Abstract
The incidence of metastatic melanoma has been increasing dramatically over the last decades. Yet, there have been many new innovative therapies, such as targeted therapies and checkpoint inhibitors, which have made progress in survival for these patients. The oncology pharmacist is part of the healthcare team and can help in optimizing these newer therapies. There will be discussion about combination therapies, the oncology pharmacist's role, and issues at the core of his interest, such as drug interactions and complementary and alternative therapies.
Keywords: : cobimetinib, complementary and alternative medicine, dabrafenib, interactions, ipilimumab, metastatic melanoma, natural products, nivolumab, pembrolizumab, pharmacist, trametinib, vemurafenib
Practice points.
Metastatic melanoma is a very chemotherapy-resistant disease with a dismal prognosis.
Nivolumab and pembrolizumab have a more distant mechanism of action than ipilimumab.
Drug resistance to anti-BRAF therapy is known to develop quite quickly.
Inhibiting the MEK kinase of the MAPK pathway, by MEK inhibitors, and by associating them with anti-BRAFV600 therapy, can delay the development of resistance.
The nature of drug–drug interactions can either be pharmacokinetic, pharmacodynamic or pharmaceutical.
During patient counseling, it is essential to do a complete medication history and medication reconciliation.
The use of complementary and alternative medicine is on the rise in the oncology population.
Natural products and herbs are not regulated in as stringent and rigorous a fashion as traditional drugs used in medicine.
The incidence of metastatic melanoma has been dramatically increasing over the last 40 years [1]. Historically, metastatic melanoma has been a very chemotherapy-resistant disease with a dismal prognosis. Prior to the development of targeted therapies and immunotherapies, survival rates were in the order of 6 months with or without treatment with chemotherapy, where agents such as dacarbazine, temozolomide or chemotherapy associations such as paclitaxel and carboplatin have been used with disappointing results [2–4].
The stimulation of the immune system for the treatment of melanoma has shown in the past a couple of success stories: IFN-α was for many years a standard of treatment for the adjuvant treatment of melanoma, and a minority of patients treated with IL-2 for metastatic melanoma showed very interesting longstanding and durable responses [3]. Unfortunately, very low response rates in the order of approximately 6% of patients, an important side effect profile, and indirect costs associated with hospitalizations, have rendered IL-2 an option for very few patients [3,4]. Yet these experiences have generated an interest in continuing to define the importance of the immune system as a potential target for the treatment of metastatic melanoma [3].
During the last decade, we have had the opportunity of witnessing dramatic improvements in the treatment of metastatic melanoma. In patients that express a BRAFV600 mutation, we have seen that targeted therapy inhibiting the MAPK pathway with either vemurafenib or dabrafenib has generated high response rates [5–7]. The use of ipilimumab, an anti-CTLA-4 inhibitor that restores T-cell function targeting tumor cells has yielded much needed overall survival improvements and durable responses of 3 years in approximately 20% of patients; certain patients achieving this milestone reach a stable plateau of response that has been reported to last up to 10 years in a certain number of patients [8–11]. Checkpoint inhibition or anti-programmed death-1 (PD-1) therapy with nivolumab or pembrolizumab that also restores T-cell function at a more peripheral level and at the tumor site was developed to try to increase the number of response rates while improving the toxicity profile of anti-CTLA-4 therapy [12–16].
In this article, we will define a recent trend in the treatment of metastatic melanoma which is the use of combination therapy: in patients with a BRAFV600 mutation, we will discuss BRAF inhibition in association with an MEK inhibitor, either trametinib or cobimetinib; we will also discuss the association of anti-CTLA-4 and anti-PD-1 therapy [2,17–19]. We will discuss the role of the oncology pharmacist in treating patients that have metastatic melanoma. We will focus our attention on pharmacy issues of drug interactions, and the use of natural products or complimentary and alternative medicine (CAM) in this patient population. We will not discuss side effect management of these newer combination therapies because this issue has been discussed extensively elsewhere [4,20–26]. We will try to make this article more of a practical tool for pharmacists and other healthcare practitioners than a theoretical one.
Combination therapy for the treatment of metastatic melanoma: Phase III trials
Immunotherapy: anti-CTLA-4 therapy & anti-PD-1 therapy
In recent years, many innovations have demonstrated benefits in the treatment of metastatic melanoma that have rendered the use of classic chemotherapy nearly obsolete [2–4]. The development of immunotherapy with ipilimumab, an anti-CTLA-4 inhibitor, or the checkpoint inhibitors which are anti-PD-1 inhibitors, such as nivolumab or pembrolizumab have made a major impact on metastatic melanoma survival [3].
Ipilimumab was the first molecule since approximately 45 years of use of dacarbazine to demonstrate an increase in progression-free and overall survival [2–4,6]. A meta-analysis has clearly shown overall survival rates of 20% at 3 years and if a patient is fortunately alive at this 3-year mark, he has a good chance at a potential long-term survival where patients have been reported alive and well 10 years post-therapy [10]. The issues with ipilimumab are that it is a very costly therapy with low response rates and no biomarker which may help us in defining beforehand which patient might well respond to this therapy [1].
Checkpoint inhibitors, or anti-PD-1 receptors, either nivolumab or pembrolizumab, have a more distant mechanism of action than ipilimumab [27–29]. Anti-PD-1 therapy restores T-cell function peripherally and at the tumor site. The idea behind this difference is that we may improve response rates with these molecules and improve the toxicity profile compared with ipilimumab [13]. These goals have been attained for nivolumab and pembrolizumab in comparative Phase III trials to ipilimumab that have been described elsewhere and are outside the scope of this article [2].
The Checkmate 067 was a 3-arm randomized Phase III trial that offered treatment to 945 patients with unresectable stage III or stage IV melanoma as a first line of therapy. Patients were randomized either to ipilimumab monotherapy (n = 315), nivolumab monotherapy (n = 316) or the combination of ipilimumab and nivolumab (n = 314). The results of this trial (see Table 1) demonstrated that the combination therapy had a statistically significant superiority over either agent alone with regard to median progression survival and objective response rates. The downside to this combination therapy is the important increase in grade 3–4 toxicity rates with the combination therapy versus either agent being used alone used as monotherapy [30].
Table 1. . Phase III combination trials for the treatment of metastatic melanoma. Checkpoint inhibition and immunotherapy: anti-CTLA-4 and anti-PD-1 combination in the Checkmate 067 study.
| Treatment | Number of patients | Response rates (%) | mPFS (months) | Grade 3–4 AEs (%) |
|---|---|---|---|---|
| Nivolumab + ipilimumab | 314 | 57 | 11.5 | 55.0 |
| Nivolumab | 316 | 44 | 6.9 | 16.3 |
| Ipilimumab | 315 | 19 | 2.9 | 27.3 |
At this time, there are no published Phase III trials involving pembrolizumab in combination therapy for the treatment of unresectable or metastatic melanoma.
Targeted therapy: BRAFV600 inhibitors & MEK inhibitors
Patients that express BRAFV600 positive, or mutated, metastatic melanoma now have the possibility of being treated with targeted therapy. Patients can now be treated with BRAFV600 inhibitors, either vemurafenib or dabrafenib, which inhibit the intracellular MAPK pathway by inhibiting the BRAF kinase which ultimately leads to cell death. Anti-BRAFV600 therapy has demonstrated in Phase III trials improvements in overall survival rates and progression-free survival compared with classic chemotherapy [2,4,6,7,4].
The first issue that must be taken into consideration with treatment with BRAFV600 inhibitors is that drug resistance to anti-BRAF therapy is known to develop quite quickly. The second issue is that once anti-BRAF therapy resistance is known, metastatic melanoma appears to progress very rapidly and it is very difficult to regain control of the disease at this point. Despite these drawbacks, anti-BRAFV600 therapy is an excellent choice in patients that present with aggressive BRAFV600-positive disease because patients usually respond quickly to anti-BRAFV600 therapy [2,4,6,7,4].
In order to overcome drug resistance which may develop over time to vemurafenib or dabrafenib therapy, the idea of targeting a different kinase of the MAP pathway may prove to be beneficial. Therefore, inhibiting the MEK kinase of the MAPK pathway, by MEK inhibitors such as trametinib or cobimetinib, and associating one of them with an anti-BRAFV600 agent may bring a benefit and delay the development of resistance to treatment. Different Phase III trials have looked at this issue [2,4,6,7,4].
The Co-BRIM trial is a Phase III trial that studied the association of vemurafenib to either cobimetinib (n = 247) or a placebo (n = 248) in patients with untreated unresectable or metastatic melanoma with BRAFV600 mutations. This trial (see Table 2) demonstrated that the association of cobimetinib to vemurafenib compared with vemurafenib alone improved the rate of progression-free survival (9.9 vs 6.2 months, p < 0.001), response rates (68 vs 45%, p < 0.001) and 9-month overall survival rates (81 vs 73%, p = 0.046) [31].
Table 2. . Phase III combination trials for the treatment of metastatic melanoma. Targeted therapy: BRAFV600 inhibitors and MEK inhibitors combination.
| Study | Treatment | Number of patients | Response rates (%) | Duration (months) | mPFS (months) | OS | >Grade 3 AEs (%) |
|---|---|---|---|---|---|---|---|
| COMBI-d | Dabrafenib + trametinib | 211 | 69 | 12.9 | 11.0 | 25.1 months | 32 |
| Dabrafenib + placebo | 212 | 53 | 10.6 | 8.8 | 18.7 months | 32 | |
| COMBI-v | Dabrafenib + trametinib | 352 | 64 | 13.8 | 11.4 | – | 52 |
| Vemurafenib | 352 | 51 | 7.5 | 7.3 | – | 63 | |
| Co-BRIM | Vemurafenib + cobimetinib | 247 | 68 | ND | 9.9 | 81%† | 65 |
| Vemurafenib + placebo | 248 | 45 | 7.3 | 6.2 | 73%† | 59 | |
The COMBI-d trial (see Table 2) looked atcombining dabrafenib to either trametinib (n = 211) or a placebo (n = 212) in previously untreated patients with unresectable stage IIIc or metastatic melanoma with BRAF mutation disease. The combination therapy demonstrated over anti-BRAFV600 monotherapy an improvement in progression-free survival (11.0 vs 8.8 months, p = 0.0004), in overall response rates (69 vs 53%, p = 0.0014) and overall survival (25.1 vs 18.7 months, p = 0.0107) [32].
The Phase III COMBI-v trial evaluated the association of dabrafenib and trametinib (n = 352) to vemurafenib monotherapy (n = 352) in patients with previously untreated BRAF-mutated metastatic melanoma. The combination therapy compared with anti-BRAFV600 monotherapy showed an improvement in response rates (64 vs 51%, p < 0.001), in progression-free survival (11.4 vs 7.3 months, p < 0.001) and 12-month overall survival rate (72 vs 65%) [33].
The role of the oncology pharmacist
The role of the oncology pharmacist in treating patients is multifold (see Table 3). The first step would be at the validation of the prescription, whether the treatment is chemotherapy, immunotherapy or targeted therapy. It is here where we validate appropriate treatment choice versus clinical indication, appropriate dose of treatment versus renal and hepatic function and if any dosage recommendations are required. A medication reconciliation should be done to make sure that the patient is not taking any other medications, which may interfere with the efficacy of the chosen antimelanoma therapy. During medication reconciliation, it is always very important to define if the patient is taking any CAM, which may also have an impact on the efficacy of treatment.
Table 3. . Roles of the oncology pharmacist in treating patients with metastatic melanoma.
| Role | Details |
|---|---|
| Prescription validation | • Validating clinical indication and appropriate therapy, validating dose, route of administration and dosing schedule, validation of dosage adjustment versus toxicities and renal or hepatic function |
| Treatment preparation | – |
| Education | • Other healthcare professionals • Patients: medication history, medication reconciliation, patient counseling, side effect prevention and management, managing drug interactions, counseling on complimentary or alternative therapies (natural products) |
| Side effect management and supportive care, if required | – |
| Facilitator role for drug access or access to compassionate programs, if required | – |
| Research and managing clinical trials | – |
| Linking with other healthcare practitioners in the community (e.g., community pharmacists) | – |
The oncology pharmacist plays an important role in the preparation of the antimelanoma treatment. Independently of what type of therapy will be given to the patient, he must make sure that the chosen treatment is prepared in the safest fashion possible and in accordance with and with respect to current manipulation of hazardous products recommendations (e.g., NIOSH) or basic aseptic technique recommendations (e.g., USP). The techniques of preparation of these products are often delegated to pharmacy technicians or technical assistants. Their tasks are delegated tasks under the supervision of an oncology pharmacist: they must well understand their responsibilities, use the most current techniques of preparation which are validated, evaluated periodically and controlled by the oncology pharmacist.
Education is an important role of the oncology pharmacist. It can be towards other healthcare professionals or residents or even students. But the most important educational role is towards the patient. Each patient starting antimelanoma therapy should be met with and have the chosen treatment explained. Issues of appropriate dosage and how to take the medication the most efficiently (e.g., in the context of oral targeted anti-BRAFV600 and anti-MEK therapy). The dosing schedule should be clear for the patient and so should be the treatment plan. It is important to discuss side effects of the chosen therapy and what can be done either to prevent, if possible, or to control them; this issue is very important with anti-CTLA-4 or anti-PD-1 therapy where the side effects are more frequent with combination therapy, they may also present at any given time and sometimes even after the treatment has stopped. All of this information should be reinforced with a written handout and clear information on where to call about any questions, issues, or worries.
During the patient counseling described above, it is essential to do a complete medication history and medication reconciliation to detect any contraindications or discrepancies with regards to the current medication list or potential drug interactions, and to validate if the patient is taking or seeking any CAM.
The oncology pharmacist is an important player in the multidisciplinary approach to optimizing treatment of the patient with metastatic melanoma; he may bring his expertise to the healthcare team and be of value to others by his skills in education, communication of knowledge to others and his thorough and keen knowledge about current therapies and how to maximize their therapeutic benefit and minimize toxicities and how to best manage them [4,34,35].
Drug interactions
During the last 20 years, we have witnessed a trend of classic chemotherapy being challenged by newer therapies such as monoclonal antibodies and targeted therapy. There has also been a trend to develop oral therapies in order to simplify therapies for patients and to try to decrease the number of patients being treated in ambulatory oncology clinics [36,37]. This is also true for the treatment of metastatic melanoma. Yet, this brings on issues of potential drug–drug interactions which need to be challenged because they are a major concern in oncology, due to a narrow therapeutic index of molecules and toxicity of anticancer therapies. This is at the heart of what an oncology pharmacist does on a daily basis whether it be during prescription validation, medication reconciliation or patient counseling [38,39].
Drug–drug interactions happen when two drugs that are either inducers, inhibitors or substrates of the same metabolic enzyme are administered together and could lead to an unexpected metabolism of one or the two drugs. This could lead potentially to a lack of efficacy or severe toxicity [40,41]. The nature of drug–drug interactions can either be pharmacokinetic, pharmacodynamic or pharmaceutical. Pharmacokinetic interactions happen when one drug alters another drug by affecting either its absorption, distribution, metabolism or excretion. Pharmacodynamic interactions occur when drugs influence each other's effect directly due to competing at a receptor site on the same physiological system. Pharmaceutical interactions take place when drugs are combined in solution or admixed during infusion that is issues of physical or chemical compatibilities [40–43].
Pharmacokinetic drug–drug interactions altering drug metabolism are common. The CYP450 is a metabolic enzyme system whose purpose is to eliminate drugs from the body. Many enzyme families have been identified within this entity; yet, the CYP3A4 is the most common involved in the metabolism of current medications. There are other isoenzymes which should be taken into consideration: CYP1A2, CYP2C9 and CYP2D6. A drug that inhibits the CYP450 enzymes will generally decrease the metabolism of a given drug, thus leading to potentially higher drug concentrations and therefore potential increased toxicity. This phenomenon happens quickly. The opposite phenomenon, where a drug induces the CYP450 system, would result in an increased clearance of the other drug, decreasing its serum concentration and potentially its efficacy. This phenomenon presents itself in a slower fashion. If a drug is metabolized to an active drug via the CYP450 system and is given with an inducer, we would see an increased serum concentration of the active metabolite, which could cause potentially serious toxicities [40,44–46].
We will try to define potential drug interactions with anti-CTLA-4, anti-PD-1, anti-BRAFV600 and anti-MEK therapies. We will try to address this issue in a practical fashion and try to offer tools that may help out oncology pharmacists in their current practice.
Immunotherapy, checkpoint inhibitors: ipilimumab, nivolumab, pembrolizumab
Ipilimumab is a monoclonal antibody and is not metabolized by the CYP450 enzyme system. A Phase I combination trial of ipilimumab and vemurafenib had been stopped prematurely due to hepatotoxicity; this association is therefore not recommended [11,47].
No formal drug interaction trials have been conducted with regard to nivolumab or pembrolizumab. The risk of drug interactions for these monoclonal antibodies is considered very low [48,49].
The question that arises frequently with immunotherapy is whether immunosuppression with corticosteroids renders the immunotherapy less effective [20]. Some authors believe that the use of prior or concomitant immunosuppression can make this happen [26]. Immunosuppression is often used to treat immune-related adverse events and studies so far have demonstrated that the use of immunosuppression has not had a negative impact on response rates or on time to response compared with patients treated with immunotherapy not requiring at any given time immunosuppression [20,24]. Recommendations from the manufacturers are that immunotherapy should not be started if the patient is on active immunotherapy with corticosteroids because of the potential interference with the pharmacodynamic activity of the immunotherapy [47–49]. Some authors will put the immunotherapy on hold while treating an immune-related adverse event and will only restart the immunotherapy once the corticosteroids are tapered down to equal or less than 10 mg/day [50].
Targeted therapy: anti-BRAFV600 & anti-MEK therapy
Vemurafenib
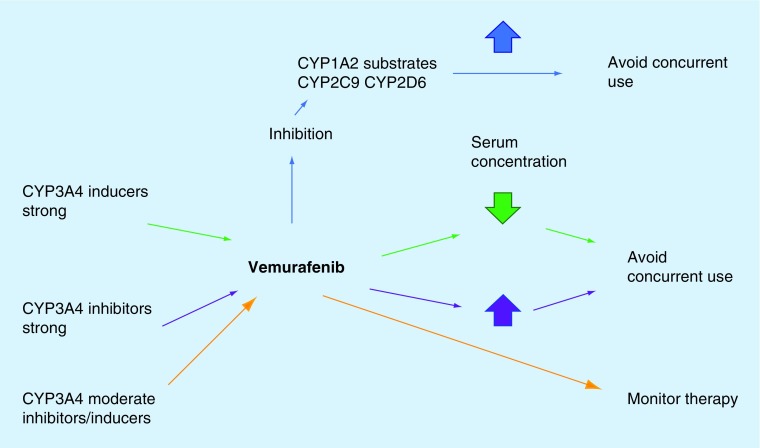
Vemurafenib is a substrate of BCRP and p-glycoprotein, and a major substrate of CYP3A4. Therefore, drugs that are either strong inducers or inhibitors of CYP3A4 (see Table 4) should be avoided if to be used concomitantly with vemurafenib; or an alternative to the inducer or inhibitor should be considered [36,51–55] Strong inducers may decrease the serum concentration of vemurafenib and have an effect on the efficacy of the molecule. Strong inhibitors would increase the serum concentration of vemurafenib and may put the patient at risk of significant toxicity (see Figure 1) [36,52,55].
Table 4. . Strong inducers and inhibitors of CYP3A4/CYP2C8.
| Category | Compound |
|---|---|
| CYP3A4 inhibitors | Amiodarone, cimetidine, clarithromycin, ciprofloxacin, diltiazem, erythromycin, fluconazole, ketoconazole, posaconazole, voriconazole, fluoxetine, fluvoxamine, grapefruit juice, indinavir, nelfinavir, lopinavir/ritonavir, saquinavir, itraconazole, norfloxacin36 |
| CYP3A4 inducers | Barbiturates, carbamazepine, dexamethasone, efavirenz, griseovulfin, phenytoin, primidone, rifabutin, St John's wort |
| CYP2C8 inhibitors | Montelukast, demfibrozil |
| CYP2C8 inducers | Rifampicin |
Figure 1. . Potential drug interactions with vemurafenib.
Vemurafenib is also known for causing inhibition of the metabolism of drugs that are substrates of the CYP1A2 (e.g., caffeine) and can cause an increase in the serum concentration of the substrate. Vemurafenib can also inhibit CYP2C9 (e.g., warfarin) and CYP2D6 (e.g., dextromethorphan). An alternative should be considered or the substrate should be avoided if it has a narrow therapeutic index [36,46,52,55].
Dabrafenib
Dabrafenib is a BRAFV600 inhibitor which is metabolized to hydroxy-dabrafenib, desmethyl-dabrafenib and carboxy-dabrafenib. The clinical activity is essentially defined by the parent molecule and the first two metabolites; the role of carboxy-dabrafenib is considered to be minimal with regard to clinical activity [56–58]. Drug interactions have the potential to affect the serum concentrations of dabrafenib and its metabolites [58].
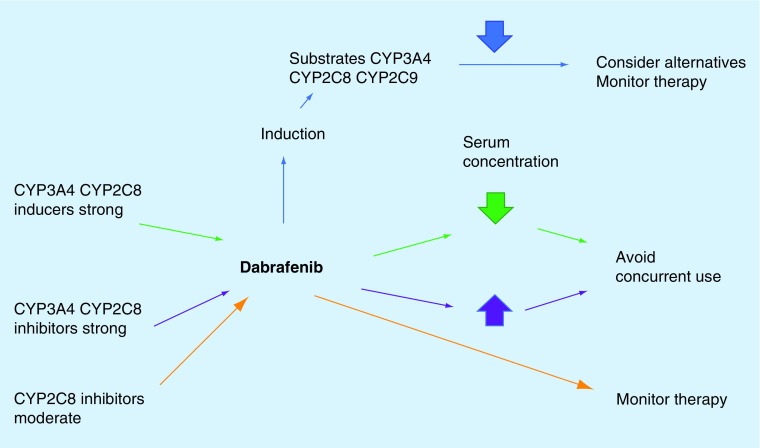
Dabrafenib is considered to be a major substrate of CYP2C8 and CYP3A4. Drugs that are either strong inhibitors or inducers of CYP2C8 should not be used concomitantly with dabrafenib (see Table 4) [36,46,52,56]. Strong CYP3A4 inducers may decrease the serum concentration of dabrafenib and one should monitor therapy closely; strong CYP3A4 inhibitors should be avoided because the may cause increased serum concentration of dabrafenib and exacerbate toxicities [52,56].
Dabrafenib is also known to be a moderate inducer of CYP2C8, CYP2C9 and CYP3A4. Dabrafenib may decrease the serum concentration of CYP2C8, CYP2C9 and CYP3A4 substrates; alternative therapies should be considered to the given substrate and concomitant therapy should be avoided; if this is not feasible, patients should be monitored closely for efficacy of the substrate [52,56]. Dabrafenib is known to decrease serum concentrations of warfarin, hormonal contraceptives and dexamethasone; substitution should be considered (see Figure 2) [58].
Figure 2. . Potential drug interactions with dabrafenib.
Trametinib
Trametinib is a MEK inhibitor that does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2D6 and CYP3A4 and the inhibition of CYP2C8, CYP2C9 and CYP2C19 which is weak occurs at much higher concentrations than the therapeutic level. Trametinib is an inducer of CYP3A4 in vitro but at a low level. Therefore, interactions with substrates are not anticipated [46,52,56,59],56.
Cobimetinib
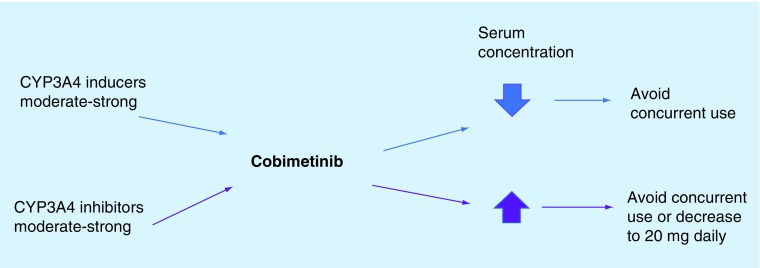
Cobimetinib is another anti-MEK molecule which is a known major substrate for CYP3A4; inducers should be avoided because they may decrease the serum concentration of cobimetinib and potentially have an impact on therapeutic efficacy. CYP3A4 inhibitors should be avoided if used concomitantly with cobimetinib because they may increase the serum concentration of cobimetinib and may lead to increased toxicity. Some authors recommend decreasing the cobimetinib dose to 20 mg daily (regular dosage is 60 mg p.o. daily; see Figure 3) [46,52,60].
Figure 3. . Potential drug interactions with cobimetinib.
Cobimetinib has been shown in vitro to be an inhibitor of CYP3A and CYP2D6 but this effect has not been seen at clinically relevant doses in clinical practice [46,60,46].
The above recommendations should be considered and individualized to the patient and his clinical condition, co-morbidities and other medications or CAM he may be taking. We recommend using the following tools in helping the oncology pharmacist in his decisional process (see Box 1).
Box 1. . Useful tools for the oncology pharmacist to help in dealing with drug interactions.
Drug monographs and package inserts
www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/UCM292362.pdf
Complementary & alternative medicine
CAM is defined as:
“A group of diverse medical and healthcare systems, practices, and products that are not generally considered to be part of conventional medicine”.
This is a very large definition and for the sake of the oncology pharmacist's interest, we will limit this definition to that of natural products that encompass herbs, vitamins and minerals, probiotics, homeopathic products [62,63]. A newer concept which is growing in popularity is that of integrative medicine where alternative therapies are integrated to science-based medicine and offer the patient the integration of two very different approaches to their treatment. The use of CAM is on the rise in the oncology population and this is probably also true in patients with metastatic melanoma [64–66].
Studies have shown that CAM use is higher in patients of the female sex, patients with a higher level of education, in non-Hispanic white race patients, in patients with diminished functional status and patients of a younger age [67]. A survey has demonstrated that fewer than half of oncologists engage discussions with their patients about herbs and supplements because of a lack of knowledge and education that inhibits an open discussion about the topic. Oncologists are yet worried about herbs and supplements interfering with the efficacy of a patient's chemotherapy treatment or increasing the risk of toxicities or unwanted complications. The oncology pharmacist is the ideal healthcare practitioner to deal with this issue because of his knowledge in pharmacotherapy, his interest in optimizing the cancer treatment and his focus on patient care [68,69].
Many issues must be taken into consideration when dealing with patients that have metastatic melanoma or any other cancer that are using CAM or natural products. These products are not regulated in a stringent and rigorous fashion as traditional drugs used in medicine. This brings on issues with regard to contamination of the products that have been reported with either metals or bacteria; issues of variations of quantity or concentrations for a same product between different lots, which could have an effect on efficacy or toxicities of a given CAM. There may also be variations between a same product made by different manufacturers [64,66,70]. There is also a lack of well-done rigorous clinical trials demonstrating in a convincing manner the efficacy of a given CAM or either documenting the given toxicity profile of CAM [63,67]. From an oncology perspective, there is also the important issue of CAM interacting with potentially curable chemotherapy regimens and leading to a lack or decrease of efficacy of the chemotherapy [69].
It is very easy as an oncology pharmacist when counseling a patient that has metastatic melanoma using CAM to take the ‘tough approach’ and simply tell the patient that these products have not demonstrated any efficacy and may be a serious waste of time. Being oncology pharmacists with the background that we have, the education and knowledge we have about drug integrity, manufacturing processes, it is in our nature to take this ‘tough approach’; for the oncology pharmacist, this is a simple way of dealing with the problem. And so the patient does not put himself at risk of a lack of efficacy of treatment, potential drug interactions caused by CAM or unwanted and unpredictable toxicities potentially attributed to CAM. Therefore, the oncology pharmacist is at ease with this approach: better safe than sorry. Yet, knowing that many cancer patients use these products and are afraid to disclose this fact with their healthcare professional for many reasons, being rigid when discussing about CAM with your patients may simply put at risk the relationship the patient has with his healthcare practitioner; and then you have jeopardized the relationship of confidence that should exist between a patient and the practitioner [66,68,71,72].
There are few clinical trials evaluating the interactions between CAM and targeted therapy or immunotherapy [73]. Some natural products are well-known inhibitors of the CYP enzyme system, such as St John's Wort that is well documented to interact with irinotecan but can interact with other substrates of the CYP system; as do garlic, Ginko Biloba, kava, ginseng, Echinacea, milk thistle and evening primrose oil [36,53,70,74,75]. These natural products most likely do not interact with immunotherapy for reasons we discussed earlier [48,49]. Yet, there is a potential for interaction with the CYP system and it would probably be safer to not use these CAM with vemurafenib, dabrafenib or cobimetinib since there are no data justifying the safe use of these combinations and also because we do not know to which intensity this interaction may take place. Grapefruit juice or derivatives are not CAM but are known to be an important inducer of the CYP system. It should be avoided in combination with vemurafenib or cobimetinib [55,56,60,73,76].
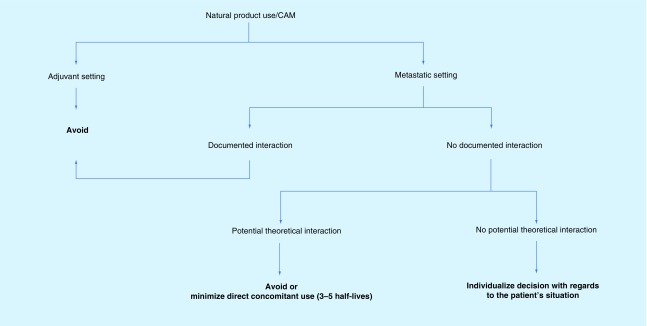
When counseling a patient receiving active therapy and the patient is considering or using CAM one must first consider if the patient is in the adjuvant versus palliative setting; if the patient is receiving curative adjuvant therapy, you probably will not want to jeopardize the efficacy of the treatment or induce unwanted toxicities by taking natural products. In the metastatic setting, you may be more tolerant with regard to natural product use, especially if there is no documented interaction. If ever there is a documented interaction (e.g., St John's Wort), you should base your decision on the available scientific literature. If you are in the ‘unknow,’ which is often the case with natural products, you may need to make your decision by individualizing the situation, keeping in mind issues such as the patient's age, co-morbidities, performance status. There is an interesting concept that we use in our practice occasionally: in a situation where a patient adamantly wants to take a natural product and requires active anticancer therapy, stopping the natural product three to five half-lives before the chemotherapy treatment and restating it three to five half-lives after the chemotherapy treatment minimizes dramatically, around 80%, of a potential pharmacodynamic interaction [73,77]. This is an easy concept to put into practice and worth mentioning; unfortunately, it is not applicable to the subject we are studying, combination therapy in metastatic melanoma, because targeted therapy is given continuously, and immunotherapies have very long half-lives. We propose an algorithm tree to help in deciding with the use of natural products and concomitant cancer therapy (see Figure 4); this process is a guide and should be adapted to each patient's situation. There are many position statements with regard to the use of CAM and natural products and useful websites which may aid the oncology pharmacist in how to counsel and give direction to the melanoma patient considering to use or taking CAM (see Box 2).
Figure 4. . Decision algorithm to help counseling patients receiving anticancer therapy wanting to use complementary and alternative medicine alongside natural products.
Box 2. . Position statements and useful websites which may help in counseling patients with regard to use of complementary and alternative medicine or natural products.
Position statements
British Columbia Cancer Agency www.bccancer.bc.ca/drug-database-site/Documents/NHPandCancerTherapy_1Jan06.pdf
Cancer Care Ontario https://archive.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13376
Clinical Oncology Society of Australia [78]
Useful Websites
www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html
-
Complementary therapies: a guide for people with cancer
-
www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs
Memorial Sloan-Kettering, about herbs, great mobile application
-
Herbs, intended uses, toxicities, and interactions with chemo, complementary and alternative therapies for cancer.
http://naturaldatabase.therapeuticresearch.com/home.aspx?cs=&s=ND&AspxAutoDetectCookieSupport=1
-
National Center for Complementary and Integrative Health
-
https://nccih.nih.gov/health/herbsataglance.htm
Herbs at a glance.
-
www.cancer.gov/publications/patient-education/thinking-about-cam
Thinking about complementary and alternative medicine.
Conclusion & future perspective
Metastatic melanoma is still considered an incurable disease with a dismal prognosis and very resistant to chemotherapy. Yet, the last decade has seen the advent of targeted therapy and immunotherapy that has rendered chemotherapy nearly obsolete for the treatment of metastatic melanoma. There have also been some gains in survival by using combination therapies: combination of anti-BRAFV600 with anti-MEK therapy in order to overcome resistance to monotherapy with anti-BRAFV600 in patients with BRAF positive disease; combination of anti-CTLA-4 with anti-PD-1 therapy in order to increase response rates seen with anti-CTLA-4 monotherapy.
In this article, we have examined the results of the Phase III clinical trials looking at the treatment of metastatic melanoma with combination therapies. We have also defined briefly the role of the oncology pharmacist and how he may help melanoma patients and bring value to a multidisciplinary team. We have examined issues that are part of the daily activities that the oncology pharmacy is faced with and are quite controversial: drug interactions and CAM. These are exciting times for the treatment of metastatic melanoma where patients have hope of increased quality of life and now even hope for longstanding overall survival responses.
Footnotes
Financial & competing interests disclosure
The author has received an honorarium as a consultant for advisory boards of Merck Canada, Bristol-Myers Squibb Canada, Novartis Canada and as a speaker for Merck Canada and Bristol-Myers Squibb Canada. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Zhu Z, Liu W, Gotlieb V. The rapidly evolving therapies for advanced melanoma – towards immunotherapy, molecular targeted therapy, and beyond. Crit. Rev. Oncol. Hematol. 2016;99:91–99. doi: 10.1016/j.critrevonc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. 2017. www.nccn.org Melanoma. Version 1.
- 3.Koller KM, Wang W, Schell TD, et al. Malignant melanoma – the cradle of anti-neoplastic immunotherapy. Crit. Rev. Oncol. Hematol. 2016;106:25–54. doi: 10.1016/j.critrevonc.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Gazzé G. Pharmacist's role in optimizing therapy of the newer agents for the treatment of metastatic melanoma. Melanoma Manag. 2015;2(1):75–82. doi: 10.2217/mmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: recent advances and future directions. Eur. J. Surg. Oncol. 2017;43(3):604–611. doi: 10.1016/j.ejso.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 6.Gibney GT, Atkins MB. Immunotherapy or molecularly targeted therapy: what is the best initial treatment for stage IV BRAF-mutant melanoma? Crit. Rev. Oncol. Hematol. 2015;13(7):1–8. [PubMed] [Google Scholar]
- 7.Han Lau PK, Ascierto PA, McArthur G. Melanoma: the intersection of molecular targeted therapy and immune checkpoint inhibition. Curr. Opin. Immunol. 2016;39:30–38. doi: 10.1016/j.coi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Wolchuk JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstone for curative therapies. Semin. Oncol. 2015;42(3):429–435. doi: 10.1053/j.seminoncol.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106(8):945–950. doi: 10.1111/cas.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno BH, Parisi G, Robert L, Ribas A. Anti-PD-1 therapy in melanoma. Semin. Oncol. 2015;42(3):466–473. doi: 10.1053/j.seminoncol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Simeone E, Grimaldi AM, Ascierto PA. Anti-PD1 and anti-PD-L1 in the treatment of metastatic melanoma. Melanoma Manag. 2015;2(1):41–50. doi: 10.2217/mmt.14.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi MS, Hoffner B, Winkelmann JL, et al. Programmed death 1 immune checkpoint inhibitors. Clin. Adv. Hematol. Oncol. 2015;13(12):858–868. [PubMed] [Google Scholar]
- 16.Moreno BH, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br. J. Cancer. 2015;112:1421–1427. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi AM, Marincola FM, Ascierto PA. Single versus combination immunotherapy drug treatment in melanoma. Expert Opin. Biol. Ther. 2016;16(4):433–441. doi: 10.1517/14712598.2016.1128891. [DOI] [PubMed] [Google Scholar]
- 18.Valpione S, Campana LG. Immunotherapy for advanced melanoma: future directions. Immunotherapy. 2016;8(2):199–209. doi: 10.2217/imt.15.111. [DOI] [PubMed] [Google Scholar]
- 19.Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr. Cancer Drug Targets. 2015;15(6):452–462. doi: 10.2174/156800961506150805145120. [DOI] [PubMed] [Google Scholar]
- 20.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann. Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]; •• It has a more practical, hands-on overview.
- 21.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetic response with ipilimumab. J. Clin. Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 22.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors. JAMA Oncol. 2016;2(10):1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 24.Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat. Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]; •• It has a more practical, hands-on overview.
- 25.Dadu R, Zobniw C, Diab A. Managing adverse events with immune checkpoint agents. Cancer J. 2016;22(2):121–129. doi: 10.1097/PPO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 26.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973–1981. doi: 10.1007/s40265-014-0314-5. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan RJ, Flaherty KT. Pembrolizumab for treatment of patients with advanced or unresectable melanoma. Clin. Cancer Res. 2015;21(13):2892–2897. doi: 10.1158/1078-0432.CCR-14-3061. [DOI] [PubMed] [Google Scholar]
- 29.Gunturi A, McDermott DF. Nivolumab for the treatment of cancer. Expert Opin. Investig. Drugs. 2015;24(2):253–260. doi: 10.1517/13543784.2015.991819. [DOI] [PubMed] [Google Scholar]
- 30.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin J, Ascierto PA, Dréno B, et al. Combined venurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 32.Long GV, Stoyakoskiy HG, Levchenko E, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014;371(20):1877–1887. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 33.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 34.Ashjian E, Redic K. Multiple myeloma: updates for pharmacists in the treatment of relapsed and refractory disease. J. Oncol. Pharm. Practice. 2016;22(2):289–302. doi: 10.1177/1078155215572036. [DOI] [PubMed] [Google Scholar]
- 35.Ministère de la Santé et des Services sociaux. Rapport du Comité de l’évolution de la pratique des soins pharmaceutiques. Government of Québec; 2016. Direction générale de cancérologie. Recommendations sur le rôle du pharmacien en oncologie dans les établissements de santé.www.msss.gouv.qc.ca [Google Scholar]
- 36.Thomas-Schoemann A, Blanchet B, Bardin C, et al. Drug interactions with solid tumor-targeted therapies. Crit. Rev. Oncol. Hematol. 2014;89:179–196. doi: 10.1016/j.critrevonc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Pajares B, Torres E, Trigo JM, et al. Tyrosine kinase inhibitors and drug interactions: a review with practical recommendations. Clin. Transl. Oncol. 2012;14:94–101. doi: 10.1007/s12094-012-0767-5. [DOI] [PubMed] [Google Scholar]
- 38.Viele CS. Managing oral chemotherapy: the healthcare practitioner's role. Am. J. Health-Syst. Pharm. 2007;64(Suppl. 5):S25–S32. doi: 10.2146/ajhp070037. [DOI] [PubMed] [Google Scholar]
- 39.Goodin S. Oral chemotherapeutic agents: understanding mechanism of action and drug interactions. Am. J. Health-Syst. Pharm. 2007;64(Suppl. 5):S15–S24. doi: 10.2146/ajhp070034. [DOI] [PubMed] [Google Scholar]
- 40.Flepisi BT, Bouic P, Sissolak G, Rosenkranz B. Drug–drug interactions in HIV positive cancer patients. Biomed. Pharmacother. 2014;68:665–677. doi: 10.1016/j.biopha.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug–drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br. J. Clin. Pharmacol. 2014;79(2):241–253. doi: 10.1111/bcp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranchon F, Vial T, Rioufol C, et al. Concomitant drugs with low risks of drug–drug interactions for use in oncology clinical trials. Crit. Rev. Oncol. Hematol. 2015;94(2):189–200. doi: 10.1016/j.critrevonc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Mani S, Ghalib M, Chaudhary I, Goel S. Alterations of chemotherapeutic pharmacokinetic profiles by drug–drug interactions. Expert Opin. Drug Metab. Toxicol. 2009;5(2):109–130. doi: 10.1517/17425250902753212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP. Drug interactions between antineoplastic and antiretroviral therapies: implications and management for clinical practice. Crit. Rev. Oncol. Hematol. 2009;72:10–20. doi: 10.1016/j.critrevonc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Yap KY, Chui WK, Chan A. Drug interactions between chemotherapeutic regimens and antiepileptics. Clin. Ther. 2008;30(8):1385–1407. doi: 10.1016/j.clinthera.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Conde-Estévez D. Targeted cancer therapy: interactions with other medicines. Clin. Transl. Oncol. 2017;19:21–30. doi: 10.1007/s12094-016-1509-x. [DOI] [PubMed] [Google Scholar]; • A more practical, hands-on review.
- 47.Bristol-Myers Squibb; Montreal: Canada: 2017. Product monograph. YERVOY ipilimumab. [Google Scholar]
- 48.Bristol-Myers Squibb; Montreal: Canada: 2017. Product monograph. OPDIVO nivolumab. [Google Scholar]
- 49.Merck Canada Inc.; Kirkland: Canada: 2017. Product monograph. KEYTRUDA pembrolizumab. [Google Scholar]
- 50.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904–1911. doi: 10.1002/cncr.30642. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An excellent therapeutic review.
- 51.Haddad A, Davis M, Lagman R. The pharmacological importance of cytochrome CYP3A4 in the palliation of symptoms: review and recommendations for avoiding adverse drug interactions. Support. Care Cancer. 2007;15:251–257. doi: 10.1007/s00520-006-0127-5. [DOI] [PubMed] [Google Scholar]
- 52.Lexicomp Online. https://online.lexi.com
- 53.Tian D, Hu Z. CYP3A4-mediated pharmacokinetic interactions in cancer therapy. Curr. Drug Metab. 2014;15:808–817. doi: 10.2174/1389200216666150223152627. [DOI] [PubMed] [Google Scholar]
- 54.Budha NR, Frymoyer A, Jin JY, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin. Pharmacol. Ther. 2012;92(2):203–213. doi: 10.1038/clpt.2012.73. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman-La Roche Limited; 2017. Product monograph. ZELBORAF Vemurafenib. [Google Scholar]
- 56.Suttle AB, Grossman KF, Ouellet D, et al. Assessment of the drug interaction potential and single- and repeat-dose pharmacokinetics of the BRAF inhibitor dabrafenib. J. Clin. Pharmacol. 2015;55(4):392–400. doi: 10.1002/jcph.437. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence SK, Nguyen D, Bowen C, Richards-Peterson L, Skordos KW. The metabolic drug–drug interaction profile of dabrafenib: in vitro investigations and quantitative extrapolation of the P450-mediated DDI risk. Drug Metab. Dispos. 2014;42:1180–1190. doi: 10.1124/dmd.114.057778. [DOI] [PubMed] [Google Scholar]
- 58.Novartis Pharmaceuticals Canada Inc; Dorval: Canada: 2017. Product monograph. TAFINLAR Dabrafenib. [Google Scholar]
- 59.Novartis Pharmaceuticals Canada Inc.; Dorval: Canada: 2017. Product monograph. MEKINIST Trametinib. [Google Scholar]
- 60.Hoffmann-La Roche Limited; MississaugaCanada: 2017. Product monograph. COTELLIC Cobimetinib. [Google Scholar]
- 61.Harvey RD, Morgan ET. Cancer, inflammation, and therapy: effects on cytochrome P450-mediated drug metabolism and implications for novel immunotherapeutic agents. Clin. Pharmacol. Ther. 2014;96(4):449–457. doi: 10.1038/clpt.2014.143. [DOI] [PubMed] [Google Scholar]
- 62.Bonacchi A, Toccafondi A, Mambrini A, et al. Complimentary needs behind complementary therapies in cancer patients. Psychooncology. 2015;24:1124–1130. doi: 10.1002/pon.3773. [DOI] [PubMed] [Google Scholar]
- 63.Bar-Sela G, Danos S, Visel B, Mashiach T, Mitnik I. The effect of complementary and alternative medicine on quality of life, depression, anxiety, and fatigue levels among cancer patients during active oncology treatment: a Phase II study. Support. Care Cancer. 2015;23:1979–1985. doi: 10.1007/s00520-014-2560-1. [DOI] [PubMed] [Google Scholar]
- 64.Gorski DH. Integrative oncology: really the best of both worlds? Nature. 2014;14:692–700. doi: 10.1038/nrc3822. [DOI] [PubMed] [Google Scholar]
- 65.Savas P, Robertson A, Beatty L, et al. Patient preferences on their integration of complementary therapy with conventional cancer care. Asia-Pac. J. Clin. Oncol. 2016;12:e311–e318. doi: 10.1111/ajco.12226. [DOI] [PubMed] [Google Scholar]
- 66.Davis EL, Byeongsang O, Butow PN, Mullan BA. Cancer patient disclosure and patient-doctor communication of complimentary and alternative medicine use: a systematic review. Oncologist. 2012;17:1475–1481. doi: 10.1634/theoncologist.2012-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baumi JM, Chokshi SC, Schapira MM, et al. Do attitudes and beliefs regarding complementary and alternative medicine impact its use among patients with cancer/a cross-sectional survey. Cancer. 2015;121:2431–2438. doi: 10.1002/cncr.29173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nightingale G, Hajjar E, Guo K, et al. A pharmacist-led medication assessment used to determine a more precise estimation of the prevalence of complementary and alternative medication (CAM) use among ambulatory senior adults with cancer. J. Geriatr. Oncol. 2015;6:411–417. doi: 10.1016/j.jgo.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Lee RT, Barbo A, Lopez G, et al. National survey of US oncologists’ knowledge, attitudes, and practice patterns regarding herb and supplement use by patients with cancer. J. Clin. Oncol. 2014;32:4095–4101. doi: 10.1200/JCO.2014.55.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arslan D, Tural D, Akar E. Herbal administration and interaction of cancer treatment. J. Palliat. Med. 2013;16(11):1466–1476. doi: 10.1089/jpm.2013.0126. [DOI] [PubMed] [Google Scholar]; •• A more practical, hands-on overview.
- 71.Stub T, Quandt SA, Arcury TA, et al. Perception of risk and communication among conventional and complementary health care providers involving cancer patients’ use of complementary therapies: a literature review. BMC Complement. Altern. Med. 2016;16(353):1–14. doi: 10.1186/s12906-016-1326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loquai C, Dechent D, Garzarolli M, et al. Risk of interactions between complementary and alternative medicine and medication for comorbidities in patients with melanoma. Med. Oncol. 2016;33(5):52. doi: 10.1007/s12032-016-0764-6. [DOI] [PubMed] [Google Scholar]
- 73.Fimognari C, Ferruzzi L, Turrini E, et al. Metabolic and toxicological considerations of botanicals in anticancer therapy. Expert Opin. Drug. Metab. Toxicol. 2012;8(7):819–832. doi: 10.1517/17425255.2012.685717. [DOI] [PubMed] [Google Scholar]
- 74.Seely D, Oneschuk D. Interactions of natural health products with biomedical cancer treatments. Curr. Oncol. 2008;15(Suppl. 2):s109.es81–s10.es6. [PMC free article] [PubMed] [Google Scholar]
- 75.Chiu J, Yau R, Epstein RJ. Complications of traditional Chinese/herbal medicines (TCM) – a guide for perplexed oncologists and other cancer caregivers. Support. Care Cancer. 2009;17:231–240. doi: 10.1007/s00520-008-0526-x. [DOI] [PubMed] [Google Scholar]
- 76.Segal EM, Flood MR, Mancini RD, et al. Oral chemotherapy food and drug interactions: a comprehensive review of the literature. J. Oncol. Pract. 2014;10(4):e255–e268. doi: 10.1200/JOP.2013.001183. [DOI] [PubMed] [Google Scholar]
- 77.Seely D. How to address potential interactions between chemotherapy and natural health products. Oncology. http://ndnr.com/oncology/how-to-address-potential-interactions-between-chemotherapy-and-natural-health-products/ Posted 20 May 2007. [Google Scholar]
- 78.Braun L, Harris J, Katris P, et al. Clinical Oncology Society of Australia position statement on the use of complementary and alternative medicine by cancer patients. Asia-Pacific J. Clin. Oncol. 2014;10(4):289–296. doi: 10.1111/ajco.12227. [DOI] [PubMed] [Google Scholar]
- 79.Frenkel M, Sierpina V. The use of dietary supplements in oncology. Curr. Oncol. Rep. 2014;16(11):411. doi: 10.1007/s11912-014-0411-3. [DOI] [PubMed] [Google Scholar]
- 80.Deng G, Cassileth B. Complementary or alternative medicine in cancer care – myths and realities. Nat. Rev. Clin. Oncol. 2013;10:656–664. doi: 10.1038/nrclinonc.2013.125. [DOI] [PubMed] [Google Scholar]