Highlights
-
•
The HSE-RFP reporter detects primarily- and secondarily-damaged neural cells in spinal cord injury.
-
•
The HSE-RFP reporter detects primarily- and secondarily-damaged neural cells in sciatic nerve injury.
-
•
Reporter-positive secondarily-injured neurons show altered physiological properties.
Abbreviations: DRG, dorsal root ganglion; FG, Fluoro-Gold; HRP, horseradish peroxidase; HSE, heat shock-response element; HSF1, heat shock factor 1; HSP, heat shock protein; IL-6, interleukin 6; M1, primary motor cortex; M2, secondary motor cortex; MPtA, medial parietal association cortex; PCR, polymerase chain reaction; PBS, phosphate buffered saline; RFP, red fluorescent protein; SCI, spinal cord injury; SNI, sciatic nerve injury; WDR, wide-dynamic range; WGA, wheat germ agglutinin
Keywords: Heat shock signaling, Cellular damage, Spinal cord injury, Sciatic nerve injury, Reporter mouse
Abstract
Spinal cord and peripheral nerve injury results in extensive damage to the locally injured cells as well as distant cells that are functionally connected to them. Both primary and secondary damage can cause a broad range of clinical abnormalities, including neuropathic pain and cognitive and memory dysfunction. However, the mechanisms underlying these abnormalities remain unclear, awaiting new methods to identify affected cells to enable examination of their molecular, cellular and physiological characteristics. Here, we report that both primary and secondary damage to cells in mouse models of spinal cord and peripheral nerve injury can be detected in vivo using a novel fluorescent reporter system based on the immediate stress response via activation of Heat Shock Factor 1. We also provide evidence for altered electrophysiological properties of reporter-positive secondarily-injured neurons. The comprehensive identification of injured, but surviving cells located both close and at distant locations from the injury site in vivo will provide a way to study their pathophysiology and possibly prevention of their further deterioration.
Introduction
Spinal cord and peripheral nerve injury are known to have substantial clinical implications beyond the local sensory and motor symptoms attributable to primary cell damage. Previous studies have reported various types of cellular and molecular responses, including microglial activation and peptide expression changes, occur widely outside the damaged tissue (Costigan et al., 2009; David and Kroner, 2011). Therefore, in addition to the local damage at the lesion sites, understanding the potential secondary effects on other parts of the nervous system is crucial. However, the secondary changes, which potentially range throughout the nervous system, are often morphologically undetectable in the early phase of the injury, making it challenging to identify the affected/vulnerable cells, in particular, in living tissue. Methods and tools for identifying such cells will help to address the underlying mechanisms prior to the manifestation of clear structural injury or resultant alterations in behavior. These understandings may provide a basis for development of prognostic biomarkers that could ultimately facilitate development of effective treatments for nerve injuries before they manifest broader clinical consequences.
Heat shock response is an evolutionarily highly conserved cytoprotective mechanism (Akerfelt et al., 2010; Hashimoto-Torii et al., 2014; Lindquist, 1986; Morimoto, 1998) that contributes to adaption to various types of stress, achieving new steady states to preserve the function and viability of at-risk cells (Ishii and Hashimoto-Torii, 2015). Heat Shock Factor 1 (HSF1) and Heat Shock Proteins (HSPs) are essential mediators of this response. Heat stress and many other stimuli can activate HSF1 through sequential protein modification events including phosphorylation, sumoylation, trimerization and nuclear translocation, triggering the transcription of HSPs and other downstream gene targets. Utilizing this cellular stress-inducible mechanism, we have recently reported the generation of a Heat Shock-response Element-Red Fluorescent Protein (HSE-RFP) reporter transgenic mouse line (Torii et al., 2017). The reporter expression in these mice enables early identification of cells that exhibit altered molecular, morphological, and behavioral properties due to the damage by prenatal or postnatal exposure to a spectrum of chemical and physical environmental insults including alcohol, methyl mercury and x-ray (Torii et al., 2017).
Up-regulation of HSPs also has been suggested to occur in the endogenous response to spinal cord injury (Kang et al., 2006; Mautes and Noble, 2000; Song et al., 2001) and peripheral nerve injury (Kim et al., 2001; Klass et al., 2008) among other responses such as the activation of endoplasmic reticulum stress response (Penas et al., 2007), and upregulation of c-fos, nitric oxide synthase, heme oxygenase, chemokines and their receptors (Hayashi et al., 2000; Kajander et al., 1996; Knerlich-Lukoschus and Held-Feindt, 2015; Mautes and Noble, 2000; Naik et al., 2006; Sharma et al., 1996). In the present study, we examined whether the new HSE-RFP reporter system can be used to identify cells that respond to the cellular damage in such traumatic insults, namely spinal cord and sciatic nerve injury, using mouse models.
We show that this system can efficiently and specifically identify both primarily- and secondarily-affected cells throughout the nervous system. The secondary damage detected by this reporter includes several previously unexpected regions. These results demonstrate the applicability and utility of this heat shock signaling-based reporter system for detecting specific reactions occurring broadly in the nervous system following spinal cord and peripheral nerve injury, to help address largely unknown mechanisms involving secondary cell damage that may contribute to various functional defects.
Experimental procedures
Animals
All animals were handled according to protocols approved by the Institutional Animal Care and Use Committees of the Children’s National Medical Center, the VA Connecticut Healthcare System, and Yale University. Generation of the HSE-RFP transgenic mice has been described previously (Torii et al., 2017). Briefly, the sequence of the Hsp70 promoter region conserved across mammalian species was amplified by polymerase chain reaction (PCR). The obtained 649 bp fragment that contains two HSF1 binding sites (HSE: Heat Shock-response Element) was inserted into the BamHI site in the multiple cloning site of the pDsRed2-1 plasmid (Clontech) to generate the HSE-RFP (DsRed2) reporter construct. DsRed2 was selected based on its high signal-to-noise ratio and low cytotoxicity (Chalfie and Kain, 2005; Yanushevich et al., 2002). Microinjection of the excised HSE-RFP fragment into the C57BL/6 J X SJL/J strain was performed by Yale animal genomics service. The founder lines were screened by PCR genotyping for RFP, and confirmed by RFP fluorescence in the neonates from alcohol-treated dams under a dissecting microscope equipped with epifluorescence. From the obtained 3 founder lines (#B9, B11 and B49), the strongest (most sensitive) reporter line, #B9, was selected for the study (Torii et al., 2017). The genetic insertion site has been defined, indicating that reporter expression is independent of the influence of transgene locus (Ishii et al., 2017). For routine genotyping, oligonucleotide PCR primers; Forward 5’-AAGGTGTACGTGAAGCACCC-3’, Reverse 5’-CCCATGGTCTTCTTCTGCAT-3’ were used for the amplification of the 250 bp partial sequence of the DsRed2 gene with Hotstar taq DNA polymerase kit (Qiagen). In all experiments in this study using these mice, we observed no sex-specific differences.
Spinal cord injury model
Adult HSE-RFP reporter mice at 8–10 weeks were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (4 mg/kg). Under sterile technique, a T9 laminectomy was performed, and dura was opened to expose the spinal cord. The dorsal vein of the spinal cord was coagulated, and the dorsal funiculus was transected using an ophthalmic scalpel (P-715; Feather Safety Co.) (Sasaki et al., 2004, Sasaki et al., 2009). The micro scalpel blade was marked for a 1.0 mm depth of cut across the entire dorsal region of the spinal cord. This surgery transects the entire dorsal funiculus, and shows consistent damage to the spinal cord from animal to animal as observed with plastic embedded semi-thin toluidine blue sections (Sasaki et al., 2004). After the transection, Gelfoam (Pharmacia and Upjohn) impregnated with 5 μl of Fluoro-Gold (FG: 4% w/v in saline, pH 7.4; Molecular Probes), was placed into the epicenter of the lesion cavity (Sasaki et al., 2009). The overlying muscles and skin were closed in layers with sutures, and the animal was allowed to recover on a 37 °C heating pad. Post-operatively, all groups were administered twice daily with subcutaneous injections of sterile saline solution (2 ml; 0.9%) and Baytril (0.3 ml; 22.7 mg/ml) for rehydration and prevention of bladder infection. Animals were also administered with buprenorphine (0.05 mg/kg/day, subcutaneous) for 48 h and ibuprofen (5 mg/kg/day, per os) for 72 h. This surgery resulted in bilaterally symmetric paraparesis in all animals, but did not impair eating, drinking or elimination as weight loss was only transient. Manual bladder expression was performed two times daily until reflex bladder emptying was established by several days. By 4 weeks after injury, animals had spontaneously regained a significant degree of locomotor function. Sham control animals underwent laminectomy only.
Sciatic nerve injury model
The sciatic nerve injury model was modified from our previous study (White and Kocsis, 2002). 8–10-week old mice were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (4 mg/kg), and the right sciatic nerves were exposed at the level of the piriform tendon. The nerves were ligated with a suture with 6.0-nylon and cut immediately distal to the ligature site. A 10 to 15 mm section of the distal nerve was removed and the distal stump was retracted. The sciatic nerve stump was sutured into a sterilized polyethylene tubing cuff (15 mm long, 3.27 mm outer diameter, 1.65 mm inner diameter, Baxter Scientific) and on the end of the cut nerve containing a small piece of sterile gelfoam permeated with the 100 μl of anterograde neurotracer wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP) (Sigma, 4% in sterile saline). Animals with unilateral sciatic nerve exposure served as sham controls. After surgery, the overlying skin and muscles were sutured, and the wound was treated with Betadine to prevent infection. Animals were administered with buprenorphine (0.05 mg/kg/day, subcutaneous) for 48 h and ibuprofen (5 mg/kg/day, per os) for 72 h. Recovery was uneventful in all cases (White and Kocsis, 2002).
Immunohistochemistry
Animals were perfused transcardially with cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde under deep anesthesia with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). Brains, spinal cords and dorsal root ganglions (DRGs) were dissected out, post-fixed in 4% paraformaldehyde overnight, and cryoprotected in 30% sucrose/phosphate-buffered saline at 4 °C. Samples were stored at −80 °C until use.
Immunohistochemistry was performed following methods previously described (Hashimoto-Torii et al., 2008; Torii et al., 2009). Briefly, Cryosections (20 μm) were cut using a cryostat and mounted on glass slides. Sections were washed in PBS-0.1% Tween 20 (PBS-T) 3 times, blocked in 5% normal goat serum/0.3 % Triton X-100 in PBS at room temperature for 30 min, and incubated in primary antibodies diluted in 5% normal goat serum/0.3% Triton X-100/PBS at 4 °C overnight. The staining was amplified using biotin-conjugated secondary antibodies, VECTASTAIN ABC system (Vector) and TSA Plus system (PerkinElmer). We used following primary antibodies; monoclonal mouse anti-Ox42 (1:100, BD Pharmingen, 550299), polyclonal chicken anti-GFAP (1:1000, Abcam, ab4674), rabbit anti-RFP-biotin (1:1000, Abcam, ab34771), anti-Fluoro-Gold (1:100 Millipore, AB153), anti-Iba1 (1:100, WAKO, 019-19741), anti-WGA (1:100, Sigma, T4144). Sections were imaged using a Zeiss LSM510 confocal microscope.
Electrophysiological recording
Recording procedures and identification of wide-dynamic range (WDR) neurons were as described in detail previously (Guan et al., 2006; Hains and Waxman, 2006). Recordings were performed at 4 weeks after sciatic nerve injury or sham surgery by an investigator blinded to the surgery condition. Animals were anesthetized with 5% isoflurane, and maintained under anesthesia with 1.5% isoflurane in a mixture of 70% N2O and 30% O2 with spontaneous ventilation for in vivo extracellular single-unit recording. Recordings were obtained with a low impedance 5 MΩ tungsten-insulated microelectrode (A–M Systems). Cells were recorded in the lumbar spinal segments (L2-3 levels). Briefly, units were isolated medially near the dorsal-root entry zone at a depth representing Rexed laminae III to VI where WDR neurons are distributed (Guan et al., 2006; Hains and Waxman, 2006). The depth of electrode in the spinal cord was controlled by a micro-positioner (KOPF Instruments). Once a cell was stably recorded, it was identified as a WDR neuron by increasing responsiveness to graded stimuli from gentle brush to incrementing strength of von Frey filaments (Guan et al., 2006; Hains and Waxman, 2006). Spontaneous discharge activity was recorded for 30 s. Recorded signals were amplified and filtered at 300–3000 Hz (DAM80; World Precision Instruments), processed and analyzed using a data collection system (CED 1401; Cambridge Instruments) and Spike 2 software (v3.13; Cambridge Electronic Design). Data management and statistical analyses were conducted using Statview (version 5.0.1; SAS Institute) and Microsoft Office Excel. Statistical test was performed using Mann-Whitney U test. Significance was set at p < 0.05.
Results
HSF1-HSP pathway activation in neural circuits affected by spinal cord injury
We first examined whether our HSE-RFP reporter mice can be utilized to detect the damaged cells that responded to the spinal cord injury (SCI). In our HSE-RFP reporter mice, activated HSF1 binds the 649 bp Heat Shock-response Element (HSE) that contains the mouse Hsp70 promoter to transcribe RFP reporter (Torii et al., 2017) (Fig. 1). Four days after the SCI (bilateral dorsal funiculus transection at the T9 level), strong reporter expression was observed in cells (neurons and glial cells) in a broad area surrounding the lesion site (Fig. 2C and D), while no RFP reporter expression was observed following sham surgery (Fig. 2A and B) or in naive control (data not shown) groups (n = 4 per group).
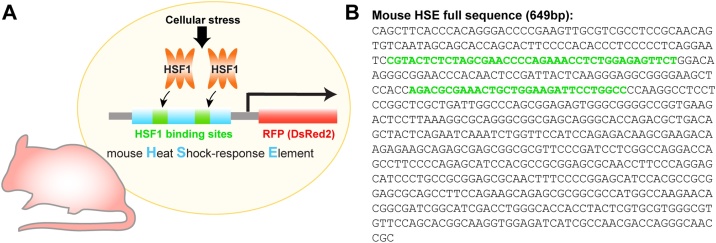
Fig. 1.
Design of the HSE-RFP reporter construct. (A) The reporter transgenic mouse harboring the Heat Shock-response Element (HSE) that contains the mouse Hsp70 promoter followed by RFP (DsRed2) coding sequence. When HSF1 is activated, it binds the HSE to transcribe RFP reporter. (B) Full sequence of HSE. Green letters indicate HSF binding domains. The details of reporter evaluation have been described previously (Torii et al., 2017). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
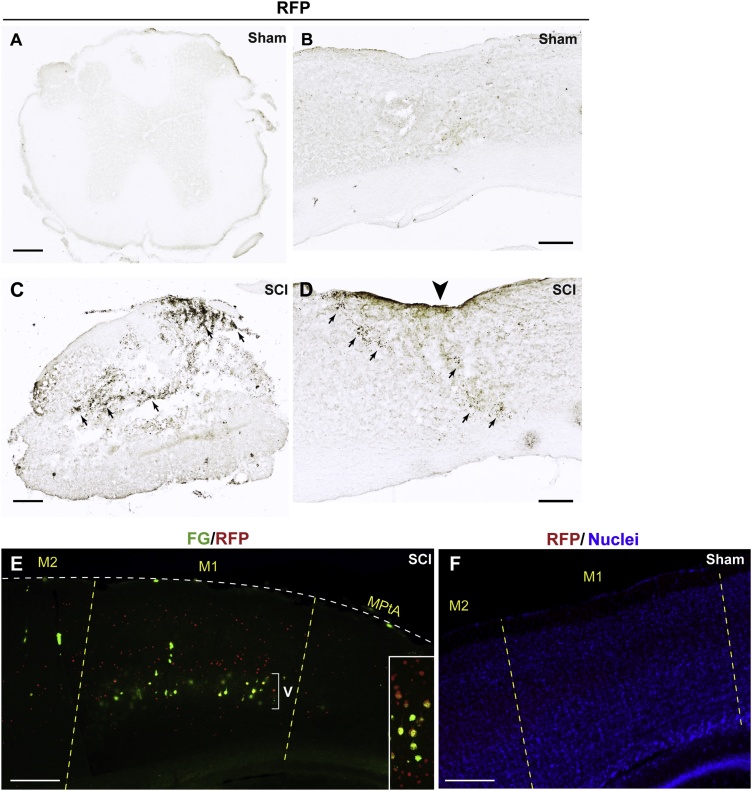
Fig. 2.
HSE-RFP reporter induction in the spinal cord and cerebral cortex following spinal cord injury. (A–D) HSE-RFP reporter expression was detected by immunohistochemistry with 3,3'-Diaminobenzidine (black) in coronal (A and C, at the T9 level) and sagittal (B and D, lateral to the lesion) sections around the lesion site 4 days after spinal cord injury (C and D) and at the same regions in the sham control (A and B). HSE-RFP reporter expression was observed in cells surrounding the lesion site (C and D). Arrows in C and D indicates the regions with the reporter expression. Note that the center of section in C is damaged due to the lesion. The arrowhead in D indicates the level of the lesion. (E) Sagittal view of the brain with immunofluorescence labeling for Fluoro-Gold (FG, green) and RFP (red) 4 days after spinal cord injury. Strong reporter expression is observed in the FG+ backfilled corticospinal projection neurons in layer V (bracket) as well as cells in other layers in the M1 cortex. The inset shows a higher magnification view (from another sample) of FG+/RFP+ neurons in layer V and RFP+ neurons above/below layer V. (F) No reporter expression was observed in the M1 in sham brain labeled for RFP (red) and nuclei (DAPI stain, blue). M1: primary motor cortex, M2: secondary motor cortex, MPtA: medial parietal association cortex. Bars = 100 (A–D) and 200 (E and F) μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
In addition to the cells at the injury site in the spinal cord, neurons in both sides of the brain showed RFP reporter expression in all of the animals that underwent SCI. Retrograde labeling with FG from the injured spinal cord site revealed that the RFP reporter is expressed in corticospinal projection neurons in layer V of the primary motor cortex (M1) (Fig. 2E). Notably, we found that the RFP reporter expression was not limited in layer V in M1 but also extended into other cortical layers that are topographically and/or functionally connected to the axotomized corticospinal tract (Fig. 2E). No reporter expression was observed in the brain of sham operated controls (Fig. 2F). These results indicate that the RFP reporter enables detection of not only the primary damaged neural cells, but also the latent damage in the second-order cells in SCI.
HSF1-HSP pathway activation in neural circuits affected by sciatic nerve injury
As another model of nerve injury in which primary structural damage occurs outside of the central nervous system, we employed a sciatic nerve ligation model (White and Kocsis, 2002) with HSE-RFP mice and traced the RFP reporter expression in the neurons connected to the injured nerves (Fig. 3) (n = 4 each per group). Primary afferents and spinal motor neurons were retrogradely and transganglionically labelled by wheat germ agglutinin (WGA) injection into the sciatic nerve. The RFP reporter expression was observed in the ipsilateral ventral horn of the spinal cord (Fig. 3A), in which RFP expression was detected in WGA-labeled motor neurons (Fig. 3A’, C and I). The RFP reporter expression was also observed in the ipsilateral DRG (Fig. 3G and I).
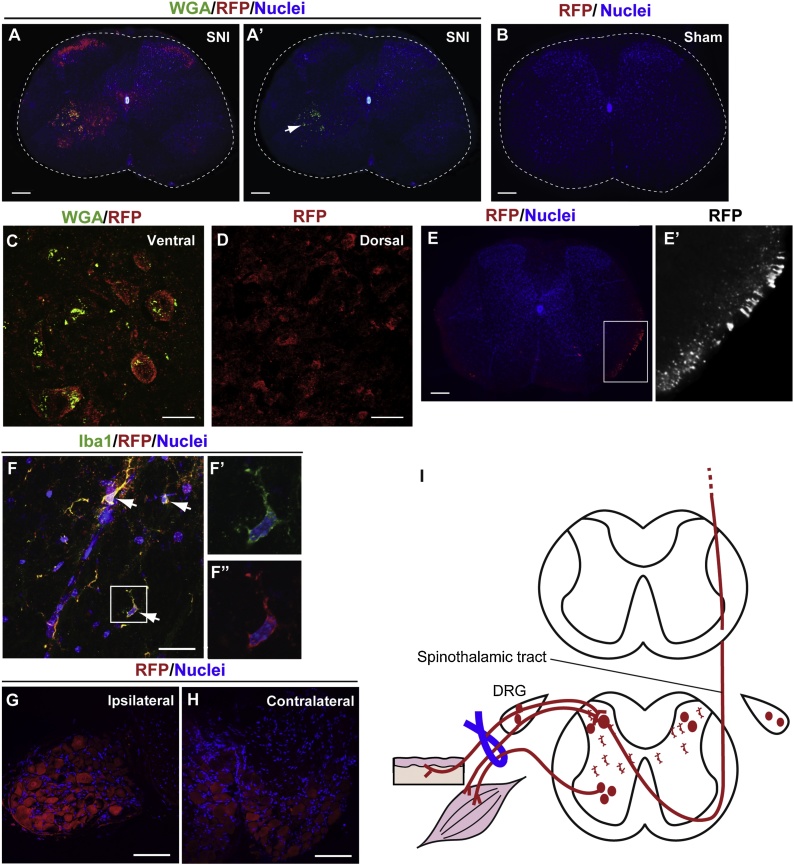
Fig. 3.
HSE-RFP reporter induction in the spinal cord and dorsal root ganglia by sciatic nerve injury. (A-F”) Coronal sections of the spinal cord (A–D and F-F”: L2-3 levels, E and E’: T4-5 levels) and DRG (G and H) were labeled for indicated markers 3 days (A–D and F-F’”) or 2 weeks (E, E’ G and H) after sciatic nerve injury (SNI) or in sham controls. The color of letters for each marker corresponds to the color of the staining. The arrow in A’ indicates the WGA labeling in the ipsilateral ventral horn of the spinal cord. HSE-RFP reporter expression was observed in motor neurons in the ipsilateral ventral horn (A and C), sensory neurons in bilateral dorsal horns [A and D (contralateral side is shown)], contralateral tract in the ventrolateral region (E), and Iba1+ microglia in the intermediate gray matter bilaterally [arrows in F (ipsilateral side is shown)] within the spinal cord. E’ and F’, F” are higher magnification views of boxed areas in E and F, respectively. Reporter expression was also observed in ipsilateral (G) and contralateral (H) DRGs. Bars = 100 (A–B and E), 10 (C and D) and 20 (F, G and H) μm. (I) Schematic drawing illustrating the SNI model and reporter expression (red) in major neural circuits connected with the injured neurons in the spinal cord and DRG. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
In addition to these primarily-damaged neurons, cell bodies of sensory neurons in the bilateral dorsal horns (Fig. 3A, D and I), contralateral tracts in the ventrolateral region of the spinal cord (Fig. 3E and E’), Iba1+ microglia (Fig. 3F-F” and I), and contralateral DRG (Fig. 3H and I) also expressed the RFP reporter. The reporter expression in these regions was observed from at least 3 days until 4 weeks post-injury. No reporter expression was observed in any cell types in the sham surgery controls (Fig. 3B) or naïve control mice (data not shown).
In addition to the DRG and spinal cord, the reporter expression was found in the brain of the mice that received sciatic nerve injury (SNI); in the contralateral red nucleus, which projects to spinal motor neurons of the injured side (Fig. 4B and E), as well as in ipsilateral red nucleus (Fig. 4A and E). No significant reporter induction was observed in other brain regions such as the cerebral cortex in SNI mice. The reporter expression in these regions was consistent across all animals that received the injury. No reporter expression was observed in the sham surgery controls (Fig. 4C and D).
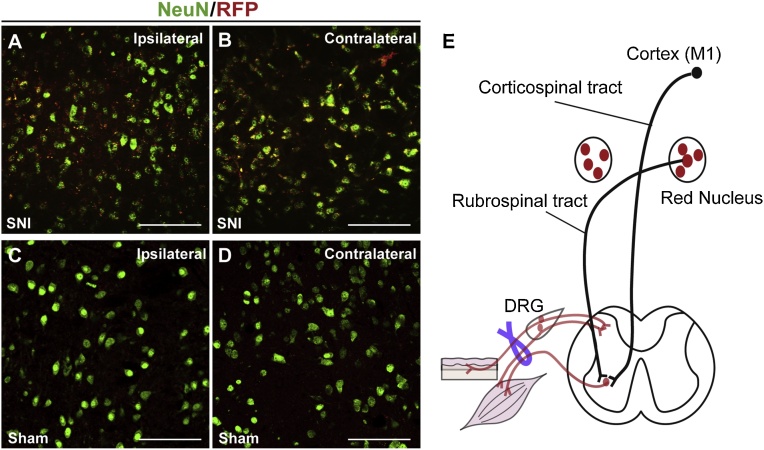
Fig. 4.
HSE-RFP reporter induction in the red nucleus by sciatic nerve injury. (A–D) HSE-RFP reporter (red) and NeuN (green) labeling in red nuclei (RN) of the ipsilateral (A and C) and contralateral (B and D) sides 2-weeks after SNI (A and B) or in sham controls (C and D). Strong reporter expression was observed in neurons in RN of both sides in SNI mice (A and B), but not in sham controls (C and D). Bars = 50 μm. (E) Schematic drawing illustrating the reporter expression (red) in the bilateral RN in SNI model. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Abnormal neuronal activity in the reporter-labeled secondarily-damaged region
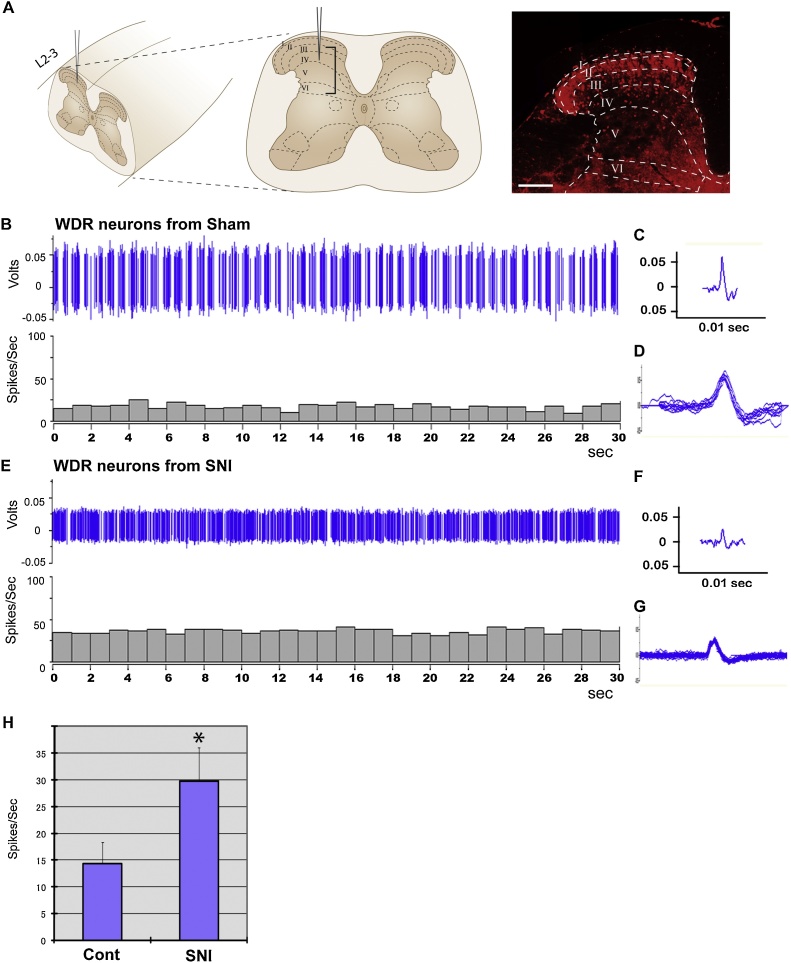
In both the SCI and SNI models, the RFP reporter expression revealed potential damage in second-order neural cells in the spinal cord and brain (Fig. 3, Fig. 4). Using our reporter system that enables detection of the affected cells in living tissue, we asked whether we can record physiological abnormalities associated with these second-order neurons. At 4 weeks after injury (SNI or sham control), we recorded spontaneous discharges of the wide-dynamic range (WDR) neurons for 30 s in vivo in the ipsilateral dorsal horns at the lumbar level (Fig. 5A), at which the naïve fluorescence of RFP was still observed in SNI mice under a dissecting microscope equipped with epifluorescence. The results revealed spontaneous hyperexcitability of these neurons in SNI mice (Fig. 5E–G and H) comparing to the WDR neurons at the corresponding region in sham operated controls (Fig. 5B–D and H) (n = 4 mice per group). Together, our results demonstrate the unique capability of our reporter system for detecting the cellular damage accompanied by physiological changes in vivo, including those in second-order neurons that are anatomically and/or functionally connected to the injured cells (Ghosh et al., 2010).
Fig. 5.
Abnormal electrophysiological properties of WDR neurons in HSE-RFP reporter-expressing dorsal horns of the spinal cord after sciatic nerve injury. (A) (left) Schematic of in vivo electrophysiological recording of WDR neurons in the lumbar spinal segments (L2-3 levels) at 4 weeks after injury. A bracket indicates the target recording region in the laminae III-VI in the dorsal horn ipsilateral to the injury. (Right) Tissue section adjacent to the recording site, showing HSE-RFP reporter expression throughout the dorsal horn, with the highest in the superficial region. Broken lines demarcate the boundaries of different laminae (I–IV). Bar = 100 μm. (B–G) Spontaneous discharge of wide-dynamic range (WDR) neurons in the ipsilateral dorsal horn of the spinal cord at the lumbar level (see the detail in Experimental Procedures) in control (B–D) and the SNI (E–G) mice 4 weeks after injury. The results are displayed in waveforms and spike-frequency histograms recording for 30 s (B and E), individual waveform (C and F), and superimposed waveforms obtained in 1 s (D and G), indicating increased firing following sciatic nerve injury. (H) Firing frequency of WDR neurons in SNI mice is significantly higher than that in sham control mice. *p < 0.05 by Mann-Whitney U test (n = 16 neurons from 4 animals per group). Mean ± SD is presented.
Discussion
We have previously shown that the HSE-based fluorescence reporter system allows early detection of cells affected by various types of prenatal and postnatal environmental and physical factors (Torii et al., 2017). The present study demonstrates that this reporter can also identify damage triggered by spinal cord or nerve injury in adulthood, not only in the directly injured cells but also in secondarily-injured cells that may be located close or distant from the place of original insult.
Our reporter detected several known as well as novel cell types and regions that are affected by SCI and SNI. For example, in the SCI model (Fig. 2), reporter expression was observed not only in the spinal cord but also in distant regions, in corticospinal projection neurons in layer V of the M1; the morphology of these cortical neurons has been reported to be remodeled in response to SCI (Lotze et al., 1999; Sasaki et al., 2009), and in the hippocampus, which is also known to be affected by SCI (Wu et al., 2014). In addition to these known regions, our reporter revealed potential damage in neurons outside of layer V in M1. Similarly, in our SNI model (Fig. 3, Fig. 4), we observed reporter expression in primarily-damaged neurons/nerves in the DRG, primary afferents and motor neurons in the ipsilateral side. In addition, reporter was expressed bilaterally in Iba1+ microglia, in which activation has been suggested to contribute to pathological pain (Gilmore and Kane, 1998; Hains and Waxman, 2006; He et al., 2012) as well as in bilateral DRGs and spinal sensory neurons. Such bilateral effects following unilateral lesions of the sciatic nerve have previously been reported (Koltzenburg et al., 1999), and suggested to be due to bilateral increases in trophic factors (Koltzenburg et al., 1999), immune interactions (Dubovy et al., 2007; Zhou et al., 1996) and/or a transneuronal mechanism (Starkey et al., 2009; Zhou et al., 1996). For example, recent studies suggest that unilateral SNI-induced increase and release of IL-6 from the associated DRG (Erta et al., 2012) may be transported via cerebrospinal fluid into remote DRG and activate gene expression (Dubovy et al., 2013, 2018). Reporter expression newly revealed that the impact of SNI in the red nucleus is also not limited to the contralateral side, which has been reported to be involved in the development of neuropathic allodynia (Jing et al., 2009; Wang et al., 2008), but is extended in the ipsilateral side. Identifying novel regions and cells affected by the injury using this reporter system will facilitate our understanding of the processes and mechanisms underlying the development of complex clinical abnormalities that are not the direct result of the primary structural lesion.
Our study suggests that HSF1-HSP signaling is a key molecular pathway activated in response to the damage by spinal cord and peripheral nerve injury. Up-regulation of HSF1-HSP signaling after injury may play important roles in enhancing the survival of specific neural cell populations and promoting the remodeling of synaptic circuits for functional recovery (Dodge et al., 2006; O’Reilly et al., 2010; Tidwell et al., 2004). Enhancement of the function of cytoprotective mechanism represented by HSF1-HSP signaling might provide a novel therapeutic strategy to minimize the cellular damage and promote recovery after nerve injuries.
An advantage of our reporter system is that reporter+ cells are detectable in living tissues and animals, enabling variable detailed biological and physiological analyses on the impact of injury. Our results of extracellular single-unit recording from WDR neurons at the reporter+ ipsilateral dorsal horn at 4 weeks after SNI (Fig. 5) showed spontaneous hyperexcitability, which has been suggested to contribute to chronic neuropathic pain (Chang and Waxman, 2010). Our SNI model, which uses a tight ligation with distal nerve section, has been shown to allow a formation of neuroma associated with ectopic axonal firing and pain (Colleoni and Sacerdote, 2010; Devor and Wall, 1976). Behavioral signs of neuropathic pain also have been reported after partial SNI models (Decosterd and Woolf, 2000; Shields et al., 2003). Our results, demonstrating the ability of the reporter to identify second-order damage associated with abnormal physiological properties in vivo, justify further analyses in such distinct injury models by distinguishing the properties of reporter+ and reporter− neurons at the single-cell level. Specific detection of cells exhibiting secondary damage in living tissue will open new avenues for isolation, physiological characterization, and manipulation of these cells. These applications will facilitate research on largely unknown processes and mechanisms underlying the broad functional impacts in patients with spinal cord or peripheral nerve injury such as cognitive and memory dysfunctions, chronic neuropathic pain, and allodynia (Chang and Waxman, 2010; Davidoff et al., 1992; Ghosh et al., 2010; Roth et al., 1989).
Identification of affected cells by our reporter system is limited to those associated with the activation of the HSF1-HSP signaling, by the nature of the reporter construct. Nevertheless, our new approach will provide a template, which can be modified to permit development of similar reporter approaches for other signaling pathways involved in secondary cell injury such as endoplasmic reticulum, c-fos, nitric oxide synthase, heme oxygenase, and chemokine pathways (Knerlich-Lukoschus and Held-Feindt, 2015; Mautes and Noble, 2000; Penas et al., 2007; Sharma et al., 1996). A fuller understanding of these multiple pathways should provide a more complete picture of the complex cellular injuries and coordinated stress responses that occur in association with spinal cord and nerve injury.
Conflicts of interest
None.
Acknowledgements
We thank Dr. Alexander Son for critical reading of the manuscript. This work was supported by National Institute of Health [R01AA025215 (K.H-T.), R01MH111674 (M.T.), R21AA024882 (K.H.-T. and M.T.), R01DA023999, R01EY002593 (P.R.)], ABMRF/The Foundation for Alcohol Research (K.H-T.), Brain & Behavior Research Foundation, Scott-Gentle Foundation (K.H-T. and M.T.), the Kavli Institute for Neuroscience at Yale (K.H-T., M.T. and P.R.), Avery Translational Research Career Development Program Award (M.T.), the JSPS KAKENHI [Grant Numbers 24890181, 25462227, 16K10794](M.S.), the AMED Translational Research Network Program (JP16lm0103003), the Medical and Rehabilitation and Development Research Services of Department of Veterans Affairs (S.G.W.); and the Biomedical Laboratory and Rehabilitation Research and Development Services of Department of Veterans Affairs [B7335R, B9260L], the NMSS [RG2135], CT Stem Cell Research Program [12-SCB-Yale-05](J.D.K.). This work was also supported by Award Number UL1TR00075 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. K.H.-T., M.S. and M.T. designed the project. K.H.-T., M.S., Y.-W.C. and H.H. performed experiments, K.H.-T., M.S. and M.T. analyzed the data. K.H.-T., M.S., S.G.W, J.D.K, P.R and M.T. wrote the manuscript.
References
- Akerfelt M., Morimoto R.I., Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Kain S.R. 2nd ed. John Wiley & Sons, Inc.; Hoboken: 2005. Green Fluorescent Protein: Properties, Applications and Protocols. [Google Scholar]
- Chang Y.W., Waxman S.G. Minocycline attenuates mechanical allodynia and central sensitization following peripheral second-degree burn injury. J. Pain. 2010;11:1146–1154. doi: 10.1016/j.jpain.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Colleoni M., Sacerdote P. Murine models of human neuropathic pain. Biochim. Biophys. Acta. 2010;1802:924–933. doi: 10.1016/j.bbadis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Costigan M., Scholz J., Woolf C.J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Davidoff G.N., Roth E.J., Richards J.S. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch. Phys. Med. Rehabil. 1992;73:275–284. [PubMed] [Google Scholar]
- Decosterd I., Woolf C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P.D. Type of sensory nerve fibre sprouting to form a neuroma. Nature. 1976;262:705–708. doi: 10.1038/262705a0. [DOI] [PubMed] [Google Scholar]
- Dodge M.E., Wang J., Guy C., Rankin S., Rahimtula M., Mearow K.M. Stress-induced heat shock protein 27 expression and its role in dorsal root ganglion neuronal survival. Brain Res. 2006;1068:34–48. doi: 10.1016/j.brainres.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dubovy P., Brazda V., Klusakova I., Hradilova-Svizenska I. Bilateral elevation of interleukin-6 protein and mRNA in both lumbar and cervical dorsal root ganglia following unilateral chronic compression injury of the sciatic nerve. J. Neuroinflammation. 2013;10:55. doi: 10.1186/1742-2094-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P., Hradilova-Svizenska I., Klusakova I., Kokosova V., Brazda V., Joukal M. Bilateral activation of STAT3 by phosphorylation at the tyrosine-705 (Y705) and serine-727 (S727) positions and its nuclear translocation in primary sensory neurons following unilateral sciatic nerve injury. Histochem. Cell Biol. 2018;150:37–47. doi: 10.1007/s00418-018-1656-y. [DOI] [PubMed] [Google Scholar]
- Dubovy P., Tuckova L., Jancalek R., Svizenska I., Klusakova I. Increased invasion of ED-1 positive macrophages in both ipsi- and contralateral dorsal root ganglia following unilateral nerve injuries. Neurosci. Lett. 2007;427:88–93. doi: 10.1016/j.neulet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Haiss F., Sydekum E., Schneider R., Gullo M., Wyss M.T., Mueggler T., Baltes C. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat. Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- Gilmore S.A., Kane C.J. Microglia, but not astrocytes, react to sciatic nerve injury in aging rats. Brain Res. 1998;806:113–116. doi: 10.1016/s0006-8993(98)00754-9. [DOI] [PubMed] [Google Scholar]
- Guan Y., Borzan J., Meyer R.A., Raja S.N. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J. Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains B.C., Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K., Torii M., Fujimoto M., Nakai A., El Fatimy R., Mezger V., Ju M.J., Ishii S. Roles of heat shock factor 1 in neuronal response to fetal environmental risks and its relevance to brain disorders. Neuron. 2014;82:560–572. doi: 10.1016/j.neuron.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K., Torii M., Sarkisian M.R., Bartley C.M., Shen J., Radtke F., Gridley T., Sestan N. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Ueyama T., Nemoto K., Tamaki T., Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J. Neurotrauma. 2000;17:203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- He W.J., Cui J., Du L., Zhao Y.D., Burnstock G., Zhou H.D., Ruan H.Z. Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav. Brain Res. 2012;226:163–170. doi: 10.1016/j.bbr.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Ishii S., Hashimoto-Torii K. Impact of prenatal environmental stress on cortical development. Front. Cell. Neurosci. 2015;9:207. doi: 10.3389/fncel.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Torii M., Son A.I., Rajendraprasad M., Morozov Y.M., Kawasawa Y.I., Salzberg A.C., Fujimoto M. Variations in brain defects result from cellular mosaicism in the activation of heat shock signalling. Nat. Commun. 2017;8:15157. doi: 10.1038/ncomms15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y.Y., Wang J.Y., Li X.L., Wang Z.H., Pei L., Pan M.M., Dong X.P., Fan G.X. Nerve growth factor of red nucleus involvement in pain induced by spared nerve injury of the rat sciatic nerve. Neurochem. Res. 2009;34:1612–1618. doi: 10.1007/s11064-009-9950-7. [DOI] [PubMed] [Google Scholar]
- Kajander K.C., Madsen A.M., Iadarola M.J., Draisci G., Wakisaka S. Fos-like immunoreactivity increases in the lumbar spinal cord following a chronic constriction injury to the sciatic nerve of rat. Neurosci. Lett. 1996;206:9–12. doi: 10.1016/0304-3940(96)12447-2. [DOI] [PubMed] [Google Scholar]
- Kang S.K., So H.H., Moon Y.S., Kim C.H. Proteomic analysis of injured spinal cord tissue proteins using 2-DE and MALDI-TOF MS. Proteomics. 2006;6:2797–2812. doi: 10.1002/pmic.200500621. [DOI] [PubMed] [Google Scholar]
- Kim D.S., Lee S.J., Park S.Y., Yoo H.J., Kim S.H., Kim K.J., Cho H.J. Differentially expressed genes in rat dorsal root ganglia following peripheral nerve injury. Neuroreport. 2001;12:3401–3405. doi: 10.1097/00001756-200110290-00050. [DOI] [PubMed] [Google Scholar]
- Klass M.G., Gavrikov V., Krishnamoorthy M., Csete M. Heat shock proteins, endothelin, and peripheral neuronal injury. Neurosci. Lett. 2008;433:188–193. doi: 10.1016/j.neulet.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F., Held-Feindt J. Chemokine-ligands/receptors: multiplayers in traumatic spinal cord injury. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/486758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M., Wall P.D., McMahon S.B. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lotze M., Laubis-Herrmann U., Topka H., Erb M., Grodd W. Reorganization in the primary motor cortex after spinal cord injury—a functional magnetic resonance (fMRI) study. Restor. Neurol. Neurosci. 1999;14:183–187. [PubMed] [Google Scholar]
- Mautes A.E., Noble L.J. Co-induction of HSP70 and heme oxygenase-1 in macrophages and glia after spinal cord contusion in the rat. Brain Res. 2000;883:233–237. doi: 10.1016/s0006-8993(00)02846-8. [DOI] [PubMed] [Google Scholar]
- Morimoto R.I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Naik A.K., Tandan S.K., Kumar D., Dudhgaonkar S.P. Nitric oxide and its modulators in chronic constriction injury-induced neuropathic pain in rats. Eur. J. Pharmacol. 2006;530:59–69. doi: 10.1016/j.ejphar.2005.11.029. [DOI] [PubMed] [Google Scholar]
- O’Reilly A.M., Currie R.W., Clarke D.B. HspB1 (Hsp 27) expression and neuroprotection in the retina. Mol. Neurobiol. 2010;42:124–132. doi: 10.1007/s12035-010-8143-3. [DOI] [PubMed] [Google Scholar]
- Penas C., Guzman M.S., Verdu E., Fores J., Navarro X., Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J. Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- Roth E., Davidoff G., Thomas P., Doljanac R., Dijkers M., Berent S., Morris J., Yarkony G. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia. 1989;27:480–489. doi: 10.1038/sc.1989.75. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Lankford K.L., Zemedkun M., Kocsis J.D. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J. Neurosci. 2004;24:8485–8493. doi: 10.1523/JNEUROSCI.1998-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Radtke C., Tan A.M., Zhao P., Hamada H., Houkin K., Honmou O., Kocsis J.D. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H.S., Westman J., Olsson Y., Alm P. Involvement of nitric oxide in acute spinal cord injury: an immunocytochemical study using light and electron microscopy in the rat. Neurosci. Res. 1996;24:373–384. doi: 10.1016/0168-0102(95)01015-7. [DOI] [PubMed] [Google Scholar]
- Shields S.D., Eckert W.A., 3rd, Basbaum A.I. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J. Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Song G., Cechvala C., Resnick D.K., Dempsey R.J., Rao V.L. GeneChip analysis after acute spinal cord injury in rat. J. Neurochem. 2001;79:804–815. doi: 10.1046/j.1471-4159.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- Starkey M.L., Davies M., Yip P.K., Carter L.M., Wong D.J., McMahon S.B., Bradbury E.J. Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury. J. Comp. Neurol. 2009;513:51–68. doi: 10.1002/cne.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell J.L., Houenou L.J., Tytell M. Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones. 2004;9:88–98. doi: 10.1379/CSC-9R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M., Hashimoto-Torii K., Levitt P., Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M., Sasaki M., Chang Y.W., Ishii S., Waxman S.G., Kocsis J.D., Rakic P., Hashimoto-Torii K. Detection of vulnerable neurons damaged by environmental insults in utero. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2367–2372. doi: 10.1073/pnas.1620641114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang J., Li X., Yuan Y., Fan G. Interleukin-1 beta of Red nucleus involved in the development of allodynia in spared nerve injury rats. Exp. Brain Res. 2008;188:379–384. doi: 10.1007/s00221-008-1365-1. [DOI] [PubMed] [Google Scholar]
- White F.A., Kocsis J.D. A-fiber sprouting in spinal cord dorsal horn is attenuated by proximal nerve stump encapsulation. Exp. Neurol. 2002;177:385–395. doi: 10.1006/exnr.2002.7996. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhao Z., Sabirzhanov B., Stoica B.A., Kumar A., Luo T., Skovira J., Faden A.I. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanushevich Y.G., Staroverov D.B., Savitsky A.P., Fradkov A.F., Gurskaya N.G., Bulina M.E., Lukyanov K.A., Lukyanov S.A. A strategy for the generation of non-aggregating mutants of Anthozoa fluorescent proteins. FEBS Lett. 2002;511:11–14. doi: 10.1016/s0014-5793(01)03263-x. [DOI] [PubMed] [Google Scholar]
- Zhou X.F., Rush R.A., McLachlan E.M. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. J. Neurosci. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]