Abstract
Background
Novel biomarkers are needed to assess response to antituberculosis therapy in smear-negative patients.
Methods
To evaluate the utility of C-reactive protein (CRP) in monitoring response to antituberculosis therapy, we conducted a post hoc analysis on a cohort of adults with symptoms of tuberculosis and negative sputum smears in a high–tuberculosis and HIV prevalence setting in KwaZulu-Natal, South Africa. Serial changes in CRP, weight, and hemoglobin were evaluated over 8 weeks.
Results
Four hundred twenty-one participants being evaluated for smear-negative tuberculosis were enrolled, and 33 were excluded. Two hundred ninety-five were treated for tuberculosis (137 confirmed, 158 possible), and 93 did not have tuberculosis. One hundred and eighty-three of 213 (86%) participants who agreed to HIV testing were HIV positive. At week 8, the on-treatment median CRP reduction in the tuberculosis group (interquartile range [IQR]) was 79.5% (25.4% to 91.7%), the median weight gain was 2.3% (−1.0% to 5.6%), and the median hemoglobin increase was 7.0% (0.8% to 18.9%); P < .0001 for baseline to week 8 comparison of absolute median values. Only CRP changed significantly at week 2 (median reduction [IQR], 75.1% [46.9% to 89.2%]) in the group with confirmed tuberculosis and in the possible tuberculosis group (median reduction [IQR], 49.0% [−0.4% to 80.9%]). Failure of CRP to reduce to ≤55% of the baseline value at week 2 predicted hospitalization or death in both tuberculosis groups, with 99% negative predictive value.
Conclusions
Change in CRP may have utility in early evaluation of response to antituberculosis treatment and to identify those at increased risk of adverse outcomes.
Keywords: C-reactive protein, HIV, response to antituberculosis therapy, tuberculosis
Tuberculosis remains a leading cause of death in high–HIV prevalence settings, notwithstanding expanding access to antiretroviral therapy (ART) and improved screening strategies and cure rates [1, 2]. Widespread implementation of a nucleic acid amplification assay, the Xpert MTB/RIF (GXP), and more recently the Xpert MTB/RIF Ultra, has enhanced rapid laboratory diagnosis of tuberculosis [3, 4]. Neither version of the GXP assay, however, can be used as a proxy measure of treatment response due to persistence of mycobacterial DNA after on-treatment culture conversion [5–7]. Empiric antituberculosis therapy in GXP-negative, HIV-positive adults has been frequent, based on nonspecific clinical findings, and is likely to persist [8, 9]. Current World Health Organization (WHO) guidelines recommend that all patients are monitored to assess response to therapy, including persistence or reappearance of symptoms of tuberculosis [9]. Sputum smear for acid-fast bacilli (AFB) remains the objective response to therapy criterion, even in the GXP era [5]. However, for patients who are sputum smear negative at baseline, which occurs frequently in HIV-positive adults, the WHO recommends only clinical monitoring, stating, “Body weight is a useful progress indicator,” without specifying criteria [10]. Case fatality rates during antituberculosis treatment are about 6-fold higher in HIV-coinfected patients and at least 2-fold higher in smear-negative cases [11, 12]. Clinicians therefore need specific tools to monitor smear-negative treatment response to detect patients who are not objectively responding to antituberculosis therapy [13].
Several immune-based biomarkers have been proposed to monitor tuberculosis treatment response, including C-reactive protein (CRP), which is an acute inflammatory protein and component of the innate immune response [14–19]. Hepatic synthesis of CRP is induced by interleukin-6 and interleukin-1β [20, 21]. Serum or whole-blood CRP concentrations are widely used in routine clinical practice and can be measured using affordable point-of-care (POC) devices. Utility of both laboratory and POC CRP testing has been shown when screening for tuberculosis in Africa [22–25], with elevated levels in >90% of HIV-infected individuals at the time of tuberculosis diagnosis [25]. Reductions in CRP concentration after 2 months on antituberculosis therapy have been described in several cohorts of tuberculosis patients [18, 26–29]. Recently, a study of 20 HIV-positive adults with multidrug-resistant tuberculosis found that sustained on-treatment CRP elevation was associated with increased risk of death [30].
To further evaluate the potential prognostic value of serial CRP measurements, we conducted a post hoc analysis of a prospective cohort of individuals with symptoms of tuberculosis and negative sputum smears or inability to produce sputum in KwaZulu-Natal, South Africa.
METHODS
Study Population
We conducted a prospective cohort study between June 2005 and February 2007 of adults (over the age of 18 years), with symptoms suggesting tuberculosis and negative sputum smears for AFB or inability to produce sputum. Potential participants were referred to the clinic-based study team for evaluation for tuberculosis by health care providers working in outpatient KwaZulu-Natal uMgungundlovu District facilities in the Edendale Hospital catchment area. HIV prevalence in this setting is 16.9%, tuberculosis incidence is 922 cases per 100 000, and the rate of HIV coinfection in patients with tuberculosis is around 70% [31–33]. Consenting adults with 3 sputum smears negative for AFB or without sputum were consecutively recruited and included in the study if, at the screening visit, constitutional symptoms or cough was reported as being present for >2 weeks or a focal disease process compatible with active tuberculosis was detected. Exclusion criteria were pregnancy, Karnofsky Performance Status (KPS) <40, tuberculous meningitis, ≥1 week of antituberculosis treatment, <3 months of antiretroviral therapy (ART), or use of a fluoroquinolone within the past 8 weeks. Additional study details can be obtained from published reports [22, 34].
At baseline, study clinicians used standardized criteria (supplementary material) with clinical evaluation, chest radiography, and, where indicated, abdominal and pericardial ultrasound scan, to diagnose smear-negative tuberculosis, with initiation of empiric antituberculosis therapy (treatment arm), or to place the participants under observation without antituberculosis therapy (observation arm). The decision of whether to initiate antituberculosis therapy was made at the baseline visit, before CRP results were available. Sputum and urine specimens for mycobacterial culture were obtained at the baseline visit. Participants’ symptoms were recorded, and individuals’ functional status was subjectively estimated using the KPS [35]. Weight was measured in kilograms on a calibrated electronic scale, and C-reactive protein (CRP) and hemoglobin were measured in venous blood at a commercial laboratory.
On-Study Clinical Review
Standardized clinical review was conducted in all participants at 2, 4, and 8 weeks after the baseline visit, which included KPS, measurement of weight, CRP, and hemoglobin concentration. A symptom score ratio (SSR) was calculated by dividing the aggregate number of symptoms compatible with tuberculosis reported as either markedly improved or resolved by the total number of tuberculosis-compatible symptoms reported at baseline. Study clinicians were not blinded to treatment group during on-study participant review. Participants who deteriorated clinically were referred for further evaluation by the Edendale Hospital Internal Medicine service; admitting clinicians did not have access to study data other than tuberculosis treatment status.
Participants in the observation arm were started on antituberculosis therapy after a pretreatment baseline evaluation if either a positive mycobacterial culture result became available or clinical deterioration occurred. These participants (re-baselined group) were then followed up on treatment on the study’s standardized clinical review for a further 8 weeks after the re-baseline visit at weeks 2, 4, and 8. Initial data obtained during the observation period in the re-baselined group were excluded from the analysis, and the analysis timeline only included the subsequent on-treatment data obtained after the re-baseline visit.
Participants who missed a scheduled appointment were contacted telephonically and rescheduled; these participants’ data were included in the analysis if their visit was within 7 days of their scheduled visit for week 2, and 14 days for weeks 4 and 8. Participants were offered HIV testing at each visit, and those with a positive test were referred for antiretroviral therapy at the week 8 visit, in keeping with national policy at the time of the study.
At the end of the 8-week follow-up period, participants were classified as either having confirmed tuberculosis (defined as a positive culture for Mycobacterium tuberculosis complex from any site or AFB with granulomata on histology), possible tuberculosis (defined as a clinical decision to initiate antituberculosis therapy without a positive culture), and as not having tuberculosis (defined as negative mycobacterial cultures and no initiation of antituberculosis therapy).
An adverse clinical event was defined as death or hospitalization for a medical condition within the first 8 weeks of antituberculosis therapy (tuberculosis treatment group) or during the 8-week observation period (not treated for tuberculosis group).
Statistical Analysis
Data were exported from the original Microsoft Access database and analyzed using Analyze-it for Microsoft Excel, version 4.51. Distribution of continuous data was determined using the Shapiro-Wilk test. Groups of unpaired nonparametric data were compared using the Wilcoxon-Mann-Whitney test. Changes in potential response to therapy measures over time were compared using the Friedman test for repeated measurements; participants with missing data points were excluded from the model. Data obtained from participants classified with an adverse outcome after week 2 were retained in the analysis. Change in CRP was calculated by subtracting the week 2 or week 8 value from the baseline value, and percent change by dividing the change by the baseline value. Change in hemoglobin concentration or weight was calculated by subtracting the baseline measurement from the week 2 or week 8 measurement and dividing the change by the baseline value. Sensitivity/specificity decision tables were generated and used to select CRP percent change cutoffs with a sensitivity of >90% for death or hospitalization. Binary nominal variables were compared using the Fisher exact test, and 2 × 2 tables were used to calculate test performance characteristics. Confidence intervals were set at 95%; those for area under the receiver operating characteristic curve were calculated using R, version 3.4.1, Vienna, Austria.
Ethics Review
The study was approved by ethics review boards of the Universities of KwaZulu-Natal and Cape Town and by the KwaZulu-Natal Department of Health. All participants provided written informed consent.
RESULTS
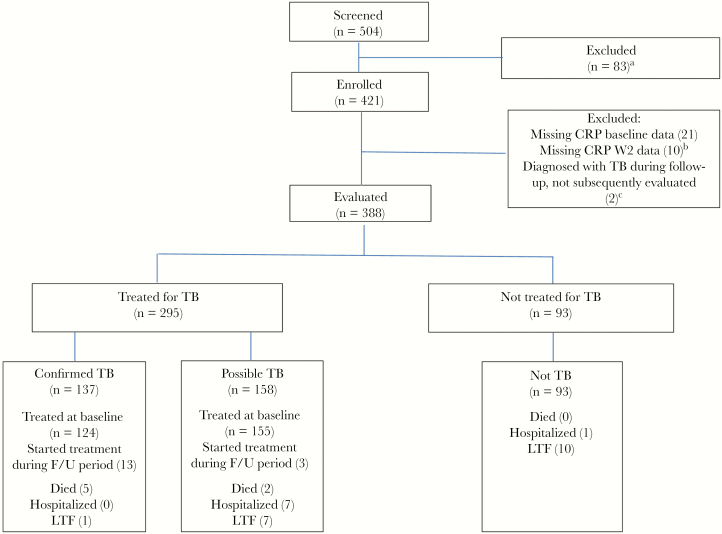
Four hundred twenty-one participants were enrolled, and 388 (92.6%) were included in this analysis. Baseline characteristics are shown in in Table 1. Five participants (1.3%) were taking antiretroviral therapy at the baseline visit. Sixteen participants initially included in the observation arm were diagnosed with tuberculosis and were started on antituberculosis treatment: 13 with confirmed tuberculosis and 3 diagnosed empirically. Participant exclusions and outcomes over the 8-week follow-up period are shown in Figure 1: 295 (76.0%) participants were started on antituberculosis therapy during the study period, and 93 (24.0%) were observed. Eighteen participants were lost to follow-up (Figure 1).
Table 1.
Baseline Characteristics of the 388 Participants Evaluated for Tuberculosis
| Characteristic | |
|---|---|
| Age, median (IQR), y | 34.4 (29.5 to 41.8) |
| Male, No. (%) | 212 (55.9) |
| HIV positive,a No. (%) | 183 (47.2) |
| CD4 count,b median (IQR), cells/µL | 139.0 (77.3 to 246.8) |
| HIV negative,a No. (%) | 30 (7.7) |
| Declined HIV testing, No. (%) | 175 (45.1) |
| Received antibiotic within 2 wk before enrollment, No. (%) | 280 (71.8) |
| Cough for >2 wk, No. (%) | 363 (93.1) |
| Weight loss, No. (%) | 295 (75.6) |
| Anorexia, No. (%) | 275 (70.5) |
| Night sweats, No. (%) | 263 (67.4) |
| Fatigue, No. (%) | 246 (63.1) |
| Fever and chills, No. (%) | 227 (58.2) |
| Chest pain, No. (%) | 221 (56.7) |
| Dyspnea, No. (%) | 154 (39.7) |
| Hemoptysis, No. (%) | 44 (11.3) |
| Peripheral lymph node swelling, No. (%) | 39 (10.1) |
| Abdominal swelling, No. (%) | 5 (1.3) |
| Pulmonary tuberculosis, No. (%) | 268 (69.1) |
| Extrapulmonary tuberculosis, No. (%) | 166 (42.8) |
| Constitutional,c No. (%) | 157 (40.4) |
| Pleural effusion, No. (%) | 87 (22.4) |
| Peripheral lymphadenopathy | 34 (8.8) |
| Mediastinal lymphadenopathy | 30 (7.7) |
| Pericardial effusion | 23 (5.9) |
| Intra-abdominal lymphadenopathy | 10 (2.6) |
| Ascitic exudate | 8 (2.1) |
Abbreviation: IQR, interquartile range.
aIncludes results of HIV tests obtained during the follow-up period, assumed to reflect baseline status.
bOne hundred thirty-nine participants (75.1%) had a CD4 result available during the study period.
cWasting (body mass index of <18.5 kg/m2 or documented weight loss of >5% body weight within a month), plus fever ≥38°C on 2 occasions or drenching sweats for >2 weeks.
Figure 1.
Participant outcomes during the 8-week follow-up period. aNot able to attend for regular review (28), no active symptoms (17), alternative medical diagnosis (14), KPS <40 (5), pneumocystis pneumonia (4), informed consent not obtained (3), sputum smear positive (3), already on antituberculosis treatment (3), other (6). bConfirmed TB (4), possible TB (2), not TB (3), TB diagnosed on culture not treated at BL visit (1). cDied before antituberculosis treatment initiation (1), referred for inpatient Rx before re-baselined week 2 visit (1). Abbreviations: CRP, C-reactive protein; F/U, follow-up; LTF, lost to follow-up; TB, tuberculosis.
Changes in Response to Therapy Parameters During the 8-Week Follow-up Period
Table 2 shows trends for CRP, weight, hemoglobin, KPS, and SSR stratified by final diagnosis. CRP showed the greatest changes from baseline and the greatest early change in the on-treatment tuberculosis groups. Only the objectively measured response to therapy parameters (CRP, weight, and hemoglobin) showed no significant change over the 8-week follow-up period in the group not treated for tuberculosis.
Table 2.
Trends in Clinical Parameters in Participants Treated for Tuberculosis, With Confirmed Tuberculosis, Possible Tuberculosis, and Not Treated for Tuberculosis
| No.a | Baseline | Week 2 | Week 4 | Week 8 | P b | ||
|---|---|---|---|---|---|---|---|
| C-reactive protein, mg/L | Treated for TB | 265 | 56.2 (17.6 to 114.2) | 13.0 (4.0 to 33.3) | 14.0 (5.0 to 28.4) | 8.6 (3.0 to 20.0) | <.0001 |
| Confirmed TB | 125 | 84.0 (45.8 to 128.3) | 15.8 (7.2 to 39.5) | 18.0 (7.0 to 35.7) | 8.0 (3.0 to 20.2) | <.0001 | |
| Possible TB | 140 | 30.4 (8.0 to 84.1) | 10.8 (3.0 to 26.8) | 9.6 (4.0 to 24.0) | 9.0 (3.0 to 20.0) | <.0001 | |
| Not treated for TB | 82 | 3.9 (2.0 to 10.0) | 3.0 (2.0 to 9.0) | 3.0 (2.0 to 10.1) | 3.4 (2.0 to 9.0) | .5 | |
| Weight, kg | Treated for TB | 270 | 56.8 (50.1 to 64.4) | 57.0 (50.6 to 64.9) | 57.9 (51.2 to 65.5) | 58.4 (52.3 to 65.8) | <.0001 |
| Confirmed TB | 128 | 55.6 (49.7 to 62.1) | 55.5 (50.2 to 62.1) | 56.6 (50.6 to 62.1) | 57.7 (52.5 to 63.5) | <.0001 | |
| Possible TB | 142 | 58.1 (50.5 to 66.0) | 58.9 (51.0 to 66.6) | 59.0 (51.9 to 66.8) | 59.9 (52.2 to 68.1) | <.0001 | |
| Not treated for TB | 82 | 59.9 (53.1 to 67.6) | 60.4 (53.9 to 68.2) | 61.0 (54.2 to 69.3) | 60.4 (54.6 to 69.8) | .4 | |
| Hemoglobin, g/dL | Treated for TB | 265 | 10.6 (9.2 to 12.1) | 10.6 (9.4 to 12.2) | 11.1 (9.8 to 12.4) | 11.7 (10.2 to 12.9) | <.0001 |
| Confirmed TB | 125 | 10.2 (8.8 to 11.7) | 10.4 (9.2 to 11.7) | 10.8 (9.7 to 12.3) | 11.7 (10.3 to 12.8) | <.0001 | |
| Possible TB | 140 | 11.1 (9.7 to 12.4) | 10.9 (9.6 to 12.4) | 11.2 (10.0 to 12.7) | 11.7 (10.0 to 13.0) | <.0001 | |
| Not treated for TB | 80 | 13.3 (11.4 to 14.4) | 13.0 (11.6 to 14.4) | 13.0 (11.3 to 14.3) | 13.2 (11.7 to 14.5) | .4 | |
| Karnofsky Performance Score | Treated for TB | 270 | 70 (60 to 80) | 70 (70 to 80) | 80 (70 to 80) | 90 (80 to 90) | <.0001 |
| Confirmed TB | 128 | 60 (50 to 70) | 70 (60 to 80) | 80 (70 to 80) | 80 (80 to 90) | <.0001 | |
| Possible TB | 142 | 70 (60 to 80) | 80 (70 to 80) | 80 (80 to 90) | 90 (80 to 90) | <.0001 | |
| Not treated for TB | 82 | 70 (70 to 80) | 80 (70 to 80) | 80 (70 to 80) | 80 (80 to 90) | <.0001 | |
| Symptom score ratio | Treated for TB | 270 | 0.0 (0.0 to 0.0) | 0.8 (0.6 to 1.0) | 1.0 (08. to 1.0) | 1.0 (1.0 to 1.0) | <.0001 |
| Confirmed TB | 128 | 0.0 (0.0 to 0.0) | 0.8 (0.6 to 1.0) | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.0) | <.0001 | |
| Possible TB | 142 | 0.0 (0.0 to 0.0) | 0.8 (0.7 to 1.0) | 1.0 (0.8 to 1.0) | 1.0 (1.0 to 1.0) | <.0001 | |
| Not treated for TB | 82 | 0.0 (0.0 to 0.0) | 0.6 (0.3 to 0.8) | 0.8 (0.5 to 1.0) | 1.0 (0.7 to 1.0) | <.0001 |
Repeat baseline and on-treatment observations were obtained for 16 participants who started antituberculosis therapy after an initial period of observation. Values at different time points are medians with interquartile ranges in parentheses.
Abbreviation: TB, tuberculosis.
aNumber of participants contributing complete data set to model.
b P value for trend.
At week 2, the median percent CRP reduction (interquartile range [IQR]) was 62.7% (19.7% to 85.1%) in the entire group treated for tuberculosis: 75.1% (46.9% to 89.2%) in the confirmed tuberculosis group and 49.0% (–0.4% to 80.9%) in the possible tuberculosis group. By week 8, the median percent CRP reduction (IQR) was 79.5% (25.4% to 91.7%) in the group treated for tuberculosis, 86.3% (74.8% to 92.4%) in the confirmed tuberculosis group, and 65.9% (–26.6% to 86.7%) in the possible tuberculosis group.
The median percent CRP reduction (IQR) was similar in participants with known HIV status who were treated for tuberculosis: HIV positive vs HIV negative 58.1% (3.9% to 84.3%) vs 23.8% (–40.4% to 90.9%) at week 2 (P = .3), and 79.9% (13.7% to 91.9%) vs 44.4% (–19.6% to 89.2%) at week 8 (P = .3).
In the group not treated for tuberculosis, the median percent CRP change (IQR) did not change significantly: 0.0% (–36.2% to 24.5%) at week 2, and 0.0% (–26.0% to 63.2%) at week 8.
Weight and hemoglobin improved significantly (P < .0001) by week 8 in the confirmed and possible tuberculosis groups, but the magnitude of change was modest, and changes at week 2 were not significant. At week 8, weight (IQR) increased by 2.3% (–1.0% to 5.6%) in the entire group treated for tuberculosis, by 3.6% (–0.7% to 7.2%) in the group with confirmed tuberculosis, 1.6% (–1.4% to 4.3%) in the group with possible tuberculosis, and 0.7% (–1.5% to 2.9%) in the group without tuberculosis. Hemoglobin increased by 7.0% (0.8% to 18.9%) in the entire group treated for tuberculosis, 10.0% (3.0% to 22.9%) in the group with confirmed tuberculosis, 3.6% (–1.8% to 15.4%) in the group with possible tuberculosis, and 0.0% (–3.2% to 5.9%) in the group not treated for tuberculosis.
The subjectively assessed KPS and SSR parameters improved both in the group treated for tuberculosis and in the group not treated for tuberculosis. In comparison with the group treated for tuberculosis, however, the week 8 KPS improved less in the group not treated for tuberculosis, and improvement in the SSR occurred at a slower rate and was less complete. To further evaluate these observations, participants in the group treated for tuberculosis and with paired data (n = 272) were compared with participants in the group without tuberculosis and with paired data (n = 82). The week 8 KPS improved by a median (IQR) of 20 (10 to 30) in the tuberculosis group and by 10 (0 to 10) in the group without tuberculosis; the week 8 SSR reached a median of 1.0 (1.0 to 1.0) in the tuberculosis group and 1.0 (0.7 to 1.0) in the group without tuberculosis (P < .0001 for both comparisons).
Change of Response to Therapy Parameters From Baseline to Week 2 as Predictors of Adverse Outcomes in the Tuberculosis Group
In the group treated for tuberculosis, 7 participants died and 7 were hospitalized (Figure 1). At week 2, both CRP and the SSR showed meaningful improvement in the group treated for tuberculosis, and percent improvement was associated with death or hospitalization (Table 3). The area under the receiver operating characteristic curve was similar for change in CRP and change in SSR (0.83 [95% confidence interval [CI], 0.70 to 0.95] vs 0.75 [95% CI, 0.60 to 0.90], difference 0.08 [95% CI, –0.12 to 0.27]).
Table 3.
Median Percent Change in RTT Parameters From Baseline to Week 2 and AROC for Predicting Death or Hospitalization in the Tuberculosis Treatment Group (n = 295)
| Percent Change From Baseline (IQR) | AROC (95% CI) for Hospitalization or Death | |
|---|---|---|
| C-reactive protein | 62.7 (19.7 to 85.1) | 0.83 (0.70 to 0.95) |
| Symptom score ratio | 80 (60 to 100) | 0.75 (0.60 to 0.90) |
| Hemoglobin | 1.1 (–3.8 to 6.9) | 0.70 (0.55 to 0.84) |
| Weight | 0.5 (–1.1 to 2.4) | 0.65 (0.49 to 0.81) |
| Karnofsky Performance Score | 14.2 (0.0 to 16.7) | 0.57 (0.40 to 0.75) |
Abbreviations: AROC, area under receiver operating characteristic curve; CI, confidence interval; IQR, interquartile range; RTT, response to therapy.
The median CRP reduction from baseline to week 2 (IQR) was 31.0 (3.3 to 77.1) mg/L in the 281 participants with an uncomplicated clinical outcome (65.1% reduction [19.9% to 85.4%]), and −6.0 (−56.5 to 1.0) mg/L in the 14 who died or were hospitalized (−33.8% reduction [−198.8% to 4.3%]; P < .0001 for both comparisons). For the SSR, the median score (IQR) was 0.8 (0.63 to 1.0) in the participants with an uncomplicated course and 0.5 (0.21 to 0.62) in those with an adverse outcome (P = .001).
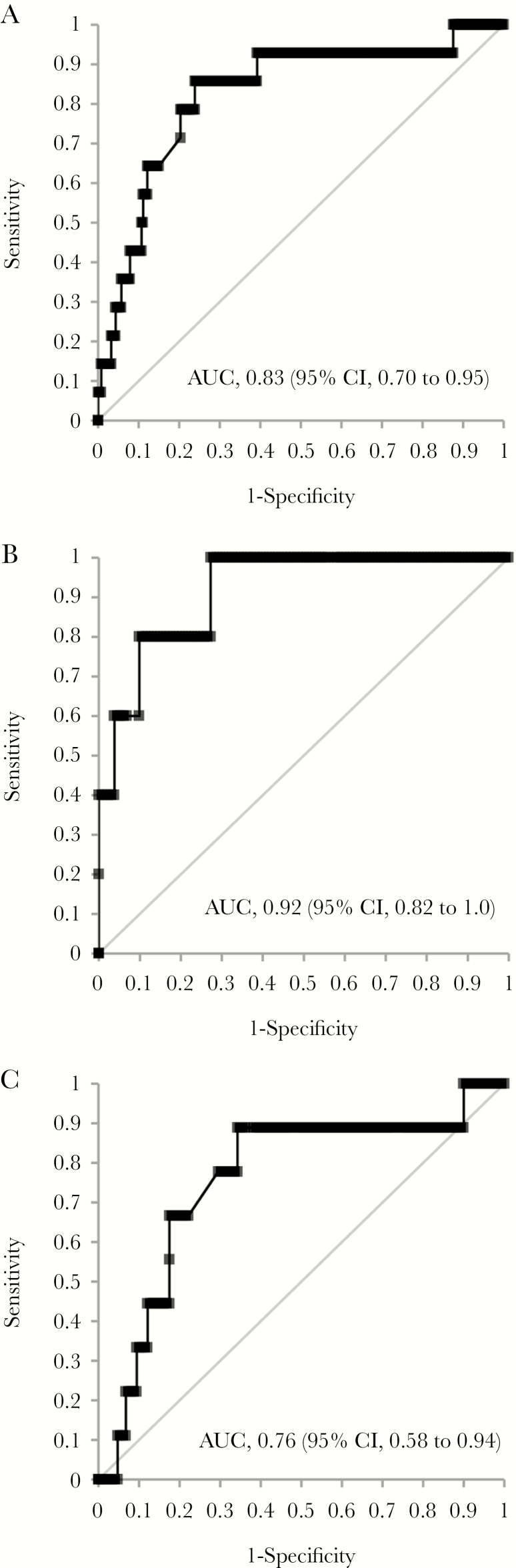
Receiver operating characteristics for the combined end point of death or hospitalization vs percent week 2 CRP change for participants treated for tuberculosis, and in the confirmed and possible tuberculosis subgroups, are shown in Figure 2. Table 4 shows the performance characteristics of the percent change in CRP at week 2, at a cutoff of ≤55%, used as a test to detect those at risk of death or hospitalization. Overall, at this cutoff, in the entire group treated for tuberculosis, the sensitivity was 92% (95% CI, 69% to 99%), specificity was 59% (95% CI, 53% to 64%), and negative predictive value was 99% (95% CI, 96% to 100%).
Figure 2.
Receiver operating characteristics for percent change in C-reactive protein and death/hospitalization. A, Participants treated for tuberculosis (n = 295). B, Participants with confirmed tuberculosis (n = 137). C, Participants with possible tuberculosis (n = 158). Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Table 4.
On-Treatment Performance Characteristics of Baseline to Week 2 Change in CRP in Predicting Death or Hospitalization at Percent Change Cutoff of ≤55%
| Treated for TB | Confirmed TB | Possible TB | |
|---|---|---|---|
| No. of participants | 295 | 137 | 158 |
| No. who died or were hospitalized | 14 | 5 | 9 |
| Sensitivity | 0.92 (0.69 to 0.99) | 1.0 (0.57 to 1.0) | 0.89 (0.57 to 0.98) |
| Specificity | 0.59 (0.53 to 0.64) | 0.71 (0.62 to 0.78) | 0.48 (0.40 to 0.56) |
| Youden’s index | 0.52 | 0.71 | 0.37 |
| Positive predictive value | 0.10 (0.08 to 0.12) | 0.1 (0.09 to 0.14) | 0.09 (0.07: 0.12) |
| Negative predictive value | 0.99 (0.96 to 1.0) | 1.0 (– to –) | 0.99 (0.92 to 1.0) |
| Positive likelihood ratio | 2.2 (1.6 to 2.7) | 3.4 (1.8 to 4.5) | 1.7 (1.1 to 2.1) |
| Negative likelihood ratio | 0.12 (0.02 to 0.53) | 0.0 (0.0 to 0.62) | 0.23 (0.04 to 0.92) |
| Odds ratio | 18.5 (3.0 to 111.8) | +∞ (3.0 to +∞) | 7.5 (1.2 to 47.2) |
Figures in parentheses are 95% confidence intervals.
Abbreviations: CRP, C-reactive protein, TB, tuberculosis.
DISCUSSION
Median CRP concentrations followed strikingly different patterns in participants treated for tuberculosis and those being observed for tuberculosis. Those in the observation group had low CRP concentrations, which remained unchanged during the study period. In contrast, participants diagnosed empirically with tuberculosis and started on treatment had higher CRP concentrations at baseline, which fell significantly by week 2 and approached the normal range by week 8. The trend was most marked in participants with confirmed tuberculosis. These findings are compatible with other studies [18, 26–30] and add additional insights into the potential for CRP to be used as a tool to evaluate response to antituberculosis therapy.
Change in CRP at week 2 predicted death or hospitalization during 8 weeks of antituberculosis therapy. The group of tuberculosis patients who died or were hospitalized within 8 weeks were less likely to experience a reduction in CRP concentration at week 2 from the baseline concentration. Overall, a decrease in CRP of ≤55% at 2 weeks predicted death or hospitalization, with a negative predictive value of 99%. However, this finding is imprecise due to the small number of events.
The other objective responses to the therapy parameters, weight and hemoglobin, have limited utility as they did not change significantly at week 2 and showed only modest improvement at week 8. The subjective responses to therapy parameters changed early and with reasonable magnitude, but their utility is limited by significant changes in participants without tuberculosis; they could have value in patients with positive rapid diagnostic tests for tuberculosis. Change in SSR at week 2 had reasonable discrimination in predicting adverse events.
Our study has a number of limitations. First, the proportion of participants with unknown HIV status was high, and the number of HIV-infected participants on antiretroviral therapy (ART) at the time of enrollment was low. ART was started only after completion of the 8-week study period, in line with guidelines when the study was done. Immune reconstitution syndrome in HIV-seropositive patients with tuberculosis at the time of ART initiation is associated with elevated CRP concentrations [36] and may limit the value of change in CRP at week 2 to predict other adverse clinical events in patients initiating ART soon after commencing antituberculosis treatment. HIV suppression on antiretroviral therapy has been associated with a reduction in CRP levels during the first year of therapy [37]; however, persistently elevated CRP during the first 24 weeks of antiretroviral therapy has been associated with HIV disease progression to WHO stage 3/4 events [38]. Tuberculosis/HIV-coinfected patients with ongoing CRP elevation after ART initiation may require ongoing targeted clinical review and adherence support. Second, drug susceptibility testing was not performed in this study, and some participants may have had drug-resistant infection not responding to firstline treatment. However, in South Africa at the time of this study, only about 2% of tuberculosis cases had multidrug resistance [39]. Third, the number of adverse outcomes in those with confirmed tuberculosis was low in comparison with those with possible tuberculosis, suggesting that diagnoses mimicking tuberculosis, including lymphoma [40], may have caused adverse outcomes in the tuberculosis treatment group. A broad-spectrum antibiotic was prescribed for 72% of participants before baseline evaluation, and falling CRP concentrations in some participants in the possible tuberculosis group may have been due to resolving bacterial infection rather than response to antituberculosis treatment. This would not, however, alter the need for further medical evaluation in this group should CRP remain elevated. Finally, this study was conducted before the implementation of GXP as the firstline diagnostic test for tuberculosis, and CRP trends may be different in GXP-negative tuberculosis cases.
This analysis provides additional information on the utility of monitoring CRP trends to assess early treatment response, with sustained CRP levels at week 2 of treatment being associated with increased risk of adverse clinical outcomes. Further evaluation in the GXP era is needed.
Supplementary Material
Acknowledgments
The authors acknowledge Edendale Hospital, the KwaZulu-Natal Department of Health, study clinicians Dr. Lindo Mbhele and Dr. Langa Ngubane, and research nurses Sr. Pat Bartman and Sr. Zanele Magcaba.
Financial support. This study was funded by BMS Secure the Future.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Global Tuberculosis Report. Geneva: World Health Organization; 2018. http://www.who.int/tb/publications/global_report/en/. Accessed 17 October 2018. [Google Scholar]
- 2. Mortality and Causes of Death in South Africa. Findings From Death Notification. Pretoria: Statistics South Africa; 2015. http://www.statssa.gov.za/publications/P03093/P030932015.pdf. Accessed 17 October 2018. [Google Scholar]
- 3. Steingart KR, Schiller I, Horne DJ, et al. . Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorman SE, Schumacher SG, Alland D, et al. ; study team Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xpert MTB/RIF Implementation Manual. Geneva: World Health Organization; 2014. http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf. Accessed 17 October 2018. [Google Scholar]
- 6. Friedrich SO, Rachow A, Saathoff E, et al. ; Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics (PanACEA) Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 2013; 1:462–70. [DOI] [PubMed] [Google Scholar]
- 7. WHO Meeting Report of a Technical Expert Consultation: Non-inferiority Analysis of Xpert MTF/RIF Ultra Compared to Xpert MTB/RIF. Geneva: World Health Organization; 2017. http://www.who.int/tb/publications/2017/XpertUltra/en/. Accessed 17 October 2018. [Google Scholar]
- 8. Churchyard GJ, Stevens WS, Mametja LD, et al. . Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 2015; 3:e450–7. [DOI] [PubMed] [Google Scholar]
- 9. Stop TB. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings. Geneva: World Health Organization; 2006. http://www.who.int/tb/publications/2006/tbhiv_recommendations.pdf. Accessed 17 October 2018. [Google Scholar]
- 10. Treatment of Tuberculosis Guidelines. 4th ed Geneva: World Health Organization; 2010. http://www.who.int/tb/publications/2010/9789241547833/en/. Accessed 17 October 2018. [Google Scholar]
- 11. Straetemans M, Glaziou P, Bierrenbach AL, et al. . Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One 2011; 6:e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang’ombe C, Harries AD, Banda H, et al. . High mortality rates in tuberculosis patients in Zomba Hospital, Malawi, during 32 months of follow-up. Trans R Soc Trop Med Hyg 2000; 94:305–9. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood N, du Bruyn E, Morris T, Wilkinson RJ. Assessment of treatment response in tuberculosis. Expert Rev Respir Med 2016; 10:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clifford V, Zufferey C, Street A, et al. . Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis (Edinb) 2015; 95:217–28. [DOI] [PubMed] [Google Scholar]
- 15. Jayakumar A, Vittinghoff E, Segal MR, et al. ; Tuberculosis Trials Consortium Serum biomarkers of treatment response within a randomized clinical trial for pulmonary tuberculosis. Tuberculosis (Edinb) 2015; 95:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee MR, Tsai CJ, Wang WJ, et al. . Plasma biomarkers can predict treatment response in tuberculosis patients: a prospective observational study. Medicine (Baltimore) 2015; 94:e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardiner JL, Karp CL. Transformative tools for tackling tuberculosis. J Exp Med 2015; 212:1759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendy J, Togun T, Owolabi O, et al. . C-reactive protein, Neopterin and Beta2 microglobulin levels pre and post TB treatment in The Gambia. BMC Infect Dis 2016; 16:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs R, Malherbe S, Loxton AG, et al. . Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget 2016; 7:57581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 2001; 38:189–97. [DOI] [PubMed] [Google Scholar]
- 21. Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem 2004; 279:48487–90. [DOI] [PubMed] [Google Scholar]
- 22. Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One 2011; 6:e15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drain PK, Mayeza L, Bartman P, et al. . Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis 2014; 18:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoon C, Davis JL, Huang L, et al. . Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr 2014; 65:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon C, Chaisson LH, Patel SM, et al. . Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2017; 21:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis 2000; 4:340–4. [PubMed] [Google Scholar]
- 27. Wilson D, Nachega J, Morroni C, et al. . Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis 2006; 10:31–8. [PubMed] [Google Scholar]
- 28. Mesquita ED, Gil-Santana L, Ramalho D, et al. . Associations between systemic inflammation, mycobacterial loads in sputum and radiological improvement after treatment initiation in pulmonary TB patients from Brazil: a prospective cohort study. BMC Infect Dis 2016; 16:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kedia K, Wendler JP, Baker ES, et al. . Application of multiplexed ion mobility spectrometry towards the identification of host protein signatures of treatment effect in pulmonary tuberculosis. Tuberculosis (Edinb) 2018; 112:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cudahy P, Warren J, Cohen T, Wilson D. Trends in CRP, D-dimer and fibrinogen during therapy for HIV associated multidrug resistant tuberculosis. Am J Trop Med Hyg. 2018. doi:10.4269/ajtmh.18-0322. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shisana O, Rehle T, Simbayi LC, et al. . South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf. Accessed 17 October 2018. [Google Scholar]
- 32. Massyn N, Day C, Peer N, Padarath A, Barron P, English R, eds District Health Barometer 2013/14. Durban: Health Systems Trust; 2014. https://www.health-e.org.za/wp-content/uploads/2014/10/DHB_2013-14.pdf. Accessed 17 October 2018. [Google Scholar]
- 33. Cohen T, Chindelevitch L, Misra R, et al. . Within-host heterogeneity of mycobacterium tuberculosis infection is associated with poor early treatment response: a prospective cohort study. J Infect Dis 2016; 213:1796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson D, Mbhele L, Badri M, et al. . Evaluation of the World Health Organization algorithm for the diagnosis of HIV-associated sputum smear-negative tuberculosis. Int J Tuberc Lung Dis 2011; 15:919–24. [DOI] [PubMed] [Google Scholar]
- 35. Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 2013; 13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narendran G, Andrade BB, Porter BO, et al. . Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8:e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wada NI, Jacobson LP, Margolick JB, et al. . The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shivakoti R, Yang WT, Berendes S, et al. ; NWCS 319 and PEARLS Study Team Persistently elevated C-reactive protein level in the first year of antiretroviral therapy, despite virologic suppression, is associated with HIV disease progression in resource-constrained settings. J Infect Dis 2016; 213:1074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ndjeka N. Multi-drug resistant tuberculosis. Strategic overview on MDRTB care in South Africa. https://www.msf.org.za/sites/msf.org.za/files/Publications/Strategic_overview_of_MDR_TB_RSA.pdf. Accessed 23 April 2018. [Google Scholar]
- 40. Breen EC, Hussain SK, Magpantay L, et al. . B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev 2011; 20:1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.