Abstract
Introduction
Human studies on low-dose resveratrol are scarce. This study aims to evaluate the safety, tolerability, and efficacy of an oral preparation of resveratrol, glucose, and malate (RGM) in slowing the progression of Alzheimer's disease (AD).
Methods
Thirty-nine subjects with mild to moderate AD who were free of life-threatening disease and who did not have contraindications to the use of the study product were screened. Progression of AD was measured by change in the cognitive portion of the Alzheimer's Disease Assessment Scale–cognitive subscale. Secondary outcomes included Clinician's Global Impression of Change, Mini–Mental State Examination, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale, and Neuropsychiatric Inventory. 15 mL of the following preparation per dose, i.e., 5 g dextrose, 5 g malate, and 5 mg resveratrol, or matching placebo was ingested with an 8 oz glass of commercial unsweetened grape juice twice a day for 1 year. Group differences in the rate of change in the outcome measures were examined using generalized estimating equations.
Results
The treatment and control groups were similar on all of the screening variables. At 12 months, change scores on Alzheimer's Disease Assessment Scale–cognitive subscale, Mini–Mental State Examination, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale, or Neuropsychiatric Inventory all showed less deterioration in the treatment than the control group; however, none of the change scores reached statistical significance. The most common AE were falls, all in the control group. None of the falls were deemed to be study related.
Conclusion
Low-dose oral resveratrol is safe and well tolerated. Interpretation of the effects on clinical outcomes trajectories remains uncertain. A larger study is required to determine whether low-dose resveratrol may be beneficial.
Trial Registration
ClinicalTrials.gov (NCT00678431), Registered 05/15/2008.
Keywords: Resveratrol, Drug therapy, Double-blind methods, Safety, Efficacy
1. Introduction
Alzheimer's disease (AD), a leading cause of morbidity and mortality in the elderly, is characterized by progressive cognitive decline and neuropathological features including amyloid plaques and neurofibrillary tangles. There is currently no cure for AD. Current US Food and Drug Administration-approved drugs for the treatment of AD include cholinesterase inhibitors such as donepezil, rivastigmine and galantamine, and memantine, an N-methyl-D-aspartate blocker. These drugs have modest symptomatic effects but do not have profound disease-modifying effects [1]. Attempts to treat amyloid toxicity are underway by a number of groups [2], [3], as are a variety of other approaches [4]. A complete review of current AD therapies can be found in a 2018 publication in this journal [5].
Resveratrol, a polyphenol, has received considerable attention based on molecular, animal, and clinical work [6]. Antiinflammatory effects of resveratrol are suggested by evidence of inhibition of TNF-α and nitric oxide (NO) in mouse microglial cell lines [7]. Resveratrol has been proposed to have antioxidant activity both through free radical scavenging [8] and through upregulation of antioxidant enzymes [6]. Finally, neuroprotection has been proposed based on reducing cell death via activation of sirtuins (including SIRT1), resulting in protection against peptide aggregate [9]. In a 6-month study on the use of resveratrol (200 mg daily) in 46 healthy overweight people aged 50-80 years, the resveratrol group showed better Auditory Verbal Learning Test scores [10] and showed significant increases in functional connectivity of the hippocampus to frontal, parietal, and occipital areas of the brain than the placebo group [11]. Despite this evidence, there have been no conclusive results on the efficacy of resveratrol in human trials [6], [12], [13]. A recent meta-analysis on the efficacy of resveratrol supplementation on cognitive performance found mixed results in currently published clinical research, with a plurality of studies reporting no significant effect on cognitive performance in the general population except a small effect in improving delayed recognition [13]. There are a few ongoing trials investigating the efficacy of resveratrol in mild cognitive impairment and moderate AD, with different routes of administration, although results from these trials are only beginning to be published [8], [14], [15], [16].
Resveratrol has been shown to be well tolerated and pharmacologically safe at doses up to 5 g/day [17]. A recent clinical trial using high-dose resveratrol (2000 mg/day) in patients with mild to moderated AD found that the agent and its metabolites were present in cerebrospinal fluid, suggesting central availability [18], [19]. Compared with the placebo group, the resveratrol group showed markedly reduced cerebrospinal fluid MMP9 levels at week 52 [19]. Cerebrospinal fluid Aβ40 and plasma Aβ40 levels declined more in the placebo group than the resveratrol-treated group at week 52; however, brain volume loss was greater in the resveratrol than placebo group [18], [19]. It is difficult to reconcile these effects as potentially beneficial, although a hypothesis has been suggested that resveratrol has potent antiinflammatory effects in the AD brain—with decreased CNS edema as the etiology of greater brain volume loss. The study also reported less decline in activities of daily living (ADLs) in the treated group, although the study was inadequately powered to determine clinical outcomes.
In an initial, double-blind, placebo-controlled, prospective clinical trial, positive effects in AD with a “metabolic enhancer” that contains low-dose resveratrol (5 mg/day), glucose, and malate (RGM) have been reported [20], [21], [22]. Glucose is the physiological precursor of substrates of oxidative metabolism in the brain, and malate is an intermediate of the energy-providing Krebs cycle. Glucose and malate can provide reducing equivalents (electrons) to regenerate the reduced form of resveratrol and do so under normal regulation of brain cell metabolism. All three ingredients are classified by the US Food and Drug Administration as generally recognized as safe. The “metabolic enhancer” also contains pharmaceutical flavorings, to mask the very sour taste of malate. The preparation is given with unsweetened grape juice because the natural sugar in grape juice is glucose. A preparation has therefore been developed, which has been designed to help the body regulate free radical metabolism rather than simply to quench free radicals. The study tested the addition of RGM to patients who were already receiving anticholinergic treatment by taking stable doses of donepezil. The results of this trial were promising. The addition of RGM to anticholinergic treatment seems to have beneficial effects on cognition without causing any significant side effects.
Based on these results, we hypothesized that this preparation may be useful as part of an evolving “AD treatment regimen” worthy of replication by more rigorous methods. Specifically, we aimed to test the hypothesis that the combination of RGM would significantly reduce clinical progression of AD and have beneficial effects over placebo on measures of ADL limitations, psychiatric and behavioral symptoms in a pilot double-blind placebo-controlled trial. The specific goals of this study were (1) to use a multisite, single-center model to test the safety and efficacy of RGM in well-characterized patients with AD by conducting the trial with exact replication of subjects, agent, outcomes, and design (i.e., 6 months of exposure) of the original study and (2) to extend the double-blind observation period to assess efficacy at 12 months.
2. Methods
2.1. Study design
This is a pilot study with placebo-controlled, parallel design. After enrollment, subjects were randomly assigned to the treatment or placebo group and followed up at 3, 6, and 12 months. Enrollment began in January 2007 and ended in September 2009. From power analysis performed based on preliminary data available at the time, 35 participants per group were expected to be needed to achieve 80% power to detect a difference in mean Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-cog) change between the active and placebo group, with α = 0.05. Enrollment target was set to be 100 (50 per group) to allow for attrition. Actual enrollment was 39, of which 32 were randomized. Cognitive, behavioral, and clinical assessments were administered at baseline and each follow-up visit. Subjects were treated with RGM or placebo for a total of 12 months. The primary outcome measure was change in ADAS-cog [23]. Secondary outcome measures were ADCS Clinician's Global Impression of Change (ADCS-CGIC) [24], Mini–Mental State Examination (MMSE) [25], Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale (ADCS-ADL) [26], and Neuropsychiatric Inventory (NPI) [27]. Study outcomes were assessed by trained clinicians who were blind to participant's treatment assignment. Randomization was centrally generated to determine group assignment with equal probability of assignment to drug and placebo, stratified by site. Safety measures included clinical and laboratory indicators. The study was approved by the local institutional review boards and registered at ClinicalTrials.gov (NCT00678431).
2.2. Participants
Patients were recruited from the Mount Sinai Alzheimer's Disease Research Center. Primary inclusion criteria were (1) a probable or possible AD diagnosis according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria [28]; (2) age 50 or older; (3) MMSE score 12-26 [25]; (4) living in the community; (5) stable medical condition and stable use of nonexcluded medications; (6) supervision available for administration of study medications; (7) availability of a study partner to accompany subject to all scheduled visits and complete informant-based assessments; (8) Modified Hachinski score < 4 [29]; (9) able to complete baseline assessments in English or Spanish; and (10) able to ingest an oral agent. Exclusion criteria included (1) active liver or renal disease; (2) active life-threatening neoplastic disease; (3) use of another investigational agent within 2 months of the screening visit; (4) history of clinically significant stroke; (5) current evidence or history in the past 2 years of seizures, head injury with loss of consciousness, and/or immediate confusion after the injury; (5) current DSM-IV diagnosis for major psychiatric disorder; and (6) blindness, deafness, language difficulties, or other disability that may interfere with assessment. Excluded medications included those with significant central anticholinergic or antihistaminic effects and experimental drugs. Psychiatric medications had to be stable. A validated Spanish version was administered by competent speakers. Written informed consent was provided by all subjects before enrollment.
2.3. Intervention
Study medication (RGM) was prepared to adhere to regimen used in the study by Blass and Gordon (2004) [20]. The active daily treatment regimen consisted of 5 g dextrose, 5 g malate, and 5 mg resveratrol per dose. It was administered twice a day in liquid form and taken in a 15 mL volume dissolved in unsweetened commercial red grape juice. Placebo contained sucrose and lemon juice and was indistinguishable by color or taste from the active preparation.
2.4. Outcomes
The primary outcome for this study was rate of change in the cognitive portion of the ADAS-cog (range = 0-70), a psychometric instrument that evaluates memory, attention, reasoning, language, orientation, and praxis [23]. Higher scores indicate more impairment. A positive change score indicates cognitive worsening. Secondary outcomes include change scores on the MMSE (range = 0-30, higher scores indicating better cognition, a positive change score indicates cognitive improvement), ADCS-ADL (range = 0-78, higher scores indicating better function, a positive change score indicates functional improvement) [26], NPI (higher scores indicating worse behavior, a positive change score indicates worsening behavior) [27], and ADCS-CGIC [24].
2.5. Safety assessments
Participants received physical and neurologic examinations and vital signs at each visit. Safety measures included standard reporting of any adverse events (AEs) or endorsement of items from a “symptom checklist” that directly inquired about known side effects of the agents. These were collected at each visit as well as at any time that the informant contacted study staff. If a participant withdrew, an early termination visit similar to a baseline visit was scheduled. An independent Data and Safety Monitoring Board reviewed data quarterly.
2.6. Statistical analysis
Baseline characteristics of the study groups were compared using Fisher's exact test for categorical variables and Wilcoxon signed-rank test for continuous variables. Change scores from baseline in ADAS-cog, MMSE, and ADCS-ADL between treatment and placebo groups were compared at each follow-up visit. Group differences in the rate of change in the outcome measures were examined using generalized estimating equations [30]. Differences in the proportion of subjects reporting worsening on the ADCS-CGIC were examined using logistics regressions. Models included age, gender, education, and baseline scores as covariates. Safety analyses were based on summary listings of AEs. Differences in the rate of adverse events were compared using Fisher's exact test. Investigators classified AEs by severity and causality. Analyses were based on an intention-to-treat population, including all randomly assigned participants with at least one postbaseline observation. All tests were two-sided. P-value of 0.05 was considered statistically significant and set a priori. Blinding of investigators was maintained until after outcomes were determined. All analyses were conducted using Stata 13 [31].
3. Results
3.1. Baseline characteristics
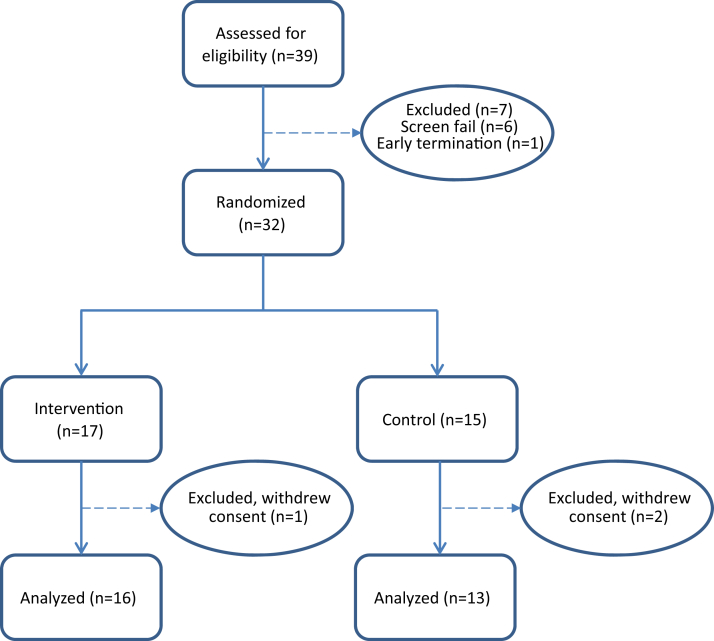
Thirty-nine subjects were screened (6 screen fail, 1 early termination). Thirty-two subjects were randomized (17 treatment and 15 control), of whom 3 subjects (1 treatment and 2 control) withdrew consent (Fig. 1 CONSORT flow diagram) [32]. The study included 29 subjects (16 treatment and 13 control) from whom data are available. Subjects were successfully randomized into treatment and placebo groups with similar characteristics on age (mean = 80 ± 7.7), gender (56.6% male), and education (mean = 15 ± 4.6) (Table 1). There were no differences between treatment and control groups on any of the screening variables.
Fig. 1.
Consort Diagram and Disposition by Treatment Group.
Table 1.
Baseline characteristics by the treatment group
| Variables | Control group (n = 13) | Treatment group (n = 16) | P-value |
|---|---|---|---|
| Age, mean ± standard deviation | 79.3 ± 6.5 | 80.5 ± 8.6 | 0.6850 |
| Male, n (%) | 8 (61.5) | 9 (56.3) | 0.7737 |
| Education, mean ± standard deviation | 14.1 ± 5.2 | 15.7 ± 4.1 | 0.3596 |
| ADAS-cog, mean ± standard deviation | 29.2 ± 8.9 | 26.4 ± 11.9 | 0.4901 |
| ADCS-ADL, mean ± standard deviation | 46.6 ± 7.6 | 49.1 ± 10.3 | 0.4805 |
| MMSE, mean ± standard deviation | 19.4 ± 3.8 | 18.1 ± 4.9 | 0.7239 |
| NPI, mean ± standard deviation | 7.9 ± 11.8 | 5.4 ± 5.9 | 0.4975 |
Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale; NPI, Neuropsychiatric Inventory.
3.2. Change scores from baseline by the treatment group
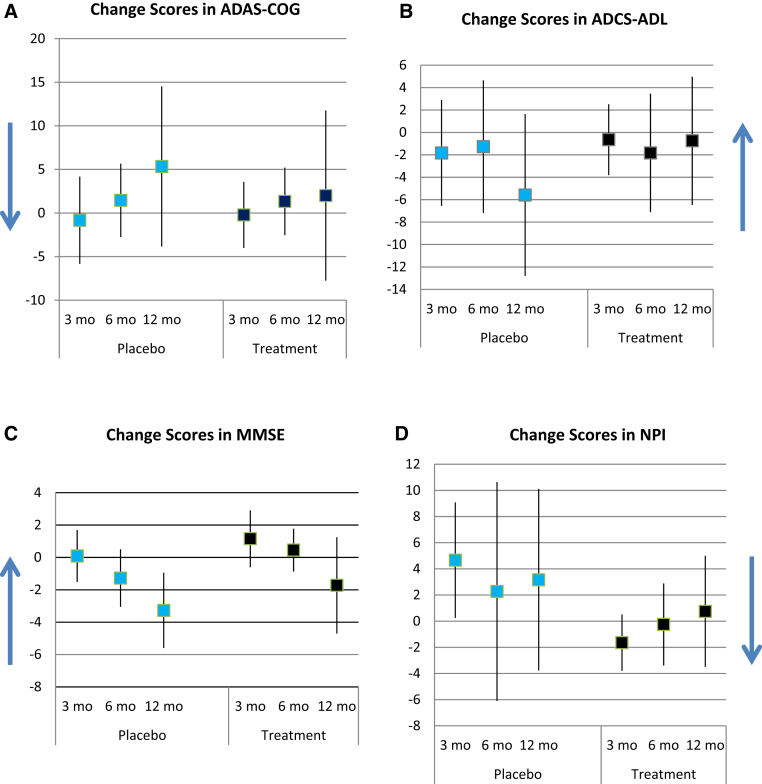
Table 2 reports scores for the outcomes by treatment and control groups at each visit. Changes in outcomes are shown graphically in Fig. 2. For ADAS-cog, mean change scores from baseline were −0.83 ± 7.88, 1.45 ± 6.27, and 5.33 ± 14.46 at month 3, 6, and 12 for the control group, and −0.21 ± 6.57, 1.33 ± 6.10, and 2.00 ± 15.36 for the treatment group. For MMSE, mean change scores from baseline were 0.09 ± 2.39, −1.27 ± 2.65, and −3.27 ± 3.47 at month 3, 6, and 12 for the control group, and 1.15 ± 2.91, 0.45 ± 1.97, and −1.73 ± 4.43 for the treatment group. For ADCS-ADL, mean change scores from baseline were −1.83 ± 7.43, −1.27 ± 8.81, and −5.58 ± 11.37 at month 3, 6, and 12 for the control group, and −0.64 ± 5.47, −1.83 ± 8.31, and −0.75 ± 9.00 for the treatment group. For NPI, mean change scores from baseline were 4.67 ± 6.95, 2.27 ± 12.46, and 3.17 ± 10.92 at month 3, 6, and 12 for the control group, and −1.64 ± 3.73, −0.25 ± 4.94, and 0.75 ± 6.69 for the treatment group. Differences were statistically insignificant.
Table 2.
Outcomes at each visit by the treatment group
| Variables | Control group (n = 13) | Treatment group (n = 16) | |||
|---|---|---|---|---|---|
| ADAS-COG | Visit | Mean | Standard deviation | Mean | Standard deviation |
| 0 | 29.23 | 8.90 | 26.44 | 11.93 | |
| 3 | 27.00 | 10.61 | 25.93 | 12.82 | |
| 6 | 27.91 | 10.12 | 26.83 | 15.14 | |
| 12 | 33.17 | 18.62 | 29.92 | 14.13 | |
| ADCS-ADL | |||||
| 0 | 46.62 | 7.57 | 49.06 | 10.26 | |
| 3 | 44.25 | 10.08 | 50.21 | 11.27 | |
| 6 | 45.09 | 12.74 | 48.83 | 10.28 | |
| 12 | 40.50 | 13.27 | 49.33 | 10.51 | |
| MMSE | |||||
| 0 | 19.42 | 3.78 | 18.07 | 4.86 | |
| 3 | 19.58 | 3.90 | 19.21 | 5.65 | |
| 6 | 18.27 | 4.98 | 19.83 | 4.84 | |
| 12 | 15.42 | 6.54 | 16.92 | 7.67 | |
| NPI | |||||
| 0 | 7.92 | 11.78 | 5.44 | 5.90 | |
| 3 | 11.67 | 17.56 | 3.36 | 6.89 | |
| 6 | 8.73 | 9.42 | 5.08 | 8.35 | |
| 12 | 10.17 | 11.03 | 6.25 | 7.51 | |
Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale; NPI, Neuropsychiatric Inventory.
Fig. 2.
Mean change scores from baseline at each follow-up visit in treatment and placebo groups. Vertical bars represent standard deviation. Blue arrow indicates direction of improvement. (A) Positive change scores in ADAS-cog indicate worsening impairment from baseline. (B) Positive change scores in ADCS-ADL indicate improvement from baseline. (C) Positive change scores in MMSE indicate improvement from baseline. (D) Positive change scores in NPI indicate worsening impairment from baseline. Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale; NPI, Neuropsychiatric Inventory.
Table 3 shows generalized estimating equation estimates of rate of change over time by the treatment group. At baseline, NPI was significantly lower in the treatment than control group (P = .025). Over time, ADAS-cog increased (P = .05) and MMSE decreased (P = .003) for the control group. There were no statistically significant differences in rate of change over time by the treatment group in the outcomes.
Table 3.
GEE estimates of rate of change in outcomes
| Variables | ADAS-COG |
P-value | ADCS-ADL |
P-value | MMSE |
P-value | NPI |
P-value |
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) [95% CI] | Estimate (SE) [95% CI] | Estimate (SE) [95% CI] | Estimate (SE) [95% CI] | |||||
| Treatment group | 2.087 (3.745) [−5.253, 9.426] | 0.577 | −0.060 (3.587) [−7.091, 6.971] | 0.987 | 1.336 (1.281) [−1.175, 3.848] | 0.297 | −6.944 (3.102) [−13.020, -0.863] | 0.025 |
| Visit | 0.680 (0.349) [−0.003, 1.363] | 0.051 | −0.387 (0.354) [−1.081, 0.307] | 0.274 | −0.367 (0.122) [−0.606, −0.128] | 0.003 | −0.089 (0.447) [−0.965, 0.787] | 0.842 |
| Treatment group × visit | −0.451 (0.612) [−1.649, 0.748] | 0.462 | 0.419 (0.491) [−0.543, 1.382] | 0.393 | 0.036 (0.191) [−0.337, 0.410] | 0.850 | 0.368 (0.485) [−0.584, 1.319] | 0.449 |
Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale; NPI, Neuropsychiatric Inventory; GEE, generalized estimating equation.
At the 3-month visit, substantially fewer subjects in the treatment group worsened marginally compared with the control group as measured by CGIC (15.4% vs. 50.0%, P = .07) (data not shown). At the 12-month visit, there were no statistically significant differences in rate of change over time by the treatment group in CGIC.
3.3. Adverse events
During the study, 7 subjects reported a total of 36 adverse events (29 AEs from 4 control subjects and 7 AEs from 3 treatment subjects) (Table 4). None of these were deemed to be study related. The most common AE was falls (in 3 control subjects). Agitation also occurred more than once (both in control subjects). Among subjects who reported AEs, fewer adverse events per subject were reported in the treatment group (2.3 vs. 7.3 events per subject, P = .059).
Table 4.
Number of adverse events by system
| System | Control group (n = 13) | Treatment group (n = 16) |
|---|---|---|
| Psychiatric disorders∗ | 6 | 8 |
| Nervous system disorders† | 4 | 4 |
| Gastrointestinal disorders‡ | 6 | 2 |
| Cardiac disorders§ | 3 | 2 |
| Injury, poisoning, or procedural complications¶ | 5 | 2 |
| Musculoskeletal and connective tissue disorders‖ | 1 | 1 |
| Skin and subcutaneous tissue disorders# | 1 | 1 |
| Renal and urinary disorders∗∗ | 0 | 1 |
| Vascular disorders†† | 1 | 0 |
| Eye disorders‡‡ | 2 | 0 |
Includes depressed mood, wandering, low energy, drowsiness, depressed mood, agitation, and altered mental status.
Facial flushing, dizziness, headache, tremors, and altered mental status.
Diarrhea, constipation, abdominal discomfort, dry mouth, hematochezia, and worsening of inguinal hernia.
Palpitations, presyncope vs. syncope, and congestive heart failure.
Fall.
Joint pain.
Rash.
Urinary discomfort.
Anemia.
Blurred vision and eye infection.
4. Discussion
This study tested the efficacy and safety of low-dose resveratrol in a cohort of well-characterized patients with AD. Despite inadequate recruitment, positive trends were noted in four clinical outcome measures. At 12 months, change scores on ADAS-cog, MMSE, ADCS-ADL, or NPI all showed less deterioration in the treatment than the control group; however, none of the change scores reached statistical significance. Results showed that low-dose oral resveratrol is safe and well tolerated. The most common AE were falls, all in the control group. None of which were deemed to be study related.
Human studies on low-dose resveratrol are scarce. We found only one trial that examined the effect of high-dose resveratrol in individuals with mild to moderate AD using data from the ADCS [18], [19]. Compared with the ADCS sample, our sample has similar levels of education (15.7 ± 4.1 years vs. 15.5 ± 3.0 years) but is older (average age 80.5 ± 8.6 vs. 69.8 ± 7.7) and has fewer females (56.3% vs. 63%). At baseline, subjects in the treatment group in our sample also have lower MMSE (18.1 ± 4.9 vs. 20.2 ± 4.4), lower ADCS-ADL (49.1 ± 10.3 vs. 63.7 ± 10.8), higher ADAS-cog (26.4 ± 11.9 vs. 25.3 ± 10.1), and lower NPI (5.4 ± 5.9 vs. 7.5 ± 7.9). Similar to our study, data from the ADCS study reported no significant effects of high-dose resveratrol on ADAS-cog, MMSE, or NPI at 52 weeks, although no actual values at week 52 were reported. Although both studies found less decline in the treatment group in ADCS-ADL, the differences were statistically significant in the ADCS study but not in the present study [18], [19]. Together, these data suggest that our sample is constituted of a typical group of AD patients with cognition and function in the mild-moderate range.
Mechanisms involved in high- and low-dose resveratrol interventions may differ. High-dose studies, such as by Turner et al., consider SIRT1 activation as a potential mechanism and point to low bioavailability but high bioactivity [33], [34], to select a maximally safe and well-tolerated dose for their study. Our model focused on metabolic enhancement via reduction of free radicals, which led to the use of low-dose resveratrol similar to that obtained in foods [35]. Glucose and malate, Krebs cycle intermediates were added to increase the rate of mitochondrial metabolism. Despite these proposed mechanistic differences, both safety and efficacy results with high- and low-dose resveratrol were not dissimilar.
This study has several limitations. The study was discontinued before recruitment goals were achieved due to difficulties in recruitment and in keeping the study product stable. The study was underpowered to detect differences in clinical outcomes between treatment and control groups. Data in this study suggest that to achieve 80% power in detecting group differences at .05 level, 48 subjects in each group are needed for ADCS-ADL, 56 subjects in each group for NPI, and 425 subjects in each group for ADAS-cog. Because the low-dose resveratrol is not unlike that obtained in foods [35], interactions with metabolic, environmental, and other nutrition intake may overwhelm the possible beneficial effects. Designing studies to take such complexity into account is challenging.
5. Conclusion
This study showed that low-dose oral resveratrol is safe and well tolerated. However, partly because the study was underpowered to detect differences in clinical outcomes between treatment and control groups, interpretation of the effects on clinical outcomes trajectories remains uncertain. A larger study is required to determine whether low-dose resveratrol may be beneficial.
Research in context.
-
1.
Systematic review: Human studies on low-dose resveratrol are scarce. This study aims to evaluate the safety, tolerability, and efficacy of an oral preparation of resveratrol, glucose, and malate (RGM) in slowing the progression of Alzheimer's disease.
-
2.
Interpretation: Results from this pilot study show less deterioration on the Alzheimer's Disease Assessment Scale–cognitive subscale, Mini–Mental State Examination, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale, and Neuropsychiatric Inventory in the treatment than the control group, although change scores were not statistically significance. No adverse effects were deemed to be study related.
-
3.
Future directions: Low-dose oral resveratrol is safe and well tolerated. Interpretation of the effects on clinical outcomes trajectories remains uncertain. A larger study is required to determine whether low-dose resveratrol may be beneficial.
Acknowledgments
Funding: This research was supported by a grant from the Alzheimer's Association (PI: M.S.) and also by the Alzheimer Disease Research Center at Mount Sinai (U01 P50 AG005138, PI: M.S.). C.W.Z., H.G., S.P., and M.S. are also supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Authors' contributions: M.S., H.G., and J.N. contributed to conception and design; H.G., J.N., S.P., A.B., and M.S. contributed to acquisition of data; X.L. and C.W.Z. contributed to analysis and interpretation of data; C.W.Z. drafted the manuscript; and all authors contributed to critical revision for important intellectual content.
Ethics approval and consent to participate: Study is approved by Mount Sinai, JJP VAMC IRBs. Written informed consent was provided by all subjects before enrollment.
Consent for publication: Not applicable.
Availability of data and material: The data sets used and analyzed during the present study are available from the corresponding author on reasonable request.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Citron M. Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci. 2004;5:677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- 2.Reisberg B., Doody R., Stoffler A., Schmitt F., Ferris S., Mobius H.J. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Reisberg B., Shao Y., Golomb J., Monteiro I., Torossian C., Boksay I. Comprehensive, Individualized, Person-Centered Management of Community-Residing Persons with Moderate-to-Severe Alzheimer Disease: A Randomized Controlled Trial. Dement Geriatr Cogn Disord. 2017;43:100–117. doi: 10.1159/000455397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hock C., Konietzko U., Streffer J.R., Tracy J., Signorell A., Muller-Tillmanns B. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 5.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molino S., Dossena M., Buonocore D., Ferrari F., Venturini L., Ricevuti G. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016;161:69–77. doi: 10.1016/j.lfs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Bi X.L., Yang J.Y., Dong Y.X., Wang J.M., Cui Y.H., Ikeshima T. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kerwin DR. Pilot Study of the Effects of Resveratrol Supplement in Mild-To-Moderate Alzheimer's Disease. NCT007437432008.
- 9.Kulkarni S.S., Canto C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lezak M., Howieson D., Loring D. 4 ed. Oxford; New York: 2004. Neuropsychological assessment. [Google Scholar]
- 11.Witte A.V., Kerti L., Margulies D.S., Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane R.L.B.M., Fink H.A., Brasure M., Davila H., Desai P., Jutkowitz E. Agency for Healthcare Research and Quality; Rockville, MD: 2017. Interventions To Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer's-Type Dementia. [PubMed] [Google Scholar]
- 13.Marx W., Kelly J.T., Marshall S., Cutajar J., Annois B., Pipingas A. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials. Nutr Rev. 2018;76:432–443. doi: 10.1093/nutrit/nuy010. [DOI] [PubMed] [Google Scholar]
- 14.BDPP Treatment for Mild Cognitive Impairment (MCI) and Prediabetes or Type 2 Diabetes Mellitus (T2DM). NCT025022532015.
- 15.Wand PH, Brody ML. Short Term Efficacy and Safety of Perispinal Administration of Etanercept in Mild to Moderate Alzheimer's Disease. NCT017166372016.
- 16.Floeel A. Effects of Dietary Interventions on the Brain in Mild Cognitive Impairment (MCI). NCT012192442016.
- 17.Patel K.R., Scott E., Brown V.A., Gescher A.J., Steward W.P., Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 18.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussa C., Hebron M., Huang X., Ahn J., Rissman R.A., Aisen P.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation. 2017;14:1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blass J., Gordon D. An Adjunct Treatment for Alzheimer Disease. NeuroBio of Aging. 2004;25:84. [Google Scholar]
- 21.Blass J. Effects of Free Radical Quenchers in Food. J Neurochem. 2005;94:75. [Google Scholar]
- 22.Blass J.P. A new approach to treating Alzheimer's disease. Ann N Y Acad Sci. 2008;1147:122–128. doi: 10.1196/annals.1427.022. [DOI] [PubMed] [Google Scholar]
- 23.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 24.Schneider L.S., Clark C.M., Doody R., Ferris S.H., Morris J.C., Raman R. ADCS Prevention Instrument Project: ADCS-clinicians' global impression of change scales (ADCS-CGIC), self-rated and study partner-rated versions. Alzheimer Dis Assoc Disord. 2006;20:S124–S138. doi: 10.1097/01.wad.0000213878.47924.44. [DOI] [PubMed] [Google Scholar]
- 25.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 27.Cummings J.L. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 28.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Hachinski V.C., Iliff L.D., Zilhka E., Du-Boulay G.H., McAllister V.L., Marshall J. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 30.Hardin J., Hilbe J. Chapman and Hall/CRC; London: 2003. Generalized Estimating Equations. [Google Scholar]
- 31.Stata Statistical Software: Release 13. StataCorp LP; College Station, Texas: 2013. [computer program]. Version 11. [Google Scholar]
- 32.Moher D., Schulz K.F., Altman D., Group C. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 33.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 34.Cottart C.H., Nivet-Antoine V., Laguillier-Morizot C., Beaudeux J.L. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 35.Truelsen T., Thudium D., Gronbaek M., Copenhagen City Heart Study Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]