Abstract
Newly discovered bacterial photoreceptors called CarH sense light by using 5′-deoxyadenosylcobalamin (AdoCbl). They repress their own expression and that of genes for carotenoid synthesis by binding in the dark to operator DNA as AdoCbl-bound tetramers, whose light-induced disassembly relieves repression. High-resolution structures of Thermus thermophilus CarHTt have provided snapshots of the dark and light states and have revealed a unique DNA-binding mode whereby only three of four DNA-binding domains contact an operator comprising three tandem direct repeats. To gain further insights into CarH photoreceptors and employing biochemical, spectroscopic, mutational, and computational analyses, here we investigated CarHBm from Bacillus megaterium. We found that apoCarHBm, unlike monomeric apoCarHTt, is an oligomeric molten globule that forms DNA-binding tetramers in the dark only upon AdoCbl binding, which requires a conserved W-X9-EH motif. Light relieved DNA binding by disrupting CarHBm tetramers to dimers, rather than to monomers as with CarHTt. CarHBm operators resembled that of CarHTt, but were larger by one repeat and overlapped with the −35 or −10 promoter elements. This design persisted in a six-repeat, multipartite operator we discovered upstream of a gene encoding an Spx global redox-response regulator whose photoregulated expression links photooxidative and general redox responses in B. megaterium. Interestingly, CarHBm recognized the smaller CarHTt operator, revealing an adaptability possibly related to the linker bridging the DNA- and AdoCbl-binding domains. Our findings highlight a remarkable plasticity in the mode of action of B12-based CarH photoreceptors, important for their biological functions and development as optogenetic tools.
Keywords: adenosylcobalamin (AdoCbl), photoreceptor, protein-DNA interaction, bacterial signal transduction, bacterial transcription, oxidative stress, Bacillus megaterium, CarH, light sensor, photoregulation, Spx
Introduction
Light is a crucial environmental factor that directly or indirectly signals diverse biological processes but can also cause damage of cellular components such as proteins, lipids, and DNA (1–6). To detect and respond to light, living organisms employ photoreceptor proteins, which are classified into distinct families based on their associated light-sensing chromophore (5, 7). A newly discovered and widespread family of bacterial photoreceptors uses as its chromophore one of the two biologically relevant forms of vitamin B12, 5′-deoxyadenosylcobalamin (AdoCbl). 4 In AdoCbl a 5′-deoxyadenosyl (5′-dAdo) group is covalently bound through its 5′-carbon to the cobalt atom in the B12 corrin ring, as the upper axial ligand. The prototype of this family, CarH, is a transcription factor that regulates light-dependent expression, typically of its own gene and of those for the synthesis of carotenoids (8–13), which quench singlet oxygen and other reactive oxygen species to mitigate photooxidative damage (2, 6). The discovery of CarH unveiled a novel biological facet of vitamin B12, that as the light-sensor of a photoreceptor (9, 11, 12), and enlarged the toolkit for optogenetics and related applications (14–17).
Details on the molecular mechanism of action of CarH photoreceptors emerged from studies of CarHMx and CarHTt from the Gram-negative bacteria Myxococcus xanthus and Thermus thermophilus, respectively (8–13, 18). Essentially, AdoCbl and light regulate the oligomeric state of CarH and its cooperative operator DNA-binding and repressor activity to directly control transcription (8, 11). In the dark, binding of AdoCbl to apoCarH, a monomer, leads to the formation of a tetramer, whose binding to its operator represses transcription; light (blue, green, UV) provokes photolysis of the AdoCbl Co-C bond and release of the 5′-dAdo group, causing tetramer disassembly to monomers and loss of operator binding, thus allowing transcription (Fig. S1A). Crystal structures of the free and DNA-bound CarHTt tetramer in the dark and the light-exposed monomer together with corroborative mutational and DNA-binding analyses, have provided atomic level insights into its architecture and mode of action (9). An autonomously folded ∼75-residue N-terminal winged-helix DNA-binding domain (DBD), of the type found in bacterial MerR family proteins, is connected by a flexible linker to an also autonomous, ∼210-residue C-terminal light-sensing oligomerization domain (Fig. S1B). The latter structurally mirrors the classical methionine synthase methylcobalamin (MeCbl)-binding module (19), consisting of a four-helix bundle and a Rossmann-fold domain, but is repurposed to bind AdoCbl, whose 5′-dAdo upper axial ligand is bulkier than the methyl group in MeCbl. Two AdoCbl-bound C-terminal domains pack as head-to-tail dimers, and two such dimers assemble as the dark-state tetramer, in which the four DBDs are splayed around the surface (Fig. S1C) (9). The consequence is a unique DNA-binding mode wherein the CarHTt tetramer uses three of its four MerR-type DBDs to contact three contiguous 11-bp (bp) direct repeats (DRs), one of which overlaps the −35 promoter element recognized by RNA polymerase (RNAP) holoenzyme with the primary σ factor σA (9). By contrast, typical MerR proteins are usually homodimers that use their DBDs to recognize operators with inverted DNA repeats (20–23). The inactive light-state monomer retains the overall dark CarHTt protomer structure. However, a large shift of its four-helix bundle subdomain (which caps the 5′-dAdo moiety of AdoCbl) relative to the Rossmann-fold provokes tetramer disassembly and loss of DNA binding (9). Intriguingly, the photochemistry underlying Co-C photolysis of CarHTt-bound AdoCbl, whose molecular basis remains elusive, differs from that known for this cofactor, free or enzyme-bound, and allows safe use of AdoCbl (by preventing release of its 5′-dAdo group as a radical) in light-dependent gene regulation (10, 24).
Whether this unusual AdoCbl- and light-dependent oligomerization, DNA binding, and photochemistry apply to other CarH homologs must be addressed for a broader understanding of the action and evolution of this new photoreceptor family. It is also relevant to their expanding use in optogenetics and other applications (14–16). Here, we report our analysis of the homolog from the Gram-positive bacterium Bacillus megaterium that we denote as CarHBm (Fig. S1D). It reveals several new insights on CarH photoreceptors, and significantly clarifies and extends previous data (25). We show that unlike the well-folded AdoCbl-bound forms, apoCarHBm is a molten globule oligomer that does not bind operator DNA, and that its AdoCbl-driven transition to a DNA-binding tetramer depends crucially on a W-X9-EH motif. CarHBm operators overlap with the −35 or −10 promoter regions and have the same design as the CarHTt operator, but are larger by one DR. Even so, CarHBm binds to the CarHTt operator in vitro. This DNA-binding flexibility may stem from the DNA- and AdoCbl-binding domains being bridged by a longer linker in CarHBm. We also identified a multipartite six-repeat operator upstream of a new CarHBm-regulated gene encoding an Spx family transcription regulator, typically involved in oxidative stress responses in the phylum Firmicutes (26, 27). Altogether, our findings indicate a plasticity, within otherwise conserved modes of AdoCbl binding, oligomerization, operator design, and DNA binding, which underlies the action of a B12-based CarH photoreceptor in light-dependent gene expression.
Results
CarHBm conserves crucial CarHTt DNA- and AdoCbl-binding motifs
CarHBm regulates light-induced carotenoid synthesis to produce a colony color change from white to pale yellow in B. megaterium strain QM B1551 (25). This also occurs with strain DSM 32 (Fig. 1A) whose CarHBm, examined in this study, is 99% identical in sequence to that in strain QM B1551 (Fig. S2A). Consistent with its role as a repressor of carotenoid synthesis, deleting carHBm in strain DSM 32 resulted in a strong yellow colony color in the dark or light (Fig. 1A). CarHBm and CarHTt have similar predicted secondary structures (Fig. S2B), which coincide well with the CarHTt crystal structure (Fig. S1, B–D). Sequence alignment (Fig. S1D) indicates that CarHBm retains: (a) almost every residue of the conserved R-X-WE-X-RY-X6-R-X5-R-X-Y motif (X: any amino acid) in the DBD involved in contacts with operator DNA in the structure of the CarHTt-DNA complex, hinting at a shared mode of DNA recognition; (b) the W-X9-EH-X32–39-E-X-H-XX-G-X41-S-X-T/V-X(22–30)-GG signature found in the C-terminal domain of putative AdoCbl-binding light-sensing modules (12). In CarHTt, the W-X9-EH motif caps the AdoCbl 5′-dAdo group and is crucial for binding to AdoCbl (9). The E-X-H-XX-G-X41-S-X-V-X(22–30)-GG corresponds to the canonical B12-binding motif first reported for MeCbl-dependent methionine synthase (19), where the conserved His replaces the dimethylbenzimidazole base as the lower axial cobalt ligand, in the so-called base off/His-on B12 binding mode (9). The presence of the AdoCbl-binding light-sensing signature in CarHBm suggests a similar role (CarAMx, a homolog that oligomerizes and acts independently of B12, lacks the Trp and four adjacent C-terminal residues; Fig. S1D) (11, 13). CarHTt C-terminal residues Arg-176 and Asp-201 are crucial for head-to-tail dimer formation, and Gly-160 and Gly-192 for tetramer assembly (9). Of these, CarHBm conserves only Gly-192 as Gly-205 (Fig. S1D), suggesting that despite conserved secondary structures, DNA- and AdoCbl-binding motifs, CarHBm and CarHTt may differ in dimer and tetramer assembly.
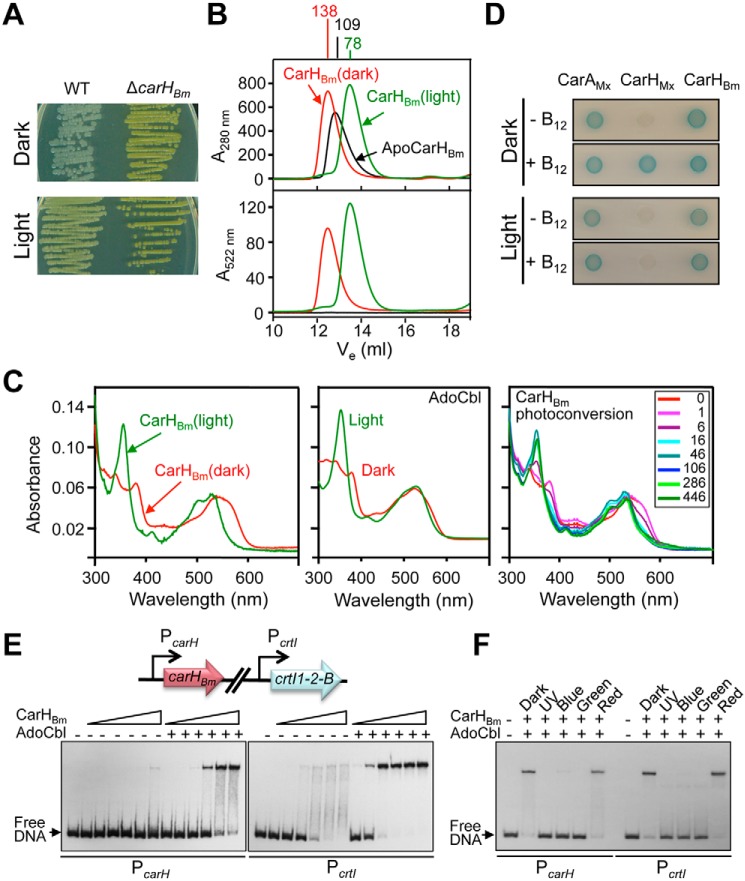
Figure 1.
Effects of B12 and light on CarHBm oligomerization and DNA binding. A, colony color of B. megaterium strain DSM 32 and its carHBm-deleted derivative grown in the dark and light. B, SEC profiles (off a Superdex200 analytical column) of CarHBm in the apo form or with AdoCbl present in the dark or after 5-min exposure to green light. Apparent molecular masses are indicated in kDa at the top. C, UV-visible absorbance spectra of the eluted AdoCbl-bound CarHBm peak (left panel) and free AdoCbl (middle panel) in the dark or after 5-min exposure to green light, and photoconversion of the CarHBm peak (right panel) eluted in the dark on stepwise illumination with green light for the times (s) indicated. D, two-hybrid analysis in E. coli with cells expressing T25 and T18 fusions of CarHBm and, as controls, CarHMx or CarAMx, spotted on plates containing X-Gal in the dark or exposed to light in the presence or absence of vitamin B12, as indicated. Blue color of the spot indicates interaction. E, representative EMSA gel for CarHBm binding to 170-bp DNA probes corresponding to the PcarH and PcrtI promoter regions (scheme on top) in the dark at increasing CarHBm concentrations (12.5, 25, 50, 100, 200, and 400 nm), with or without a 5-fold molar excess of AdoCbl present, as indicated. F, representative EMSA gel of the effects of UV (360 nm), blue (405 nm), green (520 nm), and red (660 nm) light on CarHBm binding to probes PcarH and PcrtI. Samples with PcarH or PcrtI and 400 or 50 nm CarHBm, respectively, plus 5-fold molar excess of AdoCbl, were incubated in the dark for 30 min and then irradiated for 5 min with the indicated light prior to EMSA.
CarHBm oligomerization, UV-visible absorbance, and DNA-binding
We assessed CarHBm oligomerization and its binding to specific cobalamins using size-exclusion chromatography (SEC). ApoCarHBm eluted with an apparent molecular mass of ∼109 kDa (Fig. 1B), compared with the 36.3-kDa monomer value calculated from sequence or determined by MS. With AdoCbl present, CarHBm eluted in the dark with a molecular mass of ∼138 kDa, corresponding to a tetramer, and when exposed to light with molecular mass of ∼78 kDa, expected for a dimer; and both forms absorbed at 522 nm, in addition to 280 nm, consistent with bound cobalamin (Cbl) (Fig. 1B). Protein and AdoCbl estimates in the eluted peaks indicated 1:1 stoichiometry. Our SEC data for AdoCbl-CarHBm (hereafter CarHBm, for simplicity) mirror those in an earlier report (25). However, we found that the UV-visible absorbance spectrum of CarHBm in the dark was distinct from that obtained upon light exposure (Fig. 1C, left panel), and not the same as reported previously (25). Moreover, the UV-visible spectra for CarHBm in the dark and light resembled, respectively, those known for intact AdoCbl and for its photolyzed form, free (Fig. 1C, middle panel) or bound to CarHTt (9–11). In addition, photoconversion of CarHBm from the dark to the light state was clearly apparent in the UV-visible spectral changes recorded upon stepwise illumination (Fig. 1C, right panel), with isosbestic points coincident with those reported for CarHTt photoconversion (10). The previous study (25) reported analytical ultracentrifugation data for dark-state CarHBm that indicated a substantial presence of a species whose sedimentation coefficient coincided with that for the light-exposed state. We therefore conclude that inadvertent photolysis of dark-state samples must account for the anomalous data reported in that study (25). Finally, we used bacterial two-hybrid analysis to check CarHBm oligomerization in vivo. Consistent with the SEC data, CarHBm self-interacted in vivo independently of light or B12 (like CarAMx), unlike CarHMx (Fig. 1D) or CarHTt (11).
Electrophoretic mobility shift assays (EMSA) using two 170-bp DNA probes, PcarH and PcrtI, for the two promoter regions containing the proposed CarHBm operators (25), indicated that CarHBm binds to both probes in the dark to yield a defined retarded band (Fig. 1E). On the other hand, apoCarHBm binds poorly, the diffuse smear with PcrtI at the higher protein concentrations hinting weak unstable binding. The data suggest higher affinity for the PcrtI probe, because ∼5-fold less protein was required to produce a retarded band of comparable intensity to that using PcarH. DNA binding was abolished in samples with CarHBm upon irradiation with near-UV, blue or green (but not red) light (Fig. 1F), indicating that the dimer formed in the light cannot bind DNA. (ApoCarHBm binds to MeCbl to form dimers in the dark that do not bind DNA, and remain as dimers in the light (Fig. S3).)
Thus, our data correct the previously reported anomalous UV-visible absorbance for the dark-state CarHBm, and confirm that AdoCbl is specifically required to transition, in the dark, from an apoCarHBm oligomer that does not bind DNA to a DNA-binding tetramer, which is disrupted by light to a dimer unable to bind DNA. Moreover, our data suggest a higher affinity of CarHBm for its operator at PcrtI than for that at PcarH.
ApoCarHBm is a molten globule
Structural differences may explain why apoCarHBm does not bind DNA, despite an oligomeric state close to that of the AdoCbl-bound tetramer. We therefore compared the apo and the dark and light CarHBm forms using circular dichroism (CD) and fluorescence spectroscopy. Far-UV CD spectra, with minima at 208 and 222 nm, indicated significant helical secondary structure for all three CarHBm forms (28). The mean molar residue ellipticity at 222 nm ([θ]222 in deg cm2 dmol−1), which correlates with helix content (29), was −15,300 (∼42% helix) for dark- or light-exposed CarHBm and somewhat lower (−11,600, ∼33% helix) for apoCarHBm (Fig. 2A). Near-UV CD spectra were similar for dark- and light-exposed CarHBm, with fine structures typical of native proteins with well-packed aromatic side chains, but were less well-defined and intense for the apo form suggesting that it is loosely packed (Fig. 2B) (30). CarHBm, with five Trp, yielded an intrinsic fluorescence emission maximum of (337 ± 2) nm in the apo and in both holo forms (Fig. 2C) that red-shifted to 356 nm for the denatured form in 6 m guanidinium chloride (GdmCl), with <20% drop in intensity relative to the holo forms. This indicates that the tryptophans are shielded from solvent in all three native forms (28).
Figure 2.
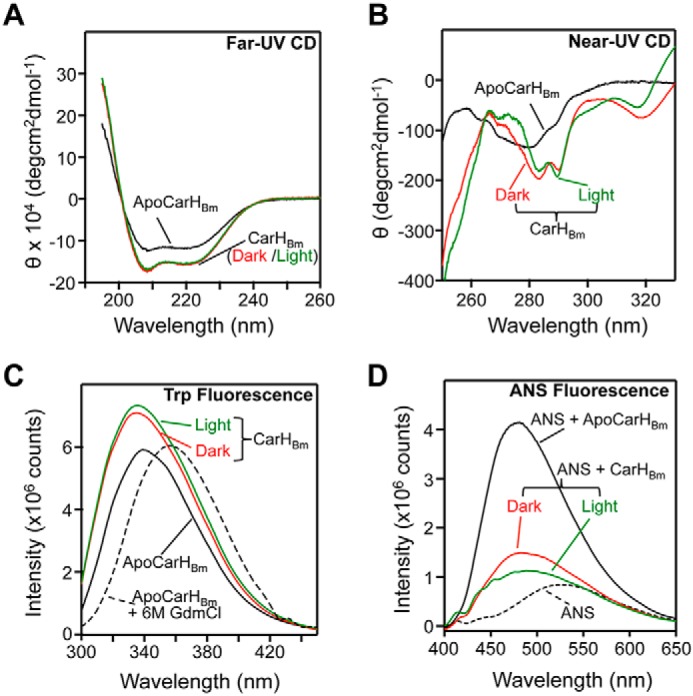
CD and fluorescence spectroscopy of CarHBm. A, far-UV CD spectra of 3–4 μm apoCarHBm (black), or CarHBm in the dark (red) or light-exposed (green). The same color code is used in B–D. B, near-UV CD spectra of apoCarHBm (16 μm), and dark or light-exposed CarHBm (27 μm). C, intrinsic Trp fluorescence emission spectra (excitation at 290 nm) of 2 μm apoCarHBm, dark- or light-exposed CarHBm, and apoCarHBm in 6 m GdmCl. D, ANS fluorescence emission spectra (400–650 nm, excitation at 370 nm) of 20 μm ANS alone, and in the presence of 2 μm apoCarHBm or CarHBm (dark- or light-exposed).
Metal or cofactor-free apoproteins often are molten globules, which are highly dynamic states with native-like secondary structure and a loosely packed, solvent-accessible protein core (30). Consequently, molten globules can interact with the indicator dye ANS (8-anilinonaphthalene-1-sulfonate) and produce a marked blue shift and enhancement in its fluorescence, low in aqueous solutions or in the presence of native proteins (31, 32). Dark- or light-exposed CarHBm blue-shifted the ANS fluorescence emission maximum from 527 to 482 and 489 nm, respectively, but with little enhancement in intensity (<30%; Fig. 2D). By contrast, apoCarHBm blue-shifted the ANS fluorescence emission to 481 nm and enhanced its intensity significantly (∼500%), consistent with apoCarHBm being a molten globule (Fig. 2D). In comparison, apoCarHTt, a monomer whose high-resolution structure is unknown, exhibited <60% ANS fluorescence enhancement relative to free ANS or to the dark or light holo forms (Fig. S4D), despite the otherwise analogous spectral (and hence, related structural) characteristics of CarHTt and CarHBm (Fig. S4, A–C). ApoCarHBm is thus an oligomeric molten globule, with a loosely folded structure distinct from that of the AdoCbl-bound form, which may explain its inability to bind operator DNA.
CarHBm requires its W-X9-EH motif for AdoCbl-binding, oligomerization, DNA-binding, and repressor activity in vivo
The 5′-dAdo group in dark-state CarHTt is in close proximity to the Trp, Glu, and His (Fig. 3A) (9) of a W-X9-EH motif that is highly conserved in CarH homologs, as mentioned earlier (12). The Trp side chain contacts one side of the 5′-dAdo ribose, which also forms a hydrogen bond with the Glu, and the His is involved in contacts at the dimer interface; and AdoCbl-binding, tetramer formation, and DNA-binding by CarHTt were impaired when the Trp or Glu was mutated to Ala, and abolished for such a mutation of the His (9). Thus far, mutational analysis of the W-X9-EH motif has been performed only with CarHTt, whose oligomerization varies from that of CarHBm. We therefore tested the effects in vitro and in vivo of mutating in the CarHBm motif (Fig. S1D) Trp-143, Glu-153, and His-154 to Ala, and Glu-153 to Asp (a conservative change that has not been analyzed in CarHTt). Far-UV CD spectra for the mutant proteins were similar to that for the WT whether or not AdoCbl was present, indicating that the mutations did not significantly affect CarHBm secondary structure (Fig. S5A). All four mutant proteins were molten globules in the apo form, like the WT protein, with blue-shifted and strongly enhanced ANS fluorescence (Fig. S5B). However, the presence of AdoCbl diminished ANS fluorescence only for the H154A mutant, suggesting that this mutant, but not the others, can transition from a molten globule to a fully folded form. Data from SEC were consistent. In the apo form, all four mutants eluted like the WT protein (Fig. 3B, left panels). Upon incubation with AdoCbl in the dark, the W143A, E153A, and E153D mutants continued to elute like the apo forms (molecular mass of ∼109 kDa), the lack of absorbance at 522 nm revealing negligible binding to AdoCbl; by contrast, the H154A mutant eluted as an AdoCbl-bound tetramer with molecular mass of ∼138 kDa, like WT CarHBm (Fig. 3B, right panels). Nonetheless, the H154A mutant was found to have a lower affinity for AdoCbl (KD ∼850 nm) than the WT protein (KD ∼210 nm, comparable with ∼250 nm for CarHTt) (10), as estimated from UV-visible absorbance titrations (Fig. S5C). Thus, AdoCbl binding was most affected by the Trp or Glu mutations in CarHBm, but by the His mutation in CarHTt (9), which may be related to apoCarHBm being a molten globule oligomer and apoCarHTt a monomer. Interestingly, the conservative E153D mutation also strongly impaired AdoCbl binding and transition to the proper tetramer fold, suggesting a strict requirement for Glu in the W-X9-EH motif.
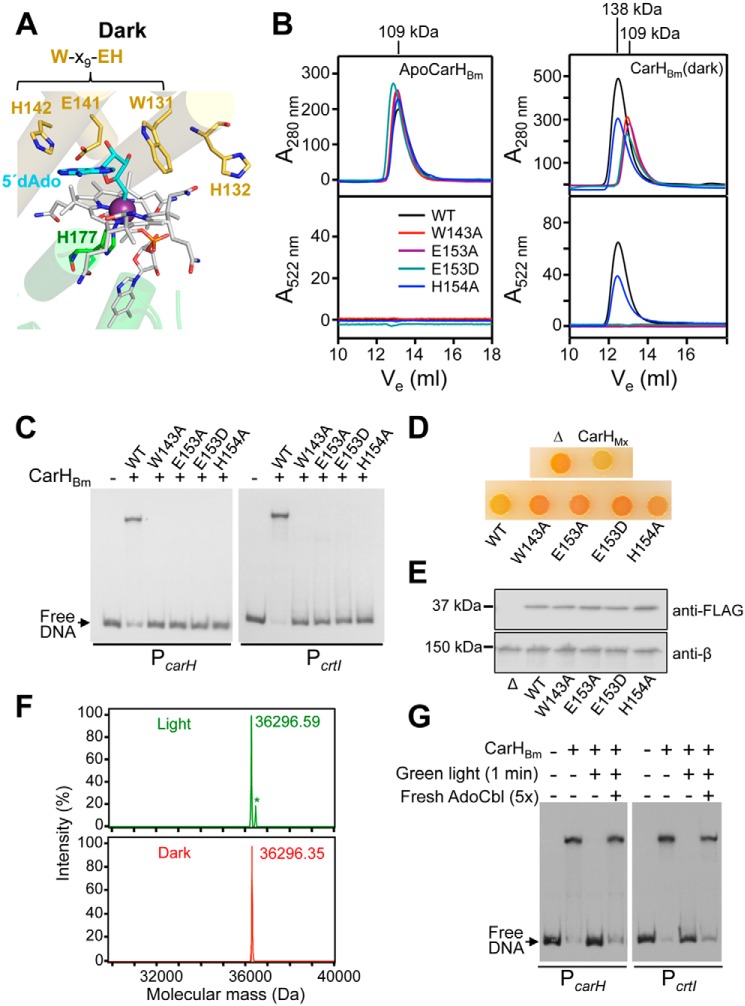
Figure 3.
Dependence of AdoCbl binding, oligomerization, DNA-binding and function on the conserved W-X9-EH motif in CarHBm. A, close-up of the B12-binding site in the dark-state CarHTt showing Trp-131, Glu-141, and His-142 of the W-X9-EH motif surrounding the 5′-dAdo group (cyan) of AdoCbl (gray with cobalt as a dark violet sphere), the lower axial His-177 cobalt ligand, and His-132, which shifts with the helix bundle in light-exposed CarHTt to provide the upper axial cobalt ligand. B, SEC elution traces for WT (WT), and the W143A, E153A, E153D, and H154A CarHBm mutants in the apo (left panels) and the dark AdoCbl-bound forms (right panels). Elution was tracked using absorbance at 280 (top panels) and 522 nm for cobalamin (bottom panels), with molecular masses of the peaks shown on the top and curve color code in the bottom left panel. C, representative EMSA for the binding of native CarHBm (WT) and its mutants (100 nm protein with 5-fold molar excess of AdoCbl) to 170-bp PcarH and PcrtI probes in the dark. D, color phenotype of M. xanthus MR2648 strain without (negative control, “Δ”) or with the gene encoding CBm (WT), its W143A, E153A, E153D, or H154A variants, or CarHMx (positive control) with an N-terminal FLAG tag under the control of a vanillate-inducible promoter. CTT-agar plates containing AdoCbl (1 μm) and vanillate (20 μm) were spotted with 10 μl of cells (grown in the dark to A550 = 0.7) and then incubated at 33 °C for 2 days in the dark. E, Western blotting of cell extracts of M. xanthus expressing WT and its variants (grown in the dark to A550 = 0.7 in CTT with 1 μm AdoCbl and 20 μm vanillate) probed with anti-FLAG antibodies (top) or, as loading control, anti-RNAP β antibodies (bottom). F, ESI-TOF mass spectra of CarHBm in the dark (bottom) and after exposure to green light (top). The same molecular mass for both forms indicates that a tightly bound Cbl adduct is not formed in the light, in contrast to CarHTt (9). The small peak with an asterisk is an unidentified impurity. G, EMSA showing that DNA binding is restored, after exposure of CarHBm (400 nm) to green light, by adding fresh AdoCbl in the dark.
In EMSA with PcarH and PcrtI, the characteristic retarded band produced in the dark and presence of AdoCbl by WT CarHBm was not observed with its W143A, E153A, or E153D mutants (Fig. 3C), consistent with these mutations impeding AdoCbl binding. The H154A mutant was also impaired in DNA binding, in line with its lower affinity for AdoCbl (Fig. 3C); and the mutants continued to bind to DNA poorly relative to WT even at higher proteins concentrations (Fig. S5D). These data therefore support the finding that AdoCbl-induced transition to the proper tetramer fold is necessary for DNA binding, and that this depends on the W-X9-EH motif. Because diminished operator binding by the mutants would reduce repressor activity, we analyzed the effects of the mutations on the regulatory action of CarHBm in vivo by performing complementation tests in M. xanthus, a heterologous host previously used for studies with CarHTt (11). The recipient strain employed (MR2648) exhibits an orange color in the dark (Fig. 3D) as a result of constitutive carotenoid synthesis, due to the lack of CarHMx and CarAMx (the B12-independent repressor of carotenogenesis). To allow control of AdoCbl supply, MR2648 also lacks PduO, the adenosyltransferase required to produce intracellular AdoCbl in M. xanthus (11). We generated MR2648-derived strains (Table S1) that express, under the control of the vanillate-inducible Pvan promoter (33), the Trp, Glu, or His mutant versions (all FLAG-tagged for immunoblot detection) in the context of a chimeric protein, CBm, which consists of the CarHBm C-terminal domain fused through its linker to the DBD of CarHMx. This was prompted by EMSA analysis showing that CarHBm binds less efficiently to the CarHMx operator than CBm (Fig. S5E). WT CBm, like CarHMx used as positive control, restored repression of carotenogenesis in the dark (on plates containing AdoCbl and the vanillate inducer) and thereby the yellow colony color of normal WT cells (Fig. 3D), which is due to a noncarotenoid pigment DKxanthene (34). By contrast, none of the four mutants was functional in vivo (Fig. 3D) despite being stably expressed, as confirmed in immunoblots with anti-FLAG antibodies (Fig. 3E). Altogether, these data demonstrate that the Trp, Glu, and His of the W-X9-EH motif in CarHBm are important for AdoCbl-dependent operator binding in vitro and repressor activity in vivo.
CarHBm lacks the ability of CarHTt to trap bound photolyzed AdoCbl via bis-His ligation
A notable feature in light-exposed CarHTt is that His-132, which flanks Trp-131 of the W-X9-EH motif, ends up as the upper cobalt axial ligand, and the resulting bis-His-ligated photolyzed Cbl cannot escape even under harsh experimental conditions (9, 10) (Fig. 3A). Bis-His formation may have roles in possible mechanisms to trap, recover, and reuse Cbl, and in CarHTt photochemistry (9, 10, 12, 24). However, His-132 is not conserved in all CarH homologs (12), including CarHBm, in which a Glu (Glu-144) replaces the His (Fig. S1D). Mass spectrometry of dark- and light-exposed CarHBm yielded the same molecular mass, in contrast to light-exposed CarHTt, whose mass exceeded that of the dark form by 1329 Da due to the tightly bis-His-ligated photolyzed Cbl (Fig. 3F) (9, 10). Also, fresh AdoCbl could replace the bound Cbl in light-exposed CarHBm and restore its DNA-binding (Fig. 3G), contrary to that observed for light-exposed CarHTt (9, 10). Thus, unlike CarHTt, CarHBm does not tightly retain the bound photolyzed AdoCbl.
CarHBm operators are larger but conserve the CarHTt operator design and binding mode
Structural and mutational analyses established that the CarHTt operator comprises three ∼11-bp tandem DRs, each with the sequence 5′-nnnnTnnACAn-3′ (n is any base) in the sense strand (9, 12) (Fig. S6A). In the central DR, the conserved TnnACA forms part of the −35 promoter element (TTGACA), which is occupied by one DBD of CarHTt to sterically block access to RNAP-σA and thereby prevent transcription initiation (9). The structure of the CarHTt-DNA complex revealed that the DNA recognition helix contacts the T of the TnnACA motif and the TGT complementary to ACA in the major groove, and mutating the T or AC in two DRs abolished DNA binding (9). In a previous report, a 28-bp imperfect palindrome with two 6-bp half-sites, separated by 16 bp, was proposed for both CarHBm operators (25). However, we could discern 11-bp tandem DRs with the characteristic TnnACA of the CarHTt operator at both CarHBm target promoters (12): four at PcarH (DR1-DR4, in the sense strand, with DR4 overlapping the −35 promoter region) and three at PcrtI (DR1–DR3, in the antisense strand, with DR3 overlapping the −10 promoter region) (Figs. S2C and S6A).
To test experimentally if the above DRs constitute the CarHBm operator, we performed DNase I, hydroxyl radical, and Exo III footprint analyses. At PcarH, CarHBm yielded a DNase I footprint of ∼50 bp precisely matching the span of DR1–DR4 (Fig. 4, A and B). Interestingly, the footprint at PcrtI was also ∼50 bp, spanning the region from DR1 to DR3 plus an additional ∼10 bp segment downstream of DR3 that was footprinted more tenuously. This additional segment has TnnTTA instead of TnnACA, and hence can be considered a pseudorepeat that we denote dr4 (Fig. 4, A and B). The presence of four DNase I-hypersensitive sites (typically indicative of local bends toward the major groove (35)) on each probe suggests that CarHBm induces DNA bending upon binding (Fig. 4, A and B). Because the hypersensitive sites occur on different strands, antisense at PcarH but sense at PcrtI, CarHBm likely binds to opposite sides of the DNA at the two operators, consistent with the DRs being oriented oppositely at the two sites (Fig. 4B). In the crystal structure, CarHTt binds to one face of the DNA using only three of its four DBDs; accordingly, hydroxyl radical footprinting revealed on each strand three evenly spaced protected tracts (∼4-bp each), which correlated with where the “wings” of the DBDs contact the minor groove (9). CarHBm binding to PcarH or PcrtI produced on both strands four hydroxyl radical footprints (∼3-bp each; Fig. 4, A and B) evenly distributed at intervals similar to those observed for CarHTt at its operator (9). This suggests that CarHBm conserves the CarHTt operator design and DNA-binding mode but binds to larger, four-repeat operators presumably using all four of its DBDs. This inference was further supported by Exo III footprinting (Fig. 4C). CarHBm binding to PcarH blocked Exo III progress at about positions −19 and −71 on the sense and antisense strands, respectively (Fig. 4, B and C; numbers relative to the transcription start site, TSS), which coincide with the limits of the DNase I footprint (Fig. 4B). At PcrtI, CarHBm arrested Exo III at about position +24 in the sense strand, and at about positions −28 (weakly) and −19 (strongly) in the antisense strand (Fig. 4, B and C), again consistent with the ends of the DNase I footprint. That Exo III can nibble into the segment from position −28 to −19 at PcrtI, corresponding to dr4, suggests that CarHBm binds weakly to this pseudorepeat.
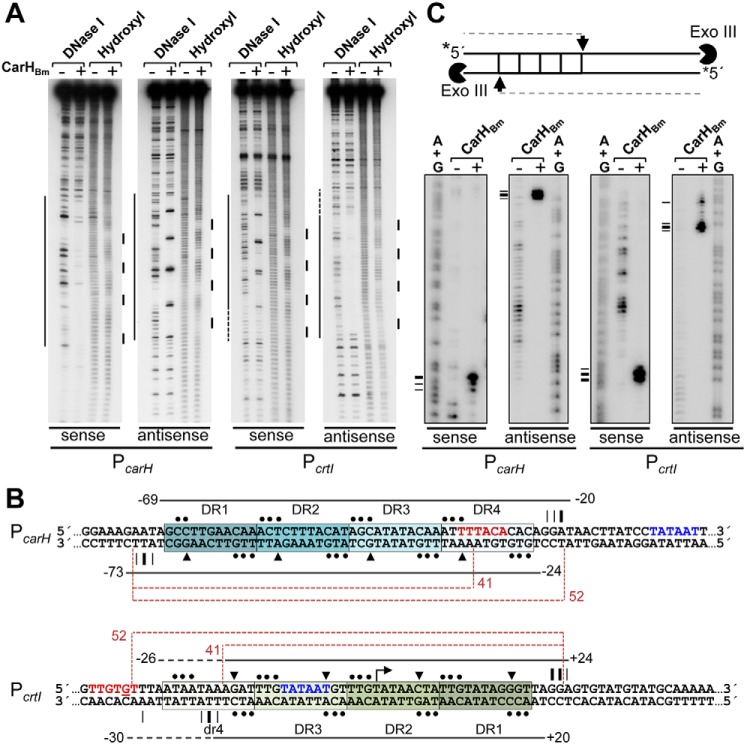
Figure 4.
CarHBm footprints at the PcarH and PcrtI promoter regions. A, representative DNase I and hydroxyl radical footprinting in the dark on the sense and antisense strands of a 170-bp PcarH or PcrtI probe without or with CarHBm (400 nm with 5-fold molar excess of AdoCbl) present. Footprinted regions, mapped using (A+G) chemical sequencing run in parallel, are indicated by lines on the left (DNase I) and right (hydroxyl radical). B, DNA sequence of the segment containing the CarHBm-binding site at PcarH and PcrtI, with footprint data depicted for each strand (sense, above; antisense, below). Horizontal lines extend over regions protected against DNase I (dashes indicate weak protection), with limits numbered relative to the TSS. Triangles point to DNase I-hypersensitive sites, and dots to the sites protected from hydroxyl radical attack. Positions at which Exo III advance is arrested, from C, are indicated by vertical lines (thicker for stronger arrest). Each DR is boxed and shaded; repeats at PcrtI are oriented opposite to transcription (dr4 in lowercase indicates a pseudorepeat described in the text). The −35 (in red) and −10 (in blue) promoter elements are indicated. Dashed brown lines indicate the span of the 52- and 41-bp probes used in Fig. 5. C, Exo III footprint data for CarHBm binding to PcarH or PcrtI. Scheme on top shows the position of Exo III arrest (arrow) and product size from a given strand (32P-labeled at the 5′-end) that would be expected if the protein binds to four DRs (squares). Radiolabeled probe, free or preincubated in the dark with CarHBm (400 nm plus 5-fold molar excess of AdoCbl), was treated with Exo III and analyzed as described under supporting data. Horizontal dashes on the left point to positions of Exo III arrest (summarized in B) mapped using the corresponding A+G sequence ladder.
EMSA using 52-bp PcarH and PcrtI DNA probes corresponding to the DNase I/Exo III footprints (the four repeats plus the 4 bp flanking each end; Fig. 4B) confirmed that this segment is both necessary and sufficient for CarHBm binding (Fig. 5A, Fig. S2D). However, DNA binding was abolished on truncating DR4 at PcarH and diminished when dr4 at PcrtI was truncated, consistent with dr4 being a weaker affinity site for CarHBm (Figs. 4B and 5A). Binding to both 52-bp probes was cooperative (Hill coefficients n >1) with dissociation constants KD of (96 ± 3) nm for PcarH and (26 ± 1) nm for PcrtI (Fig. 5B, Fig. S6B). Thus, CarHBm binds with higher affinity to its operator at PcrtI, despite weaker binding at dr4. By comparison, CarHTt can bind to a 41-bp probe corresponding to its three-repeat operator, and its KD of (67 ± 2) nm (9) falls in the range observed for CarHBm at its two operators.
Figure 5.
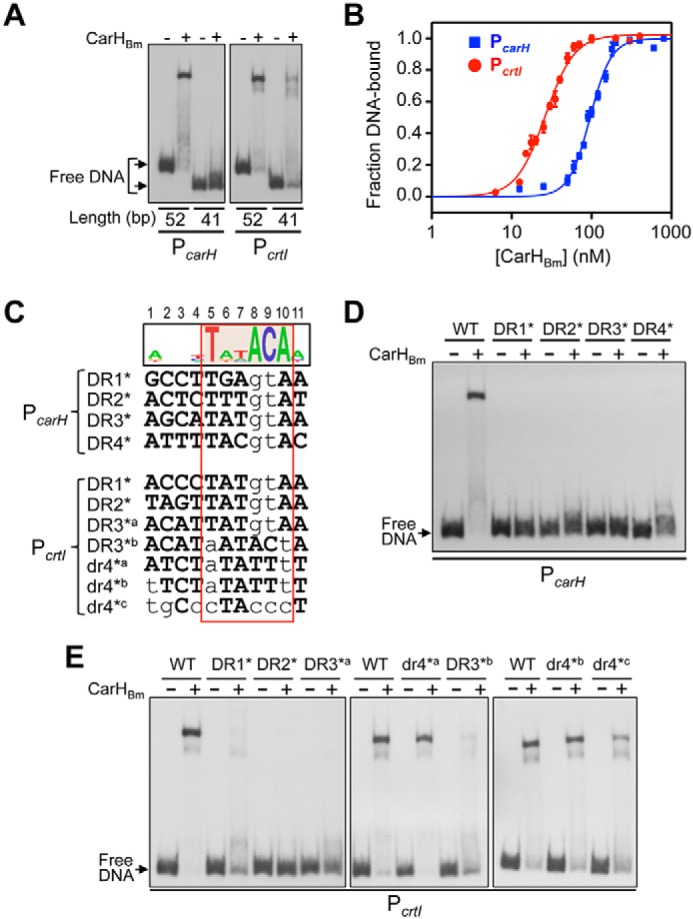
Analysis of CarHBm binding to its operators at PcarH and PcrtI. A, EMSA of CarHBm binding to 52- and 41-bp PcarH and PcrtI probes corresponding, respectively, to all four repeats or only the first three (DR1 to DR3; Fig. 4B). CarHBm was at 400 nm with PcarH and at 50 nm with PcrtI, and AdoCbl was at a 5-fold molar excess. B, CarHBm binding affinities to the 52-bp PcarH (blue) and PcrtI (red) probes from EMSA titrations (representative EMSA in Fig. S6B). Lines are fits of the data to the Hill equation with KD, the dissociation constant, and Hill coefficient being, respectively, (96 ± 3) and (3.3 ± 0.3) nm for PcarH, and (26 ± 1) and (2.39 ± 0.2) nm for PcrtI. Mean values and standard errors from three EMSA are shown. C, CarHBm operator repeats at PcarH and PcrtI aligned with mutations (lowercase) tested in D and E. Sequence logo on the top (with positions numbered) is based on the four DRs of PcarH and DR1 to DR3 of PcrtI. The TnnACA motif is shaded orange in the logo and outlined by the box in red. D, EMSA of CarHBm binding to 52-bp PcarH mutant DNA probes. E, EMSA of CarHBm binding to 52-bp PcrtI mutant DNA probes. CarHBm in D and E was 400 nm and 50 mm, respectively, with a 5-fold molar excess of AdoCbl.
To test the role of interactions with individual bases, we performed EMSA with probes bearing specific mutations. Mutating AC in any one of the PcarH TnnACA motifs was sufficient to abrogate CarHBm binding, emphasizing the importance of this AC and of each of the four DRs at this operator for binding (Fig. 5, C and D). Likewise, mutating AC of DR1, DR2, or DR3 at PcrtI markedly impaired CarHBm binding (Fig. 5, C and E). Because AC is not conserved in dr4 at PcrtI, we mutated the first T and the last A of its TnnTTA motif (dr4*a; Fig. 5C). But, surprisingly, this had no effect, unlike the equivalent mutation in DR3 (DR3*b), which did diminish CarHBm binding (Fig. 5E); nor did an additional change of the A at the first position of dr4 to T (dr4*b; Fig. 5, C and E). Because dr4 is the most AT-rich of the PcarH or PcrtI repeats, we also tested the effect of modifying it to be more GC-rich (dr4*c; note that this GC-rich segment corresponds to that immediately downstream of the three-repeat CarHTt operator, as will be discussed below). This decreased CarHBm binding somewhat (Fig. 5E), suggesting that the contribution of dr4 may rest on its AT-rich nature.
In sum, the above data indicate that CarHBm and CarHTt share the same basic operator design, comprising tandem 11-bp DRs with a TnnACA motif, and DNA-binding mode. However, both CarHBm operators are larger than that of CarHTt by one repeat or pseudorepeat.
CarHBm recognition of the smaller CarHTt operator suggests flexible DNA binding
CarHBm operators have four DRs, compared with three DRs for CarHTt (9). Yet, EMSA revealed that CarHBm binds to PcarH(Tt), and CarHTt to both PcarH and PcrtI (Fig. 6A). We analyzed this further by DNase I and Exo III footprinting, using PcarH(Tt) and PcarH. In contrast to its ∼50-bp DNase I footprint at PcarH, CarHBm yielded an ∼41-bp footprint at PcarH(Tt), similar to that produced by CarHTt (9) (Fig. 6, B and C); Exo III footprints were also very similar at PcarH(Tt) and matched the limits of the DNase I footprints (Fig. 6, C and D). Thus, CarHBm can recognize the CarHTt operator even though its natural operators are larger by one DR or pseudorepeat. Either CarHBm, like CarHTt, uses only three of its four DBDs to bind to the CarHTt operator or, alternatively, the fourth DBD interacts with an adjacent segment nonspecifically, eluding detection by footprinting. In favor of the first possibility is that the GC-rich segment immediately downstream of DR3 at the CarHTt operator does not appear to contribute to CarHBm binding, because using it to replace dr4 at PcrtI (mutant dr4*c; Fig. 5E) had the same effect as deleting dr4 (Fig. 5A).
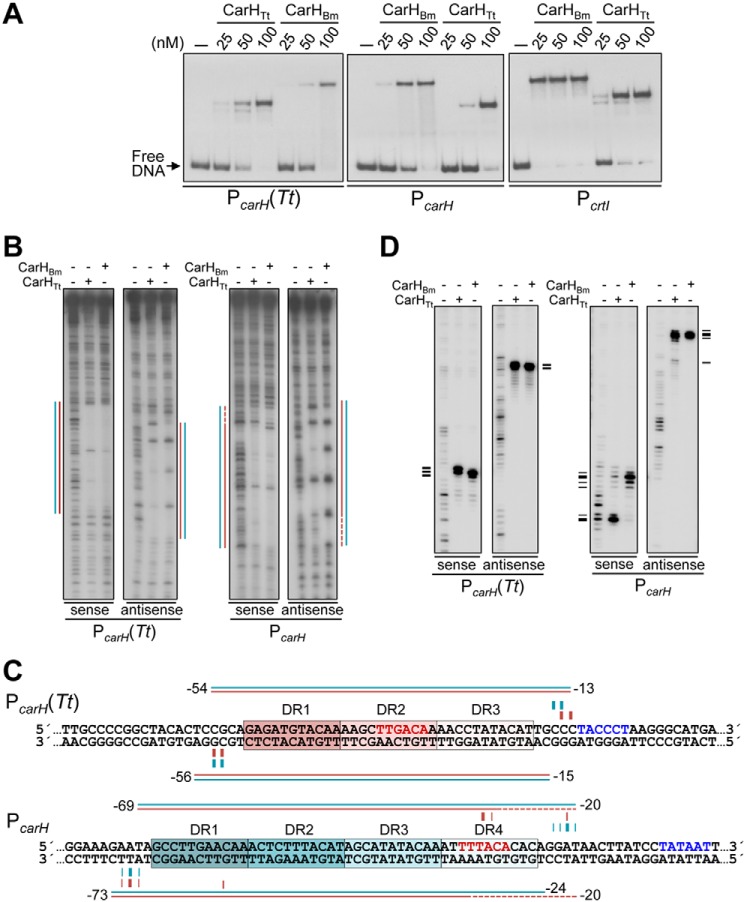
Figure 6.
Binding of CarHBm to the CarHTt operator and CarHTt to the CarHBm operators. A, EMSA for CarHBm and CarHTt binding in the dark to the 170-bp operator probes PcarH(Tt), PcarH, and PcrtI at the indicated protein concentrations and with AdoCbl at a 5-fold molar excess. Slower mobility of the DNA complex for CarHBm may be due to size, charge, and shape differences from CarHTt. B, DNase I footprints at PcarH (Tt) and PcarH generated by the binding of CarHBm or CarHTt (400 nm protein with 5-fold molar excess of AdoCbl present in the dark). Lines indicating the footprint for CarHBm (cyan) and CarHTt (pink) were mapped using (A+G) chemical sequence ladder of the corresponding strand. C, sequences with footprint data summarized (cyan for CarHBm, pink for CarHTt) above and below the corresponding strands, with horizontal lines spanning the DNase I footprint (dashes indicating weak protection) and vertical lines at positions with Exo III blocked (thicker for stronger arrest). The −35 and −10 promoter elements are in red and blue, respectively, and each DR is boxed and shaded. D, Exo III footprint data for the binding of CarHBm or CarHTt (400 nm, 5-fold molar excess of AdoCbl) to PcarH(Tt) and PcarH. Horizontal dashes on the left align with the positions of Exo III arrest mapped using the corresponding A+G sequence ladder and summarized in C.
CarHTt binding at PcarH yielded a clear DNase I footprint covering the ∼41-bp segment from DR1 to DR3, and a faintly protected region extending over DR4 (Fig. 6, B and C). Accordingly, CarHTt produced strong Exo III arrests at the limits of the ∼41-bp DNase I footprint, and weak ones delimiting the stretch from DR2 to DR4 (Fig. 6, C and D). This suggests that CarHTt continues to bind to three DRs even when a fourth is available and, interestingly, with a marked preference for the three upstream DRs, DR1 to DR3. To further reinforce these conclusions, we designed an artificial operator with four DRs derived from PcarH(Tt), PcarH(Tt)4r (Fig. S7A). EMSA showed that both CarHBm and CarHTt bind to PcarH(Tt)4r, whereas DNase I and Exo III footprinting results closely mirrored those obtained with PcarH (Fig. S7, B–D).
Taken together, the data suggest that CarHBm binds to four DRs when available but can also recognize just three, whereas CarHTt binds primarily to three DRs even when a fourth is present, with a preference for the three upstream. The latter suggests positioning determinants for binding, whose nature remains to be established.
Interdomain linker effects on operator DNA binding
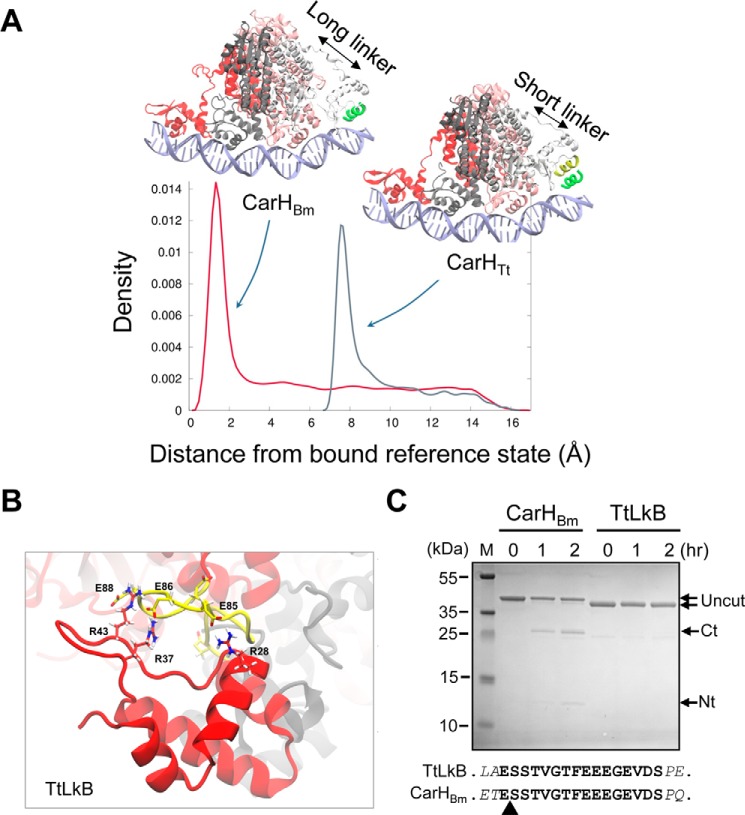
Why CarHTt binds only to three repeats at a time, whereas CarHBm can bind more flexibly to three or four repeats may be related to its longer linker between the DNA- and the AdoCbl-binding domains. Compared with the 4-residue CarHTt linker (QEVR, residues 76–79), sequence alignment and the available CarHTt structures (Fig. S1D) indicate a 16-residue linker segment (ESSTVGTFEEEGEVDS, residues 76–91) in CarHBm. Both linkers are predicted as unstructured (Fig. S2B), as was observed in the CarHTt structures (9). To check for linker effects we performed steered MD simulations (36) of the CarHTt- and CarHBm-DNA complexes (supporting data). For this, CarHBm and its PcarH operator DNA were modeled to preserve the mode of interaction between the DBDs and DNA, as well as the DNA curvature observed in the CarHTt-DNA structure (PDB code 5C8E). In the steered MD simulations, exploration of the configurational space for the DNA-bound and unbound states of the fourth DBD binding to DR4 indicated that CarHBm can bind DR4 through its fourth DBD as a consequence of its longer linker, as manifested by the peak in density close to the reference bound state (∼1 Å; Fig. 7A). By contrast, CarHTt was unable to bind DR4 due to its shorter linker, with configurations displaying maximum density far from the reference bound state (∼8 Å; Fig. 7A).
Figure 7.
Linker effects on CarHBm, CarHTt, and TtLkB. A, density plots showing the probability to find the fourth DBD in the configurational space between 0 and 15 Å of root mean square deviation relative to the reference bound state defined by the DNA recognition helix (green) for CarHTt and CarHBm, as indicated. B, snapshot of the last 50 ns of the 500-ns MD simulation of TtLkB showing H-bond interactions formed between the DBD Arg residues (red) and the Glu residues in the interdomain linker (yellow), as indicated. C, CarHBm and TtLkB digested with Glu-C at the times (in hours) indicated. The uncut protein (TtLkB, 34.3 kDa; CarHBm, 36.3 kDa) and the GluC cleavage products (Ct corresponds to the C-terminal domain of CarHBm (∼25 kDa) and Nt to the His-tagged N-terminal domain (∼12 kDa)); M, size markers. The sequence of the CarHBm linker (boldface) with the two flanking residues (italics) in CarHBm and TtLkB is shown below; the arrowhead points to the GluC-cleavage site in the linker in CarHBm identified by N-terminal sequencing.
To experimentally test linker effects on DNA binding, we generated CarHBm with 12 C-terminal residues of its linker truncated so as to be of the same length as in CarHTt, or with its linker swapped for that of CarHTt, and CarHTt with its linker swapped for that of CarHBm. Both CarHBm variants with four-residue linkers tended to precipitate. This precluded their further analysis but nonetheless indicated that the linker is not a passive tether but can affect CarHBm behavior in solution. On the other hand, CarHTt with its linker swapped for that of CarHBm (hereafter denoted TtLkB) was well behaved in solution and, like native CarHTt, formed AdoCbl-bound tetramers in the dark and monomers in the light or in the apo form (Fig. S8, A–D). We therefore tested its DNA binding to PcarH(Tt) and PcarH. Strikingly, no retarded band was detected in the EMSA for TtLkB binding to either probe, in contrast to CarHTt (Fig. S8E) or CarHBm (Fig. 6A).
On probing linker effects by unrestrained MD simulations of the AdoCbl-bound TtLkB and CarHBm systems, we observed that the DBD residues Arg-28, Arg-37, and Arg-43, which are known to interact with DNA (9), were sequestered by the highly acidic linker (calculated pI = 3.4) in the TtLkB system (Fig. 7B, Fig. S9), and could thereby prevent binding to DNA. By contrast, these sequestering interactions were not observed with the CarHBm system (Fig. S9). Moreover, comparison of the H-bonds formed between the interdomain linker and the rest of the protein during the simulation indicated, on average, 57 ± 5 H-bonds (of which 8 ± 1 were salt bridges) for CarHBm, but a much higher 75 ± 6 H-bond interactions (17 ± 2 salt bridges) for TtLkB. This may rationalize, at least in part, why the linker in CarHBm does not interfere with DNA binding, whereas the same linker in TtLkB prevents DNA binding possibly by interacting strongly with the DBD. Such charge interactions would be expected to shield the linker in TtLkB, more than in CarHBm, from proteolysis. We tested this by treating AdoCbl-bound TtLkB and CarHBm with Staphylococcus aureus endoproteinase GluC, which preferentially cleaves accessible peptide bonds C-terminal to Glu residues. CarHBm and TtLkB have many Glu residues (29 and 34, respectively) distributed along the sequence (including those in the linker), but structural constraints may protect most of them from GluC cleavage. Consistent with interactions protecting the linker in TtLkB, no cleavage was apparent on GluC treatment of TtLkB; in contrast, GluC cleaved at the linker in CarHBm to yield detectable levels of the C-terminal AdoCbl-binding and N-terminal DNA-binding domains, as confirmed by N-terminal sequencing (Fig. 7C). Altogether, these data indicate that linker length, composition and the context in which it occurs are important determinants of the properties of CarH photoreceptors and, importantly, of their binding to DNA.
A multipartite CarHBm operator for photoregulated expression of an Spx global redox-response transcription factor
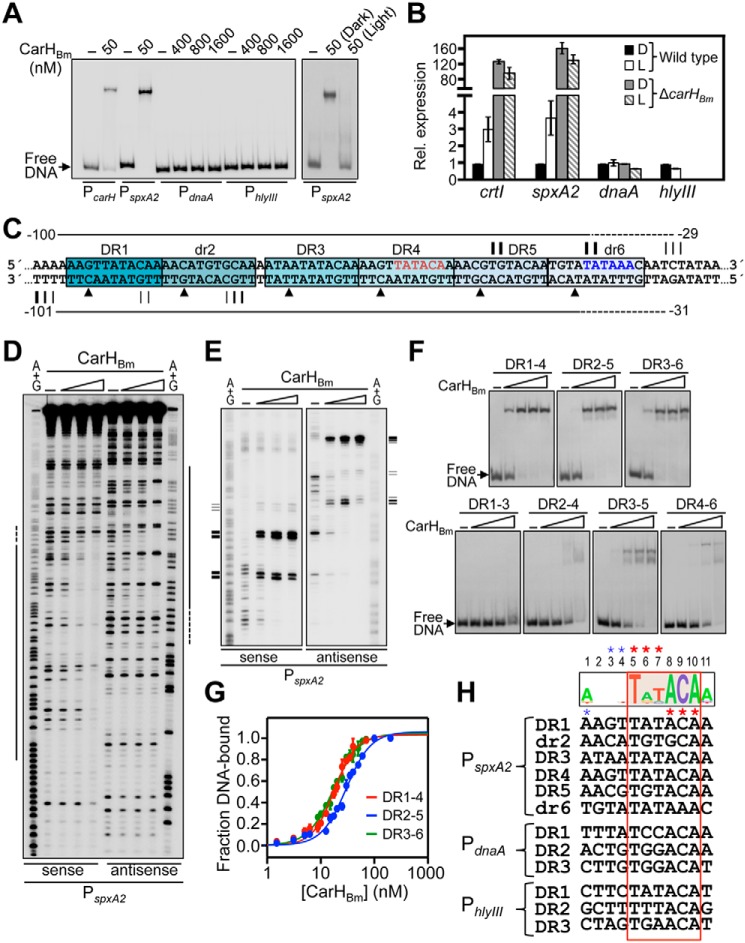
The data above showed that CarHBm can recognize three or four 11-bp tandem DRs, each with a conserved TnnACA motif. We therefore scanned the B. megaterium genome in silico for sites corresponding to three tandem repeats of the sequence 5′-nnnnTnnACAn-3′. This yielded, besides the two known sites at PcarH and PcrtI, three new hits (Fig. S10A). One of these hits was 43 bp upstream of an isolated gene encoding an Spx family global RNAP-binding transcription regulator homolog, which is highly conserved in the phylum Firmicutes and is implicated in oxidative and other stress responses (26, 27). We refer to this gene as spxA2 to distinguish it from the paralog, also annotated spxA, that we denote as spxA1 and whose product is 86% identical in sequence to Bacillus subtilis Spx (Fig. S10B). The second new hit was 193 bp upstream of the gene for replication initiator protein DnaA, and the third was 208 bp upstream of a gene for a putative hemolysin III family protein and 161 bp upstream of a gene for a putative helix-turn-helix protein transcribed in the opposite direction. We first tested if the new hits were genuine CarHBm-binding sites by EMSA using 170-bp DNA probes (PspxA2, PdnaA, and PhlyIII). Whereas PspxA2 was completely retarded by 50 nm CarHBm and light disrupted the binding, no binding was detected to the other two probes even at CarHBm concentrations as high as 1.6 μm (Fig. 8A). These results suggested that, of the three new hits, only PspxA2 may be subject to light-dependent regulation by CarHBm. Consistent with this, quantitative RT-PCR (qRT-PCR) analysis revealed that expression of spxA2, but not of dnaA or hlyIII, was induced by light to levels comparable with the positive control crtI (Fig. 8B). Moreover, in a carHBm-deleted mutant, spxA2 exhibited high-level expression in the dark or light, like crtI and unlike dnaA (Fig. 8B). This further supports that CarHBm mediates the photoregulated expression of spxA2. These results thus validated the hit at PspxA2. On the other hand, they also indicated that three tandem 5′-nnnnTnnACAn-3′ repeats as query is not sufficiently stringent to identify CarHBm binding sites.
Figure 8.
CarHBm binding to a multipartite operator upstream of photoregulated spxA2. A, representative EMSA for CarHBm binding to 170-bp probes PspxA2, PdnaA, and PhlyIII. Binding to PcarH is shown for comparison. The effect of green light on binding at PspxA2 is shown in the right panel. Protein concentrations indicated on top have AdoCbl at a 5-fold molar excess. B, qRT-PCR analysis of crtI (positive control), spxA2, dnaA, and hlyIII expression in WT and carHBm-deleted B. megaterium strains grown in the dark (D) and light (L), as indicated. Mean ± S.E. of three independent measurements are shown. C, sequence of the CarHBm-binding site at PspxA2 summarizing footprint data on each strand from D and E. Horizontal lines span the DNase I footprint (dashes for weak footprint) and triangles point to hypersensitive sites (from D). Vertical lines align with positions at which CarHBm blocks Exo III progress, with thicker lines for stronger arrest (from E). Tentative −35 (red) and −10 (blue) promoter elements are indicated. Each DR is boxed and shaded. D, DNase I footprints in the dark on the sense and antisense strands of PspxA2 without and with CarHBm (50, 100, and 400 nm and a 5-fold molar excess of AdoCbl). Lines on the side correspond to the footprint mapped using the respective (A+G) chemical sequencing ladder. E, Exo III footprint data for the binding of CarHBm (concentrations as in D) to PspxA2. Horizontal lines on the side mark the positions of Exo III arrest (thicker lines for stronger arrest) mapped using the corresponding A+G sequence ladder, as summarized in C. F, EMSA for CarHBm binding to PspxA2 probes with four (top gels) or three (bottom gels) repeats. Each probe corresponds to the segment extending over the repeats indicated, as numbered, plus the 4-bp flanking each end. Increasing CarHBm concentrations (25, 50, 100, and 400 nm) and a 5-fold molar excess of AdoCbl were used. G, EMSA titration data for CarHBm-binding to the four-repeat probes in F. Lines are fits of the data to the Hill equation. KD and Hill coefficients are, respectively (17.7 ± 0.8) and (2.14 ± 0.15) nm for DR1–4; (29.8 ± 1.8) and (1.9 ± 0.2) nm for DR2–5; and (17.3 ± 0.7) and (1.8 ± 0.1) nm for DR3–6. Mean ± S.E. of three experiments are shown. H, alignment of repeat hits at PspxA2, PdnaA, and PhlyIII, with the sequence logo based on the PcarH, PcrtI, and PspxA2 repeats indicated on top; the TnnACA motif is boxed in red, with positions numbered on top. Asterisks indicate major groove (red) and minor groove (blue) contacts, in the sense and antisense strands, in the CarHTt-DNA structure (9).
DNase I footprinting analysis of CarHBm binding to PspxA2 yielded, surprisingly, an ∼70 bp footprint (with six hypersensitive sites in the antisense strand), about twice the expected size for a three-repeat site, and also larger than the footprints observed at PcarH and PcrtI (Fig. 8, C and D). Besides the three tandem DRs identified in the genome-wide search (DR3, DR4, DR5; Fig. 8C, Fig. S10A), we discerned in the footprint an additional DR (DR1) and a pseudorepeat (dr2) upstream of DR3, and another pseudorepeat (dr6) downstream of DR5. DR1 is identical to DR4; dr2, spaced 1-bp away from DR3, resembles DR5 except at positions 4 and, importantly, 8, where G replaces the first A of the TnnACA motif; and dr6, footprinted more weakly, has A instead of C of the motif (Fig. 8, C and H). Because the four DBDs of a CarHBm tetramer can bind at most four DRs at a time, we investigated further how CarHBm recognizes the composite site at PspxA2. First, CarHBm blocked Exo III (Fig. 8, C and E) in the sense strand within DR5 (strongly) and dr6 (strongly), and downstream of dr6 (weakly); and in the antisense strand upstream of DR1 (strongly), and within DR1 (weakly) and dr2 (modestly). This suggested that CarHBm can bind to three four-repeat segments at PspxA2, from DR1 to DR4, dr2 to DR5, and DR3 to dr6. Consistent with this, CarHBm could bind well to probes corresponding to each of these four-repeat segments (Fig. 8F, top gels), and with comparable affinities (Fig. 8G): KD of (17.9 ± 0.8) and (29.8 ± 1.8) nm, respectively, for DR1–4 and DR2–5, despite the 1-bp spacing between dr2 and DR3 in both 53-bp probes, and of (17.3 ± 0.7) nm for the 52-bp probe DR3–6. As expected, light disrupted this binding (Fig. S10C). By contrast, binding was weaker with probes having only three repeats, being most evident with the 42-bp probes DR1–3, DR2–4, and DR4–6, which have two DRs and one pseudorepeat, and less so with the 41-bp DR3–5 probe, with three DRs (Fig. 8F, bottom gels). The CarHBm operator at PspxA2 thus appears to be multipartite, with three overlapping binding sites, each one made up of three DRs and one pseudorepeat. Notably, the change in register expected from the 1-bp spacing between two contiguous repeats in two of the sites does not impair CarHBm binding. Importantly, all three sites would include the putative −35 promoter element (assigned tentatively based on comparison with PcarH and PcrtI), with DR3-dr6 also including the putative −10 promoter element (Fig. 8C).
As with the three sites at PspxA2, we could identify a pseudorepeat in tandem downstream of DR3 at PdnaA, and a DR separated by 1 bp downstream of DR3 at PhlyIII (Fig. S10A). Yet, even at high CarHBm concentrations no binding was detected at PdnaA or PhlyIII, as noted before (Fig. 8A), suggesting that this cannot be attributed to lack of a fourth DR or pseudorepeat. Hence, we reasoned that binding or otherwise to CarHBm depends on additional sequence information within the DRs, besides the conserved TnnACA motif. In other words, the less conserved bases also contribute, as was observed with CarHTt in the structure of its complex with DNA (9). Aligning the DRs at PspxA2 with those at PcarH and PcrtI yielded AnnTATACAA (less conserved bases not in bold), as a more refined consensus repeat sequence for CarHBm recognition (Fig. 8H). The deviations observed at these less conserved positions from the above consensus in one or more DRs at PdnaA and PhlyIII (for example, positions 6 and 7 in all three DRs at PdnaA, or position 1 in all four DRs at PhlyIII, among others; Fig. 8H) could explain why these, despite having the TnnACA motif, do not bind CarHBm.
Discussion
Photoregulation of transcription by CarH is determined by the interplay between AdoCbl and light to modulate the oligomeric state, cooperative operator DNA binding, and repressor activity of CarH. High-resolution structural-mutational studies of T. thermophilus CarHTt revealed many unique facets of this photoreceptor and provided fundamental insights into its AdoCbl-binding, quaternary structure, operator design, and DNA-binding mode (9, 11). Our present analysis of B. megaterium CarHBm reveals a conserved mode of action, with an underlying plasticity. CarHBm binds to its operators only as an AdoCbl-bound tetramer, like CarHTt, but differs in the oligomeric states of the apo and light-exposed AdoCbl-bound forms, neither of which bind DNA. ApoCarHBm is an apparent tetramer and light-exposed CarHBm is a dimer (Fig. 9), whereas the corresponding inactive forms of CarHTt are monomers (11). Our data suggest that despite an oligomeric state akin to the AdoCbl-bound tetramer, apoCarHBm is a molten globule with structural differences, which may explain why it cannot bind operator DNA but must associate with AdoCbl to acquire the right tetramer conformation for operator recognition.
Figure 9.
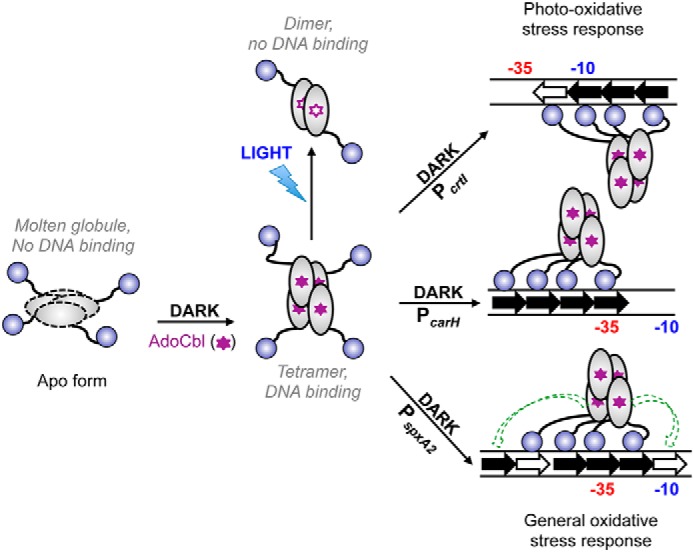
Model for AdoCbl- and light-dependent CarHBm oligomerization, operator design, and DNA-binding mode. The oligomeric apoCarHBm molten globule does not bind DNA. Its binding to AdoCbl (filled purple stars) in the dark produces the tetramer with the correct fold for DNA binding. Light photolyzes CarHBm-bound AdoCbl (unfilled purple stars) and induces tetramer collapse to dimers unable to bind operator DNA. Filled arrows indicate repeats with the TnnACA motif and unfilled arrows are pseudorepeats. Repeats orient in the same direction as the promoter (−35 and −10 elements indicated) at PcarH (middle right) and PspxA2 (bottom right), and in the opposite sense at PcrtI (top right). Consequently, the CarHBm tetramer binds to one DNA face at PcarH, whose operator includes the −35 promoter element, and at PspxA2, whose multipartite operator includes both the −35 and −10 promoter elements, but to the opposite face at PcrtI, whose operator includes the −10 promoter element. The dashed green arrows for CarHBm binding at PspxA2 indicates two additional modes of binding to four tandem repeats on either side of that depicted at this multipartite, six-repeat operator.
AdoCbl binds to CarHTt via crucial contacts of its 5′-dAdo upper axial moiety with a W-X9-EH motif (9), and the ensuing allosteric changes must determine assembly of apoCarHTt monomer to a tetramer. Mutating the equivalent Trp, Glu, or His in CarHBm also impaired its binding to AdoCbl and function, emphasizing the critical role of this motif. It is remarkable that AdoCbl drives the transition from a monomer to the active tetramer in CarHTt, but from a preformed inactive tetramer to the appropriately folded tetramer in CarHBm. This highlights plasticity in how AdoCbl binding modulates oligomerization and activity of this family of photoreceptors, even as its requirement for the W-X9-EH motif is conserved. As in the dark, plasticity is also observed in the transition to the light-exposed state. Light disrupts both CarHBm and CarHTt tetramers by photolysis of the bound AdoCbl and loss of its 5′-dAdo group, but yields CarHBm dimers versus CarHTt monomers, with both proteins retaining the photolyzed Cbl. In light-exposed CarHTt, the trapped Cbl resists exchange because it is held in a very tight bis-His-cobalt linkage via a nonconserved His (next to the Trp of the W-X9-EH motif) that is not required for tetramer disassembly in the light, or assembly in the dark (9, 12). This bis-His linkage cannot form in light-exposed CarHBm, however, because the equivalent residue is a Glu and, accordingly, the bound Cbl was exchangeable with intact AdoCbl in vitro. Although retention of photolyzed Cbl may have biological relevance in the recovery and reuse of the biologically expensive AdoCbl in some species like T. thermophilus (9, 12), lack of the bis-His linkage in B. megaterium CarHBm (and in the homologs of some other species that also lack the His) can facilitate photoreceptor regeneration simply through exchange with fresh AdoCbl, allowing for more rapid cycling between dark-light states.
Our data establish that CarHBm conserves the unique operator design and DNA-binding mode first discovered for the CarHTt tetramer. However, the CarHBm tetramer binds to a larger operator using all four DBDs (Fig. 9). Interestingly, CarHBm affinity for its site at PcarH, with four tandem 11-bp DRs, is lower (KD 4-fold higher) than for the site at PcrtI or for any of those at PspxA2, all with 3 DRs and a pseudorepeat; and no binding was detected at PdnaA and PhlyIII, even though both have three DRs plus a pseudorepeat (at PdnaA) or another DR (at PhlyIII). This suggests that the less conserved bases of the nnnnTnnACAn consensus DR are also important for DNA recognition. Thus, it appears that the information content, from sequence variations around the conserved elements in the DRs, affords considerable plasticity in determining DNA-binding affinities. Such a plasticity would allow combinatorial use of differentially optimized DRs and/or pseudorepeats to fine-tune relative affinities and hence occupancies at different target operators, as a physiologically relevant strategy for regulation in vivo.
Like CarHTt, CarHBm binds to one face of the DNA, determined by the orientation of the DRs, which is the same relative to the promoter at PcarH and PspxA2, but opposite at PcrtI (Fig. 9). In line with a common DNA-binding mode, CarHBm recognizes the three-DR CarHTt operator and CarHTt the larger four-DR CarHBm operators. However, even when a fourth DR is available, CarHTt still appears to bind to only three DRs, preferring the three upstream ones, hinting at positioning determinants whose nature remains to be established. That the CarHBm operators house the −35 (PcarH), −10 (PcrtI), or both promoter elements (possibly at PspxA2) suggests that CarHBm binding to either region can block access to RNAP to repress transcription. This mirrors the behavior reported for the winged-helix multiple antibiotic resistance (MarR) transcriptional factor MepR dimer, which can also bind to opposite faces of inverted repeat operator DNA overlapping the −35 and/or the −10 (and the TSS) element (37).
Modeling and MD simulations suggested that the CarHBm tetramer could use its fourth DBD to contact an additional repeat because its interdomain linker is longer than in CarHTt. The flexible, structurally disordered linker is free of contacts in the CarHTt structures (9) and varies considerably in length and sequence among CarH homologs (12). Although this may suggest that the linker is a passive (albeit necessary) nonspecific tether to physically join the two functional domains, we observed profound effects of the length, sequence, and protein context of the linker on CarH solution properties and DNA binding. Understanding linker effects at a molecular level depends on obtaining high-resolution experimental data, a challenging task for flexible, structurally disordered regions. Moreover, heterogeneity in linker length and sequence among CarH homologs also complicates comparative analysis. Hence, a more detailed analysis of linker effects will have to be pursued in future studies. Linkers, which necessarily relay allosteric effects between the structured functional domains they connect, can modulate DNA binding specificity and affinity, even when distant from the interacting surfaces, through transient and dynamic contacts with DNA (38–40). This can facilitate the search for specific sites and the final binding, as has been proposed for the DNA binding of bacterial nucleoid-associated protein H-NS (41) and eukaryotic transcription factors Oct1 and Pax6 (42, 43). Alternatively, linkers can diminish DNA binding via competing and unfavorable interactions through the presence of many acidic residues and/or phosphorylation that increase their negative charge, or through the entropic costs incurred on binding due to greater length and flexibility of linkers (38, 44). These factors may be in play in our observed linker effects on CarH binding to DNA, as was inferred from the MD simulations for CarHTt with the highly acidic CarHBm linker versus CarHBm.
In B. megaterium, as in T. thermophilus and M. xanthus, CarH orchestrates light-dependent regulation of carotenoid biosynthesis. Carotenoids are known to be an important line of defense against singlet oxygen, the primary source of photooxidative stress in microorganisms (2, 6). The discovery in this study of a CarHBm operator upstream of spxA2, which encodes an Spx-type regulator whose expression is activated by light, not only revealed an intriguing multipartite operator but also a new target gene for regulation by CarHBm. Spx proteins are highly conserved factors in the phylum Firmicutes (multiple paralogs often exist and B. megaterium has at least one more) that interact with the RNAP α subunit to activate expression of genes involved in cellular defense against oxidative stresses (such as disulfide and peroxide stress) (27, 45). Complex regulatory mechanisms at the transcriptional and post-translational levels (use of multiple promoters, dependence on σA or alternative σ factors, transcriptional regulators, regulated proteolysis) have been shown to control expression of spx genes (26, 27) in B. subtilis and other Firmicutes lacking a CarH homolog. Our study reveals the first example known of an spx gene whose expression is photoregulated. Simultaneous regulation of carotenoid synthesis (to quench singlet oxygen and related reactive oxygen species) and of spx expression (to activate general oxidative stress response pathways) by CarHBm would enable these two lines of defense to converge as a more effective strategy to combat oxidative cellular damage caused by light. CarHBm has comparable affinities for each of the three sites comprising the multipartite operator at PspxA2 and for that at PcrtI but, interestingly, a 4–5–fold lower affinity for the site at PcarH. If these relative affinities correlate directly with in vivo occupancy, then CarHBm would control its own expression less tightly than that of its other target genes. This may reflect an effective regulatory strategy in B. megaterium for CarHBm to control target genes present at loci unlinked to that of its own gene, in contrast to M. xanthus or T. thermophilus where the known target genes occur at the same locus. The physiological basis for multiple recognition sites at PspxA2 is unclear. It may be related to a possibly complex spx transcription, as in some other bacteria, and perhaps to achieve finer modulation of signal sensitivity and expression levels, as has been invoked for some other repressors (46). Future work can provide more details into these aspects and on how CarH-mediated photoregulation of Spx expression impinges on cellular physiology upon exposure to light.
Altogether, our study provides new insights into the specific binding to AdoCbl, its determinants, and oligomerization of CarH photoreceptors, and their mode of operator DNA binding. It has also uncovered a previously unsuspected role of the interdomain linker in modulating DNA binding. Moreover, the study has identified a multipartite light-dependent operator that controls expression of a global regulator of general oxidative stress responses, thereby linking it to the photooxidative stress response in B. megaterium. Plasticity in oligomerization, operator architecture, and DNA binding, within a conserved mode of action, thus appears to underpin how B12-based CarH photoreceptors direct light-dependent gene regulation.
Experimental procedures
Strains, plasmids, growth conditions, and stock solutions
Bacterial strains and plasmids used in this study are listed in Table S1. E. coli strain DH5α was used for plasmid constructions and BL21 DE3 for protein overexpression. Escherichia coli and B. megaterium strain DSM 32/ATCC 14581 (DSMZ, Germany) were grown in LB (Luria-Bertani) liquid medium or LB-agar (1%) plates at 37 or 25/18 °C for inducing overexpression of His6-tagged proteins in E. coli. M. xanthus was grown at 33 °C in CTT (casitone-Tris) medium (11). Growth media were supplemented as required with vitamin B12 or AdoCbl (Sigma), antibiotics (50 μg ml−1 ampicillin; 40 μg ml−1 kanamycin), or vanillate (20 μm). Concentrations of Cbl stock solutions in aqueous buffer were estimated as described previously (11), and that of ANS (Fluka) from the absorbance at 350 nm using an extinction coefficient (ϵ) of 5000 m−1 cm−1 (47). Ultrapure guanidinium chloride was obtained from ICN Biochemicals. Plates or liquid cell cultures were illuminated as required with white light (three 18-watt fluorescent lamps, 10 Wm−2).
Plasmid and strain construction
Coding sequences for His6-tagged CarHBm or mutants for overexpression in E. coli were cloned into the NdeI and BamHI sites of the pET15b vector. The carHBm gene was PCR-amplified from B. megaterium genomic DNA. Genes encoding mutant versions of CarHBm and its chimera CBm (with the N-terminal segment spanning residues 1 to 74 replaced by that of CarHMx) were synthesized (GenScript). For functional analysis in vivo, the coding sequence for CBm and its variants, and for CarHMx, fused to an N-terminal FLAG tag (for protein detection using immunoblot analysis), were cloned into plasmid pMR3679 (which confers kanamycin resistance, KmR) to enable their conditional expression from a vanillate-inducible promoter (33). Each construct was electroporated into the M. xanthus strain MR2648, and transformants with the plasmid integrated at a heterologous site by homologous recombination were selected by their KmR. All constructs were verified by sequencing. Each strain was grown in the dark in liquid CTT media with vanillate, AdoCbl, and kanamycin, spotted on plates of the same medium and incubated in the dark or light, colony color being used to assess if the CarHBm variant was functional.
For targeted deletion of carHBm in B. megaterium, we used the E. coli-Bacillus shuttle vector pUCTV2 (48), which replicates stably in B. megaterium at 30 °C but not at 42 °C. Using genomic DNA as template and appropriate primers, ∼1 kb segments upstream and downstream of carHBm were PCR-amplified as BamHI-XhoI and XhoI-BamHI products, respectively, and then cloned into the BamHI site of pUCTV2. The construct was introduced into B. megaterium protoplasts by PEG-mediated transformation and selected on LB/tetracycline (10 μg ml−1) plates at 30 °C, as described (49). Transformants were picked for growth at 42 °C in LB/tetracycline for plasmid integration into the genome by homologous recombination, and then in LB at 42 °C for allele exchange and loss of plasmid. Colonies bearing the carHBm deletion were easily identified by their strong yellow color in the dark and verified by PCR.
Two-hybrid analysis
Bacterial two-hybrid analysis of light and B12-dependent oligomerization was carried out as described previously (11). The PCR-amplified gene was cloned into the XbaI and BamHI sites of pKT25 or pUT18C; given pairs of constructs were introduced into E. coli strain BTH101 (cya−) by electroporation. CarHMx, which depends on B12 to self-interact, and CarAMx, which does not, served as controls (11), and pairs with only one fusion protein as negative controls. Interaction was assessed qualitatively from the blue color that developed on LB plates containing 40 μg/ml of X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactoside) with or without 1 μm vitamin B12 (cyanocobalamin), in the dark or light (11).
Western blot analysis
Immunoblot analysis of M. xanthus whole cell extracts expressing FLAG-tagged proteins was carried out as described elsewhere (50). Cell pellets from 1-ml samples from cultures grown in the dark to A550 ∼0.7 in CTT, 1 μm AdoCbl, 20 μm vanillate were suspended in 300 μl of buffer containing cOmplete protease inhibitor (Roche Applied Science), 1 mm each benzamidine and phenylmethylsulfonyl fluoride (Sigma) and lysed with SDS/chloroform. After isolating the supernatant by centrifugation and estimating total protein (protein assay kit, Bio-Rad), aliquots with the same total protein were resolved in 10% SDS-PAGE gels, transferred to Hybond-ECL membranes, and probed using anti-FLAG M2 monoclonal antibodies and the ECL system (F3165, Sigma), with the RNAP β subunit probed with monoclonal anti-RNAP β antibody (8RB13; ThermoFisher) serving as the loading control.
Protein purification
His6-tagged CarHBm, CarHTt, or variants were overexpressed using pET15b constructs in E. coli BL21 DE3 and purified as native apoproteins using protocols and buffers detailed in previous studies (9, 11), except for the presence of 2 mm β-mercaptoethanol in the buffers used for CarHBm and its variants. Proteins purified in a final SEC step (in 150 mm NaCl, 50 mm phosphate buffer, pH 7.5, 2 mm β-mercaptoethanol) were concentrated and aliquots were frozen at −70 °C in buffer alone or with 50% glycerol. Purified protein identities were verified by SDS-PAGE and by electrospray ionization-TOF MS, as described in our previous study (9). AdoCbl in stocks and when protein-bound was always handled under red or dim light and periodically checked by UV-visible spectroscopy to rule out inadvertent photolysis. When required, samples in 1.5-ml tubes were irradiated for specific times (1–5 min) with white light (10–12 Wm−2 from 18-watt fluorescent lamps), at specific wavelengths (405/465 nm, blue-violet; 520 nm, green; 660 nm, red) using a computer-controlled LED (light-emitting diode) array with regulatable 0–20 Wm−2 intensities, or at 360 nm for UV using a Nikon Eclipse 80i epifluorescence microscope with UV-2E/C filter (8, 11). Light intensities were estimated using an 1815-C optical power meter equipped with an 818-SL detector (Newport). Protein concentrations were determined using the Bio-Rad protein assay kit and absorbance at 280 nm in a Cary 60 UV-visible spectrophotometer with molar extinction coefficients ϵ280 (in m−1 cm−1, calculated from protein sequences (http://web.expasy.org/protparam) of 37,900 for CarHTt and variants, 39,420 for CarHBm and variants (33,920 for the Trp mutant), and with 22,500 added for AdoCbl in the holoprotein (9).
Analytical SEC
A Superdex200 HR 10/30 column (GE Life Sciences) equilibrated with 150 mm NaCl, 50 mm phosphate buffer, pH 7.5, 2 mm β-mercaptoethanol and an AKTA HPLC unit were used for analytical SEC. Calibration of the column as described previously (9, 11) yielded log Mr = 7.885–0.221 Ve, where Mr is the apparent molecular mass and Ve the elution volume. Pure protein (100 μl, 50–100 μm), alone or after 15-min incubation with a 5-fold molar excess of AdoCbl or MeCbl, was injected into the column in the dark or after 5-min exposure to light and the elution at a flow rate of 0.4 ml/min was tracked by absorbance at 280, 361, and 522 nm. Peaks were analyzed for Mr and by UV-visible spectroscopy and SDS-PAGE.
UV-visible absorption, CD, and fluorescence spectroscopy
UV-visible absorption spectra were recorded at room temperature in a Cary 60 UV-visible spectrophotometer. Far-UV and near-UV CD spectra were recorded at 25 °C in an Applied Photophysics (UK) Pistar unit with Peltier temperature control/Neslab RTE-70 unit and calibrated with (+)-10-camphorsulfonic acid. Fluorescence spectra were recorded at 25 °C in a Jobin-Yvon fluorimeter. Difference UV-visible absorbance (ΔA) was used to determine the binding affinity (KD) for AdoCbl of CarHBm or its H154A variant, as reported previously (10). Details of the spectroscopic measurements and analysis are provided under supporting data.
DNA-binding assays
All in vitro DNA-binding assays were performed under similar solution conditions in the dark or after irradiation with light, at least three times for each condition. DNA probes (170 bp) were PCR-amplified using primers, with one 32P-labeled at the 5′-end with T4 polynucleotide kinase (Takara) prior to PCR. For shorter DNA probes, complementary HPLC-purified synthetic oligonucleotides (Sigma) were mixed with one 5′-end 32P-labeled using T4 polynucleotide kinase and the other unlabeled and present at a 2-fold excess, incubated at 100 °C for 2 min and hybridized by slow cooling. EMSAs, their analysis to estimate KD, the apparent DNA-protein equilibrium dissociation constant, and DNase I and hydroxyl radical footprinting assays used procedures identical to those described in our previous study (9). Details for these and for Exo III footprinting are provided under the supporting data.
GluC proteolysis assay
Pure CarHBm or TtLkB (0.3 μg/μl) were incubated in the dark with 5-fold molar excess AdoCbl in EMSA buffer lacking BSA for 30 min at 37 °C. Samples were then treated with GluC endopeptidase (Promega) at a 1:50 ratio (w/w) in 150 μl of total volume at 30 °C. Aliquots of 50 μl were withdrawn at 0, 1, and 2 h, and the reaction was stopped by adding phenylmethylsulfonyl fluoride to 1 mm. From each aliquot, 20 μl were mixed with 4 μl of 5× SDS-PAGE buffer, heated in a boiling water bath for 5 min, electrophoresed in 12% bis-Tris gels (Bio-Rad), and the bands were visualized by Coomassie Blue staining. The rest of each sample was also subject to SDS-PAGE and electrotransferred to an ImmobilonPSQ membrane (Millipore, Bedford, MA) for N-terminal sequencing.
qRT-PCR
A 10-ml cell culture in LB grown overnight in the dark at 37 °C was diluted 1:100 with fresh LB and grown in the dark or light for 5 h to an optical density at 600 nm of ∼0.8. Cells from 5 ml of this culture were pelleted and stored at −70 °C. These were resuspended in 200 μl of RNase-free lysis buffer (10 mm Tris, pH 8.0, 1 mm EDTA, 10 mm NaCl, 1% SDS) and lysed in a Mini-beadbeater (BioSpec). RNA was purified using the PureLink RNA Minikit (ThermoFisher Scientific) and recommended protocols for TRIzol/chloroform extraction, isopropyl alcohol precipitation, and 70% ethanol wash. This RNA was treated with Turbo DNase I (Ambion), checked in native 1% agarose gels, and quantified in NanoDrop ND-1000 (Thermo Fisher Scientific). RNA (5 μg/20 μl) was reverse transcribed to cDNA with SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific). qRT-PCR was performed in a StepOne instrument (Applied Biosystems) using the 1-Step RT-PCR program cycle for samples containing 1 μl of cDNA, 10 μl of SYBR Green PCR Master Mix (Bio-Rad), and the required primers (400 nm; Table S2), which were designed using the Primer Express 3.0 software to amplify an ∼50–150 bp region within each transcript (sigA served as the reference gene). A sample with an equivalent volume of RNA served as the control to check the lack of contaminant DNA. Melting and dissociation curves were determined at 60–95 °C, 30 s, and 95 °C, 15 s. Each primer pair was tested for RT-PCR analysis on a standard curve generated from five 10-fold serial dilutions of cDNA. Only primer pairs with efficiency close to 100% were used and data were analyzed using the Applied Biosystems software. Target gene expression is reported relative to the WT level of the gene in the dark as calibrator, as the mean ± S.E. from at least three independent experiments.
In silico modeling and analysis
Details for in silico modeling and analysis are provided under Figs. S9 and S11. The systems built were: CarHTt tetramer in complex with DNA, and with its linker replaced by the longer one from CarHBm; CarHBm tetramer free or bound to DNA (both homology modeled based on the CarHTt structures, because our data indicate that CarHBm has a similar secondary structure and also binds to DNA as a tetramer). AMBER ff14SB (51) was employed to simulate the protein and PARMBSC1 (52) force field parameters to describe DNA. Parameters for base-off His-on AdoCbl were derived consistent with the AMBER ff. All systems were solvated with truncated octahedral boxes of TIP3P (53) waters with a buffer of 12 Å in all directions, and adding K+ ions to neutralization with excess 150 mm KCl. Molecular dynamics (MD) simulations were performed using the AMBER 14 package (54) at constant pressure (1 atm) and temperature (300 K) in 2-fs steps, applying periodic boundary conditions and state-of-the-art simulation protocols. Unrestrained MD simulation (total 500 ns) was performed for each system to study the behavior of the linkers in the context of CarHTt and CarHBm. For steered MD simulations (36), we built a CarHTt system bound to DNA with a fourth DNA repeat (DR4) modeled, and a homology model of CarHBm bound to the same DNA to assess the binding of its DBD to DNA. Genome-wide scan of putative CarHBm DNA-binding sites was carried out using Artemis (55).
Author contributions
J. F.-Z., R. P.-C., J. A., F. C., M. C. P., S. P., and M. E.-A. formal analysis; J. F.-Z., R. P.-C., J. A., F. C., M. C. P., S. P., and M. E.-A. validation; J. F.-Z., R. P.-C., J. A., F. C., and M. C. P. investigation; J. F.-Z., R. P.-C., J. A., F. C., M. C. P., and M. O. methodology; J. F.-Z., R. P.-C., J. A., F. C., M. C. P., M. O., S. P., and M. E.-A. writing-review and editing; M. O., S. P., and M. E.-A. conceptualization; M. O., S. P., and M. E.-A. resources; M. O., S. P., and M. E.-A. supervision; M. O., S. P., and M. E.-A. funding acquisition; S. P. and M. E.-A. writing-original draft; S. P. and M. E.-A. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Marco Jost (University of California, San Francisco) for critical reading of the manuscript, and Dr. Rebekka Biedendieck (Technische Universität Braunschweig) for plasmid pUCTV2 and helpful advice. We also thank Dr. Javier Varela (CIB-CSIC) for N-terminal sequencing and from the University of Murcia, Dr. Alejandro Torrecillas, Dr. Cesar Flores-Flores, and Prof. Senena Corbalán for technical support, Ana de la Fuente for her contribution, and José Antonio Madrid and Victoria López Egea for assistance.
This work was supported by Ministerio de Economía y Competitividad-Spain Grants BFU2015-67968-C2-1-P co-financed with the European Union Fondo Europeo de Desarrollo Regional (FEDER) funds (to M. E.-A.), BFU2015-67968-C2-2-P (to S. P.), and BIO2015-64802-R (to M. O.), and Ph.D. fellowships (to J. F.-Z and R. P.-C.), a Juan de la Cierva contract (to J. A.), the European Research Council grant ERC SimDNA (to M. O.), a European Union Marie Curie fellowship (to F. C.), and Fundación Séneca-Spain Grant 19429/PI/14 (to M. E.-A.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supporting data, Tables S1 and S2, and Figs. S1–S11.
- AdoCbl
- 5′-deoxyadenosylcobalamin
- 5′-dAdo
- 5′-deoxyadenosyl
- ANS
- 8-anilinonaphthalene-1-sulfonate
- DBD
- DNA binding domain
- Cbl
- cobalamin
- CTT
- casitone-Tris
- DR
- direct repeat
- EMSA
- electrophoretic mobility shift assay
- GdmCl
- guanidinium chloride
- MeCbl
- methylcobalamin
- Km
- kanamycin
- MD
- molecular dynamics
- RNAP
- RNA polymerase
- SEC
- size-exclusion chromatography
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- TSS
- transcription start site
- qRT
- quantitative reverse transcription.
References
- 1. Elías-Arnanz M., Padmanabhan S., and Murillo F. J. (2011) Light-dependent gene regulation in nonphototrophic bacteria. Curr. Opin. Microbiol. 14, 128–135 10.1016/j.mib.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 2. Glaeser J., Nuss A. M., Berghoff B. A., and Klug G. (2011) Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 58, 141–173 10.1016/B978-0-12-381043-4.00004-0 [DOI] [PubMed] [Google Scholar]
- 3. Li Z., Wakao S., Fischer B. B., and Niyogi K. K. (2009) Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 10.1146/annurev.arplant.58.032806.103844 [DOI] [PubMed] [Google Scholar]
- 4. Palczewski K. (2012) Chemistry and biology of vision. J. Biol. Chem. 287, 1612–1619 10.1074/jbc.R111.301150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Purcell E. B., and Crosson S. (2008) Photoregulation in prokaryotes. Curr. Opin. Microbiol. 11, 168–178 10.1016/j.mib.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 6. Ziegelhoffer E. C., and Donohue T. J. (2009) Bacterial responses to photo-oxidative stress. Nat. Rev. Microbiol. 7, 856–863 10.1038/nrmicro2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Möglich A., Yang X., Ayers R. A., and Moffat K. (2010) Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61, 21–47 10.1146/annurev-arplant-042809-112259 [DOI] [PubMed] [Google Scholar]
- 8. Díez A. I., Ortiz-Guerrero J. M., Ortega A., Elías-Arnanz M., Padmanabhan S., and García de la Torre J. (2013) Analytical ultracentrifugation studies of oligomerization and DNA-binding of TtCarH, a Thermus thermophilus coenzyme B12-based photosensory regulator. Eur. Biophys. J. 42, 463–476 10.1007/s00249-013-0897-x [DOI] [PubMed] [Google Scholar]
- 9. Jost M., Fernández-Zapata J., Polanco M. C., Ortiz-Guerrero J. M., Chen P. Y., Kang G., Padmanabhan S., Elías-Arnanz M., and Drennan C. L. (2015) Structural basis for gene regulation by a B12-dependent photoreceptor. Nature 526, 536–541 10.1038/nature14950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kutta R. J., Hardman S. J., Johannissen L. O., Bellina B., Messiha H. L., Ortiz-Guerrero J. M., Elías-Arnanz M., Padmanabhan S., Barran P., Scrutton N. S., and Jones A. R. (2015) The photochemical mechanism of a B12-dependent photoreceptor protein. Nat. Commun. 6, 7907 10.1038/ncomms8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz-Guerrero J. M., Polanco M. C., Murillo F. J., Padmanabhan S., and Elías-Arnanz M. (2011) Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. U.S.A. 108, 7565–7570 10.1073/pnas.1018972108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padmanabhan S., Jost M., Drennan C. L., and Elías-Arnanz M. (2017) A new facet of vitamin B12: gene regulation by cobalamin-based photoreceptors. Annu. Rev. Biochem. 86, 485–514 10.1146/annurev-biochem-061516-044500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pérez-Marín M. C., Padmanabhan S., Polanco M. C., Murillo F. J., and Elías-Arnanz M. (2008) Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol. Microbiol. 67, 804–819 [DOI] [PubMed] [Google Scholar]
- 14. Chatelle C., Ochoa-Fernandez R., Engesser R., Schneider N., Beyer H. M., Jones A. R., Timmer J., Zurbriggen M. D., and Weber W. (2018) A green-light-responsive system for the control of transgene expression in mammalian and plant cells. ACS Synth. Biol. 7, 1349–1358 10.1021/acssynbio.7b00450 [DOI] [PubMed] [Google Scholar]
- 15. Kainrath S., Stadler M., Reichhart E., Distel M., and Janovjak H. (2017) Green-light-induced inactivation of receptor signaling using cobalamin-binding domains. Angew. Chem. Int. Ed. Engl. 56, 4608–4611 10.1002/anie.201611998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang R., Yang Z., Luo J., Hsing I. M., and Sun F. (2017) B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release. Proc. Natl. Acad. Sci. U.S.A. 114, 5912–5917 10.1073/pnas.1621350114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García-Moreno D., Polanco M. C., Navarro-Avilés G., Murillo F. J., Padmanabhan S., and Elías-Arnanz M. (2009) A vitamin B12-based system for conditional expression reveals dksA to be an essential gene in Myxococcus xanthus. J. Bacteriol. 191, 3108–3119 10.1128/JB.01737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takano H., Kondo M., Usui N., Usui T., Ohzeki H., Yamazaki R., Washioka M., Nakamura A., Hoshino T., Hakamata W., Beppu T., and Ueda K. (2011) Involvement of CarA/LitR and CRP/FNR family transcriptional regulators in light-induced carotenoid production in Thermus thermophilus. J. Bacteriol. 193, 2451–2459 10.1128/JB.01125-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drennan C. L., Huang S., Drummond J. T., Matthews R. G., and Lidwig M. L. (1994) How a protein binds B12: a 3.0 Å x-ray structure of B12-binding domains of methionine synthase. Science 266, 1669–1674 10.1126/science.7992050 [DOI] [PubMed] [Google Scholar]
- 20. Heldwein E. E., and Brennan R. G. (2001) Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409, 378–382 10.1038/35053138 [DOI] [PubMed] [Google Scholar]
- 21. Newberry K. J., and Brennan R. G. (2004) The structural mechanism for transcription activation by MerR family member multidrug transporter activation, N terminus. J. Biol. Chem. 279, 20356–20362 10.1074/jbc.M400960200 [DOI] [PubMed] [Google Scholar]
- 22. Philips S. J., Canalizo-Hernandez M., Yildirim I., Schatz G. C., Mondragón A., and O'Halloran T. V. (2015) Allosteric transcriptional regulation via changes in the overall topology of the core promoter. Science 349, 877–881 10.1126/science.aaa9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe S., Kita A., Kobayashi K., and Miki K. (2008) Crystal structure of the [2Fe-2S] oxidative-stress sensor SoxR bound to DNA. Proc. Natl. Acad. Sci. U.S.A. 105, 4121–4126 10.1073/pnas.0709188105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jost M., Simpson J. H., and Drennan C. L. (2015) The transcription factor CarH safeguards use of adenosylcobalamin as a light sensor by altering the photolysis products. Biochemistry 54, 3231–3234 10.1021/acs.biochem.5b00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takano H., Mise K., Hagiwara K., Hirata N., Watanabe S., Toriyabe M., Shiratori-Takano H., and Ueda K. (2015) Role and function of LitR, an adenosyl B12-bound light-sensitive regulator of Bacillus megaterium QM B1551, in regulation of carotenoid production. J. Bacteriol. 197, 2301–2315 10.1128/JB.02528-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newberry K. J., Nakano S., Zuber P., and Brennan R. G. (2005) Crystal structure of the Bacillus subtilis anti-α, global transcriptional regulator, Spx, in complex with the α C-terminal domain of RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 102, 15839–15844 10.1073/pnas.0506592102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zuber P. (2009) Management of oxidative stress in Bacillus. Annu. Rev. Microbiol. 63, 575–597 10.1146/annurev.micro.091208.073241 [DOI] [PubMed] [Google Scholar]
- 28. Schmid F. X. (1997) Optical spectroscopy to characterize protein conformation and conformational changes. in Protein structure: a practical approach (Creighton T. E., ed) pp. 261–297, Oxford University Press, Oxford, UK [Google Scholar]
- 29. Rohl C. A., and Baldwin R. L. (1997) Comparison of NH exchange and circular dichroism as techniques for measuring the parameters of the helix-coil transition in peptides. Biochemistry 36, 8435–8442 10.1021/bi9706677 [DOI] [PubMed] [Google Scholar]
- 30. Baldwin R. L., and Rose G. D. (2013) Molten globules, entropy-driven conformational change and protein folding. Curr. Opin. Struct. Biol. 23, 4–10 10.1016/j.sbi.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 31. Goto Y., and Fink A. L. (1989) Conformational states of beta-lactamase: molten-globule states at acidic and alkaline pH with high salt. Biochemistry 28, 945–952 10.1021/bi00429a004 [DOI] [PubMed] [Google Scholar]
- 32. Semisotnov G. V., Rodionova N. A., Razgulyaev O. I., Uversky V. N., Gripas A. F., and Gilmanshin R. I. (1991) Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31, 119–128 10.1002/bip.360310111 [DOI] [PubMed] [Google Scholar]
- 33. Iniesta A. A., García-Heras F., Abellón-Ruiz J., Gallego-García A., and Elías-Arnanz M. (2012) Two systems for conditional gene expression in Myxococcus xanthus inducible by isopropyl-β-d-thiogalactopyranoside or vanillate. J. Bacteriol. 194, 5875–5885 10.1128/JB.01110-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meiser P., Bode H. B., and Müller R. (2006) The unique DKxanthene secondary metabolite family from the myxobacterium Myxococcus xanthus is required for developmental sporulation. Proc. Natl. Acad. Sci. U.S.A. 103, 19128–19133 10.1073/pnas.0606039103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Craig M. L., Suh W. C., and Record M. T. Jr. (1995) HO· and DNase I probing of Eσ70 RNA polymerase- λPR promoter open complexes: Mg2+ binding and its structural consequences at the transcription start site. Biochemistry 34, 15624–15632 10.1021/bi00048a004 [DOI] [PubMed] [Google Scholar]
- 36. Grubmüller H., Heymann B., and Tavan P. (1996) Ligand binding: molecular mechanics calculation of the streptavidin-biotin rupture force. Science 271, 997–999 10.1126/science.271.5251.997 [DOI] [PubMed] [Google Scholar]
- 37. Birukou I., Seo S. M., Schindler B. D., Kaatz G. W., and Brennan R. G. (2014) Structural mechanism of transcription regulation of the Staphylococcus aureus multidrug efflux operon mepRA by the MarR family repressor MepR. Nucleic Acids Res. 42, 2774–2788 10.1093/nar/gkt1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuxreiter M., Simon I., and Bondos S. (2011) Dynamic protein-DNA recognition: beyond what can be seen. Trends Biochem. Sci. 36, 415–423 10.1016/j.tibs.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 39. Vuzman D., and Levy Y. (2012) Intrinsically disordered regions as affinity tuners in protein-DNA interactions. Mol. Biosyst. 8, 47–57 10.1039/C1MB05273J [DOI] [PubMed] [Google Scholar]
- 40. Motlagh H. N., Wrabl J. O., Li J., and Hilser V. J. (2014) The ensemble nature of allostery. Nature 508, 331–339 10.1038/nature13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao Y., Foo Y. H., Winardhi R. S., Tang Q., Yan J., and Kenney L. J. (2017) Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells. Proc. Natl. Acad. Sci. U.S.A. 114, 12560–12565 10.1073/pnas.1716721114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Leeuwen H. C., Strating M. J., Rensen M., de Laat W., and van der Vliet P. C. (1997) Linker length and composition influence the flexibility of Oct-1 DNA binding. EMBO J. 16, 2043–2053 10.1093/emboj/16.8.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vuzman D., Polonsky M., and Levy Y. (2010) Facilitated DNA search by multidomain transcription factors: cross talk via a flexible linker. Biophys. J. 99, 1202–1211 10.1016/j.bpj.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flock T., Weatheritt R. J., Latysheva N. S., and Babu M. M. (2014) Controlling entropy to tune the functions of intrinsically disordered regions. Curr. Opin. Struct. Biol. 26, 62–72 10.1016/j.sbi.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 45. Barendt S., Lee H., Birch C., Nakano M. M., Jones M., and Zuber P. (2013) Transcriptomic and phenotypic analysis of paralogous spx gene function in Bacillus anthracis Sterne. Microbiologyopen 2, 695–714 10.1002/mbo3.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park D. M., and Kiley P. J. (2014) The influence of repressor DNA binding site architecture on transcriptional control. mBio 5, e01684–01614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weber G., and Young L. B. (1964) Fragmentation of bovine serum albumin by pepsin: I. the origin of the acid expansion of the albumin molecule. J. Biol. Chem. 239, 1415–1423 [PubMed] [Google Scholar]
- 48. Wittchen K. D., and Meinhardt F. (1995) Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl. Microbiol. Biotechnol. 42, 871–877 10.1007/BF00191184 [DOI] [PubMed] [Google Scholar]
- 49. Biedendieck R., Borgmeier C., Bunk B., Stammen S., Scherling C., Meinhardt F., Wittmann C., and Jahn D. (2011) Systems biology of recombinant protein production using Bacillus megaterium. Methods Enzymol. 500, 165–195 10.1016/B978-0-12-385118-5.00010-4 [DOI] [PubMed] [Google Scholar]
- 50. Gallego-García A., Iniesta A. A., González D., Collier J., Padmanabhan S., and Elías-Arnanz M. (2017) Caulobacter crescentus CdnL is a non-essential RNA polymerase-binding protein whose depletion impairs normal growth and rRNA transcription. Sci. Rep. 7, 43240 10.1038/srep43240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maier J. A., Martinez C., Kasavajhala K., Wickstrom L., Hauser K. E., and Simmerling C. (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 10.1021/acs.jctc.5b00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ivani I., Dans P. D., Noy A., Pérez A., Faustino I., Hospital A., Walther J., Andrio P., Goñi R., Balaceanu A., Portella G., Battistini F., Gelpí J. L., González C., Vendruscolo M., et al. (2016) Parmbsc1: a refined force field for DNA simulations. Nat. Methods 13, 55–58 10.1038/nmeth.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., and Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 10.1063/1.445869 [DOI] [Google Scholar]
- 54. Case D. A., Babin V., Berryman J. T., Betz R. M., Cai Q., Cerutti D. S., Cheatham T. E., Darden T. A., Duke R. E., Gohlke H., Goetz A. W., Gusarov S., Homeyer N., Janowski P., Kaus J., et al. (2014) Amber 14, University of California, San Francisco, CA [Google Scholar]
- 55. Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Rajandream M. A., and Barrell B. (2000) Artemis: sequence visualization and annotation. Bioinformatics 16, 944–945 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.