/CD24– | Negative | ||||||
| Positive | 0.002 | 3.07 | 1.51-6.22 | 0.197 | 2.85 | 0.58-14.06 | |
| ALDH1 | Negative | ||||||
| Positive | 0.896 | 1.06 | 0.44-2.57 | 0.250 | 0.59 | 0.24-1.44 | |
*Fine & Gray subdistribution hazards model and Wald test P<0.05.
Discussion
One of the major limitations in the treatment of breast cancer is the heterogeneity of this disease. In the last fifteen years, much progress has been made in understanding the molecular subtypes of breast cancer, which has helped to explain the intertumoral heterogeneity. In addition, it is known that this heterogeneity not only happens between tumors but also happens inside the tumor; therefore in the same tumor two or more distinct populations of cells can be present, with very different molecular profiles, which further complicates the understanding of this disease.19
The CSC theory was postulated in an attempt to better understand this heterogeneity and explain the mechanism of recurrence and metastasis. The expression of CD44 and CD44v6 has been associated with the potential of progression and metastasis. CD44 is a glycoprotein transmembrane involved in many cellular processes including migration and adhesion, and hyaluronic acid is its principal ligand.20 A single gene located at 11p13 chromosome that has 20 exons encodes CD44. The first five and the last five exons are constant, while the 10 exons located between these regions are subject to alternative splicing, resulting in the formation of a variable region that produces a wide range of proteins. 21,22 The standard isoform (CD44s) is the most common, but approximately 20 variant isoforms have been described. The CD44v6 is one of the most common and best-studied CD44 variant isoforms and has been described as playing a role in cell migration and proliferation and, consequently, in cancer progression and metastasis. 23,24 In this study we included the isoform CD44v6 expression in the identification of the CD44+/CD24- phenotype to try to achieve the two major isoforms that have prognostic implications and improve the identification of the CSC phenotype.
A major point in our study was the possibility to compare the two principal CSC markers in breast cancer, ALDH1 and CD44+/CD24- phenotype, since they possibly identify different groups of stem cells, and both are important in the evaluation of CSC. Most of the previous studies evaluated just one stem cell marker. Recently another study also evaluated both cancer stem cells markers and identified that high CD44/CD24 ratio was mainly in charge of self-renewal, proliferation, and tumor growth, while ALDH1+ represented a stronger capability for invasion and metastasis. The authors also demonstrated that these two markers performed different functions during tumor progression and metastasis, corroborating that a single CSC marker alone was not enough to characterize the stem properties of breast cancer.25
Our study found that grade III tumors and the locally advanced tumors (T3-T4) were associated with a greater probability of expression of ALDH1 with a significant result, as previously described in other studies.9,26 In this study, the CD44+/CD24 and the ALDH1 were not associated with the lymph node status. One explanation for this fact is that these tumors exhibit a more aggressive behavior and tend to disseminate more frequently hematologically rather than lymphatically.
In this cohort we observed a significant association between CSC markers and the molecular subtypes, with the HER2 subtype more frequently expressed in both markers. Several studies correlated the CD44+/CD24- phenotype to the more undifferentiated subtypes as Basal-like and Claudin-low.11,26,27 The basal-like subtype is possibly related to the most primitive cell lines, so it was expected that the CSC phenotype would be enriched in this kind of tumor.11,27-29 The CD44+/CD24- phenotype has been described in association with the HER2 molecular subtype, only in some in vitro studies.30 In our study we classified the molecular subtypes according to the clinicopathological criteria, and considered the Ki67 expression to be part of the Luminal B group definition, as recommended in the St. Gallen consensus,18 which resulted in more cases classified as Luminal B that would had been classified as Luminal A for other authors.27,31 The Luminal A group is the best prognosis group and, in our sample, it was negatively associated with the CSC markers. Some studies included in their samples other histological types, which made them more heterogeneous.19,27 In our study, only the invasive breast carcinoma, no special type was included.
It was recently demonstrated that CSC could possibly play an important role in the metastasis process, due to their self-renewal capability and potential to differentiate and adapt to different organ microenvironments32 and an association between CSC and metastasis of breast cancer lines has been demonstrated in vitro.32,33 In our study an association between the CSC markers and overall survival was not demonstrated. The CD44+/CD24- phenotype was initially associated with a greater risk of relapse in the univariate analysis along with CD24 negativity, molecular subtype, histological grade, and lymph node status, but in the multivariate analysis, it was not demonstrated as an independent prognostic factor. Contradicting our study recently was showed significant correlate with 5-year relapse in ER-positive breast cancer patients during adjuvant tamoxifen treatment, suggesting that CD24 expression may reflect tamoxifen resistance.34 The CD44+/CD24- phenotype was previously correlated to metastasis-free survival by other authors,35,36 but there are still many controversies about the true role of these markers, with very conflicting results in the studies.37,38 According to Riaz et al., the androgen receptor (AR), and CD24 significantly correlated with favorable clinicopathological features and an improved survival whereas CSC markers such as CD44+, CD44+/CD24−, and ALDH1+ were not effective prognostic indicators for outcome prediction.39 Zhong et al. considered the ALDH1 a better predictor of relapse than the CD44+/CD24– phenotype, and demonstrated that the ALDH1 expression was associated with a high rate of metastasis or recurrence.32 An issue that could influence these heterogeneous results is the lack of standardization in the analysis of these markers that define CD44+/CD24- phenotype, and the use of multiple cut-off criteria. We used a 5% cut-off for CD44, CD44v6 and CD24, as previously described by other authors.12 Ricardo et al. considered a score that evaluates the percentage of cells immunostaining positive: CD44 was considered positive when it has ≥10% of positivity, and CD24 was defined as negative or low when it scored up to 25% of positivity.11 Adamczyk et al. considered a 10% cut-off for both CD44 and CD24 expressions.40
The CSC markers study, is also of extreme relevance in understanding the mechanism of multidrug resistance, that decrease the chemotherapeutic agents’ efficacy in breast cancer treatment. Some studies have revealed some potential biomarkers of doxorubicin resistance in breast cancer stem cells, such as STAT3 that was recently described as a promising chemoresistance biomarker associated with the CD44(+/high)/CD24(-/low)/ALDH(+) BCSCslike subset of the triple-negative breast cancer (TNBC), classified as a claudin-low subtype.41 The progress in this field is of major importance for the improvement of breast cancer treatment.
Our study presents some limitations that need to be highlighted. First, we did not have complete information of lymphovascular space invasion and we could not make the correlation with the several markers used in the present study. Second, a simple immunostaining was employed to establish the CD44+/CD24- negative phenotype, as previously described by other authors,26-28 instead of double immunolabeling. Even though, Ricardo et al., compared the simple and the double immunostaining to establish the CD44+/CD24- phenotype in the same sample and did not find any difference between those techniques.11
There are still many controversies about the prognostic value of CSC markers that has to be elucidated. Our study demonstrated that despite these markers being associated with some indicators of bad prognosis they cannot be considered as independent prognostic factors. Considering the CSC theory, and the accessibility of these CSC markers, it is important to better understand their role in breast cancer to be able to develop in the future new target therapies for this cell population.
Acknowledgments
We would like to thank Mrs. Marina de Azevedo and Ms. Ana Paula Camargo for skillful technical assistance; Dr. Rafael Malagoli Rocha (AC Camargo Pathology Laboratory) for carrying out the CD44 immunohistochemistry and Dr. Marcia Olandoski for statistical analysis and all the support to make the tables and figures.
References
- 1.Allegra A, Alonci A, Penna G, Innao V, Gerace D, Rotondo F, et al. The cancer stem cell hypothesis: a guide to potential molecular targets. Cancer Invest 2014;32:470-95. [DOI] [PubMed] [Google Scholar]
- 2.Qiu H, Fang X, Luo Q, Ouyang G. Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci 2015;72:3411-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuthapisith S, Eremin J, El-Sheemey M, Eremin O. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol 2010;19:27-32. [DOI] [PubMed] [Google Scholar]
- 4.Nakshatri H, Srour EF, Badve S. Breast cancer stem cells and intrinsic subtypes: controversies rage on. Curr Stem Cell Res Ther 2009;4:50-60. [DOI] [PubMed] [Google Scholar]
- 5.Moore N, Lyle S. Quiescent, slowcycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol 2011;2011: 396076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol 2000;12:450-8. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645-8. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito- Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact 2003;143-144:5-22. [DOI] [PubMed] [Google Scholar]
- 11.Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol 2011; 64:937-46. [DOI] [PubMed] [Google Scholar]
- 12.Tsang JY, Huang YH, Luo MH, Ni YB, Chan SK, Lui PC, et al. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat 2012;136:407-17. [DOI] [PubMed] [Google Scholar]
- 13.Elston CW. Classification and grading of invasive breast carcinoma. Verh Dtsch Ges Pathol 2005;89:35-44. [PubMed] [Google Scholar]
- 14.Brierley JD, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours Union for International Cancer Control (UICC). 8th ed. Hoboken: J. Wiley & Sons; 2017. [Google Scholar]
- 15.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010;6:195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. [DOI] [PubMed] [Google Scholar]
- 17.Sebastião A. Estudo da expressão de fenótipo basal com marcacão imunoistoquímica para EGFR, CK5/6 e CK14 em carcinomas ductais da mama luminais A e triplo negativos. Curitiba: Federal University of Paraná; 2010. [Google Scholar]
- 18.Goldhirsch A Wood WC Coates AS Gelber RD Thurlimann B Senn HJ.. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camerlingo R, Ferraro GA, De Francesco F, Romano M, Nicoletti G, Di Bonito M, et al. The role of CD44+/CD24-/low biomarker for screening, diagnosis and monitoring of breast cancer. Oncol Rep 2014; 31:1127-32. [DOI] [PubMed] [Google Scholar]
- 20.Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringner M, et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer 2011; 11:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33-45. [DOI] [PubMed] [Google Scholar]
- 22.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci 2004;117:373-80. [DOI] [PubMed] [Google Scholar]
- 23.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991;65:13-24. [DOI] [PubMed] [Google Scholar]
- 24.Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol 1998; 51:191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Ma H, Zhang J, Zhu L, Wang C, Yang Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep 2017;7:13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CY, Barry-Holson KQ, Allison KH. Breast cancer stem cells: are we ready to go from bench to bedside? Histopathology 2016;68:119-37. [DOI] [PubMed] [Google Scholar]
- 27.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res 2008;10:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chekhun SV, Zadvorny TV, Tymovska YO, Anikusko MF, Novak OE, Polishchuk LZ. D44+/CD24- markers of cancer stem cells in patients with breast cancer of different molecular subtypes. Exp Oncol 2015;37:58-63. [PubMed] [Google Scholar]
- 29.Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res 2010;16:876-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang KH, Kao AP, Chang CC, Lee JN, Hou MF, Long CY, et al. Increasing CD44+/CD24(-) tumor stem cells, and upregulation of COX-2 and HDAC6, as major functions of HER2 in breast tumorigenesis. Mol Cancer 2010;9:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Jung MJ, Ryu DW, Lee CH. Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer 2014;17:121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Y, Shen S, Zhou Y, Mao F, Guan J, Lin Y, et al. ALDH1 is a better clinical indicator for relapse of invasive ductal breast cancer than the CD44+/CD24- phenotype. Med Oncol 2014;31:864. [DOI] [PubMed] [Google Scholar]
- 33.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 2009;13:2236-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon YW, An HJ, Koo JS, Kim GM, Han H, Park S, et al. CD44/CD24 and aldehyde dehydrogenase 1 in estrogen receptor-positive early breast cancer treated with tamoxifen: CD24 positivity is a poor prognosticator. Oncotarget 2018;9:2622-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 2007;356:217-26. [DOI] [PubMed] [Google Scholar]
- 36.Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, et al. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Patho. 2012;43:364-73. [DOI] [PubMed] [Google Scholar]
- 37.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, et al. The clinicopathologic and prognostic significance of CD44+/CD24(-/low) and CD44- /CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 2008;39:1096-102. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, et al. A CD44(-)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 2012;133:979-95. [DOI] [PubMed] [Google Scholar]
- 39.Riaz N, Idress R, Habib S, Azam I, Lalani EM. Expression of Androgen Receptor and Cancer Stem Cell Markers (CD44(+)/CD24(-) and ALDH1(+)): Prognostic Implications in Invasive Breast Cancer. Transl Oncol 2018;11:920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamczyk A, Niemiec JA, Ambicka A, Mucha-Malecka A, Mitus J, Rys J. CD44/CD24 as potential prognostic markers in node-positive invasive ductal breast cancer patients treated with adjuvant chemotherapy. J Mol Histol 2014;45:35-45. [DOI] [PubMed] [Google Scholar]
- 41.Moreira MP, da Conceicao Braga L, Cassali GD, Silva LM. STAT3 as a promising chemoresistance biomarker associated with the CD44(+/high) /CD24(-/low)/ALDH(+) BCSCs-like subset of the triple-negative breast cancer (TNBC) cell line. Exp Cell Res 2018;363:283-90. [DOI] [PubMed] [Google Scholar]
- 42.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736-50. [DOI] [PMC free article] [PubMed] [Google Scholar]


