Abstract
Background.
During the 2014–2015 US influenza season, 320 cases of non-mumps parotitis (NMP) among residents of 21 states were reported to the Centers for Disease Control and Prevention (CDC). We conducted an epidemiologic and laboratory investigation to determine viral etiologies and clinical features of NMP during this unusually large occurrence.
Methods.
NMP was defined as acute parotitis or other salivary gland swelling of >2 days duration in a person with a mumps- negative laboratory result. Using a standardized questionnaire, we collected demographic and clinical information. Buccal samples were tested at the CDC for selected viruses, including mumps, influenza, human parainfluenza viruses (HPIVs) 1–4, adenoviruses, cytomegalovirus, Epstein-Barr virus (EBV), herpes simplex viruses (HSVs) 1 and 2, and human herpes viruses (HHVs) 6A and 6B.
Results.
Among the 320 patients, 65% were male, median age was 14.5 years (range, 0–90), and 67% reported unilateral parotitis. Commonly reported symptoms included sore throat (55%) and fever (48%). Viruses were detected in 210 (71%) of 294 NMP patients with adequate samples for testing, ≥2 viruses were detected in 37 samples, and 248 total virus detections were made among all samples. These included 156 influenza A(H3N2), 42 HHV6B, 32 EBV, 8 HPIV2, 2 HPIV3, 3 adenovirus, 4 HSV-1, and 1 HSV-2. Influenza A(H3N2), HHV6B, and EBV were the most frequently codetected viruses.
Conclusions.
Our findings suggest that, in addition to mumps, clinicians should consider respiratory viral (influenza) and herpes viral etiologies for parotitis, particularly among patients without epidemiologic links to mumps cases or outbreaks.
Keywords: non-mumps viral parotitis, non-mumps parotitis, parotitis
Acute, viral non-mumps parotitis (NMP) is an infrequently recognized illness that occurs sporadically and has been associated with multiple etiologic agents, including adenoviruses, enteroviruses (coxsackieviruses, echoviruses), Epstein-Barr virus (EBV), human herpes virus (HHV) 6A and 6B, influenza A(H3N2) and influenza B viruses, human parainfluenza viruses (HPIVs) 1–3, and parvovirus B-19 [1–9]. While there is no systematic surveillance for NMP, results of several studies have suggested EBV is the most frequently detected virus among patients with NMP, followed by HPIV3, HPIV2, and adeno-viruses [6–9]. During January 2015, approximately 17 cases of NMP were reported to the Centers for Disease Control and Prevention (CDC) from several Midwestern states. Although small in number, these temporally related reports represented an unusual occurrence of viral NMP.
The only known cause of epidemic parotitis among humans is mumps, a vaccine-preventable disease caused by mumps virus, a member of the Rubulavirus genus of the Paramyxoviridae family [10]. Prior to the licensure of mumps vaccine in 1967 and its subsequent routine use in the United States, mumps was one of the most frequently reported diseases during childhood [11, 12]. Since 1990, the Advisory Committee on Immunization Practices to the CDC has recommended children routinely receive 2 doses of measles–mumps–rubella vaccine (MMR) [13]; the effectiveness against mumps following 2 doses of MMR is approximately 88% (range, 66%–95%) [14, 15]. This vaccine recommendation had a powerful impact on reducing mumps occurrence from more than 150 000 cases reported annually during the 1960s to a nadir of approximately 250 cases reported annually during 2000–2005 [16].
Laboratory testing includes serologic assays to detect mumps immunoglobulin M (IgM), virus culture, or conventional or real-time reverse transcription polymerase chain reaction (RT-PCR) to detect mumps viral RNA. However, confirming mumps virus infection can be challenging among persons with immunity induced by prior vaccination or infection. Upon infection, patients with prior immunity may not mount an IgM response or may have low viral load, thus a negative RT-PCR or serologic test result does not rule out mumps in a patient with compatible signs and symptoms [17].
Because of the unusual occurrence of viral NMP during the 2014–2015 influenza season and the importance of pursuing laboratory confirmation when acute parotitis occurs and mumps is suspected, enhanced understanding of the epidemiologic and clinical features of acute NMP would improve the accuracy of diagnosis among clinicians evaluating suspected mumps cases and result in more timely treatment and public health action when appropriate. During February 2015, we initiated a multistate epidemiologic and laboratory study to describe the etiologic, demographic, epidemiologic, and clinical features of all reported cases of NMP and a parallel multistate case-control study to examine risk factors for the occurrence of NMP caused by influenza A(H3N2) viruses that circulated during the 2014–2015 season. Here, we present the results of the epidemiologic and laboratory study.
METHODS
Case Ascertainment and Epidemiologic Investigation
On 22 December 2014, the CDC’s Influenza Division was notified by the Indiana State Department of Health of a cluster of patients with influenza-associated parotitis. On 9 January 2015, after additional state health departments reported similar occurrences of viral NMP, the Influenza Division notified state and local health departments of the occurrence of influenza-associated parotitis through the Epidemic Information Exchange and requested notification when a patient with nonmumps parotitis associated with influenza was identified and the illness met the case definition [18]. On 4 February, state and local health departments and public health laboratories were invited to participate in a multistate investigation of NMP. States could participate in the case-control study of influenza-associated parotitis and/or the epidemiologic and laboratory investigation of NMP regardless of etiology. The methods and results of the case-control study are presented elsewhere [18].
For the epidemiologic and laboratory investigation of NMP, a case was defined as clinical signs or symptoms compatible with acute parotitis or other salivary gland swelling of >2 days duration in a patient with illness onset from 1 October 2014 through 31 May 2015, who had no known epidemiologic linkage to a laboratory-confirmed case of mumps, did not have a laboratory-confirmed diagnosis of mumps infection (was either mumps-negative or not tested for mumps), and either had a laboratory-confirmed non-mumps viral infection (using a recommended test, including RT-PCR or viral culture) or had a buccal swab specimen available for viral testing at the CDC. Surveillance methods for eligible patients varied among states; methods included contacting clinicians using the Health Alert Network, using clinician email listservs, enhancing existing influenza surveillance activities, and passive reporting of suspected mumps cases.
Case ascertainment and investigation were designated as public health surveillance and were given a nonresearch determination by a CDC institutional review board. A questionnaire was administered by telephone to consenting eligible persons or their guardians. Information collected included patient demographic information; signs and symptoms; testing for mumps, influenza, and other viral agents; past medical history; self-reported current and previous seasonal influenza vaccination; self-report of MMR vaccination; hospitalization during the past 12 months; and recent travel.
Laboratory Testing and Analysis
The CDC Division of Viral Disease laboratories conducted testing for mumps virus, HPIV 1–4, adenoviruses, and herpes family viruses, including cytomegalovirus (CMV), EBV, herpes simplex virus (HSV) 1 and 2, HHV6A, and HHV6B. The CDC Influenza Division laboratories conducted testing for influenza viruses.
Mumps Virus
The real-time RT-PCR assays to detect mumps RNA were performed as previously described [19].
Herpes Family Viruses
HHV6
A conventional PCR method coupled with gel electrophoresis was used to screen samples for the presence of HHV6. The primers are from the immediate early gene, U90, and are designed to discriminate HHV6A from HHV6B based on a deletion in the U1102 strain. HHV6A-positive samples are determined by a band size of 325 bp; whereas a band size of 553 bp is the result for HHV6B-positive samples [7, 20].
EBV
Specimens were screened using a real-time florescence resonance energy transfer (FRET)–based PCR method that uses 2 florescent probes, the anchor and the detector. When the target is present, these bound probes are in close proximity and release a detectable signature florescence. The target for this method is the BamHI region of EBV [7].
HSV1/2
FRET technology was used to discriminate HSV-1 from HSV-2 in this real-time PCR method targeting the glycoprotein B, UL27, gene. This 2-probed system can discriminate type 1 from type 2 based on melt curve analysis. A sample is considered HSV-1 positive if the melt temperature (Tm) is 56°C and HSV-2 positive if the Tm is 63°C (CDC, unpublished method).
Influenza Viruses
Influenza virus infection was confirmed and typed/subtyped using RT-PCR with standard protocols or next-generation sequencing. RT-PCR assays to detect influenza viral RNA were performed as previously described [21] or next-generation sequencing was conducted using a MiSeq platform and the Iterative Refinement Meta Assembler [22]. Study sequences were compared to viral reference sequences and sequences from other circulating viruses.
Adenoviruses and Human Parainfluenza Viruses 1–4
Sample nucleic acid extracts were tested using CDC singleplex real-time RT-PCR assays for adenoviruses and parainfluenza types 1–4 [23]. Threshold cycle values were determined by manually adjusting the fluorescence baseline to fall within the exponential phase of the amplification curves and above any background signal. A positive test result was considered a well-defined curve that crossed the threshold cycle within 40 cycles.
Statistical Analyses
Statistical analyses included use of χ2 test to compare proportions and Wilcoxon rank-sum tests to compare medians. Analyses were performed with SAS® version 9.3 (SAS Institute, Cary, NC).
RESULTS
Virus Detections
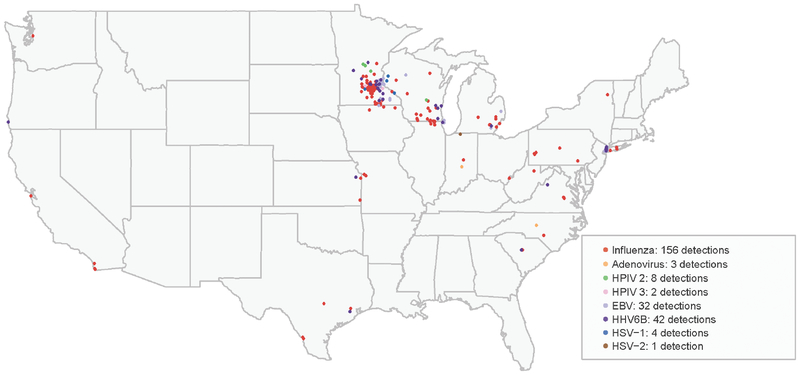
From 1 October 2014 through 31 May 2015, 323 cases of NMP among residents of 21 states were reported to the CDC. The geographic distribution of viruses detected during testing at the CDC of buccal samples from 294 NMP patients from 19 states is summarized in Figure 1 and Table 1. Influenza A(H3N2) virus was detected in 156 (53%) patient samples, including ≥1samples from all 19 states with reported cases. HHV6B was detected in 42 (14%) samples from 10 states. Six other viruses were detected, including adenovirus (1%), HPIV2 (3%), HPIV3 (0.7%), EBV (13%), HSV-1 (1.4%), and HSV-2 (0.3%), primarily in specimens from Midwestern and Northeastern states (Table 1). Multiple viruses were detected in 13% of samples. Mumps virus, CMV, HHV6A, HPIV1, and HPIV4 were not detected in any sample.
Figure 1.
Geographic distribution of viruses detected among patients with non-mumps viral parotitis, with samples tested at the Centers for Disease Control and Prevention, United States, 1 October 2014–31 May 2015. Abbreviations: EBV, Epstein-Barr virus; HHV, human herpes virus; HPIV, human parainfluenza virus; HSV, herpes simplex virus.
Table 1.
Viruses Detected in Samples from Patients With Non-mumps Viral Parotitis Tested at the Centers for Disease Control and Prevention and Number of Detections by Virus and State, United States, 1 October 2014–31 May 2015
| State | Influenza A(H3N2) Virus (N = 156) | Adenovirus (N = 3) | HPIV2 (N = 8) | HPIV3 (N = 2) | HHV6B (N = 42) | EBV (N = 32) | HSV1 (N = 4) | HSV2 (N = 1) |
|---|---|---|---|---|---|---|---|---|
| California | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Indiana | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Maine | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Massachusetts | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Michigan | 10 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| Minnesota | 71 | 0 | 3 | 2 | 17 | 16 | 4 | 0 |
| Missouri | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| New Hampshire | 6 | 0 | 1 | 0 | 2 | 1 | 0 | 0 |
| New Jersey | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Yorka | 4 | 1 | 2 | 0 | 9 | 9 | 0 | 0 |
| North Carolina | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oregon | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Pennsylvania | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| South Carolina | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Texas | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virginia | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Washington | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| West Virginia | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wisconsin | 25 | 0 | 1 | 0 | 5 | 3 | 0 | 0 |
Abbreviations: EBV, Epstein-Barr virus; HHV, human herpes virus; HPIV, human parainfluenza virus; HSV, herpes simplex virus.
Two viruses were codetected in each of 36 samples. The codetection pairings included 19 samples with influenza A(H3N2) virus and HHV6B detected, 10 with influenza A(H3N2) virus and EBV, 2 with EBV and HHV6B, 2 with influenza A(H3N2) virus and HSV-1, 1 with EBV and HPIV2, 1 with HHV6B and HPIV2, and 1 with HPIV2 and HSV-2. Three viruses were detected in 1 sample: influenza A(H3N2) virus, EBV, and HHV6B. Eighty-four samples from patients with non-mumps parotitis that were tested at the Centers for Disease Control and Prevention had no viruses detected. These samples were from patients from 8 states: Indiana, 1; Michigan, 2; Minnesota, 38; New Hampshire, 1; New York, 20 (19 from New York City and 1 from New York State); Texas, 1; Virginia, 1; and Wisconsin, 20.
Represents New York City (36 samples) and New York State (9 samples).
Patient Characteristics
Data regarding demographic and clinical features and exposure and vaccination history from 320 NMP patients (with sufficient data) are summarized in Tables 2–4. Among these patients, most were male (65%), median age was 14.5 years (range, <1–90; [interquartile range (IQR), 8–30 years]), and 64% were aged <20 years (Table 2). There were significant differences in sex and median age by virus detection category, which includes single virus detected in the sample (4 categories: influenza A(H3N2), other respiratory viruses, HHV6B, and EBV), virus codetection, and no virus detected (Table 2). The percentage male was greatest among patients with influenza virus single detections and codetections (32 of 37 codetections included influenza virus) and least among patients with no virus detected. Younger median age was associated with HHV6B detection, other respiratory virus detection (HPIV2/3 and adenovirus), and virus codetection, while older median age was associated with EBV and no virus detected (Table 2).
Table 2.
Demographic Features, Self-reported Signs and Symptoms, and Other Clinical Characteristics Among 320 Patients With Non-mumps Parotitis by Virus Detection Category, United States, 1 October 2014–31 May 2015
| Variable | Total Non-Mumps Parotitis (N = 320) | Influenza A(H3N2) Virusa (N = 124) | Other Respiratory Virusa,b (N = 10) | Virus Codetectionc (N = 37) | Human Herpes Virus 6Ba (N = 19) | Epstein-Barr Virusa (N = 18) | No Virus Detected (N = 84) |
|---|---|---|---|---|---|---|---|
| Demographic features, n (%)d | |||||||
| Male | 207 (65) | 92 (75)e | 5 (50) | 27 (73) | 12 (63) | 10 (56) | 43 (51)e |
| Age, median years | 14.5 | 14 | 7f | 10f | 5f | 35f | 26f |
| Range | <1–90 | <1–84 | 1–12 | 2–77 | <1–37 | <1–90 | 1–74 |
| Interquartile range | 8–30 | 8–23 | 6–8 | 6–18 | 2–9 | 16–60 | 11–40 |
| Self-reported signs and symptoms, n (%)d | |||||||
| Influenza-like illnessh | 129 (40) | 64 (52)e | 3 (30) | 13 (35) | 5 (26) | 4 (22) | 23 (27) |
| Fever/feverishi | 153 (48) | 73 (59)e | 6 (60) | 14 (38) | 5 (26) | 5 (28) | 33 (39) |
| Chills | 116 (38) | 56 (45) | 8 (80) | 13 (35) | 4 (21) | 5 (28) | 24 (29) |
| Muscle ache | 100 (31) | 40 (33) | 4 (40) | 9 (24) | 2 (11) | 4 (22) | 28 (33) |
| Headache | 124 (39) | 49 (40) | 5 (50) | 15 (41) | 3 (16) | 5 (28) | 13 (15) |
| Cough | 118 (37) | 62 (50) | 4 (40) | 19 (51) | 4 (21) | 3 (16) | 11 (13) |
| Wheeze | 39 (12) | 17 (14) | 2 (20) | 4 (11) | 1 (5) | 2 (11) | 8 (10) |
| Sore throat | 177 (55) | 67 (55) | 4 (40) | 18 (49) | 9 (47) | 5 (29) | 47 (56) |
| Runny nose | 98 (31) | 37 (30) | 5 (50) | 17 (46) | 1 (6) | 2 (11) | 18 (21) |
| Ear pain | 121 (38) | 46 (37) | 6 (60) | 7 (19) | 10 (53) | 5 (29) | 34 (40) |
| Rash | 24 (8) | 8 (7) | 0 (0) | 3 (8) | 4 (21) | 1 (6) | 5 (6) |
| Facial swelling | 239 (75) | 97 (79) | 7 (70) | 28 (76) | 11 (58) | 15 (83) | 62 (74) |
| Gland swelling | 213 (67) | 80 (65) | 4 (40) | 26 (70) | 11 (58) | 10 (56) | 61 (73) |
| Unilateral parotitis | 215 (67) | 84 (68) | 7 (70) | 29 (78) | 16 (84) | 11 (61) | 49 (58)e |
| Bilateral parotitis | 91 (28) | 37 (30) | 2 (20) | 6 (16) | 2 (11) | 7 (39) | 28 (33) |
| Clinical characteristics, n (%)d | |||||||
| Hospitalized during illness | 34 (11) | 9 (7) | 1 (10) | 5 (14) | 2 (11) | 4 (22)e | 9 (11) |
| Experienced complication from illnessg | 16 (5) | 3 (3) | 0 (0) | 3 (9) | 0 (0) | 1 (6) | 7 (8) |
| Received antibiotics during illness | 150 (46) | 62 (50) | 4 (40) | 16 (43) | 8 (42) | 5 (28) | 40 (48) |
Included are 210 patients with non-mumps parotitis (NMP) who had buccal specimens from which 1 or more viruses were detected. These patients were subsequently defined as having non-mumps viral parotitis. Also included are 84 patients with NMP who had specimens from which viruses were not detected.
Unless otherwise noted, virus detection indicates single detections of a virus in a sample.
Other respiratory viruses include human parainfluenza virus 2 (HPIV2) detected in 5 samples, HPIV3 detected in 2 samples, and adenovirus detected in 3 samples.
Two viruses were codetected in each of 36 samples. The codetection pairings included 19 samples with influenza A(H3N2) virus and human herpes virus 6B (HHV6B) detected, 10 with influenza A(H3N2) virus and Epstein-Barr virus (EBV), 2 with EBV and HHV6B, 2 with influenza A(H3N2) virus and herpes simplex virus-1 (HSV-1), 1 with EBV and HPIV2, 1 with HHV6B and HPIV2, and 1 with HPIV2 and HSV-2. Three viruses were detected in 1 sample: influenza A(H3N2) virus, EBV, and HHV6B.
Unless otherwise noted.
The χ2 test P value < .05. Reference group consists of patients not in the category; for example, patients with HHV6B detected were compared with patients with samples in which HHV6B was not detected.
Wilcoxon rank-sum test P value < .05. Reference group consists of patients not in the category; for example, patients with HHV6B detected were compared with patients with samples in which HHV6B was not detected.
Self-reported complications included ear infections, testicular pain, pneumonia, and abdominal pain.
Influenza-like illness defined as fever (temperature ≥100°F) or feeling feverish and cough or sore throat.
Temperature ≥100°F or self-report of feeling feverish.
Table 4.
Underlying Medical Conditions, Exposure History, and Vaccination History Among Patients With Non-mumps Parotitis and Non-mumps Viral Parotitis by Virus Detection Category, United States, 1 October 2014–31 May 2015
| Variable | Total Non-Mumps Parotitis (N = 320) | Influenza A(H3N2)a (N = 124) | Other Respiratory Virusa,b (N = 10) | Virus Codetectionc (N = 37) | Human Herpes Virus 6Ba (N = 19) | Epstein-Barr Virusa (N = 18) | No Virus Detected (N = 84) |
|---|---|---|---|---|---|---|---|
| Underlying medical condition, n (%)d | |||||||
| Had any underlying medical condition | 141 (44) | 61 (50) | 4 (40) | 11 (30) | 3 (16)e | 8 (44) | 40 (48) |
| Asthma | 72 (23) | 29 (24) | 4 (40) | 5 (14) | 2 (11) | 4 (22) | 21 (25) |
| Chronic obstructive pulmonary disease or chronic lung condition | 7 (2) | 2 (2) | 0 (0) | 1 (3) | 0 (0) | 2 (11) | 1 (1) |
| Cardiovascular condition | 13 (4) | 6 (5) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 2 (2) |
| Diabetes | 14 (4) | 4 (3) | 0 (0) | 2 (5) | 1 (5) | 2 (11) | 4 (5) |
| Renal condition | 3 (0.9) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Immunosuppressive condition | 7 (2) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) |
| Chemotherapy in past year | 5 (2) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neurologic/neurodevelopmental condition | 13 (4) | 8 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Rheumatoid arthritis | 8 (3) | 2 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 4 (5) |
| Sjogren’s syndrome | 2 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Obesity | 55 (18) | 20 (17) | 0 (0) | 5 (14) | 2 (11) | 2 (11) | 20 (24) |
| Other conditionf | 16 (5) | 6 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 8 (10) |
| Exposure history, n (%)d | |||||||
| History of mumps virus infection | 19 (6) | 3 (3) | 0 (0) | 5 (14) | 0 (0) | 2 (11) | 8 (10) |
| History of parotitis | 18 (6) | 2 (2) | 0 (0) | 3 (9) | 1 (6) | 2 (11) | 7 (8) |
| Strep throat in past year | 41 (13) | 22 (18) | 3 (30) | 1 (3)e | 3 (16) | 1 (6) | 10 (12) |
| Respiratory syncytial virus or mononucleosis in past year | 2 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 0 (0) |
| Hospitalization for other illness in past year | 26 (8) | 8 (7) | 1 (10) | 3 (8) | 0 (0) | 5 (28)e | 1 (1) |
| Dentist/oral surgeon visit within 2 weeks before illness | 8 (3) | 3 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 3 (4) |
| Sinus procedure within 2 weeks before illness | 1 (0.3) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Aware of others with parotitis/mumps | 44 (14) | 6 (5) | 0 (0) | 4 (12) | 1 (11) | 2 (11) | 10 (12) |
| Travel within 2 weeks before illness | 24 (8) | 19 (16) | 2 (20) | 4 (12) | 2 (6) | 1 (6) | 11 (13) |
| Vaccination history, n (%)d | |||||||
| Influenza vaccine: 2013–2014 season | 195 (62) | 84 (69) | 7 (70) | 21 (58) | 10 (53) | 11 (61) | 45 (54) |
| Influenza vaccine: 2014–2015 seasong | 179 (56) | 67 (55) | 5 (50) | 18 (49) | 14 (74) | 10 (56) | 47 (56) |
| Measles-mumps-rubella vaccinationh | 280 (89) | 113 (93) | 10 (100) | 33 (89) | 18 (95) | 11 (61)e | 69 (82) |
Unless otherwise noted, virus detection indicates single detections of a virus in a sample.
Other respiratory viruses include human parainfluenza virus 2 (HPIV2) detected in 5 samples, HPIV3 detected in 2 samples, and adenovirus detected in 3 samples.
Two viruses were codetected in each of 36 samples. The codetection pairings included 19 samples with influenza A(H3N2) and human herpes virus 6B (HHV6B) detected, 10 with influenza A(H3N2) and Epstein-Barr virus (EBV), 2 with EBV and HHV6B, 2 with influenza A(H3N2) and herpes simplex virus 1 (HSV-1), 1 with EBV and HPIV2, 1 with HHV6B and HPIV2, and 1 with HPIV2 and HSV-2. Three viruses were detected in 1 sample: influenza A(H3N2), EBV, and HHV6B.
Unless otherwise noted.
The χ2 test P value < .05. Reference group consists of patients not in the category; for example, patients with HHV6B detected were compared with patients with samples in which HHV6B was not detected.
Other conditions included hepatic disease.
Received influenza vaccine at least 2 weeks before symptom onset.
Reported receiving at least 1 dose of the measles–mumps–rubella vaccine.
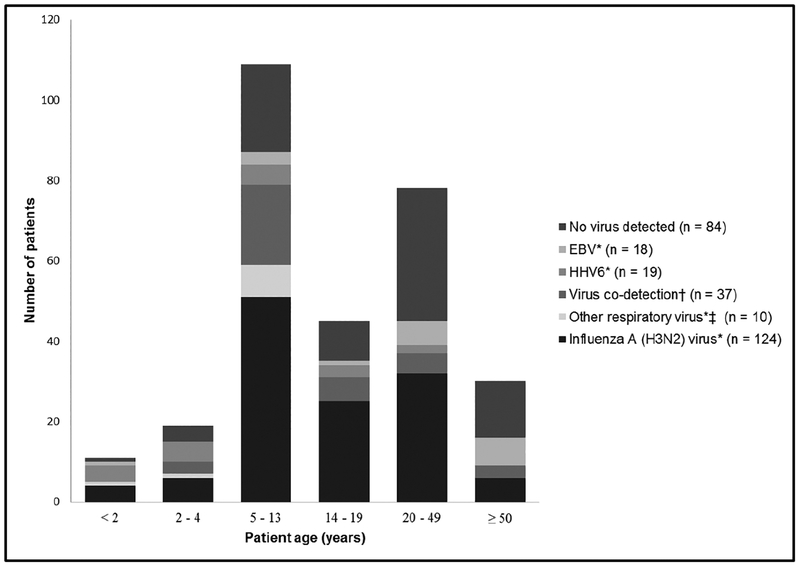
Among the 294 patients with buccal samples tested, 232 (79%) were aged 5–49 years. Single detections of influenza A(H3N2) virus occurred in all age groups, but 76 (61%) occurred among patients aged 5–19 years (Figure 2). Among single detections of other viruses, 14 (74%) HHV6B detections and all other respiratory virus detections occurred among younger patients (aged <14 years), and 14 (78%) EBV detections occurred among older patients (aged >19 years). Among patients with no virus detected, 47 (56%) were aged >19 years (Figure 2, Table 2).
Figure 2.
Virus detection among patients with non-mumps parotitis by age group, United States, 1 October 2014–31 May 2015. * Unless otherwise noted, virus detection indicates single detections of a virus in a sample. †Two viruses were codetected in each of 36 samples. The codetection pairings included 19 samples with influenza A(H3N2) virus and human herpes virus 6B (HHV6B) detected, 10 with influenza A(H3N2) virus and Epstein-Barr virus (EBV), 2 with EBV and HHV6B, 2 with influenza A(H3N2) virus and herpes simplex virus1 (HSV-1), 1 with EBV and human parainfluenza virus 2 (HPIV2), 1 with HHV6B and HPIV2, and 1 with HPIV2 and HSV-2. Three viruses were detected in 1 sample: influenza A(H3N2) virus, EBV, and HHV6B. ‡ Other respiratory viruses include HPIV2 detected in 5 samples, HPIV3 detected in 2 samples, and adenovirus detected in 3 samples. Abbreviations: EBV, Epstein-Barr virus; HHV, human herpes virus.
Among all patients, 67% reported unilateral parotitis and 40% reported influenza-like illness (ILI; fever [temperature ≥100°F] or feling feverish and cough or sore throat). Unilateral parotitis was less frequent among patients with no virus detected. Patients with influenza virus detection more frequently reported ILI and other symptoms preceding parotitis onset than patients with EBV or no virus detected (Tables 2 and 3). Among all patients, 46% reported receiving antibiotics during their illnesses; with the exception of patients with EBV detection, little difference in percentage with antibiotic receipt was noted by virus detection category (Table 2). Among all patients, 5% reported complications and 11% were hospitalized; hospitalization was most frequent (22%) among patients with EBV detection, although numbers were small.
Table 3.
Timing of Sample Collection and Symptom Onset Among 320 Patients With Non-mumps Parotitis by Virus Detection Category, United States, 1 October 2014–31 May 2015
| Variable | Total Non-Mumps Parotitis (N = 320) | Influenza A(H3N2) Virusa (N = 124) | Other Respiratory Virusa,b (N = 10) | Virus Codetectionc (N = 37) | Human Herpes Virus 6Ba (N = 19) | Epstein-Barr Virusa (N = 18) | No Virus Detected (N = 84) |
|---|---|---|---|---|---|---|---|
| Timing of sample collection, n (%)d | |||||||
| ≤2 days after any symptom onset | 148 (46) | 48 (39) | 4 (40) | 14 (38) | 12 (63) | 13 (72) | 37 (44) |
| ≤2 days after parotitis onset | 222 (69) | 89 (72) | 7 (70) | 29 (78) | 14 (74) | 13 (72) | 44 (52)e |
| Timing of symptom onset, n (%)d | |||||||
| Symptom onset preceded parotitis onset | 154 (55) | 71 (63)e | 5 (50) | 23 (62) | 6 (32) | 4 (27)e | 24 (29)e |
| Symptom onset at same time as parotitis onset | 106 (38) | 38 (34) | 3 (20) | 8 (22) | 6 (32) | 7 (47) | 38 (45) |
| Parotitis onset preceded other symptom onset | 8 (3) | 0 (0) | 2 (20) | 0 (0) | 0 (0) | 2 (13) | 5 (6) |
Included are 210 patients with non-mumps parotitis (NMP) who had buccal specimens from which 1 or more viruses were detected. These patients were subsequently defined as having non-mumps viral parotitis. Also included are 84 patients with NMP who had specimens from which viruses were not detected.
Unless otherwise noted, virus detection indicates single detections of a virus in a sample.
Other respiratory viruses include human parainfluenza virus 2 (HPIV2) detected in 5 samples, HPIV3 detected in 2 samples, and adenovirus detected in 3 samples.
Two viruses were codetected in each of 36 samples. The codetection pairings included 19 samples with influenza A(H3N2) virus and human herpes virus 6B (HHV6B) detected, 10 with influenza A(H3N2) virus and Epstein-Barr virus (EBV), 2 with EBV and HHV6B, 2 with influenza A(H3N2) virus and herpes simplex virus 1(HSV-1), 1 with EBV and HPIV2, 1 with HHV6B and HPIV2, and 1 with HPIV2 and HSV-2. Three viruses were detected in 1 sample: influenza A(H3N2) virus, EBV, and HHV6B.
Unless otherwise noted.
The χ2 test P value < .05. Reference group consists of patients not in the category; for example, patients with samples in which HHV6B detected were compared with patients with samples in which HHV6B was not detected.
Most samples (69%) were collected within 2 days after parotitis onset. Specimens with no viruses detected were more likely to be collected >2 days after parotitis onset than specimens with viruses detected (Table 3).
Overall, 141 (44%) patients reported having an underlying medical condition, and asthma (23%) and obesity (18%) were the most common reported conditions. There was little difference in frequency of underlying conditions by virus detection category (Table 4).
There was little difference in history of mumps virus infection, parotitis, respiratory syncytial virus infection, or mononucleosis during the past year by virus detection category (Table 4). History of strep throat during the past year was more frequently reported among patients with virus codetection, although numbers were small (Table 4). Prior hospitalization during the past year was more frequent among patients with EBV detection.
Except for lower frequency of MMR vaccination among patients with EBV detected, there were no significant differences in MMR or influenza vaccination history by virus detection category (Table 4).
DISCUSSION
We report the largest known survey of sporadic cases of NMP and influenza-associated NMP, including 294 patients with interviews and sufficient samples available for further testing. Influenza viruses and viruses in the herpes family were commonly detected among these patients. All illness onsets occurred during the 2014–2015 US influenza season (October–May). Eight viruses were detected, and 210 (71%) of the samples tested were positive for at least 1 virus. The most frequently detected viruses were influenza A(H3N2), 156 detections in patients from 19 states; HHV6B, 42 detections; and EBV, 32 detections. Codetections of influenza A(H3N2) virus with HHV6B or EBV viruses were also common.
Investigators in other Northern Hemisphere countries have also reported on viral etiologies of NMP during the 2014–2015 influenza season [24–26]. While 2 of these investigations [25, 26] restricted their laboratory investigation to influenza viruses, a survey in the United Kingdom tested children for a broad panel of respiratory viruses, identifying influenza A(H3N2) virus in 16 (15%) samples and respiratory syncytial virus A with codetection of influenza A/H3 in 1 sample [26]. Similarly, we found an increased occurrence of influenza A(H3N2) virus–associated parotitis in this cohort, which might be an artifact of enhanced surveillance and case-finding efforts or a reflection of the dominance of influenza A(H3N2) virus in North America and Europe during the 2014–2015 season [27].
When included in the test panels, EBV was the most frequently detected virus in studies investigating etiologies of NMP prior to the 2014–2015 influenza season. Among 5 such studies, the average EBV detection rate was 20% (range, 6%–25%); however, EBV test methods varied [6–9, 28, 29]. We detected EBV in 13% of samples; however, the lower frequency reported here might have resulted from the initial surveillance focus on influenza-associated NMP.
The second most commonly detected virus among patients tested during this 2014–2015 study was HHV6B. Investigators in the United States and Finland screened patients with NMP during 2009–2011 and 1993–1998 for HHV6, respectively, with comparable results [7, 8]. The US study used a PCR-based molecular assay, while the Finnish study used a serologic assay; the HHV6B detection rate was 4%–10%. In the US study, the median age of patients with HHV6B detection was 6 years (range, 0–35), while testing for HHV6 was limited to children aged <4 years in the Finnish study. In our investigation, patients who had NMP with HHV6B had an age distribution similar to the range in the prior US study and were predominately male. HHV6 is found to infect almost all individuals during early childhood and, similar to other herpes viruses, is capable of reactivation in both normal and immunocompromised persons [30]. Interestingly, HHV6 appears to persist in salivary glands and viral DNA can be routinely detected in saliva using PCR [30]. Furthermore, HHV6B is the predominant strain found in both normal and immunocompromised hosts, which might explain the high frequency of HHV6B detection in our case series.
Of note, our rate of codetection of viruses in patient samples was 14%, and each of the 37 codetection samples included a herpes virus (HHV6B, EBV, HSV1, or HSV2) with either a respiratory virus (influenza A(H3N2) virus, 32 samples or HPIV2, 3 samples) or another herpes virus (2 samples). Results of 1 study included codetection with EBV and respiratory viruses [9]; another reported codetection with respiratory viruses, with codetection rates ranging from 2% to 8% [6]. Results of prior studies suggest that infection with influenza and other respiratory viruses might reactivate herpes family viruses [31, 32]. In our study, patients with codetections did not report higher frequency of complications, hospitalizations, or underlying medical conditions compared with patients having samples with single virus detection.
It is challenging to determine the etiologic agents among sporadic cases of parotitis occurring in regions with a low incidence of mumps. Information regarding parotitis onset and timing of sample collection is important when interpreting laboratory results. In our study, 69% of oral samples were collected during the first 2 days following parotitis onset; among those samples, viruses were detected in 156 (70%), and the most frequent viruses detected were influenza A(H3N2) virus, HHV6B, and EBV. Further, detection of mumps virus by RT-PCR decreases >2 days following onset of swelling independently of the vaccination status. In one study, the sensitivity of RT-PCR for mumps virus detection decreased from 87% among oral samples collected during day 1 of swelling and 78% among samples collected during day 2 to 41% among samples collected during day 3 [22]. To enhance detection and diagnostic accuracy among patients with NMP, public health laboratories should consider additional respiratory virus panel and herpes family viral testing if resources permit.
Our investigation is subject to multiple limitations. First, it did not include testing for bacterial causes and noninfectious causes of parotitis, such as parotid stones [10]. This was intentional because the study focus was to characterize and describe viral etiologies of NMP. Second, while buccal swab specimens are the best diagnostic samples for suspected mumps, they are less sensitive than nasopharyngeal (NP) swab specimens for detection of respiratory viruses [33, 34]. This suboptimal sampling using buccal specimens might have resulted in an underestimation of the true prevalence of these viruses among our study population. Third, detection of remnant genetic material from previous infections could result in overestimation of the prevalence of certain viruses. Fourth, viruses that were not tested for, including echoviruses and parvovirus B19, which are known but rarely reported etiologies of viral NMP, potentially could have contributed to clinical presentations among these patients. Fifth, only samples associated with completed patient questionnaires were analyzed. This might have resulted in underreporting of certain viruses. Sixth, our initial case-finding strategy focused on influenza-associated viral NMP. This might have resulted in a higher-than-previously-reported occurrence of cases associated with influenza A(H3N2) virus and a lower-than-previously-reported frequency of NMP cases associated with EBV.
In conclusion, we investigated a large occurrence of nonmumps parotitis during the 2014–2015 US influenza season. Possible viral etiologic agents other than mumps virus were detected in a high proportion of samples tested. These detections resulted, in part, from enhanced surveillance, including additional respiratory testing at state public health laboratories and active case-finding efforts. To correctly exclude mumps virus as the etiology of parotitis with mumps-negative RT-PCR results, obtaining additional NP swabs for viral testing within 2 days of parotitis onset should also be considered, particularly among patients without epidemiologic links to mumps cases or outbreaks. Testing for illnesses that mimic mumps might result in more timely and appropriate treatment, including antibiotic cessation and public health response. Additional investigations of NMP are warranted to better understand the etiologic, clinical, and epidemiologic features of outbreak-related and sporadically occurring cases.
Acknowledgments.
Dr. Jeffrey P. Davis was the Wisconsin State Epidemiologist and Chief Medical Officer for the Division of Public Health, Bureau of Communicable Diseases. He served in these positions for more than 40 years. Dr. Davis was passionate about the field of public health and protecting the health of Wisconsin residents. He led many important public health investigations. Dr. Davis’ contributions to the fields of Infectious Disease, Epidemiology and Public Health are reflected in his over 250 publications. He was a mentor to many in public health, and a kind and wise friend. Tiffany Wallin, Amie Worthington (Kansas Department of Health and Environment); Vicki Rea (Maine Department of Health and Human Services); Emily Banerjee, Dave Boxrud, Larry Carroll, Kate Engels, Cynthia Kenyon, Brian Nefzger, Kirk Smith, Angie Taylor, Jason Wotton (Minnesota Department of Health); Jessica Bauer, C. Jon Hinkle (Missouri Department of Health and Senior Services); Lisa Hubbert, Laura Kresl, Lisa Mertz, Beckie Chebet Rono (City of Kansas City Health Department); Elizabeth Daly, Pamela Hill, Maureen MacDonald (New Hampshire Department of Public Health Services); Pinar Erdogdu, Annmarie Haldeman, Lindsay Hamilton, Natalie Kratz, Erica Rauch, Deepam Thomas (New Jersey Department of Health); Saima Abedin, Melissa Chacko, Jie Fu (New York City Department of Health and Mental Hygiene); Helen Blanchette, Donna Gowie, Emily Haner, Lauren Lopano, Angela Maxted, Kathryn Sen, Christine Waters, Shelley Zansky (New York State Department of Health); Keila Castillo, Crystal Van Cleave, Heather Cooks-Sinclair, Vivienne Heines, Johnathan Ledbetter, Anita Lewis, Peter So, Reynol Vela, Rachel Wiseman (Texas Department of State Health Services); Andrea Alvarez, Jonathan Falk, Marilyn Bibbs Freeman, Kathleen Gregory, Jasmin Howard, Sean Kelly, Heather Masri, Bethany McCunn, Carolyn Palmer, Megan Price, Okey Utah, Kim Whetzel, Laura Young (Virginia Department of Health); Claire Leback, Wes Robertson, Nailah Smith, Amanda Thoma (Wisconsin Division of Public Health); Christi Clark, Shannon McBee, Joyce Nicola (West Virginia Department of Health and Human Resources); and Matthew Biggerstaff, Kristine Bisgard, Joseph Bresee, Stephen Lindstrom, Gregory Wallace (Centers for Disease Control and Prevention [CDC]).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Howlett JG, Somlo F, Kalz F. A new syndrome of parotitis with herpangina caused by the Coxsackie virus. Can Med Assoc J 1957; 77:5–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom HH, Johnson KM, Jacobsen R, Channock RM. Recovery of parainfluenza viruses from adults with upper respiratory illness. Am J Hygiene 1961; 74:50–9. [Google Scholar]
- 3.Zollar LM, Mufson MA. Acute parotitis associated with parainfluenza 3 virus infection. Am J Dis Child 1970; 119:147–8. [DOI] [PubMed] [Google Scholar]
- 4.Buckley JM, Poche P, McIntosh K. Parotitis and parainfluenza 3 virus. Am J Dis Child 1972; 124:789. [DOI] [PubMed] [Google Scholar]
- 5.Brill SJ, Gilfillan RF. Acute parotitis associated with influenza type A: a report of twelve cases. N Engl J Med 1977; 296:1391–2. [DOI] [PubMed] [Google Scholar]
- 6.Barrabeig I, Costa J, Rovira A, et al. Viral etiology of mumps-like illnesses in suspected mumps cases reported in Catalonia, Spain. Hum Vaccin Immunother 2015; 11:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barskey AE, Juieng P, Whitaker BL, et al. Viruses detected among sporadic cases of parotitis, United States, 2009–2011. J Infect Dis 2013; 208:1979–86. [DOI] [PubMed] [Google Scholar]
- 8.Davidkin I, Jokinen S, Paananen A, Leinikki P, Peltola H. Etiology of mumps-like illnesses in children and adolescents vaccinated for measles, mumps, and rubella. J Infect Dis 2005; 191:719–23. [DOI] [PubMed] [Google Scholar]
- 9.Hatchette TF, Mahony JB, Chong S, LeBlanc JJ. Difficulty with mumps diagnosis: what is the contribution of mumps mimickers? J Clin Virol 2009; 46:381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hviid A, Rubin S, Mühlemann K. Mumps. Lancet 2008; 371:932–44. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Mumps surveillance. Report No.2. CDC: Atlanta, GA, 1972. [Google Scholar]
- 12.Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 2009; 27: 6186–95. [DOI] [PubMed] [Google Scholar]
- 13.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34. [PubMed] [Google Scholar]
- 14.Harling R, White JM, Ramsay ME, Macsween KF, van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005; 23:4070–4. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580–9. [DOI] [PubMed] [Google Scholar]
- 17.Rota JS, Turner JC, Yost-Daljev MK, et al. Investigation of a mumps outbreak among university students with two measles-mumps-rubella (MMR) vaccinations, Virginia, September-December 2006. J Med Virol 2009; 81:1819–25. [DOI] [PubMed] [Google Scholar]
- 18.Rolfes MA, Millman AJ, Talley P, et al. Influenza-associated parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018; 67:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rota JS, Rosen JB, Doll MK, et al. Comparison of the sensitivity of laboratory diagnostic methods from a well-characterized outbreak of mumps in New York City in 2009. Clin Vaccine Immunol 2013; 20:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Mukai T, Kondo K, Yamanishi K. Variation of DNA sequence in immediate-early gene of human herpesvirus6 and variant identification by PCR. J Clin Microbiol 1994; 32:473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Wentworth DE. Influenza A virus molecular virology techniques In: Kawaoka Y, Neumann G, eds. Influenza virus: methods and protocols. New York City, NY: Humana Press, 2012:174–92. [Google Scholar]
- 22.Shepard SS, Meno S, Bahl J, Wilson MM, Barnes J, Neuhaus E. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genomics 2016; 17:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakthivel SK, Whitaker B, Lu X, et al. Comparison of fast-track diagnostics respiratory pathogens multiplex real-time RT-PCR assay with in-house singleplex assays for comprehensive detection of human respiratory viruses. J Virol Methods 2012; 185:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shepherd SJ, MacLean AR, Aitken C, Gunson RN. Letter to the editor: there is a need to consider all respiratory viruses in suspected mumps cases. Euro Surveill 2015; 20:21210. [DOI] [PubMed] [Google Scholar]
- 25.Chambers C, Skowronski DM, Sabaiduc S, et al. Detection of influenza A(H3N2) clade 3c.2a viruses in patients with suspected mumps in British Columbia, Canada, during the 2014/15 influenza season. Euro Surveill 2015; 20:pii=30015. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CI, Ellis J, Galiano M, Ramsay M, Brown KE, Zambon M. Detection of influenza A(H3N2) virus in children with suspected mumps during winter 2014/15 in England. Euro Surveill 2015; pii: 21203. [DOI] [PubMed] [Google Scholar]
- 27.Appiah GD, Blanton L, D’Mello T, et al. ; Centers for Disease Control and Prevention (CDC). Influenza activity—United States, 2014–15 season and composition of the 2015–16 influenza vaccine. Morb Mortal Wkly Rep 2015; 64:583–90. [PMC free article] [PubMed] [Google Scholar]
- 28.Guy RJ, Andrews RM, Kelly HA, et al. Mumps and rubella: a year of enhanced surveillance and laboratory testing. Epidemiol Infect 2004; 132:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magurano F, Baggieri M, Marchi A, Bucci P, Rezza G, Nicoletti L. Mumps clinical diagnostic uncertainty. Eur J Public Health 2018; 28:119–23. [DOI] [PubMed] [Google Scholar]
- 30.Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev 2015; 28:313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinde B Herpesviruses: latency and reactivation—viral strategies and host response. J Oral Microbiol 2013; 5:2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonizzoli M, Arvia R, di Valvasone S, et al. Human herpesviruses respiratory infections in patients with acute respiratory distress (ARDS). Med Microbiol Immunol 2016; 205:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R, Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest 2010; 138:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginocchio CC, McAdam AJ. Current best practices for respiratory virus testing. J Clin Microbiol 2011; 49:44–8. [Google Scholar]