Abstract
In this study we examined if sleep time, caloric intake and energy expenditure are important contributors to development of ovariectomy-induced obesity in mice fed control or high fat diet (HFD). Twelve female mice at 6 weeks of age were divided into 2 groups: Sham (n=5) and ovariectomized (OVX, n=7). Mice were fed control diet for 9 weeks and shifted to HFD for additional 9 weeks. Food intake and body weight were measured daily and body composition was measured weekly by EchoMRI. Energy expenditure (EE), oxygen consumption (VO2), motor activity (MA) and sleep time were monitored at week 9 during control diet and HFD. OVX did not alter caloric intake, body weight or body composition, MA, sleep time or fasting blood glucose, but slightly reduced EE compared to Sham mice on control diet. After HFD feeding, OXV mice had similar caloric intake, lean mass, MA, and blood glucose levels but had significantly greater weight gain (8.2±1.0 vs. 4.8±1.2 g, p<0.05), increased fat mass and sleep time, and reduced EE (3.3±0.4 vs. 5.5±0.2 kcal/hr) and VO2 (1.12±0.01 vs. 1.83±0.05 ml/min) compared to Sham group. Daytime blood pressure was higher while nighttime heart rate was lower in OVX group. These results suggest that OVX may not substantially alter body weight or body composition in mice fed a normal diet, but when combined with HFD it increases sleep time and reduces EE, leading to greater weight gain and adiposity without altering food intake.
Keywords: Adiposity, food intake, body composition, blood pressure, sex hormones
Introduction
Ovarian hormones, including estrogens and progesterone, play an important role in many physiological functions including reproduction, cardiovascular and body weight regulation (Ashraf and Vongpatanasin, 2006, Brown and Clegg, 2010, Mauvais-Jarvis et al. , 2013, Waraich and Mauvais-Jarvis, 2013). Reduction in ovarian hormones, especially estrogens, may predispose women to increased adiposity and metabolic dysfunction including type-2 diabetes (Brown and Clegg, 2010, Zhang et al. , 2002). Obesity and associated metabolic abnormalities are more prevalent in postmenopausal compared to premenopausal women (Brown and Clegg, 2010, Zhang et al., 2002). However, diet-dependent and independent mechanisms by which loss of ovarian function may increase central adiposity and causes metabolic abnormalities are still unclear.
Experimental studies in ovariectomized (OVX) rodents have yielded conflicting results on the mechanisms by which ovarian hormones regulate energy balance. For instance, Seidlova- Wuttke et al (Seidlova-Wuttke et al. , 2012) demonstrated that OVX leads to increased body weight and adiposity lasting for 12 months after surgery while other studies showed that OVX does not affect body weight or fat mass at 8 to 10 months after surgery (Gilbert et al. , 2014). Rogers et al (Rogers et al. , 2009) reported that OVX in mice caused increased adiposity that was associated with decreased energy expenditure but no change in energy intake whereas other studies indicate that OVX causes hyperphagia due mainly to loss of estrogens (Liang et al. , 2002, Meli et al. , 2004). Studies by Bhatia et al (Bhatia and Wade, 1989, 1991, 1993) showed that estradiol plus progesterone replacement in OVX Syrian hamsters reduced body weight and fat mass without a significant change in food intake, suggesting that ovarian hormones may play an important role in regulating energy expenditure but no major effect on food intake. Differences in diet may have contributed to some of these discrepancies. Therefore, further studies are needed to assess the mechanisms by which loss of ovarian hormones influence energy balance and cause metabolic abnormalities in animals fed normal and obesogenic diets.
Sleep disorders are associated with increases in body weight and adiposity, and postmenopausal women have higher prevalence of sleep difficulties than younger women (Jehan et al. , 2015). However, whether sleep time and reduced energy expenditure contribute to development of OVX-induced obesity is still unclear.
In the present study we examined whether caloric intake, motor activity, energy expenditure and sleep time may contribute to development of OVX-induced obesity in mice fed a control or a high fat diet (HFD). We found that when mice were fed a normal control diet OVX did not alter caloric intake, body weight, body composition, sleep time or fasting blood glucose and insulin concentrations, but reduced energy expenditure and oxygen consumption. However, OVX mice fed a HFD had greater weight gain, increased fat mass and sleep time compared to Sham mice, despite similar caloric intake, lean mass, motor activity and fasting blood glucose and insulin levels. We also observed that daytime blood pressure was higher while nighttime heart rate was lower in OVX mice. Thus, our data suggest that OVX may not substantially alter body weight or adiposity in mice fed a control diet, however, when combined with a HFD it is associated with reduced energy expenditure and increased sleep time, leading to more pronounced weight gain and greater adiposity.
Materials and Methods
All experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center, Jackson, Mississippi. Mice were placed in a 12-h dark (6:00 pm to 6:00 am) and light (6:00 am to 6:00 pm) cycle and given free access to food and water throughout the study.
Animals
Twelve female B6/129 mice at 6 weeks old were divided into two groups: 1) Sham-operated (n=5) and ovariectomized (OVX, n=7). When fed a high fat diet, B6/129 mice exhibit many of the metabolic alterations found in humans with obesity (Fengler et al. , 2016).
Ovariectomy
At 3 weeks of age, female mice were weaned, individually housed, and fed a control diet. Three weeks later, they were randomized to two groups. Under 2% isoflurane anesthesia, one group underwent sham operation (Sham), whereas mice in the other group underwent dorsal ovariectomy (OVX). Nine weeks after the surgery, OVX and Sham control mice were switched to a HFD for an additional 9-week period.
Body Weight Composition and Food Intake Measurements from Weaning to Adulthood
Mice were individually housed and fed a control diet (Harlan Teklad/ENVIGO, CA 8640, 3.0 kcal/g, 13% fat) starting at 6 weeks of age and continuing until 15 weeks of age. The diet was then switched to a HFD (Harlan Teklad/ENVIGO, TD-0881, 4.7 kcal/g, 45% fat) until the experiments were completed at 25 weeks of age. Body weight was measured twice per week during the normal and high fat diets. Weekly changes in body composition were analyzed using magnetic resonance imaging (4in1 EchoMRI-900TM, Echo Medical System, Houston, TX). To minimize potential stress caused by single housing, we used environmental enrichment in the cages of the mice.
Blood Pressure and Heart Rate Measurements
At 23 weeks of age the mice were anesthetized with 2% isoflurane and, under aseptic conditions, a telemetry probe (TA11PA-C10, Data Science, MN) was implanted in the left carotid artery and advanced into the aorta. Ten days after recovery from surgery, mean arterial pressure (MAP) and heart rate (HR) were measured 24 hr/day for 5 consecutive days using computerized methods for data collection as previously described (do Carmo et al. , 2011a, do Carmo et al. , 2014a). Daily MAP and HR were obtained from the average of 12:12 hr light:dark recording using a sampling rate of 1000 Hz with a duration of 10 seconds every 10–minute period.
Oxygen Consumption, Motor Activity and Sleep Time
In separate experiments, mice at 22-24 weeks of age (n=12) were placed individually in metabolic cages (Promethion Metabolic Measurement System, Sable International, NV) equipped with oxygen sensors to measure oxygen consumption (VO2) and carbon dioxide production (VCO2). Respiratory quotient (RQ) was calculated as the ratio of VCO2/VO2. Ambulatory spontaneously motor activity was determined using infrared light beams mounted in the cages in X, Y and Z axes.
We also quantified sleep time using duration of immobility of 40s or greater as a surrogate of sleeping as previously described (Fisher et al. , 2012). The very small movements of the body associated with breathing during sleep were ignored in these measurements. The XYZ arrays consist of high resolution (1 cm pacing) infrared beam arrays that are invisible to the animal (wavelength ca. 900 nm). The intensity of these beams are measured in rapid succession and movements are easily detected and a centroid-algorithm calculates the animal position and movements.
Acute Air-jet Stress Test
To determine whether OVX alters MAP and HR responses to acute stress, female mice fed a HFD and implanted with BP telemeters were placed in special cages used for air-jet stress testing as previously described (do Carmo et al., 2011a). Mice were allowed to acclimate to the cages for at least 4 hours, then MAP and HR were continuously measured for 30 minutes. The air-jet stress was then administered in 5-second pulses every 10 seconds for 5 minutes aimed at the forehead of the mice. Changes in BP and HR to acute stress were measured by subtracting the average baseline measurement from the average values recorded during acute stress.
Heart Rate Variability
To assess the impact of OVX combined with a HFD on heart rate variability (HRV), the RR intervals were extracted using a detection algorithm based on peak-picking of systolic blood pressure continuously measured for 2 hours (10:00-12:00 am). We excluded one animal with extreme values of the RR intervals (<70 and >500 ms) and other outliers on the basis of the means and standard deviations (SD) of nearby RR values. Time and frequency domain measures were calculated for each second of normal RR interval data using Kubios HRV software (version 2.1). In the time domain, the mean RR intervals, standard deviation of normal R-R intervals (SDNN) and root mean square of the successive differences (RMSSD) were calculated. The coefficient of variance (CV %), defined as the ratio SDNN/mean RR which reflect the variability due to combination of long, intermediate and short term components, was also calculated. In the frequency domain, the power spectral density of RR interval time series was computed, and two different frequency-domain measures of HRV were calculated, low-frequency (LF) 0.4-1.5 Hz and high frequency range (HF) 1.5-4.0 Hz, with data expressed as absolute and arbitrary units. These measurements were taken at the end of the experiment (week 9 of HFD).
Acute Leptin Injection
To determine whether OVX reduces the acute anorexic effects of leptin, caloric intake was measured 2, 4, 15 and 24 hr after an intraperitoneal leptin (5 mg/kg) or vehicle injection (saline, 0.3 ml) between 5:00 and 6:00 pm in separate groups of non-fasted Sham and OVX mice fed control or HFD diet.
Food Intake and Cardiovascular Responses to Fasting/Refeeding
To determine whether refeeding responses after prolonged fasting were affected by OVX in mice fed a HFD, the mice were subjected to a 24-hr fasting followed by a 3-day period where food was available ad libitum. Food intake, body weight, BP and HR were measured at baseline, fasting, and during 3 days of refeeding.
Plasma Hormones and Glucose Measurements.
Plasma leptin and insulin concentrations were measured with ELISA kits (R&D Systems and Crystal Chem Inc., respectively), and plasma glucose concentrations were determined using the Beckman glucose analyzer 2.
Statistical Analyses
Data are expressed as mean ± SEM. Significant differences between the two groups were determined by unpaired Student’s t-test or 2-way ANOVA followed by Bonferroni’s post hoc test when appropriate. One-way ANOVA with repeated measures followed by Dunnett’s post hoc test was used for comparisons between control and experimental values within each group when appropriate. A p value of <0.05 indicates a significant difference.
Results
Uterine Weight
To confirm the efficacy of the OVX surgery, uterine weight was measured at the end of the experiment. Uterine weight was significantly reduced in OXV compared to Sham control mice (OVX: 0.05±0.01 vs. Sham: 0.21±0.03 g).
Body Weight, Lean Mass, Visceral Fat, Glucose and Hormone Concentration Responses to OVX
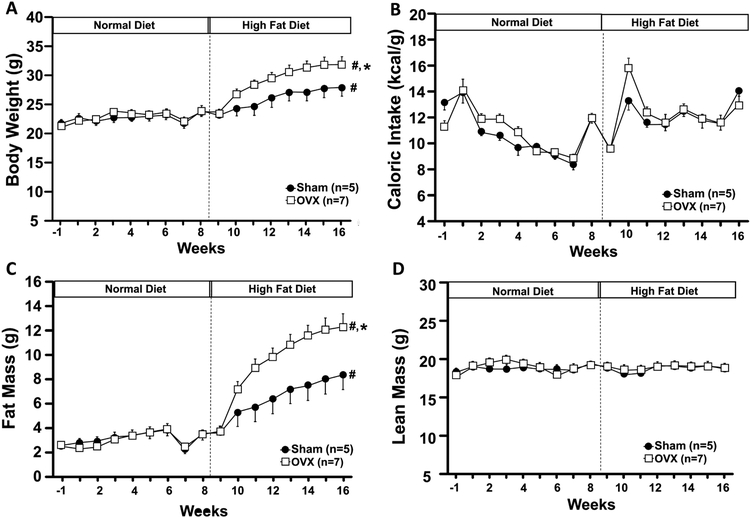
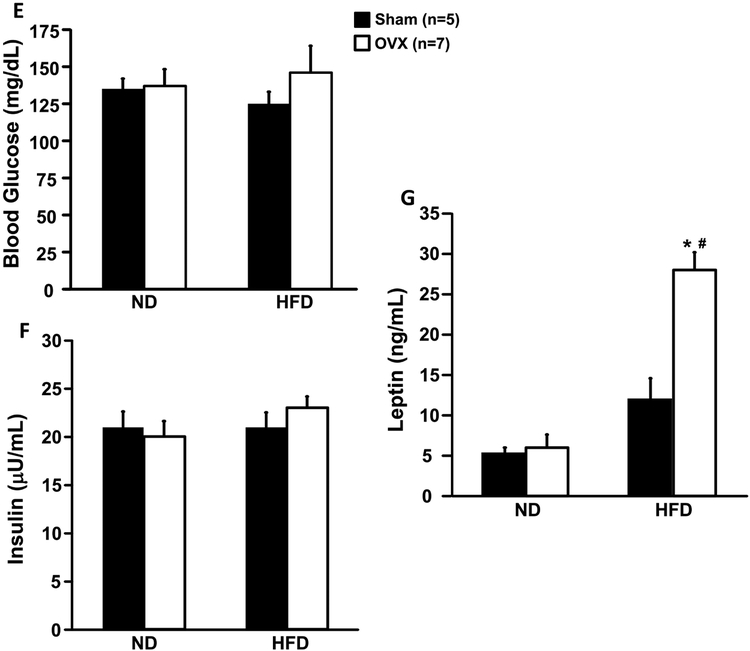
When mice were fed a control diet starting at 6 weeks of age and continuing until 15 weeks of age, there were no significant differences in body weight, caloric intake, fat or lean mass of OVX compared to Sham control mice (Figs. 1A-D). When fed a HFD OVX mice showed significantly greater weight gain despite similar caloric intake compared to Sham mice (Figs. 1A and B). The increase in body weight was mainly accounted for by increased fat mass (Fig. 1C) as total lean mass was not altered by OVX in mice fed a control or HFD (Fig. 1D). The HFD did not significantly alter fasting glucose and insulin concentrations (Figs. 1E and F). Plasma leptin concentration was increased in Sham mice fed a HFD compared to control diet, although the difference was not statistically significant (Fig. 1G). However, plasma leptin concentration was significantly increased in OVX mice fed a HFD compared to control diet and Sham group (Fig. 1G).
Figure 1.
Body weight (A), caloric intake (B), fat mass (C), lean mass (D), blood glucose (E), plasma insulin (F) and leptin levels (G) in ovariectomized (OVX) or Sham-operated control mice. ND, normal diet; HFD, high fat diet. *p<0.05 compared to Sham group. #p<0.05 compared to normal diet.
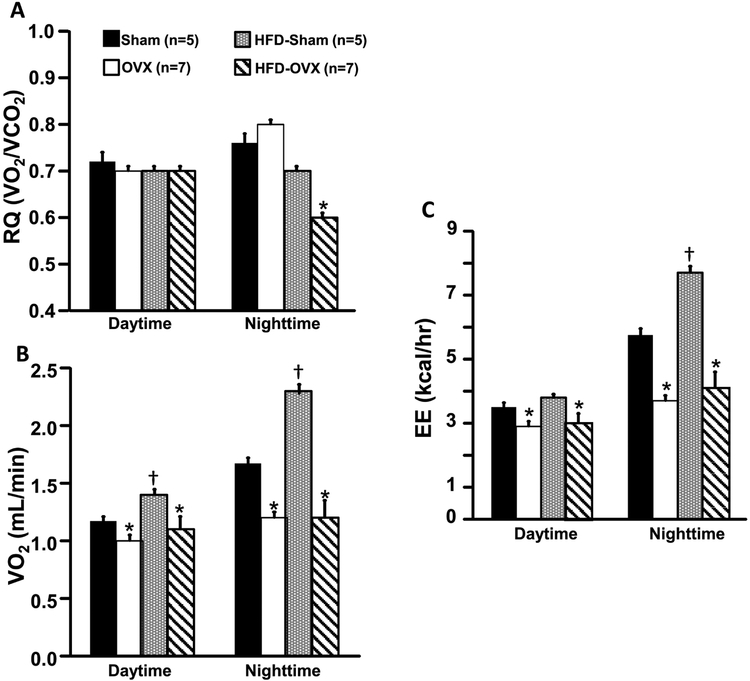
Respiratory Quotient (RQ), Oxygen Consumption (VO2) and Energy Expenditure EE) Responses to OVX
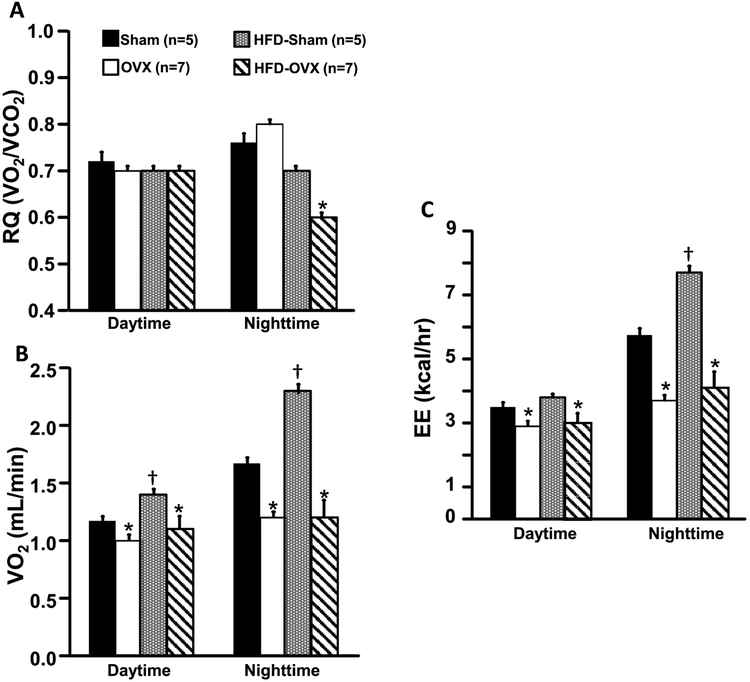
Ovariectomy did not alter RQ in mice fed a control diet, however VO2 and EE were significantly reduced in OVX compared to Sham control mice (Figs. 2A-C). Nighttime RQ was significantly reduced in OVX mice fed a HFD (Fig. 2A). VO2 and EE were also significantly reduced in OVX mice compared to Sham mice fed a HFD (Figs. 2B and C). RQ was not altered in Sham mice fed a HFD compared to control diet (Fig. 2A), although daytime and nighttime VO2 and nighttime EE were significantly increased in Sham mice fed a HFD (Figs. 2B and C).
Figure 2.
Respiratory quotient (RQ, A), oxygen consumption (VO2, B) and energy expenditure (EE, C) in ovariectomized (OVX) or Sham-operated control mice fed a normal or high fat diet.*p<0.05 compared to its respective Sham group. † p<0.05 compared to Sham-operated control mice fed a control diet.
Effects of OVX on Sleep Time and Motor Activity
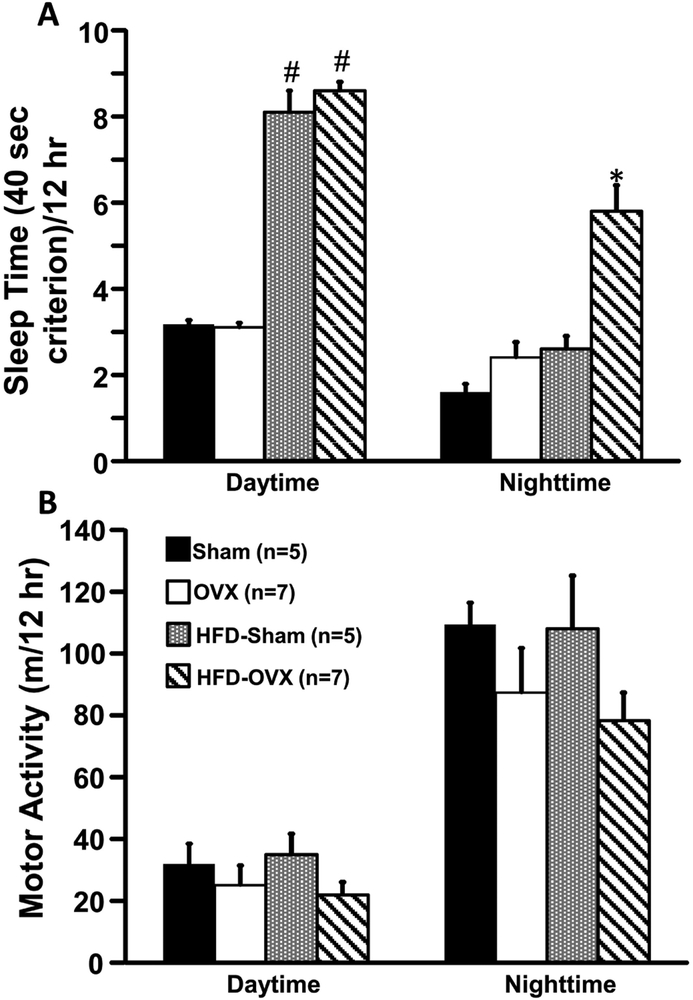
OVX was associated with significantly greater sleep time during nighttime in mice fed a HFD (Fig. 3A). OVX mice also showed a tendency for reduced motor activity when fed a control or HFD but these changes were not statistically significant (Fig. 3B).
Figure 3.
Sleep time (A) and motor activity in ovariectomized (OVX) or Sham-operated control mice fed a normal or high fat diet. *p<0.05 compared to its respective Sham group. # p<0.05 compared to Sham or OVX groups on normal diet.
MAP, HR and HRV Responses to OVX
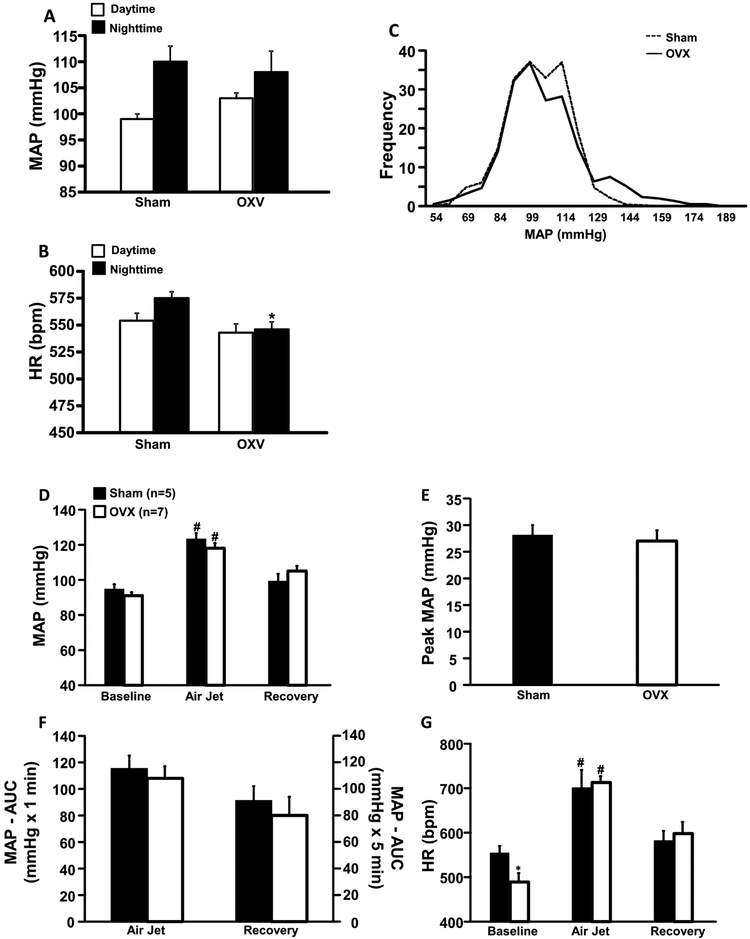
Compared to Sham group, OXV mice fed HFD had similar daytime and nighttime MAP (Fig. 4A), whereas HR was significantly reduced in OVX mice during nighttime (Fig. 4B). We also observed that MAP frequency distribution was slightly, but not significantly, shifted to the right in OVX mice compared to Sham, highlighting their elevated daytime MAP. However, the overall frequency distribution of MAP was not different between groups (Fig. 4C). Pre stress resting measurements of MAP were not significantly different in OVX compared to Sham mice (Fig. 4D). In response to acute stress, MAP of OVX and Sham mice increased by 27±2 and 28±2 mmHg, respectively (Fig. 4E). The total area under the curve of the MAP during the air-jet stress was also similar between groups (Fig. 4F). At baseline, HR was significantly lower in OVX mice compared to Sham controls (Fig. 4G) and acute air-jet stress raised HR equally in OVX and Sham mice (Fig. 4G).
Figure 4.
Baseline mean arterial (MAP, A), heart rate (HR, B), frequency distribution of MAP (C), changes in MAP (D), area under curve (AUC) for blood pressure during acute air jet stress and recovery period (E), peak MAP during air jet stress (F) and HR during baseline, air jet stress and recovery periods in ovariectomized (OVX) or Sham-operated control mice fed a high fat diet. *p<0.05 compared to Sham group. #p<0.05 compared to baseline.
To determine whether OVX plus HFD alters HRV, we analyzed time and frequency domain measures for each second of normal RR interval. Combined OVX plus HFD had no significant effect on RR interval, SDNN, CV% or RMSSD when compared to Sham group (Table 1). However, we did find significant decrease in LF (which reflects cardiac sympathetic activity) and increase in HF (which reflects cardiac parasympathetic activity) components of HR in OVX mice, suggesting that OVX combined with HFD is associated with increased parasympathetic tone to the heart (Table 2).
Table 1 –
Heart rate variability (HRV) in Sham and OVX mice.
| Measure | Sham | OVX |
|---|---|---|
| RR (ms) | 111.3±1.8 | 110.4±3.1 |
| SDNN (ms) | 10.8±1.1 | 9.9±0.9 |
| CV% | 9.6±0.8 | 8.9±0.7 |
| RMSSD (ms) | 8.1±2.0 | 5.7±1.4 |
Data are mean±SEM, RR, the intervals from systolic peak; SDNN, standard deviation of all R-R intervals; CV%, coefficient of variance (100 X (SDNN/mean RR)); RMSSD, root mean square of successive difference.
Table 2 –
Frequency domain analysis on Heart rate variability (HRV) in Sham and OVX mice.
| Power (ms) | Power (%) | Power (nu) | LF/HF | ||||
|---|---|---|---|---|---|---|---|
| LF | HF | LF | HF | LF | HF | ||
| (0.04–0.15 Hz) | (0.15–0.40 Hz) | (0.04–0.15 Hz) | (0.15–0.40 Hz) | (0.04–0.15 Hz) | (0.15–0.40 Hz) | ||
| Sham | 16.5±4.7 | 11.0±3.5 | 20.4±2.7 | 13.2±2.3 | 60.9±1.3 | 38.9±1.3 | 1.6±0.1 |
| OVX | 9.2±2.7 | 11.2±2.5 | 11.3±1.8* | 14.8±1.1 | 41.8±2.7* | 58.0±2.7* | 0.7±0.1* |
Values are mean±SEM, LF, low-frequency; HF, high-frequency; nu, normalized units (represent the relative value of each power component). P<0.05 compared to Sham group.
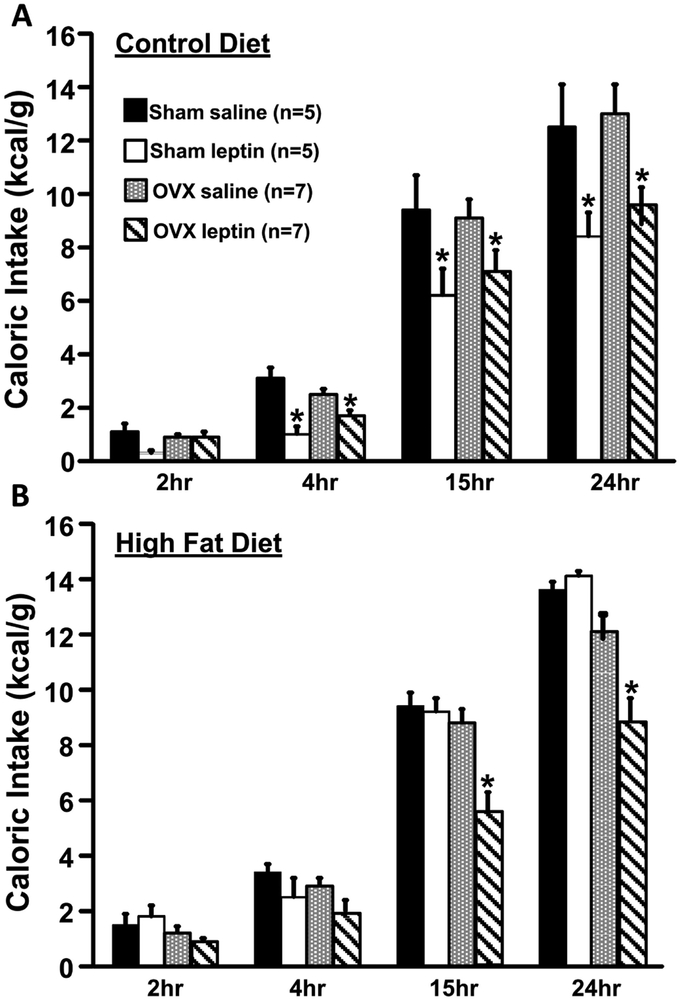
Impact of OXV on Food Intake Responses to Leptin
To test whether the acute anorexic effect of leptin is altered in OVX mice fed a control or HFD, leptin was injected and food intake was measured for 24 hours. Compared to saline injection as baseline control, leptin injection resulted in similar 24-hr reductions in food intake in OVX mice (~26%) and Sham controls (~33%) fed a control diet (Fig. 5A). The anorexic effect of leptin was observed as early as 2 hours post-injection in Sham controls and 4 hours in the OVX group. During HF feeding, however, the anorexic effect of leptin was abolished in Sham control mice, whereas leptin injection still reduced food intake in OVX mice at 15 (~37%) and 24 hr (~27%) post-injection (Fig. 5B).
Figure 5.
Caloric intake in mice fed a normal diet (A) and caloric intake in mice fed a high fat diet (B) in response to leptin injection (5 mg/kg, IP) in ovariectomized (OVX) or Sham-operated control mice. *p<0.05 compared to saline injection.
Impact of OVX on Food Intake, Body Weight and Cardiovascular Responses to Fasting and Refeeding
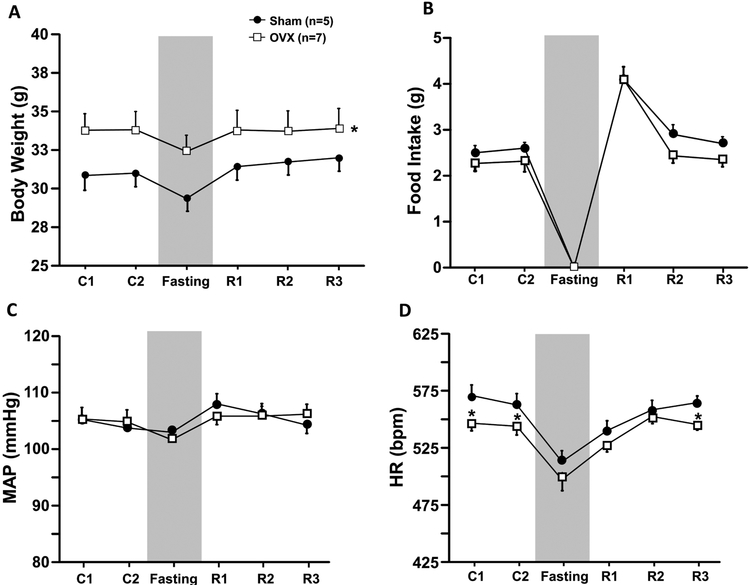
Prolonged (24 hr) fasting resulted in similar weigh weight loss in Sham (~5%) and OVX mice (~4%) (Fig. 6A). Food intake was significantly increased by 78% in OVX and 59% in Sham control mice on the first day of refeeding (Fig. 6B) and then returned to baseline values 48 hours after normal access to feed was restored. No significant changes were observed in MAP and HR between groups during the refeeding period (Figs. 6C and D).
Figure 6.
Body weight (A), food intake (B), mean arterial pressure (MAP, C) and heart rate (HR, D) in responses to fasting and refeeding in ovariectomized (OVX) or Sham-operated control mice fed a high fat diet. *p<0.05 compared to Sham group.
Discussion
An important goal of this study was to test the hypothesis that caloric intake, energy expenditure and sleep time are important contributors to development of OVX-induced obesity in mice fed a HFD. Our study indicates that OVX does not significantly alter body weight or body composition in mice fed a control diet, but when combined with HFD, OVX is associated with increased sleep time, reduced energy expenditure, increased body weight, greater adiposity and elevated plasma leptin concentration. The obesity and associated metabolic changes in OVX mice fed a HFD occurred in the absence of increases in caloric intake or significant changes in fasting glucose or insulin concentrations. Another important, albeit surprising, finding of our study was that OVX did not alter the acute anorexic effect of leptin, HRV or MAP and HR responses to fasting/refeeding and air-jet stress in mice fed a HFD. To our knowledge whether OVX-induced obesity in mice fed a HFD is associated with increased sleep time and reduced energy expenditure has not been previously reported.
Loss of ovarian hormones, especially estrogens, is associated with alterations in energy homeostasis and increased abdominal fat in women (Zhang et al., 2002). Litwak et al (Litwak et al. , 2014) showed that OVX caused a transient increase in food intake that was reversed by estrogen replacement. In addition, they found that hyperphagia or reduced energy expenditure did not fully account for changes in metabolism and development of obesity after OVX (Litwak et al., 2014). Reduced spontaneously physical activity and lean mass have also been implicated in contributing to increased adiposity after OVX (Mosti et al. , 2016, Zoth et al. , 2010). Our observations suggest that OVX does not alter caloric intake, body weight or even motor activity, but it does reduce energy expenditure in mice fed a control diet. These results are surprising if one assumes that reduced energy expenditure contributes to weight gain after OVX (Park et al. , 2011, Tolson et al. , 2014). A possible explanation for differences in the effects of OVX on body weight in various studies may be differences in genetic background of the mice used. In our experiments B6 mice were used while most experiments that showed increased body weight after OVX were performed in female C57BL/6J (Park et al., 2011, Tolson et al., 2014).
Our finding that OVX did not cause obesity in mice fed a control diet is consistent with the possibility that loss of ovarian hormones may not substantially alter leptin signaling during a normal diet. Leptin-mediated activation of various CNS pathways, including proopiomelanocortin neurons, is an important regulator of food intake and energy expenditure (Bohler et al. , 1991). Chen et al, however, showed that increased body weight after OVX was not due to leptin resistance in rats (Chen and Heiman, 2001). Consistent with this observation, we also found normal anorexic responses to leptin injections in OVX mice fed a control diet. Furthermore, the anorexic effects of leptin were preserved in OVX mice fed a HFD, suggesting that loss of ovarian hormones does not substantially attenuate the effects of leptin on food intake regulation.
Since OVX did not alter body weight in mice fed a normal diet, we challenged the mice with a HFD for 9 weeks and characterized their metabolic and cardiovascular responses. We found that OVX mice exhibited greater body weight gain mainly due to increased fat mass when compared to Sham controls despite similar caloric intake. We also observed that these mice had lower nighttime RQ, suggesting that OVX caused a shift toward lipid metabolism when combined with a HFD. In addition, oxygen consumption and energy expenditure were also significantly reduced in OVX mice compared to Sham controls, and these changes occurred in the absence of changes in motor activity. We also observed that RQ was not altered in Sham mice fed a HFD compared to control diet, although daytime and nighttime VO2 and nighttime EE were significantly increased in these mice, which could contribute to the difference in body weight compared to OVX mice fed a HFD. Although total EE in OVX mice fed a HFD did not decrease below levels observed in OVX fed a normal diet, it is important to note that the OVX mice fed a HFD had greater body weight and more fat mass than OVX mice fed a normal diet. Therefore, EE/gram body weight was reduced in OVX mice fed a HFD compared to OVX mice fed a normal diet.
Although previous studies showed that OVX-induced increased plasma glucose and insulin levels, likely due to insulin resistance (Tawfik et al. , 2015, Vieira Potter et al. , 2012), in the present study we found only a slight increase in fasting glucose and insulin concentrations. However, we observed a much greater increase in plasma leptin concentration in OVX mice fed a HFD and this was associated with a much greater increase in fat mass. A possible explanation for these findings is that high plasma leptin concentration in OXV mice may have prevented the development of glucose intolerance and insulin resistance since we (do Carmo et al. , 2011b, do Carmo et al. , 2014b, do Carmo et al. , 2016, do Carmo et al. , 2008) and others (Kamohara et al. , 1997, Morton and Schwartz, 2011, Petersen et al. , 2002) showed that leptin has potent antidiabetic effects.
Studies in rats suggest that BP increases after OVX and this elevation can be reversed by estrogen replacement therapy (Dubey et al. , 1998, Hernandez et al. , 2000, Mercier et al. , 2002). In double estrogen receptor (α and β) knockout mice, however, BP was similar to that of wildtype controls. In addition, treatment with 17β estradiol or estrogen pellets for 60 days did not alter BP in either wild type or double knockout mice (Doevendans et al. , 1998, Karas et al. , 2001). We observed higher daytime BP in OVX compared to Sham control mice on a HFD. However, nighttime HR was significantly reduced in OVX suggesting increased vagal tone which was confirmed using frequency domain analysis and lower LF/HF ratio (index of sympathovagal balance) to the heart. Campos et al (Campos et al. , 2014) showed no major changes in LF, HF and LF/HF ratio in OVX compared to estrogen treatment group, despite improvement on cardiac autonomic control. They also found increased HRV with estrogen replacement. We found no evidence that OXV alters HRV in mice. Furthermore, OVX did not enhance the HR and BP responses to an acute air jet stress in mice, suggesting that OVX is not associated with enhanced BP or HR responses to an acute stress.
Sleep disorders are associated with metabolic and cardiovascular dysfunction. However the potential mechanisms underlying the association between sleep duration and cardiometabolic dysfunction are not well understood. A meta-analysis of prospective studies showed that the association between sleep duration and risk of type 2 diabetes followed a U-shaped curve (Shan et al. , 2015). Both short and long sleep durations were associated with significantly greater risk of type 2 diabetes. Our results indicate that increased sleep time is associated with greater weight gain in OVX mice fed a HFD. The 40 sec criterion we used to assess sleep is a non-invasive and inexpensive method that provides high correlation with simultaneous EEG/EMG assessment of sleep (Fisher et al., 2012). To our knowledge this is the first study that has explored the potential impact of sleep time in contributing to altered body weight regulation in OVX mice fed a HFD.
Caloric restriction increases appetite, reduces metabolic rate and decreases sympathetic nervous system activity to the heart, kidneys and brown adipose tissue (Muralidhara and Shetty, 1987, Overton et al. , 2001, Williams et al. , 2002). Caloric deprivation also decreases HR and BP (do Carmo et al., 2014b). In the present study we examined whether OVX alters the food intake and cardiovascular responses to caloric deprivation. We found that OVX mice fed a HFD did not exhibit altered food intake and body weight responses to refeeding after 24 hours of fasting. We also did not find significant changes in BP and HR responses to fasting or refeeding. Taken together, our findings suggest that ovarian hormones are not required for normal metabolic and cardiovascular responses to caloric deprivation.
Overall, our results suggest that OVX does not alter body weight homeostasis in mice fed a normal diet, but when combined with a HFD it reduces energy expenditure and increases sleep time and plasma leptin levels without altering food intake or fasting glucose or insulin levels. Our findings suggest that OVX exacerbates the adverse effects of obesity induced by a HFD mainly due to a reduction in energy expenditure and increased sleep time. Although our observations indicate that OVX mice fed a control diet did not have greater weight gain than control mice despite reduced energy expenditure, OVX mice fed a HFD had reduced energy expenditure and increased sleep time associated with greater weight gain without changes in caloric intake. The mechanisms for the increased susceptibility of OVX mice to HFD-induced obesity remain to be elucidated. However, our findings may have important implications for women who have deficiency of ovarian hormones due to menopause or ovariectomy. Further studies are needed to determine whether these findings are relevant to humans and whether therapeutic strategies may be developed to avoid deleterious metabolic effects of a HFD and prolonged sleep time in postmenopausal or ovariectomized women.
Acknowledgments
The authors were supported by grants from the National Heart, Lung, and Blood Institute (P01 HL51971) and the National Institute of General Medical Sciences (P20 GM104357 and U54 GM115428) of the National Institutes of Health.
Footnotes
Competing Interests
No conflicts of financial interests are declared by the authors.
Supplementary information is available at International Journal of Obesity’s website.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–76. [DOI] [PubMed] [Google Scholar]
- Bhatia AJ, Wade GN. Progesterone can either increase or decrease weight gain and adiposity in ovariectomized Syrian hamsters. Physiology & behavior. 1989;46:273–8. [DOI] [PubMed] [Google Scholar]
- Bhatia AJ, Wade GN. Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters. The American journal of physiology. 1991;260:R153–8. [DOI] [PubMed] [Google Scholar]
- Bhatia AJ, Wade GN. Energy balance in pregnant hamsters: a role for voluntary exercise? The American journal of physiology. 1993;265:R563–7. [DOI] [PubMed] [Google Scholar]
- Bohler HC, Jr., Tracer H, Merriam GR, Petersen SL. Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology. 1991;128:1265–9. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. The Journal of steroid biochemistry and molecular biology. 2010;122:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C, Casali KR, Baraldi D, Conzatti A, Araujo AS, Khaper N, et al. Efficacy of a low dose of estrogen on antioxidant defenses and heart rate variability. Oxid Med Cell Longev. 2014;2014:218749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. American journal of physiology Endocrinology and metabolism. 2001;280:E315–22. [DOI] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011a;57:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011b;57:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, et al. Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes. 2014a;38:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, et al. Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. International journal of obesity. 2014b;38:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A, et al. Regulation of Blood Pressure, Appetite, and Glucose by Leptin After Inactivation of Insulin Receptor Substrate 2 Signaling in the Entire Brain or in Proopiomelanocortin Neurons. Hypertension. 2016;67:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic-vagal balance and baroreflex sensitivity in diabetic rats. American journal of physiology Heart and circulatory physiology. 2008;295:H1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res. 1998;39:34–49. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK, Keller PJ. 17Beta-estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension. 1998;31:522–8. [DOI] [PubMed] [Google Scholar]
- Fengler VH, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Muller R, et al. Susceptibility of Different Mouse Wild Type Strains to Develop Diet-Induced NAFLD/AFLD-Associated Liver Disease. PloS one. 2016;11:e0155163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Godinho SI, Pothecary CA, Hankins MW, Foster RG, Peirson SN. Rapid assessment of sleep-wake behavior in mice. J Biol Rhythms. 2012;27:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert EL, Mathis KW, Ryan MJ. 17beta-Estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension. 2014;63:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez I, Delgado JL, Diaz J, Quesada T, Teruel MJ, Llanos MC, et al. 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. American journal of physiology Regulatory, integrative and comparative physiology. 2000;279:R1599–605. [DOI] [PubMed] [Google Scholar]
- Jehan S, Masters-Isarilov A, Salifu I, Zizi F, Jean-Louis G, Pandi-Perumal SR, et al. Sleep Disorders in Postmenopausal Women. J Sleep Disord Ther. 2015;4. [PMC free article] [PubMed] [Google Scholar]
- Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–7. [DOI] [PubMed] [Google Scholar]
- Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, et al. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circulation research. 2001;89:534–9. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:1103–9. [DOI] [PubMed] [Google Scholar]
- Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, et al. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology. 2014;155:4447–60. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–21. [DOI] [PubMed] [Google Scholar]
- Mercier I, Pham-Dang M, Clement R, Gosselin H, Colombo F, Rouleau JL, et al. Elevated mean arterial pressure in the ovariectomized rat was normalized by ET(A) receptor antagonist therapy: absence of cardiac hypertrophy and fibrosis. Br J Pharmacol. 2002;136:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosti MP, Ericsson M, Erben RG, Schuler C, Syversen U, Stunes AK. The PPARalpha Agonist Fenofibrate Improves the Musculoskeletal Effects of Exercise in Ovariectomized Rats. Endocrinology. 2016;157:3924–34. [DOI] [PubMed] [Google Scholar]
- Muralidhara DV, Shetty PS. Cold-induced thermogenesis in rats nutritionally deprived early in life. Indian J Physiol Pharmacol. 1987;31:149–58. [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME. Central leptin infusion attenuates the cardiovascular and metabolic effects of fasting in rats. Hypertension. 2001;37:663–9. [DOI] [PubMed] [Google Scholar]
- Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, et al. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. The Journal of clinical investigation. 2011;121:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. The Journal of clinical investigation. 2002;109:1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Nguyen BT, Wuttke W. Long-term effects of ovariectomy on osteoporosis and obesity in estrogen-receptor-beta-deleted mice. Comp Med. 2012;62:8–13. [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–37. [DOI] [PubMed] [Google Scholar]
- Tawfik SH, Mahmoud BF, Saad MI, Shehata M, Kamel MA, Helmy MH. Similar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem Res Int. 2015;2015:567945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. The Journal of clinical investigation. 2014;124:3075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, et al. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology. 2012;153:4266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraich RS, Mauvais-Jarvis F. Paracrine and intracrine contributions of androgens and estrogens to adipose tissue biology: physiopathological aspects. Horm Mol Biol Clin Investig. 2013;14:49–55. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. American journal of physiology Regulatory, integrative and comparative physiology. 2002;282:R1459–67. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, et al. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women : the strong heart study. Diabetes Care. 2002;25:500–4. [DOI] [PubMed] [Google Scholar]
- Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. The Journal of steroid biochemistry and molecular biology. 2010;122:100–5. [DOI] [PubMed] [Google Scholar]