Abstract
The orphan G protein-coupled receptor 6 (GPR6) displays unique promise as a therapeutic target for the treatment of neuropsychiatric disorders due to its high expression in the striatopallidal neurons of the basal ganglia. GPR6, along with closely related orphan receptors GPR3 and GPR12, are phylogenetically related to CB1 and CB2 cannabinoid receptors. In the current study, we performed concentration-response studies on the effects of three different classes of cannabinoids: endogenous, phyto-, and synthetic, on both GPR6-mediated cAMP accumulation and β-arrestin2 recruitment. In addition, structure-activity relationship studies were conducted on cannabidiol (CBD), a recently discovered inverse agonist for GPR6. We have identified four additional cannabinoids, cannabidavarin (CBDV), WIN55212-2, SR141716A and SR144528, that exert inverse agonism on GPR6. Furthermore, we have discovered that these cannabinoids exhibit functional selectivity toward the β-arrestin2 recruitment pathway. These novel, functionally selective inverse agonists for GPR6 can be used as research tools and potentially developed into therapeutic agents.
Keywords: Biochemistry, Cell biology
1. Introduction
G protein-coupled receptors (GPCRs) comprise a large family of genes that are popular targets for pharmaceutical intervention [1, 2]. Of the commercially available medications currently on the U.S. market, about 30–50% target GPCRs [3]. Within the GPCR family, there are numerous orphan receptors which have no known endogenous ligands and offer considerable potential for novel drug discovery. GPR6 is no exception, showing unique opportunity as a target for the treatment of neurological and neuropsychiatric disorders [4, 5].
GPR6, along with GPR3 and GPR12, make up a subfamily of three closely related orphan receptors with approximately 60% shared amino acid sequence identity [6, 7]. GPR6, specifically, was originally cloned from rats in 1994 [8] and humans in 1995 [6, 9]. GPR6 was initially called rCNL3 by its discoverer in 1994 [8]. It is predominantly located in the central nervous system (CNS), particularly the striatum [8], and to a lesser extent in frontal cortex, hippocampus, and hypothalamus [9].
GPR6 is constitutively active and couples with Gs proteins to activate adenylyl cyclase [10, 11, 12]. Functionally, GPR6 expression up-regulates cAMP accumulation in neurons and enhances neurite outgrowth [13]. The role of G protein-independent pathways in GPR6 signaling is less understood. Traditionally, the β-arrestin pathway has been thought to only participate in receptor desensitization and internalization [14]. However, new evidence suggests the capacity for β-arrestin-mediated signal transduction [14]. Recently, it was shown that a cannabinoid ligand inhibited GPR6-mediated, constitutive β-arrestin2 recruitment [15]. However, to date the physiological function of GPR6-mediated β-arrestin2 signaling has not been reported.
The aforementioned cloning experiments showed GPR6 is phylogenetically related to the shingosine-1-phosphate (S1P) receptor family. Both Uhlenbrock, Gassenhuber, and Kostenis [12] and Ignatov et al. [16] showed that S1P activates GPR6. However, Yin et al. found that S1P does not activate GPR6 [17]. Further work is needed to clarify the controversy and discrepancy between the different laboratories, to determine whether S1P is indeed an endogenous ligand for GPR6.
Notably, GPR6, along with GPR3 and GPR12, is also phylogenetically related to the CB1 and CB2 cannabinoid receptors, sharing 35% amino acid sequence identity in the transmembrane regions [18]. In fact, Marchese et al. called these orphans the “cannabinoid orphan receptors” due to their degree of shared sequence identity [18]. Cannabidiol (CBD), one of the major non-psychotropic components of Cannabis sativa L. [19, 20], is reported to have a wide range of medical applications, including treatment of cancer [21], inflammation [22], epilepsy [23, 24], neurodegenerative disorders [25, 26], and psychiatric diseases [27]. However, CBD has been shown to bind with low orthosteric affinity to CB1 and CB2, and act as a negative allosteric modulator at each [28, 29, 30]. Our recent report revealed that GPR6 is a novel molecular target for CBD, which inhibit GPR6-mediated β-arrestin2 recruitment [15].
In the current study, we investigated compounds from all three cannabinoid classes: endogenous, phyto-, and synthetic cannabinoids (agonists and antagonists) in order to search for candidate GPR6 ligands that may prove to be useful either therapeutically or as research tools. To better understand the actions of cannabinoid ligands on GPR6, concentration-response studies were performed with all tested cannabinoid compounds in two pathways of GPR6 signaling, cAMP accumulation and β-arrestin2 recruitment. In addition, we characterized the structure-activity relationships for actions of CBD on GPR6.
2. Materials & methods
2.1. Materials
Dulbecco's Modified Eagles's Medium (DMEM), penicillin/streptomycin, L-glutamine, trypsin, and geneticin were purchased from Mediatech (Manassas, VA). Fetal bovine serum was obtained from Atlanta Biologicals (Lawrenceville, GA). Dichlorodimethylsilane for silanizing glass tubes was purchased from Sigma-Aldrich (St. Louis, Mo). 384-well, round bottom, low volume white plates were purchased from Grenier Bio One (Monroe, NC). The homogenous time-resolved fluorescence (HTRF) cAMP Hirange kits were purchased from CisBio International (Bedford, MA). The PathHunter™ Chinese hamster ovary (CHO)-K1 β-arrestin2 human GPR6 eXpress kits were purchased from DiscoverX (Fremont, CA). Cannabinoid ligands were purchased from Cayman Chemical (Ann Arbor, MI).
2.2. Cell-based HTRF cAMP assay
The HTRF cAMP assay was performed as previously published with modifications [31]. In brief, GPR6-HEK cells were plated in 384-well plates in DiscoverX cell plating reagent1 for 48 hours in a humidified atmosphere at 37 °C and 5% CO2. The cells were then treated with the phosphodiesterase inhibitor Ro 20-1724 (2 μM). Ligands were diluted in DiscoverX cell plating reagent1 containing 2.5% fatty acid free bovine serum albumin. Cells were treated with ligands for an hour in a humidified incubator at 37 °C and 5% CO2 followed by incubation with d2-conjugated cAMP and Europium cryptate-conjugated anti-cAMP antibody for an hour at room temperature. The fluorescent output was measured using a TECAN GENios Pro microplate reader.
2.3. Pathhunter™ β -arrestin2 recruitment assay
The GPR6 mediated β-arrestin2 recruitment was measured by using the PathHunter™ Chinese hamster ovary (CHO)-K1 β-arrestin2 human GPR6 eXpress kits. In this cell line, GPR6 receptors are fused with a β-galactosidase N-terminal fragment termed ProLink 1 (GPR6-PK1), and β-arrestin2 are fused to an N-terminal deleted version of β-galactosidase (EA-β-arrestin2). Activation of the receptor induces β-arrestin2 recruitment, causing complementation of the two β galactosidase enzyme fragments. Levels of the active enzyme are the direct result of β-arrestin2 recruitment caused by receptor activation and quantified using the PathHunter™ detection reagent containing β-galactosidase substrates. The assays were performed following manufacturer's instructions for the PathHunter eXpress kits. Briefly, cells were plated in 384-well plates in DiscoverX cell plating reagent1 for 48 hours in a humidified atmosphere at 37 °C and 5% CO2. Cannabinoids were serially diluted in cell plating reagent1. After incubation with ligand at 37 °C following manufacturer's instructions, the cells were incubated with detection reagent for 1 hour at room temperature in the dark. Subsequently, the chemiluminescence signal, measured as relative luminescence units, was detected using a TECAN GENios Pro microplate reader.
2.4. Data analysis
For cAMP accumulation assays, data analyses were performed based on the ratio of fluorescence intensity of each well at 620 nm and 665 nm. Data are expressed as ΔF%, which is defined as [(standard or sample ratio – ratio of the negative control)/ratio of the negative control] x 100. The standard curves were generated by plotting ΔF% versus cAMP concentrations using non-linear least squares fit (Prism software, GraphPad, San Diego, CA). Unknowns are determined from the standard curve as nanomolar concentrations of cAMP. Ligand-induced changes in cAMP accumulation were calculated by dividing cAMP levels in the presence of different concentrations of ligands by basal cAMP levels, times 100.
For β-arrestin2 recruitment assays, ligand-induced changes in β-arrestin2 recruitment were calculated by dividing luminescence readings in the presence of different concentrations of ligands by basal luminescence readings, times 100.
For both cAMP accumulation and β-arrestin2 recruitment assays, data were subject to non-linear regression analysis using GraphPad Prism (GraphPad Software, San Diego, CA) and the graphs were also generated using GraphPad Prism. Data points represent the mean ± SEM obtained from three independent experiments performed in quadruplicate. Statistical analyses were performed using t test, or one-way ANOVA followed by Bonferroni's post-test. p-values of <0.05 were considered significant.
3. Results & discussions
GPR6 is an orphan GPCR and a promising therapeutic target. Regarding potential ligands, the cannabinoids hold particular promise since phylogenetic analyses have identified the CB1 and CB2 cannabinoid receptors as the closest relative subfamily with known ligands [18]. With this in mind, we designed our present study by testing three cannabinoid classes, as defined by origin – endogenous, phyto- (plant-derived), and synthetic.
3.1. Constitutive activity of GPR6
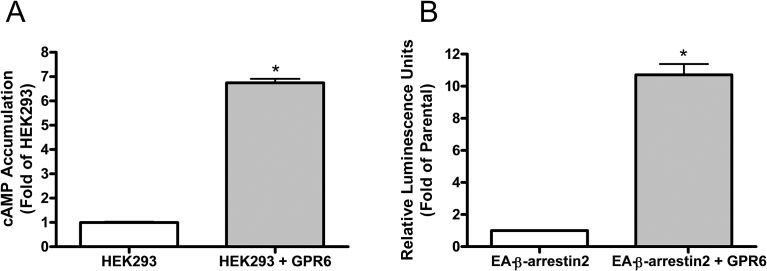
In the first set of experiment, we simply examined constitutive activity of GPR6. Our data showed that HEK293 cells stably expressing GPR6 had 6.5-fold higher cAMP levels compared to parental HEK293 cells, and thus the receptor exhibits constitutive activity in the cAMP accumulation assay (Fig. 1A). These results are consistent with previous studies showing that GPR6 is constitutively coupled to the Gs protein [12, 16]. We also found that GPR6 constitutively recruits β-arrestin2, as CHO cells co-expressing GPR6-PK1 and EA-β-arrestin2 had an 11-fold higher luminescence signal compared to the parental cells expressing only EA-β-arrestin2 (Fig. 1B). To our knowledge, this is the first time that GPR6 is shown to be coupled constitutively to the β-arrestin2 recruitment pathway.
Fig. 1.
Constitutive activity of GPR6. (A) Constitutive activity of GPR6 in the cAMP accumulation assays. The open bar represents parental HEK293 cells, while the striped bar represents HEK293 cells stably expressing GPR6. *Significant difference from parental HEK293 cells (p < 0.05, t test). (B) Constitutive activity of GPR6 in the β-arrestin2 recruitment assays. The open bar represents CHO parental cells expressing the EA-β-arrestin2, while the striped bar represents CHO cells co-expressing both GPR6-PK1 and EA-β-arrestin2. *Significant difference from parental cells (p < 0.05, t test).
3.2. Effects of endogenous and phyto-cannabinoids on GPR6-mediated cAMP accumulation and β-arrestin2 recruitment
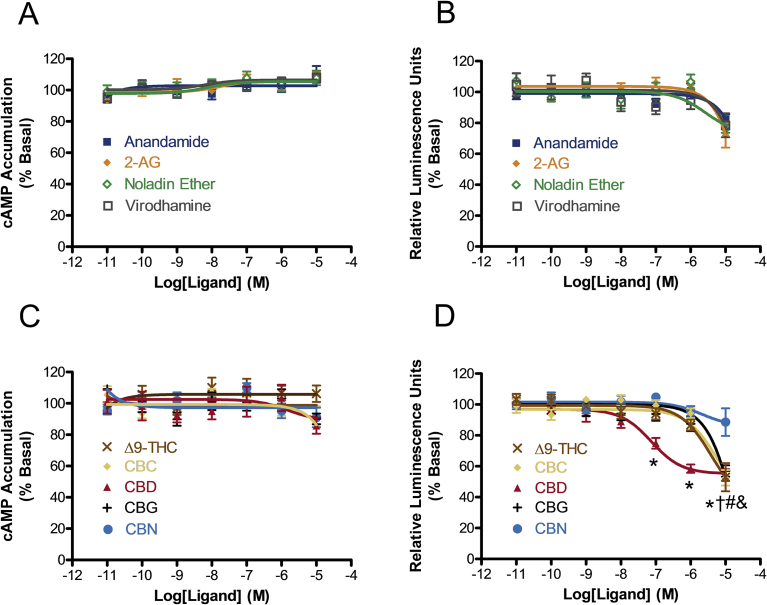
We then performed concentration-response relationship studies on four endocannabinoids – anandamide, 2-arachidonoylglycerol (2-AG), noladin ether, and virodhamine (Fig. 2), using both the cAMP accumulation and β-arrestin2 recruitment assays. All four endocannabinoids failed to significantly alter either cAMP accumulation or β-arrestin2 recruitment at concentrations ranging from 10 pM–10 μM (Fig. 3A and B). Although negative, these findings provide valuable information nevertheless. Our negative data indicates that the endogenous ligands for GPR6 have structures different from those of endocannabinoids that we tested.
Fig. 2.
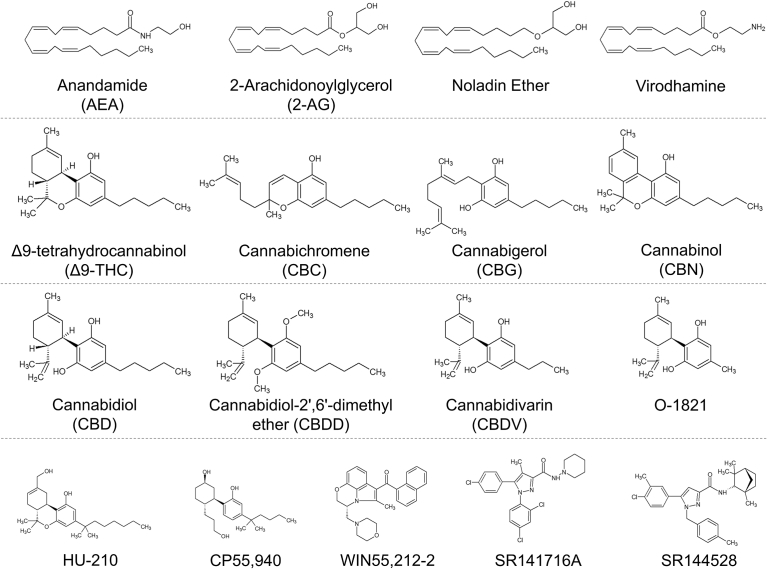
Chemical structures of the cannabinoid compounds used in this study.
Fig. 3.
Effects of endogenous and phyto-cannabinoids on GPR6-mediated cAMP accumulation and β-arrestin2 recruitment. (A) Effects of endocannabinoids on cAMP accumulation. (B) Effects of endocannabinoids on β-arrestin2 recruitment. (C) Effects of phytocannabinoids on cAMP accumulation. (D) Effects of phytocannabinoids on β-arrestin2 recruitment. For cAMP accumulation experiments, HEK293 cells stably expressing GPR6 were treated with different concentrations of cannabinoids for 1 hour. Results are expressed as percent of basal cAMP accumulation. For β-arrestin2 recruitment experiments, CHO cells co-expressing both GPR6-PK1 and EA-β-arrestin2 were cultured for 48 hours and subject to stimulation with cannabinoids. Results are expressed as percent of basal relative luminescence units. Data shown represent the mean ± SEM of three experiments performed in quadruplicate. Significant differences from vehicle (p < 0.05, one-way ANOVA with Bonferroni's post-test) are shown for *CBD, #Δ9-THC, †CBC, and &CBG. For phytocannabinoids, F-test results for each concentration were 0.1 μM: F(5,82) = 9.468, p < 0.05; 1 μM: F(5,90) = 18.69, p < 0.05; 10 μM: F(5,76) = 15.78, p < 0.05.
Our recent discovery of CBD as a novel inverse agonist for GPR6 was shown exclusively in the β-arrestin2 recruitment assay. It remained unclear, however, if CBD also exerts inverse agonism in the GPR6-mediated cAMP accumulation assay through the Gs dependent pathway. Thus, our next set of experiments sought to compare the effects of CBD in both assays. At the same time, we examined full concentration-response relationships of four other phytocannabinoids in both assays as well: the tricyclic cannabinoids Δ9-tetrahydrocannabinol (Δ9-THC) and cannabinol (CBN), the bicyclic cannabichromene (CBC), and the monocyclic cannabigerol (CBG) (Fig. 2). While none of the five compounds tested were able to significantly alter GPR6-mediated cAMP accumulation at concentrations up 10 μM, four of the five showed a reduction in GPR6-mediated β-arrestin2 recruitment at 10 μM (Fig. 3C and D). Notably, CBD displayed markedly higher potency compared to the other phytocannabinoids with an EC50 (95% CI) value of 74.8 (29.8–188) nM (reduction of β-arrestin2 recruitment of 44.85 (38.12–51.57%)). This suggests that the CBD chemical scaffold is the most suitable for interacting with GPR6. Since CBD is effective in reducing GPR6-mediated β-arrestin2 recruitment but not cAMP accumulation, it exhibits functional selectivity (biased signaling) toward the β-arrestin2 recruitment pathway.
3.3. CBD structure-activity relationship at GPR6
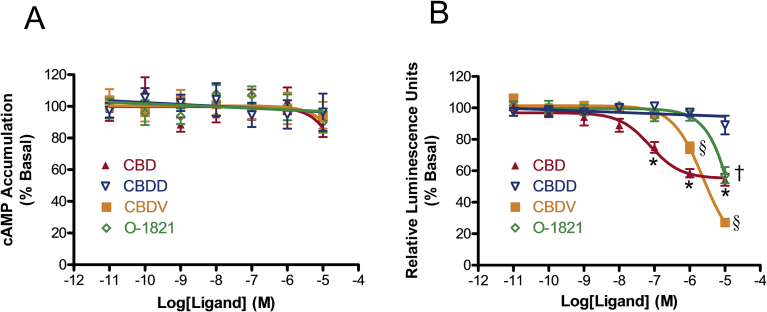
In the next set of experiments, we explored the structure-activity relationship of CBD as an inverse agonist for GPR6. Fig. 4A demonstrates neither CBD nor its analogues altered cAMP accumulation levels at concentrations up to 10 μM in cells stably expressing GPR6. Cannabidiol-2′,6′-dimethyl ether (CBDD) is a CBD analogue in which the 2′,6′- hydroxyl groups are substituted with methoxy groups (Fig. 2). We found that CBDD was unable to alter β-arrestin2 recruitment (Fig. 4B), demonstrating that free hydroxyl groups on the benzene ring are crucial for CBD to exert its inverse agonistic effects at GPR6. Shortening the length of the pentyl side chain of CBD also altered the activity of the ligand on GPR6, as seen with the derivatives cannabidavarin (CBDV) and O-1821, which have propyl and methyl side chains respectively (Fig. 2). Although CBDV was less potent compared to CBD, it was a more efficacious inverse agonist for β-arrestin2 recruitment, which decreased by 90.62(78.97–102.27)% (EC50 (95% CI) 2.40 (1.56–3.71) μM) (Fig. 4B). With regard to O-1821, shortening the side chain by 4 methylene bridges resulted in a marked decrease of the ability of the ligand to inhibit GPR6-mediated β-arrestin2 recruitment (Fig. 4B). Taken together, our results indicate that by modifying the aliphatic side chains on CBD, it is possible to change the potency and/or efficacy of the inverse agonistic effects of the ligands for GPR6.
Fig. 4.
CBD structure-activity relationship at GPR6. (A) Effects of CBD derivatives on cAMP accumulation. HEK293 cells stably expressing GPR6 were treated with different concentrations of CBD and CBD derivatives for 1 hour. Results are expressed as percent of basal cAMP accumulation. (B) Effects of CBD and CBD derivatives on β-arrestin2 recruitment. CHO cells co-expressing both GPR6 and EA-β-arrestin2 were cultured for 48 hours and subject to stimulation with CBD and CBD derivatives. Results are expressed as percent of basal relative luminescence units. Data shown represent the mean ± SEM of three experiments performed in quadruplicate. Significant differences from vehicle (p < 0.05, one-way ANOVA with Bonferroni's post-test) are shown for *CBD, §CBDV, †O-1821. F-test results for each concentration were 0.1 μM: F(4,65) = 17.06, p < 0.05; 1 μM: F(4,67) = 46.26, p < 0.05; 10 μM: F(4,64) = 59.93, p < 0.05.
3.4. Effects of prototypical synthetic cannabinoid agonists and antagonists on GPR6-mediated cAMP accumulation and β-arrestin2 recruitment
In the final set of experiments, we compared the effects of synthetic cannabinoid agonists and antagonists on GPR6-mediated cAMP accumulation and β-arrestin2 recruitment.
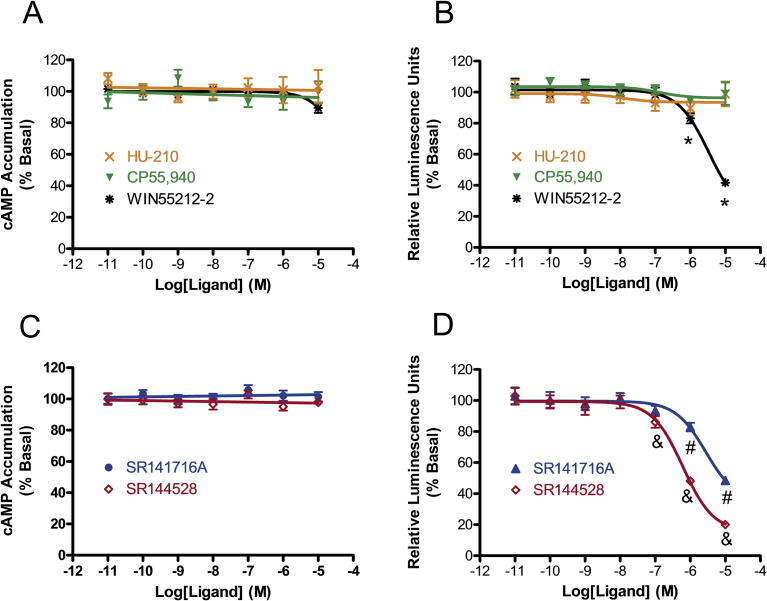
Three prototypical synthetic cannabinoid agonists from different chemical classes were tested: a classical cannabinoid agonist HU-210, a nonclassical bicyclic cannabinoid agonist CP55,940, and an aminoalkylindole cannabinoid agonist WIN55,212-2 [32] (Fig. 2). None of these cannabinoid agonists had any effects on the cAMP accumulation assay at concentrations up to 10 μM, whereas WIN55,212-2 alone displayed a significant reduction in the β-arrestin2 recruitment assay, decreasing by 78.36(54.94–101.79)% (EC50 value (95% CI) 3.34 (1.33–8.42) μM) (Fig. 5A and B). Our data indicate that the interaction with GPR6 requires chemical structures different from those of classical (HU-210) and nonclassical bicyclic (CP55,940) synthetic cannabinoids that we tested.
Fig. 5.
Effects of synthetic cannabinoid agonists and antagonists on GPR6-mediated cAMP accumulation and β-arrestin2 recruitment. (A) Effects of synthetic cannabinoid agonists on cAMP accumualtion. (B) Effects of synthetic cannabinoid agonists on β-arrestin2 recruitment. (C) Effects of synthetic cannabinoid antagonists on cAMP accumualtion. (D) Effects of synthetic cannabinoid antagonists on β-arrestin2 recruitment. For cAMP accumulation experiments, HEK293 cells stably expressing GPR6 were treated with different concentrations of synthetic cannabinoids for 1 hour. Results are expressed as percent of basal cAMP accumulation. For β-arrestin2 recruitment experiments, CHO cells co-expressing both GPR6-PK1 and EA-β-arrestin2 were cultured for 48 hours and subject to stimulation with synthetic cannabinoids. Results are expressed as percent of basal relative luminescence units. Data shown represent the mean ± SEM of three experiments performed in quadruplicate. Significant differences from vehicle (p < 0.05, one-way ANOVA with Bonferroni's post-test) are shown for *WIN55,212-2, #SR141716A and &SR144528. For synthetic agonists, F-test results for each concentration were 1 μM: F(3,54) = 5.813, p < 0.05; 10 μM: F(3,53) = 47.23, p < 0.05. For synthetic antagonists, F-test results for each concentration were 0.1 μM: F(2,58) = 4.721, p < 0.05; 1 μM: F(2,61) = 150.3, p < 0.05; 10 μM: F(2,61) = 359.9, p < 0.05.
Next, we tested SR141716A and SR144528, prototypical CB1 and CB2 antagonists respectively [33] (Fig. 2). These synthetic cannabinoids were unable to alter cAMP levels, but showed a marked ability to inhibit β-arrestin2 recruitment to GPR6 (Fig. 5C and D). These results demonstrate that, as novel inverse agonists for GPR6, both SR141716A and SR144528 have a functional selectivity toward the β-arrestin2 recruitment pathway. Both cannabinoid antagonists had similar efficacy; SR14716A showed a reduction of 65.68 (49.55–81.81)% and SR144528 showed a reduction of 84.53 (76.27–92.79)%. Of the two, SR144528 was more potent (EC50 (95% CI) 0.62 (0.40–0.96) μM) than SR141716A (EC50 value (95% CI) 2.77 (1.21–6.34) μM). These data demonstrate that the diphenylpyrazole structure of SR144528 is a promising synthetic chemical scaffold for developing highly potent and efficacious ligands for GPR6. On an additional note, SR141716A and SR144528 are generally thought to be specific for CB1 and CB2, respectively, and are often used experimentally to define the involvement of CB1 and CB2. Our data suggest that SR141716A and SR144528 can also act on GPR6, at least at micromolar concentrations. Thus, caution should be applied when interpreting the results involving the usage of these cannabinoid antagonists, especially at high concentrations.
3.5. Implications and significance
GPR6 is expressed predominately in the CNS, with only trace amounts in the periphery [8]. Of the central tissues, the striatopallidal neurons exhibit the highest GPR6 protein levels [4, 5, 8, 34]. Functionally, the striatum, as the principal input structure of the basal ganglia, modulates the motor control system, participates in the reward pathway, and influences learning and memory [35]. Pathophysiologically, the striatum has been implicated in a large number of neurodegenerative diseases and neuropsychiatric disorders, most notably Parkinson's Disease, Huntington's Disease, amyotrophic lateral sclerosis, autism, addiction, and schizophrenia [35].
Biased ligands are defined as compounds that preferentially signal through one pathway over the other [14]. Pharmaceutical companies have already begun clinical trials for specific pathway-targeted therapies; the biotech company Trevena has successfully developed a drug that provides post-surgery pain relief through activation of biased signaling in μ opioid receptors [36]. In the context of our current study, the functional selectivity that we discovered for the novel GPR6 inverse agonists may have therapeutic implications for the treatment of neuropsychiatric pathologies associated with the striatum, such as schizophrenia [4, 5].
The dopamine hypothesis of schizophrenia posits that striatal hyperdopaminergia causes the psychopathological symptoms. In support of this hypothesis is the fact that many clinically effective antipsychotics are known to antagonize the interaction between Dopamine D2 receptors and β-arrestin2 [37]. Previously, it has been shown that GPR6 modulates dopamine signal transduction [38], motor activity [38] and instrumental learning [34]. Also, GPR6 is heavily expressed in the indirect pathway of striatum and is a potential target for the treatment of schizophrenia [4, 5, 34]. Interestingly, CBD has recently been shown to be an effective antipsychotic agent for the treatment of schizophrenia [39, 40]. Since we have discovered that non-psychoactive cannabinoid CBD selectively target GPR6-mediated β-arrestin2 recruitment, it is worth investigating in future whether GPR6 plays a role in the antipsychotic effect of CBD.
CBD and CBDV have chemical structures different from that of WIN55212-2, SR141716A and SR144528. However, all of these compounds displayed functionally selective inverse agonistic activity at GPR6. Further studies using structural biology techniques as well as molecular modeling are needed to elucidate the molecular mechanisms for these cannabinoid ligands to act on GPR6.
In conclusion, in this study we have discovered that CBD and CBDV, two non-psychoactive components of marijuana, WIN55,212-2, a prototypical aminoalkylindole cannabinoid agonist, as well as SR141716A and SR144528, CB1 and CB2 receptor antagonists respectively, are novel inverse agonists for GPR6 that preferentially block signaling through β-arrestin2 recruitment pathway. Since GPR6 is a candidate drug target for the treatment of neurological and neuropsychiatric disorders, our discovery paves new ways to the development of potent and efficacious ligands for GPR6 as research tools and as potential therapeutic agents.
Declarations
Author contribution statement
Zhao-Hui Song: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alyssa Laun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sarah Shrader: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the National Institutes of Health Grant (ES11564, DA11551 and EY13632), University of Louisville Research Infrastructure Fund (R5385), and University of Louisville Integrated Program Biological Science Fellowship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Thomsen W., Frazer J., Unett D. Functional assays for screening GPCR targets. Curr. Opin. Biotechnol. 2005;16:655–665. doi: 10.1016/j.copbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins A.L., Groom C.R. The druggable genome, Nature reviews. Drug discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnadottir T.K., Gloriam D.E., Hellstrand S.H., Kristiansson H., Fredriksson R., Schioth H.B. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Komatsu H. Novel therapeutic GPCRs for psychiatric disorders. Int. J. Mol. Sci. 2015;16:14109–14121. doi: 10.3390/ijms160614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alavi M.S., Shamsizadeh A., Azhdari-Zarmehri H., Roohbakhsh A. Orphan G protein-coupled receptors: the role in CNS disorders. Biomed. Pharmacother. 2018;98:222–232. doi: 10.1016/j.biopha.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 6.Song Z.H., Modi W., Bonner T.I. Molecular cloning and chromosomal localization of human genes encoding three closely related G protein-coupled receptors. Genomics. 1995;28:347–349. doi: 10.1006/geno.1995.1154. [DOI] [PubMed] [Google Scholar]
- 7.Laun A.S., Shrader S.H., Brown K.J., Song Z.H. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2018 doi: 10.1038/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z.H., Young W.S., 3rd, Brownstein M.J., Bonner T.I. Molecular cloning of a novel candidate G protein-coupled receptor from rat brain. FEBS Lett. 1994;351:375–379. doi: 10.1016/0014-5793(94)00888-4. [DOI] [PubMed] [Google Scholar]
- 9.Heiber M., Docherty J.M., Shah G., Nguyen T., Cheng R., Heng H.H., Marchese A., Tsui L.C., Shi X., George S.R. Isolation of three novel human genes encoding G protein-coupled receptors. DNA Cell Biol. 1995;14:25–35. doi: 10.1089/dna.1995.14.25. [DOI] [PubMed] [Google Scholar]
- 10.Martin A.L., Steurer M.A., Aronstam R.S. Constitutive Activity among Orphan Class-A G Protein Coupled Receptors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad B.M., Hollins B., Lambert N.A. Methods to detect cell surface expression and constitutive activity of GPR6. Methods Enzymol. 2010;484:179–195. doi: 10.1016/B978-0-12-381298-8.00010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlenbrock K., Gassenhuber H., Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 2002;14:941–953. doi: 10.1016/s0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S., Ishii K., Kasai K., Yoon S.O., Saeki Y. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J. Biol. Chem. 2007;282:10506–10515. doi: 10.1074/jbc.M700911200. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopal S., Rajagopal K., Lefkowitz R.J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laun A.S., Song Z.-H. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophys. Res. Commun. 2017;490:17–21. doi: 10.1016/j.bbrc.2017.05.165. [DOI] [PubMed] [Google Scholar]
- 16.Ignatov A., Lintzel J., Kreienkamp H.J., Schaller H.C. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem. Biophys. Res. Commun. 2003;311:329–336. doi: 10.1016/j.bbrc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., Nguyen D.G., Caldwell J.S., Chen Y.A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchese A., George S.R., Kolakowski L.F., Jr., Lynch K.R., O'Dowd B.F. Novel GPCRs and their endogenous ligands: expanding the boundaries of physiology and pharmacology. Trends Pharmacol. Sci. 1999;20:370–375. doi: 10.1016/s0165-6147(99)01366-8. [DOI] [PubMed] [Google Scholar]
- 19.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Zuardi A.W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev. Bras. Psiquiatr. 2008;30:271–280. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 21.Massi P., Solinas M., Cinquina V., Parolaro D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013;75:303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito G., Filippis D.D., Cirillo C., Iuvone T., Capoccia E., Scuderi C., Steardo A., Cuomo R., Steardo L. Cannabidiol in inflammatory bowel diseases: a brief overview. Phytother Res. 2013;27:633–636. doi: 10.1002/ptr.4781. [DOI] [PubMed] [Google Scholar]
- 23.Gloss D., Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst. Rev. 2012;6:CD009270. doi: 10.1002/14651858.CD009270.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Scuderi C., Filippis D.D., Iuvone T., Blasio A., Steardo A., Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 2009;23:597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- 25.Iuvone T., Esposito G., De Filippis D., Scuderi C., Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci. Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Ruiz J., Sagredo O., Pazos M.R., Garcia C., Pertwee R., Mechoulam R., Martinez-Orgado J. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013;75:323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuardi A.W., Crippa J.A., Hallak J.E., Bhattacharyya S., Atakan Z., Martin-Santos R., McGuire P.K., Guimaraes F.S. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr. Pharmaceut. Des. 2012;18:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- 28.McPartland J.M., Duncan M., Di Marzo V., Pertwee R.G. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Pinilla E., Varani K., Reyes-Resina I., Angelats E., Vincenzi F., Ferreiro-Vera C., Oyarzabal J., Canela E.I., Lanciego J.L., Nadal X., Navarro G., Borea P.A., Franco R. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017;8:744. doi: 10.3389/fphar.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laprairie R.B., Bagher A.M., Kelly M.E., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P., Song Z.H. Identification of raloxifene as a novel CB2 inverse agonist. Biochem. Biophys. Res. Commun. 2013;435:76–81. doi: 10.1016/j.bbrc.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howlett A.C., Barth F., Bonner T.I., Cabral G., Casellas P., Devane W.A., Felder C.C., Herkenham M., Mackie K., Martin B.R., Mechoulam R., Pertwee R.G. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 33.Barth F., Rinaldi-Carmona M. The development of cannabinoid antagonists. Curr. Med. Chem. 1999;6:745–755. [PubMed] [Google Scholar]
- 34.Lobo M.K., Cui Y., Ostlund S.B., Balleine B.W., Yang X.W. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat. Neurosci. 2007;10:1395–1397. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd G.M. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kingwell K. Pioneering biased ligand offers efficacy with reduced on-target toxicity. Nat. Rev. Drug Discov. 2015;14:809–810. doi: 10.1038/nrd4784. [DOI] [PubMed] [Google Scholar]
- 37.Masri B., Salahpour A., Didriksen M., Ghisi V., Beaulieu J.M., Gainetdinov R.R., Caron M.G. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oeckl P., Hengerer B., Ferger B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson's disease. Exp. Neurol. 2014;257:1–9. doi: 10.1016/j.expneurol.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Rohleder C., Muller J.K., Lange B., Leweke F.M. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front. Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatry. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]