Abstract
In the vertebrate eye, limiting oxidation of proteins and lipids is key to maintaining lens function and avoiding cataract formation. A study by Serebryany et al. identifies a surprising contributor to the eye's oxidative defense in their demonstration that γD-crystallin (HγD) functions as an oxidoreductase and uses disulfide exchange to initiate aggregation of mutant crystallins that mimic oxidative damage. These insights suggest a mechanism by which a dynamic pool of closely packed proteins might avoid oxidation-driven protein-folding traps, providing new avenues to understand the basis of a human disease with global impact.
The eye lens walks on the wild side of life. Lens proteins (1) do not turn over as in other cells, and, indeed, carbon-dating of eye proteins is often used to determine the age of a particular animal. One consequence of this literal lifetime is that any damage that occurs accumulates over a multiyear timeline. To avoid damage, the lens has an impressive oxidation defense system, including the highest concentrations of glutathione (GSH) found anywhere in the body, as well as biosynthetic and regenerating systems to maintain this high GSH concentration (2). However, as we age, a chemical barrier develops to prevent GSH diffusion from the cortex (outside) into the nucleus (center), isolating the oldest, and arguably most vulnerable, cells in the nucleus of the lens (see Ref. 2 and references therein). As a result, the effectiveness of this defense system erodes (2) as GSH breaks down (3), leading to increases of oxidized thiols in the form of disulfides, both mixed (3) and intermolecular (see Ref. 2 and references therein), novel post-translational modifications, and cross-links based upon cysteine oxidation (4). This eventually leads to the loss of lens function due to protein aggregation and cataract formation, which remains the major cause of blindness worldwide (http://www.who.int/blindness/causes/priority/en/index1.html, accessed September 6, 2018). 3
Why would the lens evolve a built-in GSH barrier designed to activate later in life if it's a major redox buffer? Is this the smoking gun for the involvement of other as yet unknown players in lenticular redox homeostasis? An answer to this question has been provided by Serebryany and colleagues (5), who have previously studied the crystallins. These are major components of the lens proteome and present in the lens at concentrations of up to 600 mg/ml in order to deliver the graded refractive index needed to focus light onto the retina. Additionally, oxidative damage to the crystallins in the form of increasing disulfide bonds is directly correlated with cataracts. The β- and γ-crystallins, two of the three major crystallin classes, belong to an ancient superfamily (6) characterized by Greek key motifs and calcium- and transition metal–binding properties and are typically thought of in terms of their structure and refractive properties. Specifically, the close packing between these proteins and their very high concentration (see Ref. 6 and references therein) underpins lens function but runs the risk of initiating aggregation. In their previous work, Serebryany and colleagues (7) explored the aggregation of the W42Q mutant of HγD, which mimics a cataract-associated mutation. They discovered that the WT isoform of HγD could promote aggregation of the W42Q mutant in a disulfide bond–dependent manner. In their new study, Serebryany et al. (5) now present evidence that this function involves protein disulfide exchange, defining an unexpected oxidoreductase activity of HγD.
HγD contains six cysteine residues, with the cysteine pair at positions 108 and 110 resembling the “CXC” motif of some thioredoxins and disulfide isomerases, as noted by Serebryany et al. (5). The authors confirmed these residues do form a disulfide bond upon oxidation and that this event is required to promote aggregation of the W42Q mutant. Furthermore, the authors explicitly demonstrated disulfide exchange between HγD and the W42Q mutant, although the newly formed disulfide occurs between Cys32 and Cys41 of the W42Q mutant. Interestingly, the same disulfide transfer does not occur between WT HγD molecules.
It is an open question as to what triggers the disulfide transfer. The authors demonstrate that the Cys108–Cys110 bond destabilizes the WT protein, so relief from this conformational strain may facilitate the reaction, but that does not explain why transfer only occurs to the mutant sequence. One possibility is that the Trp to Gln mutation adjacent to Cys41 lowers the redox potential of the Cys32–Cys41 disulfide, driving electron flow from the Cys32,Cys41 pair to the Cys108–Cys110 bond (Fig. 1). The authors also suggest conformational changes in the mutant could facilitate disulfide transfer and additionally promote aggregation of oxidized W42Q but not WT HγD. The impact of protein concentration has not been considered as the experiments were conducted at protein concentrations two orders of magnitude less than in the eye lens. Nevertheless, in their model, the authors suggest that a “hot potato” competition is in play with the oxidation of Cys108–Cys100 leading to a less stable W42Q HγD and predisposing it to aggregation (7). The model also implies that WT HγD can act like a catalyst, converting many molecules of mutant HγD. Prevention of HγD oxidation might block aggregation of the W42Q mutant. What, however, should we make of these results in the absence of mutations or pre-existing damage?
Figure 1.
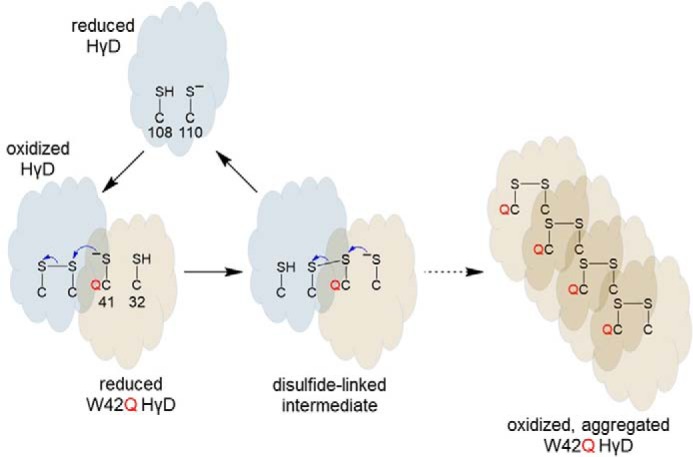
Model for disulfide transfer from oxidized HγD to reduced W42Q HγD. The Cys108–Cys100 and Cys32–Cys41 potential disulfides in HγD are shown. The Trp to Gln mutation adjacent to Cys41 is shown in red.
To us, these data lend themselves to a larger scale “hot potato” model in which β- and γ-crystallins are continually rubbing shoulders within a high-protein concentration, close-packed, disulfide-sharing, dynamic protein network. It is akin to the annual emperor penguin huddle in Antarctica, but on a completely different length and temperature scale! The oxidoreductase function provides the capability to resolve undesired contacts, i.e. disulfide bonds, by sharing them within the crystallin protein network as though they are at a never-ending “meet and greet” party. Deleterious electrons are either removed by appropriate sinks elsewhere or return so slowly that they do not interfere with normal function. As humans (and mice) age, however, disulfide formation shifts from intra- to intermolecular configurations, a trend that is strongly linked to cataractogenesis (2). For example, an experiment meant to mimic aging demonstrated that in an oxidized HγD containing an N-terminal methionine, Cys111 (equivalent to Cys110) forms an intermolecular disulfide with either Cys111 or Cys19 (i.e. Cys18) in an adjacent HγD chain (8). In these cases, the “hot potato” cannot be passed further and aggregation occurs.
It is possible that other γ-crystallins could have similar oxidoreductase-like properties as HγD, building the possible avenues by which disulfides could be dispersed. For example, although it is not expressed at the same high levels as HγD, γA-crystallin has the same cysteine arrangement. In human γS-crystallin (HγS), there is an N-terminal β-strand DCDCDC (Cys23,25,27) sequence. HγS also has the potential to form intermolecular disulfides as oxidation of Cys83 in HγS along with one of these N-terminal cysteines has been shown to encourage its dimerization with β-crystallins due to a conserved dimer interface (9). It will be fascinating to learn more about these proteins and, through them, aging compensation mechanisms more generally.
In conclusion, the Serebryany et al. (5) paper demonstrates an exciting new function to add to the repertoire for the crystallin family. It emphasizes further the importance of oxidative mechanisms in lens cataractogenesis. The article also provides new impetus to explore oxidation chemistry–based solutions to the global challenge of treating cataracts, particularly in parts of the world where advanced surgery and laser treatments are unavailable, but also where limited medical resources could be deployed more efficiently in favor of pharmacological solutions. Indeed, recent reports have shown the efficacy of redox-active lipoic acid to prevent presbyopia (10) and an antioxidant mixture to ameliorate cataractogenesis in animal models (11), providing new opportunities to augment the redox balance in the eye lens.
Footnotes
This work was supported by Fight for Sight UK Grants 1584/1585 and the LDLensRad project, which has received funding from the Euratom Research and Training Program 2014–2018 in the framework of the CONCERT grant agreement 662287 (to R. A. Q.) and National Health and Medical Research Council Research Fellowship 1110219 (to P. J. H.). The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
References
- 1. Stewart D. N., Lango J., Nambiar K. P., Falso M. J., FitzGerald P. G., Rocke D. M., Hammock B. D., and Buchholz B. A. (2013) Carbon turnover in the water-soluble protein of the adult human lens. Mol. Vis. 19, 463–475 [PMC free article] [PubMed] [Google Scholar]
- 2. Fan X., Monnier V. M., and Whitson J. (2017) Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 156, 103–111 10.1016/j.exer.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedrich M. G., Wang Z., Schey K. L., and Truscott R. J. W. (2018) DehydroalanylGly, a new post translational modification resulting from the breakdown of glutathione. Biochim. Biophys. Acta Gen. Subj. 1862, 907–913 10.1016/j.bbagen.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z., and Schey K. L. (2018) Quantification of thioether-linked glutathione modifications in human lens proteins. Exp. Eye Res. 175, 83–89 10.1016/j.exer.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serebryany E., Yu S., Trauger S. A., Budnik B., and Shakhnovich E. I. (2018) Dynamic disulfide exchange in a crystallin protein in the human eye lens promotes cataract-associated aggregation. J. Biol. Chem. 293, 17997–18009 10.1074/jbc.RA118.004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slingsby C., and Wistow G. J. (2014) Functions of crystallins in and out of lens: Roles in elongated and post-mitotic cells. Prog. Biophys. Mol. Biol. 115, 52–67 10.1016/j.pbiomolbio.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serebryany E., and King J. A. (2014) The βγ-crystallins: Native state stability and pathways to aggregation. Prog. Biophys. Mol. Biol. 115, 32–41 10.1016/j.pbiomolbio.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramkumar S., Fan X., Wang B., Yang S., and Monnier V. M. (2018) Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3595–3604 10.1016/j.bbadis.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagar V., Chaturvedi S. K., Schuck P., and Wistow G. (2017) Crystal structure of chicken γS-crystallin reveals lattice contacts with implications for function in the lens and the evolution of the βγ-crystallins. Structure 25, 1068–1078.e2 10.1016/j.str.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garner W. H., and Garner M. H. (2016) Protein disulfide levels and lens elasticity modulation: Applications for presbyopia. Invest. Ophthalmol. Vis. Sci. 57, 2851–2863 10.1167/iovs.15-18413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kador P. F., Guo C., Kawada H., Randazzo J., and Blessing K. (2014) Topical nutraceutical Optixcare EH ameliorates experimental ocular oxidative stress in rats. J. Ocul. Pharmacol. Ther. 30, 593–602 10.1089/jop.2014.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]


