Abstract
The glucocorticoid (GC) rhythm is entrained to light-dark (LD) cycles via a molecular clock in the suprachiasmatic nucleus (SCN) and is maintained by an adrenal clock synchronized by SCN-dependent signals. Targeted deletion of the core clock gene Bmal1 can disrupt adrenal clock function. The requirement of the adrenal clock to stabilize the circadian GC rhythm during exposure to aberrant LD cycles was determined using novel aldosterone synthase (AS)Cre/+::Bmal1Fl/Fl mice in which Bmal1 deletion occurred during postnatal adrenal transdifferentiation. To examine whether adrenal Bmal1 deletion results in loss of the adrenal clock, mice were crossed with mPER2::Luciferase (mPER2Luc/+) mice. Adrenals from ASCre/+::Bmal1+/+::PER2Luc/+ [control (CTRL)] mice show mPER2Luc rhythms ex vivo, whereas slices from ASCre/+::Bmal1Fl/Fl::PER2Luc/+ [knockout (KO)] mice show dampened rhythms. To monitor corticosterone rhythmicity, mice were implanted with subcutaneous microdialysis probes and sampled at 60-minute intervals for up to 3 days under 12:12-hour [τ (T) 24] LD or 3.5:3.5-hour (T7) LD cycles. Corticosterone rhythms were entrained to T24 LD in CTRL and KO mice. Under T7 LD, circadian corticosterone rhythms persisted in most CTRL mice but not KO mice. Hyperadrenocorticism also was observed in KO mice under T7 LD, reflected by increased corticosterone peak amplitude, total daily corticosterone, and responses to ACTH. Analysis of dysregulated adrenal genes in KO mice exposed to aberrant light identified candidates involved in cholesterol metabolism and trafficking, including steroidogenic acute regulatory protein, which could increase steroidogenesis. Our results show that the adrenal clock functions to buffer steroidogenic responses to aberrant light and stabilize circadian GC rhythmicity.
Circadian rhythms are controlled by a molecular clock in the suprachiasmatic nucleus (SCN) of the hypothalamus that is entrained daily by photic input transmitted by intrinsically photosensitive retinal ganglion cells, synchronizing metabolism, cognition, and behavior to the environmental day-night cycle (1). Because light is the most potent entrainer of the SCN clock, rapid resetting to changing day-night cycles is required during shift work and jet lag to prevent desynchronization of circadian rhythms (2). Conversely, the misalignment of circadian rhythms that occurs chronically in shift work and jet lag contributes to adverse health effects (3). The SCN clock consists of interlocking feedback loops of gene transcription and translation (4). The “positive” limb involves CLOCK and BMAL1, which heterodimerize and activate transcription of the genes, period (Per) and cryptochrome (Cry). The protein products, PER and CRY, function as the “negative limb” by acting on the CLOCK:BMAL1 complex to repress their transcription. The feedback loops are self-regulating with a ∼24-hour periodicity. The molecular clock is found in most tissues (5), providing a peripheral clock mechanism that subserves tissue-specific functional rhythms (6). Thus, shifts in the light-dark (LD) cycle require resetting of the SCN clock that in turn synchronizes the timing of peripheral clocks (7, 8).
Adrenal secretion of glucocorticoids (GCs; cortisol in humans and corticosterone in rodents) is controlled by the hypothalamic-pituitary-adrenal axis and exhibits a prominent circadian rhythm that provides optimal GC exposure throughout the day. Because circadian GC rhythms entrain central (9, 10) and peripheral clocks (11, 12), altered GC rhythms will have major effects on metabolic, hemodynamic, and cognitive function. The adrenal cortex expresses a molecular clock that induces the rhythmic expression of the clock-controlled gene, steroidogenic acute regulatory protein (StAR) (13), and has been implicated in gating adrenal sensitivity to ACTH (14). Surprisingly, adrenal cortex–selective clock disruption has resulted in no alteration in the circadian corticosterone rhythm under 12:12-hour [τ (T) 24] LD (13) and inconsistent responses under constant dark (DD) (13, 15), suggesting that the adrenal clock is not required for maintaining circadian GC rhythms. Instead, the SCN clock may be sufficient for synchronizing circadian GC rhythms under T24 LD and DD. Whether the adrenal clock is important for maintaining GC rhythmicity during chronic exposure to an aberrant LD cycle is unknown. To address this possibility, we have generated, to our knowledge, a novel adrenal cortex–specific Bmal1 knockout (KO) mouse and examined the hypothesis that the adrenal clock is required to maintain the circadian GC rhythm during aberrant light exposure produced by an ultradian 3.5:3.5-hour (T7) LD cycle (16, 17).
Using chronic microdialysis sampling to obtain the temporal resolution required to characterize the circadian GC rhythm in mice (18), we determined that exposure to T7 LD disrupts the circadian GC rhythm and that the adrenal clock acts to buffer aberrant light-induced GC responses. Adrenal Bmal1 KO mice also show increased corticosterone responses to ACTH challenge under T7 LD, suggesting that aberrant light-induced changes in corticosterone may result from increased responsiveness to ACTH.
Methods and Materials
Animal welfare assurance
Mice (7 to 16 months old) were housed on a 12-hour light/12-hour dark cycle (12:12 hours, T24 LD; lights on at 0600 hours). Zeitgeber times (ZTs) of ZT0 and ZT12 were used as the lights-on and lights-off times, respectively. The light intensity at the surfaces of the cages was ∼200 lux. Mice were fed normal, commercial rodent chow and provided with water ad libitum. Prior to tissue collection, mice were humanely euthanized by decapitation or carbon dioxide exposure followed by cervical dislocation, and all efforts were made to minimize suffering. Animals were maintained and cared for in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Experimental procedures were approved by the University of Minnesota Animal Care and Use Committee.
Experimental animals
We generated adrenal cortex–specific clock Bmal1 KO mice by intercrossing aldosterone synthase (AS)–Cre recombinase (ASCre/+, Cyp11b2tm1.1(cre)Brit) mice (19) and Bmal1Fl/Fl mice (Jackson Laboratory, Bar Harbor, ME). Mice expressing ASCre/+ show transdifferentiation of cells from the outer zona glomerulosa (zG) to the cells of the inner zona fasciculata (zF) postnatally (19). By crossing ASCre/+::Bmal1Fl/Fl mice with the Gt(ROSA26Sortm4(ACTB-tdTomato, EGFP)Luo/J) mTomato/mGFP Cre reporter line (20) (Jackson Laboratory), we generated ASCre/+::Bmal1Fl/Fl::R26RmTom/mGFP/+ mice and monitored mGFP expression to determine if Bmal1 deletion altered postnatal transdifferentiation in the adrenal cortex. We also bred homozygous ASCre/Cre::Bmal1Fl/Fl::R26RmTom/mGFP/+ mice to ensure complete recombination (19) for use in gene analysis experiments. To examine whether Bmal1 deletion results in loss of the adrenal clock gene rhythm, we bred ASCre/+::Bmal1Fl/Fl mice with mPER2 Luc/+ (mPER2Luc) mice and monitored ex vivo rhythms in adrenal bioluminescence as a reflection of the mPER2 clock protein rhythm (21, 22). For the above experiments, we used ASCre/+::Bmal1+/+::R26RmTom/mGFP/+ and ASCre/+::Bmal1+/+::mPER2Luc/+ mice.
Immunohistochemistry
Immunohistochemistry of adrenal slices was performed as described previously (23). Adrenals were removed, cleaned of fat, and immersion fixed in 4% paraformaldehyde in PBS overnight; adrenals were stored in PBS at 4°C. Each adrenal was sectioned at 100 μm on a Vibratome 1000 (Vibratome, St. Louis, MO). Free-floating slices were blocked and permeabilized overnight at 4°C in PBS supplemented with 10% donkey serum and 0.5% Triton X-100. The sections were then incubated in primary antibody for 2 to 3 days at 4°C, rinsed with PBS, and incubated overnight in secondary antibody at 4°C. After rinsing in PBS for 1 hour, sections were mounted directly to glass slides and covered with the antifade agent Vectashield (Vector Laboratory, Burlingame, CA). The primary antibody was rabbit anti-BMAL1 (1:1000) (NOVUS Biologicals, Littleton, CO) (24). We used donkey antirabbit cyanine 5 or donkey antirabbit Alexa-594 as secondary antibodies (Invitrogen, Grand Island, NY; 1:500). Confocal z stacks were acquired using an Olympus FluoView FV1000 confocal microscope (Center Valley, PA). Editing of images was limited to adjusting the brightness and contrast levels using ImageJ software (NIH, Bethesda, MD).
Real-time monitoring of bioluminescence
Animals were euthanized by CO2 asphyxiation/decapitation 3.5 to 4.0 hours before lights out (at ZT 8 to 8.5). Adrenals were rapidly excised and placed in cold Hank’s balanced salt solution. After removing the adherent fat, tangential cuts (∼300 μm) were made through the adrenal to produce slices containing only outer cortical tissue or both cortical and medullary tissue. Tissue was placed on Millicell organotypic inserts in a 35-mm Petri dish with 1.5 mL of warmed culture media (DMEM without phenol red) supplemented with luciferin and penicillin/streptomycin as described previously (7, 21). Dishes were sealed with circular glass coverslips and silicon grease. Cultures were maintained at 36°C, and bioluminescence was measured for 1 minute at 7.5-minute intervals for 1 week using photomultiplier tubes in an Actimetrics Lumicycle (Actimetrics, Evanston, IL). Data from the first day of recording were omitted from analysis due to transient bioluminescent activity (8). The remaining data were smoothed and detrended using a 2-hour and 24-hour running average, baseline subtracted, and fit to a damped sine wave using Lumicycle Analysis software (Actimetrics).
In vivo microdialysis sampling
Mice under ketamine/xylazine (100/10 mg/kg intramuscularly) anesthesia were implanted subcutaneously in the dorsal neck region with a microdialysis probe (CMA20 Elite: membrane polyarylethersulfone, 20-kDa cutoff, 10-mm length, 0.5-mm diameter; Harvard Apparatus, Holliston, MA) as described previously in rats (25). Following tethering to a liquid swivel (Instech Laboratories, Plymouth Meeting, PA) and counterbalance arm system (Instech Laboratories), mice were housed separately in Plexiglas mouse cages with food and water ad libitum. This system allows free movement throughout the cage and unrestricted access to food and water. Probes were perfused continuously with sterile saline (0.9%) at 2 μL/min using a syringe pump (Harvard Apparatus). Sampling was initiated at 2 days postsurgery, when mice resumed nesting behavior.
Dialysate corticosterone analysis
Corticosterone was determined by I125 radioimmunoassay using a commercially available kit (MP Biomedical, Solon, OH). Free corticosterone values in a dialysate pool diluted in parallel with the corticosterone standard curve (data not shown); dialysate samples were diluted 1:3 to ensure that values fell on the linear part of the standard curve and final values were corrected for dilution. The intra-assay and interassay coefficient of variation for dialysate corticosterone were 11% and 15%, respectively.
Experimental design
Postnatal transdifferentiation
Adrenals were collected from mice between 7 and 9 months of age. To examine whether postnatal transdifferentiation of zG to zF cells occurred following deletion of Bmal1 in zG cells, adrenocortical mGFP expression was monitored in adrenals from male and female control (CTRL) and KO mice. Confocal z stacks were acquired using an Olympus FluoView FV1000 confocal microscope. Editing of images was limited to adjusting the brightness and contrast levels using ImageJ software (NIH). The pixel area of the total cortex and the cortex expressing GFP was calculated for adrenal slices (n = 4 per adrenal) using ImageJ; to determine the extent of postnatal transdifferentiation, the area of cortical GFP expression was calculated as a percentage of total cortical area.
Circadian locomotor activity
Mice were housed singly in individual cages equipped with running wheels (11.5 cm diameter) that were maintained in ventilated chambers that allowed control of LD cycles. Following housing under T24 LD cycles for 10 to 14 days, mice were exposed to DD for 10 to 14 days. Other mice housed under T24 LD cycles were exposed to a T7 LD (3.5-hour light/3.5-hour dark) cycle (16) for 10 to 14 days. Activity data were collected continuously at 1-minute intervals using a PC system and analyzed using Clocklab software (Actimetrics). The free-running period was calculated over a 10- to 14-day duration under DD or T7 LD using a χ2 periodogram; the amplitude of the circadian (24-hour) and ultradian (7-hour) components was estimated from a normalized Fourier spectrum, and the total daily wheel-running activity (revolutions/d) was obtained for an interval of 10 to 14 days. Following exposure to DD or T7 LD, mice were weighed and euthanized by decapitation; adrenals were harvested, cleaned of fat, and weighed. Adrenals also were harvested from mice under T24 LD that were housed in cages without running wheels.
Subcutaneous microdialysis sampling
To evaluate changes in corticosterone rhythms, CTRL and KO mice exposed to T24 or T7 LD for 3 weeks were implanted with subcutaneous microdialysis probes. Following a 2-day recovery period, dialysate samples were collected continuously at 60-minute intervals for 48 to 72 hours. To examine responsiveness to ACTH, mice then were injected with a maximal dose of ACTH [100 μL, 3.0 μg/kg body weight (BW), subcutaneous (26)], and sampling continued at 30-minute intervals for 3 hours.
Data analysis of corticosterone rhythms
Circadian rhythmicity in dialysate corticosterone and the peak phase (center of gravity) and amplitude of the rhythm were determined for individual mice using CircWave v1.4 (Dr. R. Hut, EUCLOCK, Munich, Germany) (27). A circadian rhythm in free corticosterone was confirmed if P < 0.05. The PULSAR peak detection algorithm (28) was used to determine the number of peaks, average peak amplitude, average peak duration, and interpulse interval over a 24-hour duration as described previously (29). In addition, the area under the curve (total corticosterone above baseline) was calculated to assess the daily corticosterone produced. The following G values were used: G(1) = 4.2, G(2) = 2.4, G(3) = 1.68, G(4) = 1.24, G(5) = 0.93, and smoothing time = 12 hours. These values were chosen based on inspection of the data as recommended in the original method (28). Using these parameters, PULSAR detected no peaks in a data series produced by assaying 50 replicates of a microdialysate pool with a mean concentration of 151.4 ± 16.4 ng/mL (or 6.1 ± 0.1 ng/mL when corrected for dilution), providing some assurance that peaks in the experimental data series are not due to false positives produced by the corticosterone RIA.
Gene expression analysis
Adrenals were collected from ASCre/Cre::Bmal1Fl/Fl KO mice in the am (ZT3 to ZT4) after exposure to T24 LD or T7 LD for 3 weeks. Total RNA was extracted from whole adrenals using a Direct-zol RNA miniprep kit (Zymo Research, Irvine, CA) following the manufacturer’s instructions.
RNA sequencing.
Adrenals were obtained from mice at 8.2 ± 1.5 (n = 4, T24) and 7.4 ± 0.7 (n = 3, T7) months of age. RNA quality was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and only samples with RNA integrity number value >7.0 were used for RNA sequencing (RNA-Seq). Libraries were prepared using a TrueSeq RNA Library Prep Kit (Illumina Inc., San Diego, CA). RNA sequencing was performed on an Illumina NextSeq 500 sequencing platform with single-end 75-bp reads. All samples were processed using an RNA-Seq pipeline implemented in the bcbio-nextgen project (30). Raw reads were examined for quality issues using FastQC (31) to ensure library generation and sequencing were suitable for further analysis. Adapter sequences, other contaminant sequences such as polyA tails, and low-quality sequences with PHRED quality scores <5 were trimmed from reads using cutadapt (32). Trimmed reads were aligned to the genome using STAR (version 2.4.1d) (33). The alignments were performed with the genome build and annotations for GRCm38. Alignments were checked for evenness of coverage, rRNA content, genomic context of alignments (e.g., alignments in known transcripts and introns), complexity, and other quality checks using a combination of FastQC (version 0.11.3) and Qualimap (version 30-03-15) (34), as well as custom tools. Counts of reads aligning to known genes were generated by featureCounts (version 1.4.4) (35). Differential expression at the gene level was called with EdgeR (36). Significantly differentially expressed genes were determined using a false discovery rate cutoff of 0.05 (P values were multiple test corrected using the Benjamini-Hochberg method).
Quantitative RT-PCR.
Adrenals were obtained from mice at 9.5 ± 1.1 (n = 8, T24) and 11.5 ± 1.3 (n = 8, T7) months of age. Complementary DNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed using TaqMan Universal PCR Master Mix and a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The TaqMan Gene Expression probes used for quantitative RT-PCR were as follows: StAR, Mm00441558_m1; Fabp7, Mm00445225_m1; Nr1d1, Mm00520708_m1; Nr1d2, Mm01310356_g1; Per3, Mm00478120_m1; Osbpl6, Mm00467461_m1; Gapdh, Mm99999915_g1; and Actb, Mm02619580_g1. Expression of the genes of interest was normalized to the average expression of two housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and β-actin (Actb), and the comparative 2−ΔΔCT method was used to calculate relative gene expression. Results are presented as n-fold changes in gene expression by adrenals from T7 LD–exposed mice compared with adrenals from T24 LD–exposed mice.
Statistical analysis
Body weight, adrenal weight, adrenal gene transcripts, and locomotor activity parameters represent mean ± SEM from groups of mice. Data from microdialysis experiments are presented as corticosterone values in a series of samples from individual mice or from groups (mean ± SEM) of mice. Statistical differences were determined using one-way ANOVA (using Tukey correction for post hoc analysis), two-way ANOVA (using Sidak correction for post hoc analysis), or unpaired Student t test where appropriate using Prism software (GraphPad Software, La Jolla, CA). Differences were considered significant if P < 0.05.
Results
Deletion of adrenocortical Bmal1 does not impair postnatal transdifferentiation
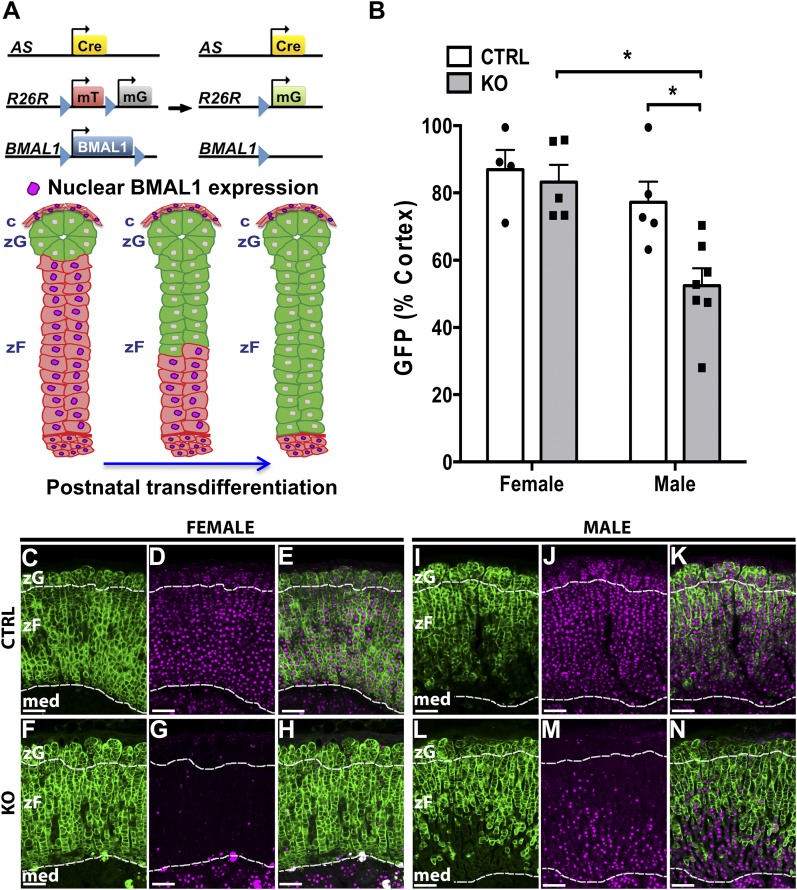
To study the impact of adrenocortical Bmal1 deletion on postnatal transdifferentiation, we generated adrenal Bmal1 KO (ASCre/+::Bmal1Fl/Fl::R26RmTom/mGFP/+) mice by intercrossing ASCre/+ mice (Cre recombinase inserted into the AS genetic locus) with the mT/mG Cre reporter (19) and the floxed Bmal1 strains (Fig. 1A) and CTRL (ASCre/+::Bmal1+/+::R26RmTom/mGFP/+) mice. In CTRL mice, Cre-dependent mGFP expression is first noted in the outer zG during early postnatal life (19). These mGFP cells then undergo continuous centripetal migration and transdifferentiation into inner zF, a process that persists throughout adult life (19). To assess whether adrenocortical Bmal1 affects the process of transdifferentiation, we examined high-resolution GFP images to determine whether the morphology of the zG and/or zF was altered by Bmal1 deletion. In adrenals from male and female CTRL and KO mice, the zG was characterized by globular nests of cells underlying the capsule, and the adjacent zF consisted of parallel radial cords of cells (Fig. 1C–1N). We found no clear differences in adrenal morphology in KO mice that would indicate an effect of Bmal1 deletion on the process of transdifferentiation as defined previously in ASCre/+ mice (19). By monitoring both mGFP and BMAL1 expression in male and female KO mice, we observed loss of nuclear BMAL1 labeling in mGFP+ cells (Fig. 1F–1N). By 7 to 9 months of age, mGFP+ cells and Bmal1 deletion extended throughout the adrenal cortex in female KO mice (Fig. 1F–1H). Analysis of mGFP expression, as a percentage of the total cortex, a proxy for the degree of transdifferentiation and centripetal migration, showed no difference between female CTRL and KO mice (Fig. 1B). These data indicate that the extent of transdifferentiation is not affected by Bmal1 deletion in female mice. In contrast, analysis of age-matched male KO mice revealed a decrease in the extent of transdifferentiation and in Bmal1 deletion (Fig. 1B and 1L–1N). Male KO mice (n = 7) showed reduced postnatal transdifferentiation compared with female KO mice and male CTRL mice (n = 5). Confirming that the AS-Cre model is specific to the adrenal cortex, Bmal1 expression was normal in the adrenal medulla (Fig. 1C–1N). Based on the above sex differences, experiments assessing responses to adrenocortical Bmal1 deletion were performed in female mice.
Figure 1.
Analysis of postnatal transdifferentiation in male and female adrenal ASCre/+::Bmal1Fl/Fl KO mouse. (A) Adrenal clock KO (ASCre/+::Bmal1Fl/Fl::R26RmTom/mGFP/+) mice were generated by breeding ASCre/+ mice with the mTomato/mGFP Cre reporter and floxed Bmal1 mice. During postnatal transdifferentiation, zG cells migrated inward and differentiated into zF cells (19). Cre-dependent deletion of Bmal1 was predicted to occur in parallel with postnatal transdifferentiation reflected by GFP expression. (B) The extent of postnatal transdifferentiation (area of cortical GFP expression/total cortical area × 100) was not different between female ASCre/+::Bmal1+/+::R26RmTom/mGFP/+ CTRL (n = 4) and KO mice (n = 6) at 8 to 9 mo of age. However, male KO mice (n = 7) showed reduced postnatal transdifferentiation compared with female KO mice and male CTRL mice (n = 5). (C, F, I, L) In adrenals from female and male CTRL and KO mice, the zG was characterized by globular nests of cells underlying the capsule, and the adjacent zF consisted of parallel radial cords of cells. No clear differences in morphology were observed in KO mice. (C–E) In a female CTRL mouse (8.0 mo old), mGFP expression and nuclear BMAL1 labeling extended throughout the adrenal cortex from the zG through the zF; 95% of cortical area expressed mGFP. (F–H) In a female KO mouse (8.6 mo old), expression of mGFP was associated with loss of nuclear BMAL1 labeling and extended throughout the adrenal cortex; 94% of cortical area expressed mGFP. (I–K) In a male CTRL mouse (7.6 mo old), expression of GFP did not extend completely through the zF; 77% of cortical area expressed mGFP. (L–N) In a male KO mouse (8.6 mo old), mGFP expression and Bmal1 deletion did not extend completely throughout the adrenal cortex; only 56% of cortical area expressed mGFP. Magenta: BMAL1 immunofluorescence labeling. Borders between zG and zF and between cortex and medulla are denoted by dashed lines. Scale bar, 100 µm. med, medulla.
Deletion of adrenocortical Bmal1 results in the loss of the adrenal clock
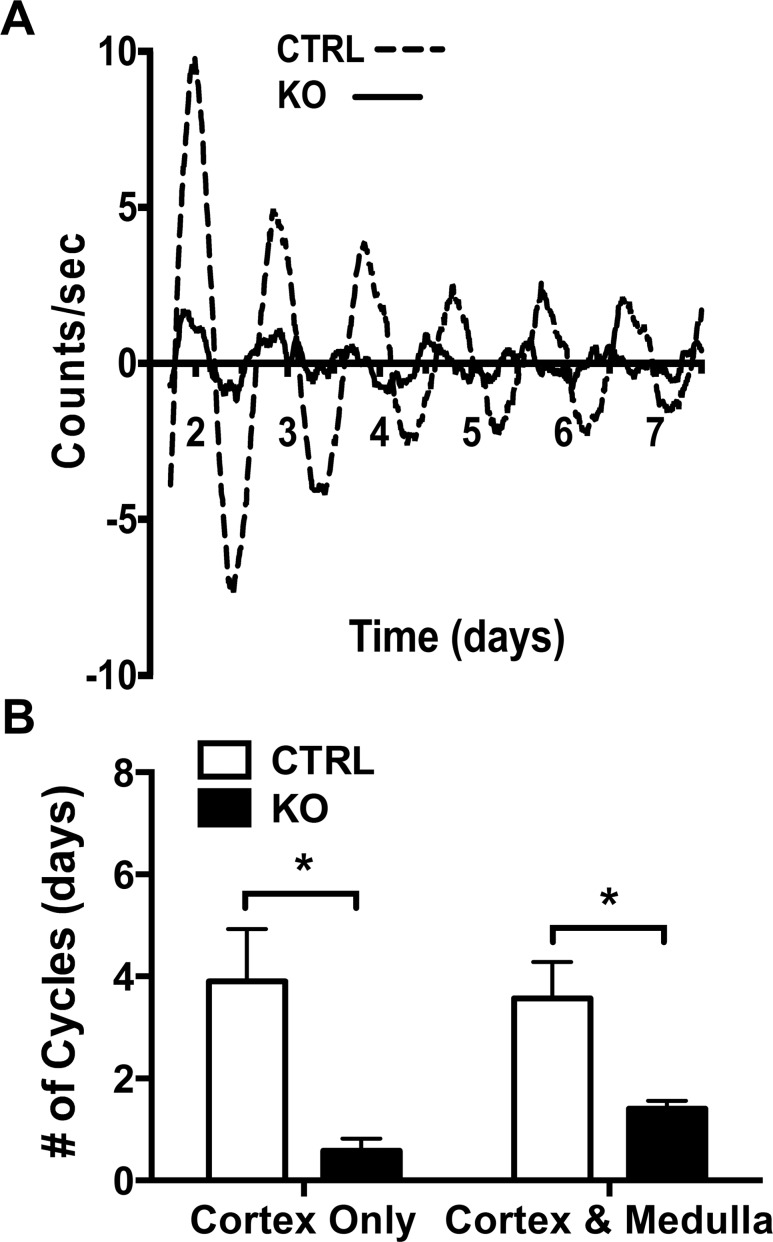
To examine whether deletion of adrenocortical Bmal1 results in the loss of a functioning adrenal clock, we crossed ASCre/+::Bmal1Fl/Fl with mPER2::Luciferase (mPER2Luc) mice in which ex vivo rhythms in adrenal bioluminescence reflect the in vivo mPER2 clock protein rhythm (22, 37). Adrenal slices consisting of cortex and medulla from ASCre/+::Bmal1+/+::PER2Luc (CTRL) mice showed mPER2Luc rhythms that persisted for 4 to 5 days ex vivo, reflecting the output of an endogenous clock mechanism. In contrast, adrenal slices from ASCre/+::Bmal1Fl/Fl::PER2Luc (KO) mice showed rapidly dampening rhythms (Fig. 2A). In KO mice, daily cycles in mPER2Luc activity were diminished in amplitude from adrenal slices consisting of either the cortex only or the cortex and medulla (Fig. 2B). Despite Bmal1 expression in the adrenal medulla (see Fig. 1), these data show that deletion of Bmal1 in the adrenal cortex results in the loss of the endogenous adrenocortical clock.
Figure 2.
Adrenal Bmal1 deletion results in reduced adrenal mPER2Luc rhythms. (A) Adrenal slices from an ASCre/+::Bmal1+/+::PER2Luc CTRL mouse show mPER2Luc rhythms that persist for ∼7 d in vitro, whereas slices from an ASCre/+::Bmal1Fl/Fl::PER2Luc KO mouse show dampened rhythms. (B) Rhythms are diminished in slices of adrenal cortex only and cortex with medulla in KO mice, reflecting the loss of the endogenous cortical clock. Mean ± SEM, n = 5 to 7 mice, *P < 0.05.
Deletion of adrenocortical Bmal1 does not affect circadian rhythms in locomotor activity during exposure to DD or to aberrant light
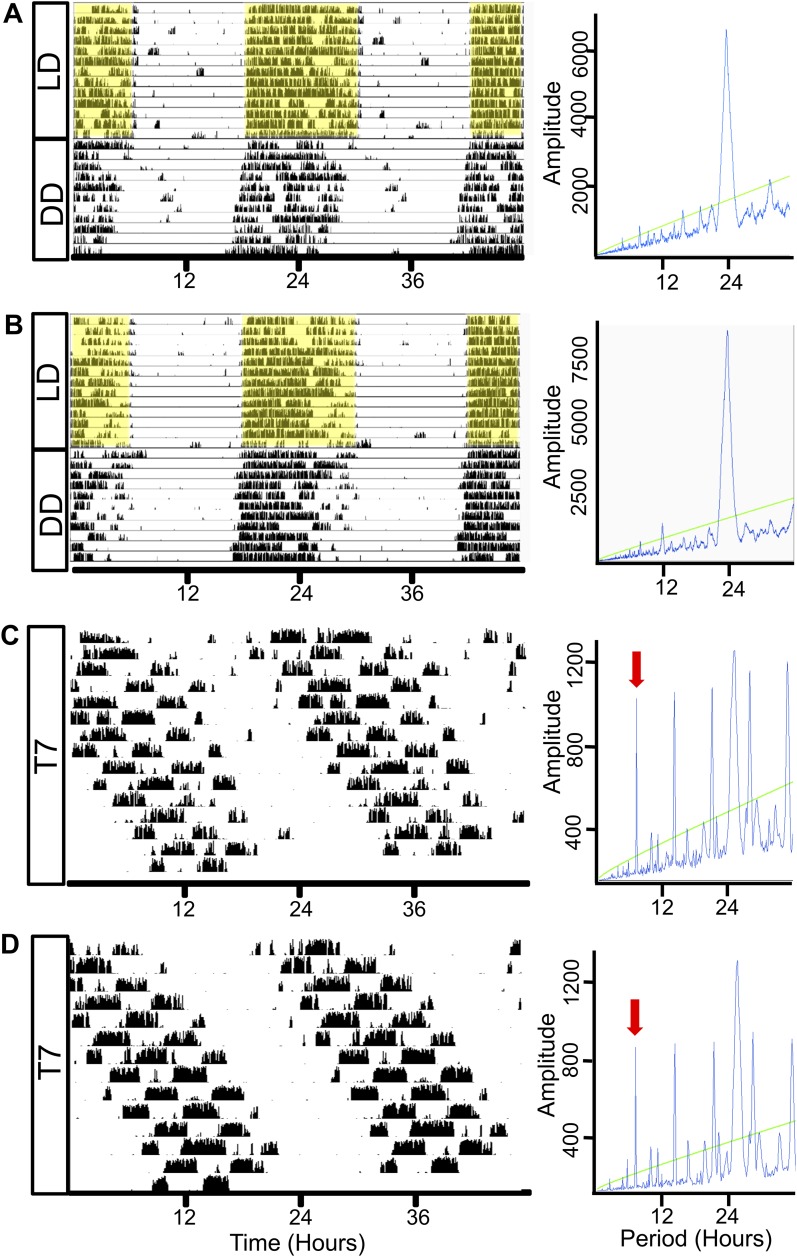
To determine whether adrenal Bmal1 deletion altered rhythms in locomotor activity, running wheel activity was monitored in different groups of mice for 10 to 14 days under T24 LD followed by DD or under T7 LD. CTRL and KO mice showed rhythms entrained to the T24 LD cycle with increased activity during the dark period (Fig. 3A and 3B). After release into DD, free-running rhythms showed a predominant circadian periodicity (Fig. 3A and 3B). Circadian period lengths (τ) and amplitudes did not differ between CTRL and KO mice (Table 1). Under T7 LD, CTRL and KO mice showed ultradian rhythms in activity (Fig. 3C and 3D); however, a prominent circadian periodicity was maintained under T7 LD in both mice (Fig. 3C and 3D). Circadian period lengths (τ), circadian amplitudes, and ultradian amplitudes did not differ between CTRL and KO mice (Table 1). Total daily activity did not differ between CTRL and KO mice under T24 LD (CTRL: 26,998 ± 2037 vs KO 29,618 ± 1002 revolutions/24 hours), DD (CTRL: 34,502 ± 1576 vs KO: 36,127 ± 2999 revolutions/24 hours), or T7 LD (CTRL: 22,913 ± 1576 vs KO: 22,832 ± 1547 revolutions/24 hours). However, total daily activity was decreased (P < 0.05) for both CTRL and KO mice under T7 LD compared with DD but not T24 LD conditions.
Figure 3.
Wheel-running activity under T24 LD, DD, and T7 LD in adrenal clock KO mice. Representative activity records from individual female (A) CTRL and (B) KO mice maintained under a T24 (12:12-h) LD cycle regimen for 10 to 14 d and then transferred to DD for 10 to 14 d. Both (A) CTRL (left panel) and (B) KO (left panel) mice demonstrate light entrainment in T24 LD as indicated by enhanced activity in the dark period. Shaded yellow regions indicate periods of darkness in T24 LD. Under DD, rhythms are free-running; periodograms (right panels) show a predominant circadian periodicity and measured periods (τ) are not different between CTRL (23.83 ± 0.02 h, n = 5) and adrenal clock KO mice (23.81 ± 0.05 h, n = 4). Activity records of (C) CTRL and (D) KO mice housed for 14 d in ultradian light cycles of T7 (3.5:3.5 h) LD. Both CTRL and KO mice are active during dark periods under T7 conditions; however, periodograms (right panels) show a lengthened circadian periodicity in CTRL (24.85 ± 0.09 h, n = 6) and KO (24.82 ± 0.15 h, n = 5) mice. Green line: cutoff for significant periodicity, P < 0.05. Red arrows: 7-h component of periodogram.
Table 1.
BW, Adrenal Weight, and Locomotor Activity Rhythms as a Function of Genotype and LD Cycle
| Genotype | LD | n | Age, mo | BW, g | AW, mg | τ, h | CIRCAmpl | ULTRAmpl |
|---|---|---|---|---|---|---|---|---|
| CTRL | DD | 5 | 7.1 ± 0.4 | 22.2 ± 0.4 | 6.7 ± 0.2 | 23.83 ± 0.02 | 7655 ± 599 | ND |
| KO | DD | 5 | 7.1 ± 0.2 | 25.4 ± 0.5a | 9.3 ± 0.4a | 23.81 ± 0.05 | 7788 ± 504 | ND |
| CTRL | T7 | 6 | 8.4 ± 0.4 | 27.9 ± 1.0 | 7.2 ± 0.3 | 24.82 ± 0.15 | 6496 ± 785 | 2646 ± 649 |
| KO | T7 | 6 | 9.0 ± 0.2 | 26.0 ± 0.8 | 9.5 ± 0.6a | 24.85 ± 0.09 | 8065 ± 314 | 2033 ± 767 |
Data are presented as means ± SEM. CTRL: ASCre/+::Bmal1+/+. KO: ASCre/+::Bmal1Fl/Fl. τ is the circadian period length of free-running activity rhythm.
Abbreviations: AW, adrenal weight; CIRCAmpl, amplitude of circadian (24-h) periodicity; ND, not detected; ULTRAmpl, amplitude of ultradian (7-h) periodicity.
P < 0.05 vs CTRL.
Deletion of adrenocortical Bmal1 increases adrenal mass independently of LD cycle
BW and adrenal weight were increased in KO mice under DD (Table 1). Adrenal weight normalized to BW also increased (CTRL: 30.3 ± 0.8 vs KO: 36.7 ± 1.3 mg/100 g BW; P < 0.05), suggesting that increased adrenal mass occurs independently of increased body growth. After exposure to T7 LD, there were no differences in BW between CTRL and KO mice, but adrenal weight remained higher in KO mice (Table 1). Potential differences in BW or adrenal weight resulting from DD vs T7 LD exposure could not be assessed due to the potential confound that older mice were used for the T7 experiment (Table 1). However, adrenal weight also was increased in another cohort of KO mice housed under T24 LD [CTRL: 7.27 ± 0.22 mg (n = 6) vs KO: 8.83 ± 0.50 mg (n = 4); P < 0.05], indicating that increased adrenal mass occurs independently of LD cycle.
Deletion of adrenocortical Bmal1 does not alter circadian GC rhythms under T24 LD
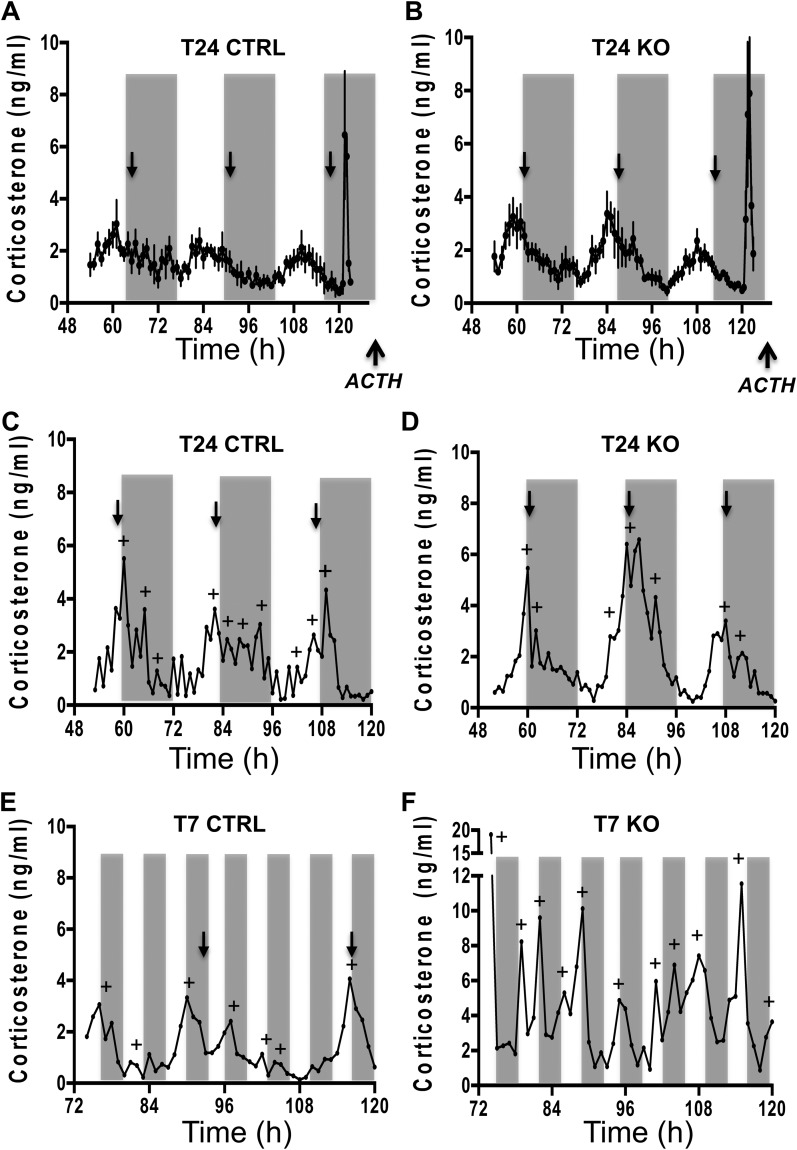
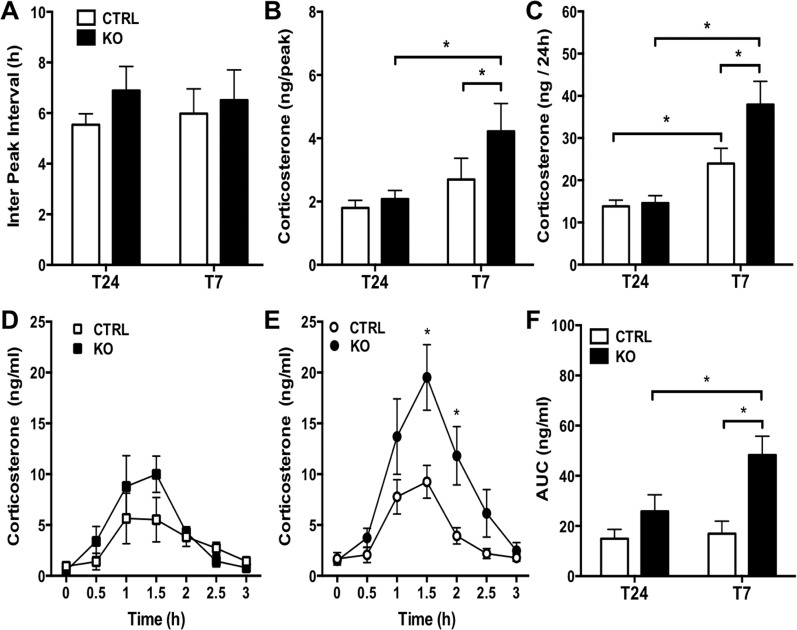
To determine how deleting adrenal Bmal1 affects GC rhythms, we used subcutaneous microdialysis sampling in mice as validated in rats (25). Due to a 20-kDa molecular weight cutoff, the microdialysis probe excludes transcortin-bound corticosterone, resulting in sample collection of free (unbound) corticosterone. Circadian rhythms in free corticosterone were entrained to T24 LD cycles in CTRL and KO mice, showing peak circadian phases near the onset of subjective night (Fig. 4A–4D). There were no differences between CTRL and KO mice in the corticosterone profile across 72 hours of sampling (Fig. 4A and 4B). A circadian rhythm was detected by CircWave in all CTRL and KO mice under T24 LD; there was no difference between genotypes in the amplitude (CTRL: 2.78 ± 0.22 vs KO: 2.42 ± 0.31) or the peak phase (CTRL: 13.6 ± 1.2 vs KO: 12.2 ± 0.5) of the circadian corticosterone rhythm. In addition, corticosterone responses to an ACTH challenge did not differ between CTRL and KO mice under T24 LD (Fig. 4A and 4B; Fig. 5D), although the integrated response (area under the curve) showed a trend (Fig. 5F; P = 0.08) to be higher in KO mice that might reach significance by increasing the sample size.
Figure 4.
Circadian corticosterone rhythmicity under T24 LD and T7 LD in adrenal ASCre/+::Bmal1Fl/Fl KO mice. Seventy-two-hour profiles [mean ± SEM (n = 5 to 6)] of subcutaneous dialysate corticosterone in (A) CTRL and (B) KO mice and in individual female (C) CTRL and (D) KO mice under T24 LD. Peak corticosterone occurred daily at the onset of subjective night in CTRL and KO mice under T24 LD. Forty-eight-hour profiles of dialysate corticosterone in individual (E) CTRL and (F) KO mice under T7 LD. Circadian rhythmicity (defined by CircWave with peak phase denoted by black arrows) was observed in CTRL and KO mice under T24 LD and in CTRL mice under T7 LD; circadian rhythmicity was lost under T7 in most KO mice. Microdialysis samples were collected at 60-min intervals. Gray bars indicate the periods of darkness. Profiles in individual mice were analyzed to detect corticosterone peaks (denoted by +) using PULSAR.
Figure 5.
Increased corticosterone responses under T7 LD in adrenal ASCre/+::Bmal1Fl/Fl KO mice. PULSAR analysis showed that increased (B) peak corticosterone amplitude, but not (A) peak frequency, occurs under T7 LD compared with T24 LD, resulting in increased (C) total daily corticosterone production. Both (B) peak amplitude and (C) total daily corticosterone increased in KO compared with CTRL mice under T7 LD. Mice were injected with ACTH (0.3 μg, subcutaneously) at the end of the sampling period. Increased corticosterone responsiveness to ACTH was observed in KO mice compared with CTRL mice on (E) T7 LD but not (D) T24 LD. (F) Total corticosterone in response to ACTH [area under the curve (AUC)] was increased in KO mice under T7LD compared with CTRL mice under T7LD and KO mice under T24 LD. Mean ± SEM, n = 5 to 6 mice, *P < 0.05.
Deletion of adrenocortical Bmal1 results in hyperadrenocorticism under T7 LD
To determine whether aberrant light induced changes in the circadian GC rhythm, mice were exposed to T7 LD for 3 weeks and then underwent microdialysis sampling for 48 hours. Under T7 LD, a circadian rhythm was detected in most (six of seven) CTRL mice (Fig. 4E); the amplitude of the circadian rhythm was not different from CTRL mice under T24 LD (T7 CTRL: 3.47 ± 0.59 vs T24 CTRL: 2.78 ± 0.22), but the peak phase under T7 LD was more variable (T7 CTRL: 14.4 ± 3.9 vs T24 CTRL: 13.6 ± 1.2; P < 0.05) as expected due to the loss of circadian photic entrainment. In contrast, KO mice showed high-amplitude corticosterone pulses that disrupted the circadian rhythm (Fig. 4F); most (four of six) KO mice showed no circadian rhythm by CircWave. Pulsar analysis showed that both peak amplitude (Fig. 5B) and total daily corticosterone production (Fig. 5C) were increased under T7 LD in KO compared with T7 LD CTRL mice, but peak frequency or interpeak interval (Fig. 5A) was not affected. A peak frequency of ∼6 hours in all groups (Fig. 5A) likely represents an average interval of ∼6 hours over a duration of 24 hours, not a 6-hour periodicity in corticosterone. Corticosterone responses to an ACTH challenge also were increased in KO mice on T7 LD (Fig. 5E and 5F) but not on T24 LD (Fig. 5D and 5F). These results suggest that loss of adrenal Bmal1 results in hyperadrenocortical response to T7 LD by augmenting corticosterone pulse amplitude.
Differential gene expression in adrenals from Bmal1 KO mice under T7 vs T24 LD
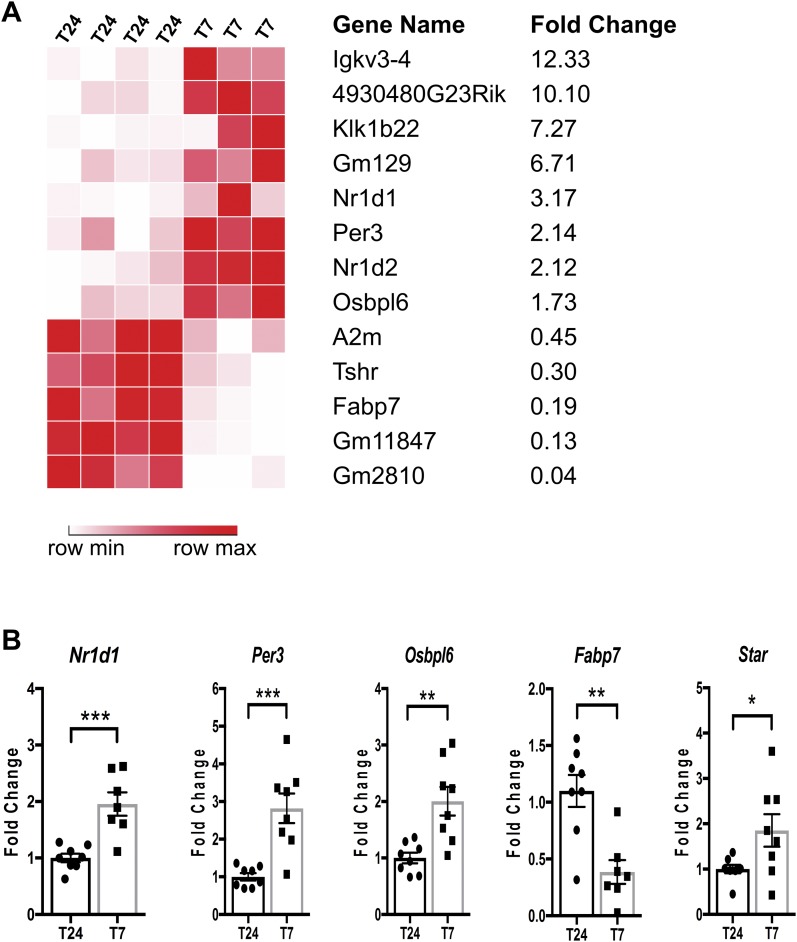
To ensure efficient recombination, we used homozygous ASCre/Cre::Bmal1Fl/Fl KO mice for these experiments, as previously described (19). To identify adrenal transcripts potentially involved in hyperadrenocortical responses to aberrant light in adrenal Bmal1 KO mice, we assessed mRNA expression profiles by RNA-Seq analysis. A total of 13 transcripts were differentially expressed in adrenals from KO mice after T7 LD compared with T24 LD, with 8 upregulated and 5 downregulated (Fig. 6A). The dysregulated transcripts included circadian clock genes like Nr1d1 (38), Nr1d2 (38), and Per3 (39); clock-controlled genes like GM129 (40); and cholesterol metabolism genes like oxysterol binding proteinlike 6 (Osbpl6) (41) and fatty acid binding protein 7 (Fabp7) (42). To validate the results of the RNA-Seq analysis, we analyzed a subset of the differentially expressed genes by quantitative RT-PCR and confirmed upregulation of Nr1d1, Nr1d2 (data not shown), Per3, and Osbpl6 and downregulation of Fabp7 (Fig. 6B). In addition, we measured increased expression of StAR mRNA in adrenals from KO mice exposed to T7 LD (Fig. 6B).
Figure 6.
Gene expression analysis of adrenals from female ASCre/Cre::Bmal1Fl/Fl KO mice under T24 LD and T7 LD. (A) RNA sequencing analysis revealed 13 genes significantly changed with a false discovery rate <0.05 and fold change >1.5 (upregulated) or <0.5 (downregulated) (n = 3 to 4). (B) Quantitative validation of genes relevant to the circadian clock and cholesterol metabolism using quantitative RT-PCR (n = 8). Mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Experiments were done to determine whether the adrenal clock is required for maintaining circadian GC rhythms during chronic exposure to aberrant light signals. Using ASCre/+::Bmal1Fl/Fl mice to delete Bmal1 selectively in the adrenal cortex and high temporal resolution sampling for corticosterone, we found that exposure to aberrant T7 LD not only resulted in loss of GC circadian rhythmicity but also produced hyperadrenocorticism, reflected by increased GC peak amplitude and augmented GC responsiveness to ACTH. These observations underscore a novel role for adrenal Bmal1 to buffer the circadian GC rhythm from changes that occur under aberrant light exposure.
To our knowledge, we generated a novel adrenal Bmal1 null mouse by taking advantage of the capability of the rodent adrenal to undergo postnatal transdifferentiation (19). As shown using AS-Cre reporter mice to trace the lineage of adrenocortical cells, outer differentiated zG cells that express AS transdifferentiate to form the inner zF cells postnatally (19). By using AS to drive Cre-recombinase and delete Bmal1 in zG cells, we hypothesized that postnatal transdifferentiation would proceed in the absence of Bmal1, and if true, loss of Bmal1 would occur in parallel with postnatal transdifferentiation to produce an adrenal cortex–selective Bmal1 null mouse. Results showed that KO mice underwent postnatal transdifferentiation comparable to that observed in CTRL mice as reflected by mGFP expression extending inward from the zG to the cortical-medullary border. Also, there were no clear morphological differences in the zG or zF, indicating that Bmal1 deletion did not prevent adrenal transdifferentiation. In addition, in KO mice, adrenal mGFP expression was paralleled by the loss of BMAL1 labeling, indicating that ASCre/+::Bmal1Fl/Fl mice could serve as an effective model for adrenal-selective Bmal1 deletion. In contrast to our previous report (19), in which complete transdifferentiation occurred within ∼3 months, in these experiments, complete transdifferentiation required a minimum of 7 months. Because no differences were observed between CTRL and KO female mice, Bmal1 deletion does not appear to be responsible for the delay. However, male KO mice did show a reduction in the extent of transdifferentiation and Bmal1 deletion compared with female KO mice when compared at 8 to 9 months. Sex differences in the rate of postnatal transdifferentiation have not been reported, although multiple factors have been implicated in the maintenance and transdifferentiation of zG cells (43), so it is possible that sex differences in expression of one or more of these factors contribute to more rapid postnatal differentiation in female mice. To circumvent these limitations, subsequent experiments were performed using 7- to 10-month-old female mice.
To examine how loss of the adrenal clock affected GC rhythms, we used chronic microdialysis sampling in mice as described in rats (25). Under T24 LD, both CTRL and adrenal clock KO mice displayed prominent circadian GC rhythms with peak corticosterone occurring at the onset of the dark phase. Loss of the adrenal clock did not alter the amplitude or timing of the rhythm, indicating that the adrenal clock is not required to maintain circadian GC rhythmicity under T24 LD. These results are consistent with previous work showing persistence of the GC rhythm during LD entrainment in MC2R-AS-BMAL transgenic mice, in which the adrenal clock is knocked down by expressing part of the BMAL1 coding region in an antisense orientation under adrenal cortex–specific control using the ACTH receptor (MC2R) promoter (13). Unlike other tissues in which selective Bmal1 deletion results in impairment of both intrinsic rhythms and organ function (44–47), loss of the adrenal clock does not consistently result in disruption of GC rhythms or impaired adrenal function. For example, MC2R-AS-BMAL transgenic mice showed a dampened corticosterone rhythm under DD, suggesting that the adrenal clock may be required to maintain GC rhythmicity in the absence of T24 LD (13). However, this effect was not replicated using a conditional Cyp11A1::Bmal1 KO mouse, despite loss of the molecular clock gene rhythm in the adrenal cortex (15). The prevailing view is that the SCN clock synchronizes the phase of peripheral clock gene rhythms that in turn act locally to maintain rhythmic function in a tissue-selective manner. Based on the current study and previous results (13, 15), this premise does not hold for adrenal clock control of GC rhythmicity. Because the GC rhythm is suppressed in global Bmal1 KO mice under T24 LD and DD (48), a functioning SCN clock is sufficient to maintain the GC rhythm.
However, the adrenal clock may be required to maintain the circadian GC rhythm during chronic exposure to aberrant light. To test this possibility, we adopted a mouse model of aberrant light exposure produced by an ultradian T7 LD cycle. The daily resetting of the SCN clock by environmental light requires an LD cycle with a period close to the endogenous τ of the SCN (∼24 hours) (49). Based on the premise that the SCN clock and peripheral clocks require synchronization to maintain timing critical for homeostasis, exposure to ultradian LD cycles have been used in mice to create internal desynchrony of circadian rhythms (50). Circadian rhythms in body temperature and SCN clock gene rhythms (17) are maintained under ultradian LD, but peripheral clock gene rhythms are disrupted (50), suggesting central-peripheral clock desynchronization. We monitored activity in CTRL and KO mice under DD and under ultradian T7 LD using running wheels to determine if adrenal Bmal1 deletion affected circadian or ultradian rhythms. Results showed no differences between CTRL and KO mice in circadian period or amplitude of activity rhythms under DD; these data confirm results using conditional Cyp11A1::Bmal1 KO mice (15) that adrenal Bmal1 deletion does not alter circadian activity rhythms. During exposure to T7 LD, CTRL and KO mice showed an ultradian (∼7 hours) periodicity in activity that likely is due to light negative masking (16) but maintained a circadian periodicity that did not differ in duration or amplitude between genotypes. These results show that activity rhythms under ultradian LD are not affected by adrenal Bmal1 deletion, underscoring the prominence of the SCN clock to maintain circadian rhythmicity during aberrant light exposure (17).
The effect of ultradian LD on adrenal GC rhythms has been inconsistent; elevated plasma corticosterone with persistent circadian rhythmicity (17) or complete loss of circadian rhythmicity (50) has been reported. Differences might be attributed to variability due to low-frequency sampling over a 24-hour period. By using hourly microdialysis sampling in individual mice over 48 hours, we characterized the corticosterone profile under T7 LD. Results showed that circadian rhythms in corticosterone were maintained in CTRL mice under T7 LD; however, the total daily corticosterone produced was increased compared with mice under T24 LD, consistent with previous work showing elevated plasma corticosterone but persistence of circadian rhythmicity (17). Because exercise can result in increased circadian peak plasma corticosterone and increased corticosterone responses to ACTH (51, 52), we measured total daily activity under different lighting conditions. Total daily activity was decreased under T7 LD compared with DD but not compared with T24 LD, indicating that increased locomotor activity likely does not contribute to elevated corticosterone in mice exposed to aberrant light. Exposure to T7 LD produced profound stimulatory effects in KO mice, including increased corticosterone peak amplitude with loss of circadian corticosterone rhythms in most mice and increased total daily corticosterone compared with CTRL mice. In addition to augmented corticosterone responses to T7 LD, we found increased corticosterone responses to an ACTH challenge in KO mice, suggesting that the hyperadrenocorticism results in part from increased responsiveness to ACTH. Aberrant light leads to altered corticosterone rhythms in adrenal Bmal1 KO mice despite maintenance of SCN-dependent circadian rhythms in activity. In contrast to T24 LD that entrains the SCN, it is possible that ultradian photic signals transmitted via intrinsically photosensitive retinal ganglion cells bypass the SCN (17, 53) to affect the adrenal. It is unclear whether ACTH or sympathetic input via the hypothalamic-spinal-adrenal circuit (54, 55) mediates aberrant light-induced alterations in GC rhythmicity in KO mice.
The loss of Bmal1 resulted in augmented steroidogenic responses to T7 LD, suggesting that adrenal Bmal1 may act as a repressor of steroidogenic activity in the adrenal cortex. In most peripheral tissues, Bmal1 deletion results in loss of clock gene rhythmicity and reduced tissue function (44–47). In contrast, Bmal1 deletion produces exaggerated chemokine responses in inflammatory monocytes (56) and in pulmonary epithelial club cells (57) when triggered by infection, indicating repression by BMAL1 in some tissues. A similar phenomenon may occur with adrenal Bmal1 deletion in which exposure to aberrant light triggers hyperadrenocortical responses, implicating Bmal1 as a repressor of steroidogenic function. Interestingly, corticosterone responses to acute stress were not affected in other mouse models in which adrenal Bmal1 was knocked down (13) or deleted (15), suggesting that chronic activation of the adrenal produced by aberrant light may be required to uncover a hyperadrenocortical phenotype. Alternatively, because previous studies were restricted to male mutant mice (13, 15), our findings in female KO mice may indicate sex differences in steroidogenic responses to adrenal Bmal1 deletion.
To identify candidate genes that could account for the hyperadrenocortical response in KO mice exposed to aberrant light, we used RNA-Seq analysis to compare gene expression between adrenals collected from adrenal Bmal1 KO mice exposed to T24 LD vs T7 LD. We found 13 differentially expressed genes that included genes associated with the circadian clock and cholesterol metabolism. We used quantitative PCR to validate the RNA-Seq results, confirming dysregulation of circadian clock genes, Nr1d1, Nr1d2, and Per3, and cholesterol metabolism and trafficking genes, Fabp7, Osbpl6, and StAR. Based on the importance of cholesterol storage and utilization in controlling steroidogenesis (58), our discussion will focus on genes that have been implicated in these cell processes. For example, Nr1d1 and Nr1d2 encode the nuclear receptors, REV-Erbα and REV-Erbβ, respectively, components of an accessory clock feedback loop that is driven by BMAL1:CLOCK and in turn represses Bmal1 transcription (59). Nuclear receptors REV-Erbα and REV-Erbβ not only act as clock genes in the brain and peripheral tissues (38, 59) but also have important regulatory effects on cholesterol metabolism in the liver (38, 60, 61). Expression of adrenal REV-Erbα displays a prominent circadian rhythm in wild-type mice (26, 62), but expression is decreased and nonrhythmic in adrenal Cyp11A1::Bmal1 mutant mice (15). Our novel finding of upregulation in adrenal Bmal1 KO mice during aberrant light exposure may suggest a role for REV-Erbα and REV-Erbβ in enhancing steroidogenesis by increasing cholesterol availability. Although most differentially expressed adrenal genes were upregulated in KO mice under aberrant light, Fabp7, which encodes FABP7, was downregulated. The function of adrenal FABP7 is unknown, but other members of the FABP family have been implicated in regulating cholesterol metabolism (63, 64); for example, liver-selective FABP1 deletion results in cholesterol accumulation in hepatocytes (65). If decreased adrenal Fabp7 results in cholesterol accumulation, it could provide increased substrate to promote steroidogenesis in KO mice exposed to aberrant light. Genome-wide expression profiling in REV-Erbα KO mice identified Fabp7 as a direct target of repression by REV-Erbα in the SCN and other brain areas (66). Our observation of adrenal Nr1d1 upregulation concomitant with Fabp7 downregulation in KO mice exposed to T7 LD supports a similar relationship between REV-Erbα and Fabp7 in the adrenal gland. Adrenal Osbpl6 also is upregulated in adrenal Bmal1 KO mice; Osbpl6 encodes the Osbpl-related protein 6 that contributes to cholesterol trafficking in macrophages and hepatocytes (41). Although a role for adrenal Osbpl-related protein 6 has not been reported, treatment of mouse Y1 adrenocortical cells with ACTH increases Osbpl6 mRNA (67), and silencing adrenal Osbpl-related protein 2 in human H295 adrenocortical cells reduces steroidogenesis (68). Finally, StAR is upregulated in KO mice exposed to aberrant light. This clock-controlled gene is expressed in a circadian fashion in the adrenal (13, 26) and is nonrhythmic and decreased in expression in adrenal Cyp11A1::Bmal1 mutant mice (15). As a cholesterol shuttling protein that is required for steroidogenesis (58, 69), upregulation of StAR could be responsible for increased corticosterone responses in adrenal Bmal1 KO mice exposed to aberrant light. Our initial screen has identified multiple differentially expressed genes that affect cholesterol uptake and trafficking, including StAR, providing potential candidates that could contribute to a hyperadrenocortical response. Additional experiments are required to determine the possible role of these genes in mediating increased steroidogenesis in adrenal Bmal1 KO mice exposed to aberrant light.
In summary, to our knowledge, we have generated a novel adrenal-selective Bmal1 null mouse and used high-frequency microdialysis sampling for corticosterone to determine the requirement of the adrenal clock to maintain circadian GC rhythmicity during chronic exposure to aberrant light. Our results confirm previous work showing that the adrenal clock is not required to maintain circadian GC rhythms under T24 LD, supporting the concept that the SCN clock is sufficient to synchronize GC rhythms during normal photoperiods. In response to aberrant T7 LD, circadian GC rhythms were maintained in most CTRL mice, whereas most KO mice were characterized by the loss of circadian GC rhythms. Loss of circadian GC rhythms in KO mice was associated with augmented GC peak amplitude and responses to ACTH, suggesting that adrenal Bmal1 acts as a repressor of adrenal steroidogenesis. An initial screen of dysregulated genes expressed in KO mice exposed to aberrant light identified multiple candidates involved in cholesterol metabolism and trafficking, including StAR, that could affect steroidogenesis. These observations underscore a novel role for the adrenal clock to prevent desynchronization of circadian GC rhythms that occur under conditions of aberrant light exposure like shift work and jet lag.
Acknowledgments
We thank Krista Koehler, Maggie Yang, and Lauren Miller, undergraduate students who provided technical assistance.
Financial Support: This work was supported in part by research grants from the National Science Foundation (IOS1025119; to W.C.E.), the Wallin Discovery Fund (to W.C.E.), the Harvard NeuroDiscovery Center (to M.E.P.), and the NIH (R01-DK100653; to D.T.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AS
aldosterone synthase
- BW
body weight
- CTRL
control
- DD
constant dark
- Fabp7
fatty acid binding protein 7
- GC
glucocorticoid
- KO
knockout
- LD
light-dark
- NIH
National Institutes of Health
- Osbpl16
oxysterol binding proteinlike 6
- RNA-Seq
RNA sequencing
- SCN
suprachiasmatic nucleus
- StAR
steroidogenic acute regulatory protein
- zF
zona fasciculata
- zG
zona glomerulosa
- ZT
zeitgeber time
References
- 1. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35(1):445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menet JS, Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr Opin Neurobiol. 2011;21(6):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014;40:80–101. [DOI] [PubMed] [Google Scholar]
- 4. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. [DOI] [PubMed] [Google Scholar]
- 5. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72(1):517–549. [DOI] [PubMed] [Google Scholar]
- 6. Sahar S, Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol Metab. 2012;23(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009;29(1):171–180. [DOI] [PubMed] [Google Scholar]
- 9. Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140(3):753–757. [DOI] [PubMed] [Google Scholar]
- 10. Woodruff ER, Chun LE, Hinds LR, Spencer RL. Diurnal corticosterone presence and phase modulate clock gene expression in the male rat prefrontal cortex. Endocrinology. 2016;157(4):1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- 12. Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120(7):2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Son GH, Chung S, Choe HK, Kim H-D, Baik S-M, Lee H, Lee H-W, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA. 2008;105(52):20970–20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163–173. [DOI] [PubMed] [Google Scholar]
- 15. Dumbell R, Leliavski A, Matveeva O, Blaum C, Tsang AH, Oster H. Dissociation of molecular and endocrine circadian rhythms in male mice lacking Bmal1 in the adrenal cortex. Endocrinology. 2016;157(11):4222–4233. [DOI] [PubMed] [Google Scholar]
- 16. Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16(4):415–429. [DOI] [PubMed] [Google Scholar]
- 17. LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kofuji P, Mure LS, Massman LJ, Purrier N, Panda S, Engeland WC. Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS One. 2016;11(12):e0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman BD, Kempna PB, Carlone DL, Shah M, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 21. Yoder JM, Brandeland M, Engeland WC. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am J Physiol Regul Integr Comp Physiol. 2014;306(6):R387–R393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Razzoli M, Karsten C, Yoder JM, Bartolomucci A, Engeland WC. Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. Am J Physiol Regul Integr Comp Physiol. 2014;307(2):R198–R205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Purrier N, Engeland WC, Kofuji P. Mice deficient of glutamatergic signaling from intrinsically photosensitive retinal ganglion cells exhibit abnormal circadian photoentrainment. PLoS One. 2014;9(10):e111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RRID:AB_10000794.
- 25. Qian X, Droste SK, Lightman SL, Reul JMHM, Linthorst ACE. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153(9):4346–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1128–R1135. [DOI] [PubMed] [Google Scholar]
- 27. Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006;21(5):350–361. [DOI] [PubMed] [Google Scholar]
- 28. Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243(4):E310–E318. [DOI] [PubMed] [Google Scholar]
- 29. Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology. 1994;59(2):97–109. [DOI] [PubMed] [Google Scholar]
- 30.RRID:SCR_004316.
- 31.RRID:SCR_014583.
- 32.RRID:SCR_011841.
- 33. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García-Alcalde F, Okonechnikov K, Carbonell J, Cruz LM, Götz S, Tarazona S, Dopazo J, Meyer TF, Conesa A. Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics. 2012;28(20):2678–2679. [DOI] [PubMed] [Google Scholar]
- 35. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2013;30(7):923–930. [DOI] [PubMed] [Google Scholar]
- 36. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engeland WC, Yoder JM, Karsten CA, Kofuji P. Phase-dependent shifting of the adrenal clock by acute stress-induced ACTH. Front Endocrinol (Lausanne). 2016;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30(2):525–536. [DOI] [PubMed] [Google Scholar]
- 40. Annayev Y, Adar S, Chiou YY, Lieb JD, Sancar A, Ye R. Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J Biol Chem. 2014;289(8):5013–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouimet M, Hennessy EJ, van Solingen C, Koelwyn GJ, Hussein MA, Ramkhelawon B, Rayner KJ, Temel RE, Perisic L, Hedin U, Maegdefessel L, Garabedian MJ, Holdt LM, Teupser D, Moore KJ. miRNA targeting of oxysterol-binding protein-like 6 regulates cholesterol trafficking and efflux. Arterioscler Thromb Vasc Biol. 2016;36(5):942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, Zhang Q, Wakelam MJO, Karpe F, Schulze A, Harris AL. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Reports. 2014;9(1):349–365. [DOI] [PubMed] [Google Scholar]
- 43. Pignatti E, Leng S, Carlone DL, Breault DT. Regulation of zonation and homeostasis in the adrenal cortex. Mol Cell Endocrinol. 2017;441:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2010;54(1):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2014;125(1):324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, Sellix MT. Conditional deletion of Bmal1 in ovarian theca cells disrupts ovulation in female mice. Endocrinology. 2016;157(2):913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leliavski A, Shostak A, Husse J, Oster H. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155(1):133–142. [DOI] [PubMed] [Google Scholar]
- 49. Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106(3):253–266. [Google Scholar]
- 50. Oishi K, Higo-Yamamoto S, Yamamoto S, Yasumoto Y. Disrupted light-dark cycle abolishes circadian expression of peripheral clock genes without inducing behavioral arrhythmicity in mice. Biochem Biophys Res Commun. 2015;458(2):256–261. [DOI] [PubMed] [Google Scholar]
- 51. Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst ACE, Reul JMHM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144(7):3012–3023. [DOI] [PubMed] [Google Scholar]
- 52. Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. J Appl Physiol (1985). 2009;106(1):66–72. [DOI] [PubMed] [Google Scholar]
- 53. Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11(5):1535–1544. [DOI] [PubMed] [Google Scholar]
- 55. Engeland WC, Arnhold MM. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28(3):325–332. [DOI] [PubMed] [Google Scholar]
- 56. Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52(12):2111–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. [DOI] [PubMed] [Google Scholar]
- 60. Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7(9):e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26(7):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feillet C, Guérin S, Lonchampt M, Dacquet C, Gustafsson JA, Delaunay F, Teboul M. Sexual dimorphism in circadian physiology is altered in LXRα deficient mice. PLoS One. 2016;11(3):e0150665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem. 2003;278(24):21429–21438. [DOI] [PubMed] [Google Scholar]
- 64. Chmurzyńska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47(1):39–48. [DOI] [PubMed] [Google Scholar]
- 65. Martin GG, Atshaves BP, Landrock KK, Landrock D, Schroeder F, Kier AB. Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Arch Biochem Biophys. 2015;580:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schnell A, Chappuis S, Schmutz I, Brai E, Ripperger JA, Schaad O, Welzl H, Descombes P, Alberi L, Albrecht U. The nuclear receptor REV-ERBα regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS One. 2014;9(6):e99883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schimmer BP, Cordova M, Cheng H, Tsao A, Morris Q. A genome-wide assessment of adrenocorticotropin action in the Y1 mouse adrenal tumor cell line. Mol Cell Endocrinol. 2007;265-266:102–107. [DOI] [PubMed] [Google Scholar]
- 68. Escajadillo T, Wang H, Li L, Li D, Sewer MB. Oxysterol-related-binding-protein related protein-2 (ORP2) regulates cortisol biosynthesis and cholesterol homeostasis. Mol Cell Endocrinol. 2016;427:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63(1):193–213. [DOI] [PubMed] [Google Scholar]