Abstract
Mucinous ovarian tumors (MOT) morphologically and epidemiologically resemble mucinous cystic neoplasms (MCN) of the pancreas, sharing a similar stroma and both occurring disproportionately among young females. Additionally, MOT and MCN share similar clinical characteristics and immunohistochemical phenotypes. Exome sequencing has revealed frequent recurrent mutations in KRAS and RNF43 in both MOT and MCN. The cell of origin for these tumors remains unclear, but MOTs sometimes arise in the context of mature cystic teratomas and other primordial germ cell (PGC) tumors. We undertook the present study to investigate whether non-teratoma associated MOT and MCN share a common cell of origin. Comparisons of the gene expression profiles of MOT (including both the mucinous borderline ovarian tumors [MBOT] and invasive mucinous ovarian carcinomas [MOC]), high-grade serous ovarian carcinomas, ovarian surface epithelium, Fallopian tube epithelium, normal pancreatic tissue, pancreatic duct adenocarcinomas, MCN, and single cell RNA-sequencing of PGCs revealed that both MOT and MCN are more closely related to PGCs than to either eutopic epithelial tumors or normal epithelia. We hypothesize that MCN may arise from PGCs that stopped in the dorsal pancreas during their descent to the gonads during early human embryogenesis, while MOT arise from PGCs in the ovary. Together, these data suggest a common pathway for the development of MCN and MOT and suggest that these tumors may be more properly classified as germ cell tumor variants.
Keywords: mucinous ovarian tumor, mucinous cystic neoplasm, primordial germ cells, pathogenesis, RHOB
Introduction
Epithelial ovarian cancers (EOC) are the leading cause of gynecologic cancer death in the developed world [1]. EOC are histologically classified into four major subtypes: serous, clear cell, endometrioid, and mucinous. Of these, the mucinous type has been the least studied, probably because of its less frequent incidence, comprising about 3% or less of EOC [2]. Mucinous tumors are morphologically distinct from all other epithelial ovarian cancers. They tend to be borderline or low grade, have an indolent course and a favorable prognosis and occur in young female smokers. Some mucinous ovarian tumors (MOTs) have been shown to arise from germ cell tumors [3]. This has raised the hypothesis that these tumors might arise from a different cell of origin than other EOC.
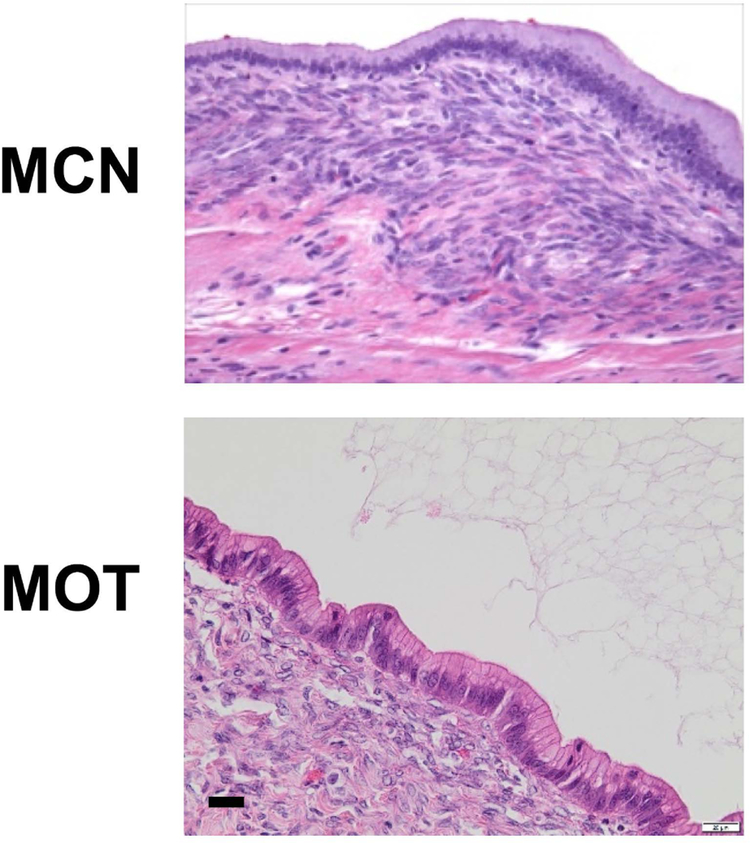
Morphologically, MOTs closely resemble a rare pancreatic tumor, mucinous cystic neoplasm (MCN) (Figure 1). MCN is distinct among pancreatic lesions by the presence of a unique ovarian-like stroma [4]. MCN, like MOT, is a typically a low grade neoplasm that occurs mainly in young women. Like MOTs, MCNs tend to be indolent, usually localized to the body and/or tail of the pancreas [5,6]. Whole exome sequencing studies have revealed molecular similarities between MOT and MCN with mutations at similar frequencies in RNF43 and KRAS [7,8]. However, if MOT and MCN are somehow related, why do MCNs arise mainly in women (sex ratio 1:10), in the pancreas (a non-gynecologic organ), and why do they have such specific anatomic localization in the body/tail of pancreas?
Figure 1. Hematoxylin and eosin sections of a mucinous cystic neoplasm (MCN) of the pancreas and a mucinous ovarian tumor (MOT).
Note the similar morphology of the overlying, mucin-filled epithelium and dense underlying stroma. Scale bar 20 μm.
In human embryos, the precursors of gametes, termed primordial germ cells (PGCs), are initially located in extragonadal regions (yolk sac) at the3rd week of development and migrate caudad [9]. In the 5-week old embryo, PGCs reach the dorsal mesentery which becomes the body, tail and isthmus of the pancreas. PGCs then continue to move laterally around both sides of the coelomic angle, pass beyond the primitive mesonephros bodies, and eventually enter the gonadal ridges at the 9th week [9]. This raises the possibility that MOT and MCN could derive from a common embryologic precursor, PGCs that stopped in the body or tail of the pancreas during their migration to the gonads.
Here we consider the possibility of a biological relationship among ovarian and pancreatic mucinous tumors based on the gene-expression patterns of tumor and normal tissue samples. We demonstrate that the closest normal cell type to either of these tumors is in fact the PGC, rather than either pancreatic or gynecologic epithelia. We suggest that MOT and MCN may be related tumors and more properly classified as unusual germ cell tumor variants.
Materials and Methods
Ethics approval
Review of patient medical records and use of archival specimens were approved by the Brigham and Women’s Hospital Institutional Review Board Protocols 2013P000553 and 2016P002742.
Isolation and gene expression profile of human PGCs
Data were pooled from two published gene expression datasets [10,11]. We used gene-expression profiles from 8–11 week human PGCs (1 male and one female) [10](E-MTAB-6851). As described by the authors, PGCs had been isolated using magnetic cell sorting technology (MACs) and indirect labeling of cells with magnetically tagged goat anti-mouse IgM antibodies toward a mouse-anti-SSEA1 antibody (Miltenyi Biotech) [10]. To analyze gene expression profiles, the Affymetrix® Human U133 Plus 2.0 GeneChip (Affymetrix, Santa Clara, CA, USA) was used. In addition, single-cell RNA-sequencing data were obtained from 5 samples of human female PGCs from week 4 to week 11 (GSE63818) (supplementary material, Table S1) [11]. For that study, human gonads from 7-week to 11-week embryos were dissected in Dulbecco’s phosphate buffered saline (DPBS) (plus 1% fetal bovine serum). The gonads were washed in DPBS twice before digestion in 250 μl of Accutase Cell Detachment Solution (Millipore #SCR005; Merck KGaA, Darmstadt, Germany) for 5 min at 37 °C. For the isolation of pure human PGCs from 7- to 11-week embryos, 100 μl of FcR Blocking Reagent and 100 μl of CD117 MicroBeads (Miltenyi Biotec #130–091-332; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were added to the 300 Ml of gonad cell suspension and mixed well by gently pipetting. After magnetic enrichment, the fraction containing PGCs (CD117-positive cells) was further sorted by BD FACSAria (BD Biosciences, Franklin Lakes, NJ, USA), and CD117-positive cells were collected for downstream analysis. The purity of PGCs was assayed by immunostaining for OCT4 and single-cell RT-qPCR for human OCT4 transcripts. For 4-week embryos, the aorta-gonads-mesonephros regions were dissected and single-cell suspension was obtained by digestion with Accutase. Then, the cell suspension was inspected under the microscope carefully and the large cells (less than 0.1%) were manually picked out with a mouth pipette. For RNA sequencing, a DNA library prep kit for Illumina® (New England Biolabs Inc., Ipswich, MA, USA) was used to prepare the sequencing library following the manufacturer’s protocol. Libraries were pooled and sequenced on Illumina® HiSeq2500 (Illumina Inc., San Diego, CA, USA) sequencers using 100-bp paired-end sequencing, as described [11].
Gene expression profile of pancreatic and ovarian samples
Data were pooled from several published gene expression datasets [12–16] (supplementary material, Table S1). We compiled gene-expression data from 16 samples of normal pancreatic tissue (GSE16515) [16], 36 pancreatic ductal adenocarcinomas (PDAC) (GSE16515) [16], the epithelial component of 7 microdissected MCNs of the pancreas (E-MTAB-6853)[13](two distinct samples from MCN4 were analyzed), the epithelial component of 8 microdissected mucinous borderline ovarian tumors (MBOT) and 9 microdissected invasive mucinous ovarian carcinoma (MOC) (E-MTAB-6844)[12], 24 microdissected samples of Fallopian tube epithelium (FT) (GSE10971)[14], the epithelial component of 13 microdissected high-grade serous ovarian carcinomas (HGSOC) (GSE10971)[14] and 6 microdissected samples of ovarian surface epithelium (OSE) (GSE40595)[15]. All array expression data were generated with Affymetrix® microarrays (Affymetrix, Santa Clara, CA, USA).
Data processing
For all array expression datasets, we recovered the raw data in the form of .CEL files, which were imported and processed in the R language and environment for statistical computing [17]. Raw data from each dataset was treated with the Robust Multi-array Average (RMA) algorithm [18], from the “Affy” BioConductor package [19] using default settings. For genes mapping to multiple probes, the mean expression was used as a gene-level summary. Batch effects were attenuated by using a previously published list of endogenous control genes [20] to correct for unwanted variation [21].
The single-cell RNAseq data (GSE63818) were obtained as RPKM estimates, then filtered to exclude genes with extremely low expression (<0.3 RPKM) or extremely low variance (bottom 1%). For every sample, the gene expression estimates were aggregated by calculating the mean expression in the isolated single cells. The RNAseq expression estimates were mean-centered against the microarray datasets, to obtain a similar distribution of values. The mean-variance plot for all common genes from both platforms was used as a quality control and confirms that the results have comparable distributions for most of the expression range.
Class comparison
Differentially expressed genes between groups were identified using the limma package with default options, with gene expression ordered by absolute log fold-change (FC) using a false discovery rate (FDR) of 0.05 or less [22]. These comparisons identified genes whose expression was significantly altered between PGCs, MCN specimens, PDACs and normal pancreatic tissue. Similarly, we identified genes whose expression was different between PGCs, MBOT, HGSOC, OSE and FT specimens. Dendrograms were created using hierarchical clustering (hclust) and a Euclidean distance metric on genes expressed by all samples (N=9,626). The most discriminative and statistically significant genes were ordered by logFC and summarized in heatmaps. During the revision of the manuscript, the MOC samples were added and a second distinct analysis was done in order to identify genes whose expression was different between PGCs, MBOT, MOC, HGSOC, OSE and FT specimens. A new gene list was also generated (N=9,626).
Gene set enrichment analysis
Common biologic pathways for the 1,000 most similarly expressed genes (as measured by absolute log-FC) between PGCs and MCN or PGCs and MBOT were examined by gene set enrichment analysis using DAVID v.6.8 [23]. The top ten gene ontology terms most overrepresented by adjusted p-value after a Bonferroni correction are shown.
Laser capture microdissection and RNA extraction for RT-qPCR
New samples were selected for validation of gene expression profiles by RT-qPCR. Sections were cut at 4 μm thickness from each sample and stained with hematoxylin and eosin (HE) for review to ensure proper tissue orientation and histology (JCT and GP). Fresh-frozen samples of HGSOC (n=5), PDAC (N=5) and normal pancreatic tissue (n=5) were macrodissected. The epithelium component from frozen samples of MCNs (n=4) and MBOTs (n=4) were laser-capture microdissected using a Leica LMD7000 instrument (Leica Microsystems, Wetzlar, Germany) (supplementary material, Figure S1). Shortly before microdissection, 10 μm sections were cut, adhered onto frame slides, immediately fixed in ethanol 75% for 2 min, stained with Hematoxylin, washed in water, dehydrated in graded alcohols then xylene and microdissected. RNA was also isolated from immortalized OSE cell lines (n=2) and immortalized FT cell lines (n=4) [24]. Total RNA was extracted from tissues or cell lines using QIAGEN® RNeasy kit (QIAGEN #74104, Valencia, CA, USA) following the manufacturer’s protocol. Measurement of total RNA concentration was performed with a Qubit fluorimeter (Thermo Fisher Scientific, Waltham, MA, USA) and quality assessed with an Agilent Bioanalyzer (Agilent Technologies, Lexington, MA, USA).
RT-qPCR
To confirm expression of identified genes, cDNA was synthesized from 150 ng of total RNA using a mix of random hexamers - oligo d(T) primers and PrimerScript reverse transcriptase enzyme (Takara Bio, Inc, Kusatsu, Japan) following the supplier’s instructions. SYBR green assays were designed using the program Primer Express v 2.0 (Applied Biosystems, Waltham, MA, USA) with default parameters. Amplicons sequences were aligned against the human genome by BLAST to ensure that they were specific for the gene being tested. Oligonucleotides were obtained from Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, USA). The efficiency of each design was tested with serial dilutions of cDNA. PCR reactions (10 μl volume) contained diluted cDNA, 2 x Power Up SYBR Green Master Mix (Thermo Fisher Scientific) and 300 nM of forward and reverse primers. PCR primers are listed in supplementary material, Table S2. PCR reactions were performed on a SDS 7900 HT instrument (Applied Biosystems) with the following parameters: 50 °C for two min, 95 °C for ten min, and 45 cycles of 95 °C for 15 s and 60°C for one minute. Each reaction was performed in three replicates on a 384-well plate. Raw CT values obtained with SDS 2.2 (Applied Biosystems) were imported in Excel (Microsoft, Redmond, WA, USA). Normalisation factor and fold-changes were calculated using the GeNorm method [25]. Target gene CT values were normalized to β-tubulin (TUBB) and β-actin (ACTB) transcripts.
Chart review of MCN and MOT
Twenty-three cases of MCN were identified by querying the Brigham and Women’s Hospital (BWH) pathology database. All cases were confirmed by a staff GI pathologist (J.L.H or L.A.D.) to be MCN and not an intraductal pancreatic mucinous neoplasm (IPMN), mucinous adenocarcinoma, or other form of pancreatic neoplasm. Medical charts for the cases were reviewed to extract clinical data after approval by the BWH Institutional Review Board (Protocol 2013P000553). MOT cases were identified from the New England Case Control (NECC) study as previously described [26]. In brief, cases were enrolled from 7/1984 – 9/1987 (NECC2), 5/1992 – 3/1997 (NECC3), 8/1998 – 4/2003 (NECC4), and 10/2003 – 11/2008 (NECC5). The four phases enrolled 2,475 cases including 2,274 with epithelial ovarian cancers, of which 287 were mucinous. Controls for NECC3 were identified by random-digit dialing supplemented with residents’ lists for older controls. About 10% of households contacted had an eligible control and of these, 421 (72%) agreed to participate.
Statistical methods
Unless specified otherwise, all statistical tests were performed in R v.3.4.3 using p-value correction for multiple comparisons to account for the false discovery rate (FDR) [17]. Clinical characteristics of patients with MCN and MOT were compared using a z-test for population proportions. Median fold-changes of gene expression from RT-qPCR data were compared using a pairwise Student’s t-test.
Results
Clinical presentation for patients with mucinous ovarian tumors or mucinous cystic neoplasms
We performed a chart review to investigate clinical similarities between patients with MOT or MCN. From BWH, we identified 23 cases of MCN. Tumors occurred exclusively in women (Table 1). Compared to the cases in the NECC study, women with MCN were similar in terms of age, smoking history and stage at diagnosis. The only notable difference appeared to be in racial distribution, as a larger proportion of MCN than MOT were diagnosed among non-white women.
Table 1.
Patient clinical characteristics.
| Mucinous ovarian tumors N=287 N (%) |
Mucinous cystic neoplasms N=23 N (%) |
P value | |
|---|---|---|---|
| Sex | |||
| Female | 287 (1000) | 23 (100) | 1.0 |
| Male | 0 (0) | 0 (0) | |
| Age | |||
| <44 | 128 (44.6) | 6 (26) | 0.12 |
| 44–53 | 69 (24.0) | 6 (26) | |
| 54–62 | 46 (16.0) | 8 (35) | |
| >62 | 44 (15.3) | 3 (13) | |
| Race | |||
| White | 273 (95.1) | 18 (78.2) | 0.008 |
| Non-white | 14 (4.9) | 5 (21.7) | |
| Ever Smoker | |||
| No | 123 (42.9) | 12 (63.1) | 0.10 |
| Yes | 164 (57.1) | 7 (36.8) | |
| Unknown | 4 | ||
| Stage* | |||
| I | 213 (88.8) | 23 (100) | 0.14 |
| II-IV | 27 (11.3) | 0 (0) |
Missing for 47 cases.
Similar gene expression profiles for MCNs of the pancreas and PGCs
Unsupervised hierarchical clustering was performed for gene expression profiles from 7 MCN and 36 PDACs, 16 normal pancreatic tissues, and 7 human PGC samples. The dendrogram in Figure 2A shows the unsupervised hierarchical clustering of all 66 samples based on the expression of 9,626 genes (the intersection of available genes over all datasets). The samples separated into two main branches. The left branch contains PGCs and MCN. The right branch includes PDAC and normal pancreatic tissues. This dendrogram suggests that PGCs are more closely related to MCN and PDAC more closely associated with normal pancreatic tissue. Importantly, a small number of PDAC samples clustered together with normal pancreatic samples, but all MCN samples were clearly distinct. The heatmap displays the most differentially expressed, statistically significant genes per sample type (Figure 2B). It clearly demonstrates important similarities between PGC and MCN samples, compared with normal pancreas and PDAC. Importantly, the single-cell PGC RNA sequencing dataset and array expression PGC dataset are highly concordant, emphasizing the consistency of these gene expression profiles. Together, these data suggest that global gene expression in MCN more closely resembles that of PGCs rather than normal pancreatic tissue or other pancreatic tumors.
Figure 2. Unsupervised hierarchical clustering of PGCs, pancreatic and MCN samples.
(A) Expression of 9,626 genes in MCN, PGCs, PDAC and normal pancreatic tissue. Dendrogram of the 66 experimental samples. Hierarchical clustering illustrates MCN specimens are closely associated with PGCs (left branch), whereas pancreatic ductal adenocarcinomas (PDAC) group together with normal pancreatic tissue (right branch). (B) Heatmap of the differentially expressed genes in MCN, PGCs, PDAC and normal pancreatic tissue. Blue= high expression, brown=low expression.
We used limma and a cut-off at a false discovery rate of 0.05 to identify common differentially expressed genes between the MCN and PGCs samples on one hand and the PDAC and normal pancreatic tissue samples on the other hand. The list of 1,000 top differentially expressed genes are listed in supplementary material, Dataset S1. Gene set enrichment analysis of the shared gene sets between MCN and PGCs showed overrepresentation of genes related to phosphoproteins (supplementary material, Table S3).
Similar gene expression profiles for MOTs and PGCs
Unsupervised hierarchical clustering was performed from microarrays of 8 MBOT, 24 FT, 6 OSE, 13 HGSOC, and 7 PGCs. The dendrogram in supplementary material, Figure S2A represents the unsupervised hierarchical clustering of all 58 samples. MBOT samples were aligned more closely with PGCs than OSE, whereas HGSOC grouped with either OSE or FT. The heat map summarizes differentially expressed genes in each sub-group of samples (supplementary material, Figure S2B). It indicates that MBOT and PGC samples have important similarities and are more distantly related to OSE, HGSOCs, and FT, respectively. MBOT are closely related to MOC and a continuum appears to be present from borderline to carcinoma, which is different from other epithelial EOC [29]. Thus, we questioned whether MOC resemble PGCs. Unsupervised hierarchical clustering was performed from microarrays of 8 MBOT, 9 MOC, 24 FT, 6 OSE, 13 HGSOC, and 7 PGCs. MBOT and MOC samples clustered together and were aligned more closely with PGCs than OSE (Figure 3A and 3B). These data suggest that global gene expression in MOT more closely resembles that of PGCs than Mullerian epithelia The normal FT cells clustered closely with HGSOC, adding further evidence to the theory that FT cells give rise to most HGSOC [24,27,28]. Genes similarly expressed among MBOT, MOC and PGCs versus HGSOC, OSE and FT were selected using limma at a false discovery rate of 0.05. A complete list of 1,000 top genes is shown in supplementary material, Dataset S2. Gene set enrichment analysis again showed a high percentage of phosphoproteins (supplementary material, Table S4).
Figure 3. Unsupervised hierarchical clustering of PGCs, ovarian, Fallopian tube, and MOT samples.
(A) Expression of 9,626 genes in MOC, MBOT, PGCs, HGSOC, OSE and FT. Dendrogram of the 67 experimental samples based on hierarchical clustering. MOC and MBOT specimens are clustering together and closely associated with PGCs, whereas high-grade serous ovarian carcinomas (HGSOC) group with normal Fallopian tube (FT) or ovarian surface epithelium (OSE). (B) Heatmap of the differentially expressed genes in MOC, MBOT, PGCs, HGSOC, OSE and FT. Blue= high expression, brown=low expression.
Shared gene expression among PCG, MCN, and MOT
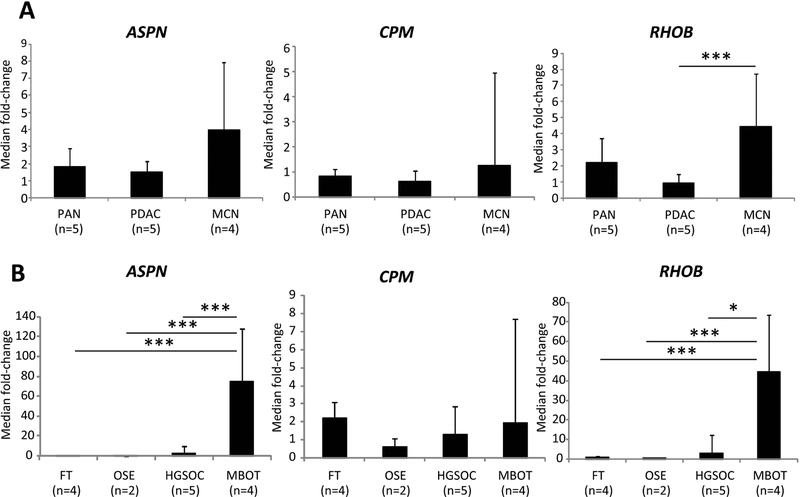
By comparing the lists of genes generated in supplementary material, Datasets S1 and S2, we observed that many genes had similar expression patterns shared among the MOT, PGCs, and MCN samples. Genes differentially expressed between MOT (MBOT and MOC), MCN and PGCs, and the pancreatic and adnexal eutopic normal and tumor samples were selected and ordered by logFC (Supplementary material, Figure S3). A complete list of the 411 most differentially expressed genes among all the mucinous tumor types and PGCs versus other tissue types is shown in supplementary material, Dataset S3. Three genes highly expressed and common among MOT (MBOT and MOC), MCN and PGCs were CPM, RHOB and ASPN. To validate the microarray results, these three genes were selected for RT-qPCR analysis. Expression levels for the three genes were determined on independent samples, not included in the microarray analyses. In agreement with our microarray data, MCN were found to express higher levels of ASPN, RHOB and CPM compared with normal pancreatic tissue or PDAC, although only RHOB compared with PDAC reached statistical significance (p=7.2×10−3) (Figure 4A). Consistent with our profiling data, MBOT samples expressed much higher levels of ASPN compared with OSE (p=10−5), FT (p=4.3×10−5) or HGSOC (p=3.14×10−3). RHOB was also strongly expressed by MBOT and this was statistically significant compared with OSE (p=3.8×10−4), FT (p=1.3×10−4) or HGSOC (p=0.01). CPM was not tissue specific (Figure 4B). Since our RT-qPCR data were obtained on specimens distinct from those of the microarray data, our observations suggest that RHOB and ASPN could be specific new markers for these mucinous neoplasms.
Figure 4. Expression of ASPN, RHOB and CPM in ovarian and pancreatic samples.
To confirm the increased expression of ASPN, RHOB and CPM in MBOT and MCN, qPCR) was performed using cDNA generated from new samples of ovarian and pancreatic tumors and normal tissues. (A) RT-qPCR confirmed an increase of RHOB in MCN compared to PDAC. (B) RT-qPCR confirmed the differential expression of ASPN and RHOB uniquely expressed in MBOT. FT: immortalized Fallopian tube cell lines. OSE: immortalized ovarian surface epithelium cell lines. HGSOC: high grade serous ovarian carcinomas. PDAC: pancreatic ductal adenocarcinomas. PAN: normal pancreas. MBOT: mucinous borderline ovarian tumors. MCN: mucinous cystic neoplasms of the pancreas. *:p=0.01; ***:p=<0.001.
Discussion
MOT and MCN are both rare, indolent mucinous neoplasms with a propensity to develop in young women who smoke. They share common epidemiologic, clinical, morphologic and genomic features. The histological presence of a unique ovarian-type stroma is mandatory to diagnose MCN and distinguishes MCN from other pancreatic neoplasms [30]. MCN nearly always occur in the body or tail of the pancreas and unlike PDAC do not communicate with the pancreatic ducts. Likewise, MOT are unique among ovarian tumors in that they usually arise within large parenchymal cysts, not along the Fallopian tube-ovarian interface. The tumors share expression of CK7, and unlike gastrointestinal tumors, have only variable expression of CK20 and do not express MUC2 [31]. The stroma of MOT and MCN also show similar expression patterns for sex hormone receptors [32]. Here we consider the possibility that MOT and MCN could derive from a common embryologic precursor, PGCs. Approximately 100 PGCs start the journey but by the time they arrive at the gonads, they number about 1,700 because they proliferate en route [33]. MCNs could arise from some PGCs that stop in the body or tail of the pancreas during their migration to the gonads. Thus, embryological remnants of PGCs that stopped in the body/tail of the pancreas would give rise to MCN, whereas MOT would develop from PGCs that reached the ovaries (Figure 5A,B).
Figure 5. Proposed model for origin of mucinous ovarian tumors and mucinous cystic neoplasms of the pancreas from primordial germ cells.
(A) Migration of primordial germ cells (PGCs) in the human embryo starts from the dorsal wall of the yolk sac near the developing allantois (III weeks). At VII weeks, PGCs migrate into the midgut and hindgut, passing through the dorsal mesentery into the gonadal ridges (VIII weeks). At IX weeks, PGCs colonize gonadal ridges [43]. (B) Mucinous cystic neoplasms (MCN) of the pancreas would arise from left embryological remnant of migrating PGCs that stopped in the body/tail of the pancreas. MCN of the liver would arise from right embryological remnant of migrating PGCs that stopped in the left lobe of the liver. In the ovaries, MOT would develop from PGCs that did not develop oogonia. These three mucinous tumors each occur only in women, have CK7+MUC2- immunohistochemical staining, and are surrounded by ovarian-like stroma.
Recent studies suggest that teratoma-associated MOT are of germ cell origin [3,34]. Using unsupervised clustering of gene-expression profiles and RNA sequencing of different ovarian and pancreatic tissues and tumors, we have shown for the first time that gene expression in non-teratoma-associated pancreatic and ovarian mucinous tumors also resembles PGCs. Validation on independent samples and by RT-qPCR of the microarray data for selected genes further strengthens our microarray analysis.
We acknowledge several limitations of our study. First, while we offer several observations to advance the theory that MOT and MCN share a common cell of origin, we cannot exclude convergent evolution of pancreatic and ovarian cells to a common PGC-like phenotype. Second, we have used bulk pancreas as a control. Microdissected pancreatic ductal epithelium would be more ideal, but this is technically challenging as pancreatic enzymes degrade the quality of RNA. Third, we did not investigate the expression of specific PGC markers. This important question needs to be addressed in future work.
This hypothesis can explain many characteristics of MCN and MOT: their rarity, their development outside the normal epithelial interfaces, and their clinical, histological and molecular similarities [30]. Although not profiled in this study, MCN of the liver and kidney have similar clinical and pathological characteristics of pancreatic MCN (almost exclusively in young women and tumor having two components: mucinous epithelium associated with ovarian-like stroma). These could similarly arise from PGCs stopping in the right part of the abdomen (specifically left lobe of the liver) or retroperitoneum [35–37]. Likewise, another rare tumor that arises mainly in young women and is located on the PGC migration trajectory is the mixed epithelial stromal tumor (MEST). These tumors develop in the kidney and rarely contain mucinous epithelium, and the epithelial elements are always PAX8 positive; however, like MCNs, MESTs are always associated with ovarian-like stroma and occur almost exclusively in middle aged females [38]. Nevertheless, several questions remained unanswered: why do MCN develop only in women since PGCs have the same migration process in male and female embryos? And why are these epithelial tumors mucinous rather than other histological subtypes? It is possible that exposure to female hormones, smoking, and other factors to be identified play an important role in pathogenesis of MCN.
Our microarray and RT-qPCR data on two independent cohorts suggest that RHOB and ASPN are common among MOT, MCN and PGCs. Deregulations of these genes have been observed in several types of tumors. RHOB codes for Ras homolog B (RhoB) protein, a Rho family GTPase that is itself a subset of the Ras superfamily. RhoB plays an important role in cell migration, membrane trafficking, cell proliferation and DNA repair. RhoB alteration seems crucial for Ras-transformed cells response to farnesyltransferase inhibitors [39]. Because KRAS is frequently mutated in MOTs [7] and MCNs [8], it would be interesting to investigate whether KRAS mutations correlate with RHOB expression.
ASPN codes for asporin, a small leucine-rich proteoglycan (SLRP). In the tumor microenvironment, asporin is mainly secreted by cancer-associated fibroblasts [40,41]. Its expression in prostate cancer samples correlates with disease progression [42]. Certainly, these data need further validation in larger cohorts and at the protein level to elucidate this relationship further.
In conclusion, we present molecular data that may provide a better understanding of the pathogenesis of mucinous ovarian and pancreatic cystic tumors. Our data support the hypothesis that MOTs resemble MCNs of the pancreas at the macroscopic, microscopic, and molecular levels, and share a possible common cell of origin in PGCs. Knowledge of the cell of origin may accelerate translational and clinical research for these rare diseases.
Supplementary Material
Acknowledgements
We thank Mrs. Laurence Zulianello for the iconographic support. We thank the Genomic platform at the faculty of Medicine of Geneva for the technical support.
Financial support
KME and RD are supported by the Honorable Tina Brozman Foundation. KME is supported by the Robert and Deborah First Family Fund, Saltonstall Research Fund, the Minnesota Ovarian Cancer Alliance, and the Reproductive Scientist Development Program from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) award K12-HD000849. RD is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Department of Defense, the Claneil Foundation, and the Run & Walk 4 Family & Friends with Cancer Foundation. JH is supported by the Ann and Sol Schreiber Mentored Investigator Award from the Ovarian Cancer Research Fund Alliance (OCRFA) and the Foundation for Women’s Wellness. SS is supported by the Ann and Sol Schreiber Mentored Investigator Award from the OCRFA. SILG is supported by the Research fund of the department of internal medicine of the Hopitaux Universitaires de Geneve and the Faculty of Medicine of Geneva; this fund receives an unrestricted grant from AstraZeneca Switzerland.
Conflict of interest: All the authors disclose no potential conflict of interest
Array databases
Our raw data are available at Array Express (https://www.ebi.ac.uk/arrayexpress/): EMTAB-6844, E-MTAB-685, E-MTAB-6853.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003; 27: 985–993. [DOI] [PubMed] [Google Scholar]
- 3.Kerr SE, Flotte AB, McFalls MJ, et al. Matching maternal isodisomy in mucinous carcinomas and associated ovarian teratomas provides evidence of germ cell derivation for some mucinous ovarian tumors. Am J Surg Pathol 2013; 37: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 4.Testini M, Gurrado A, Lissidini G, et al. Management of mucinous cystic neoplasms of the pancreas. World J Gastroenterol 2010; 16: 5682–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008; 247: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas 2011; 40: 67–71. [DOI] [PubMed] [Google Scholar]
- 7.Ryland GL, Hunter SM, Doyle MA, et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol 2013; 229: 469–476. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of thepancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011; 108: 21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Felici M Origin, migration and proliferation of human primordial germ cells. In: Oogenesis. Springer: London, 2013; 19–37. [Google Scholar]
- 10.Pashai N, Hao H, All A, et al. Genome-wide profiling of pluripotent cells reveals a unique molecular signature of human embryonic germ cells. PLoS One 2012; 7: e39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Yan L, Guo H, et al. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 2015; 161: 1437–1452. [DOI] [PubMed] [Google Scholar]
- 12.Wamunyokoli FW, Bonome T, Lee JY, et al. Expression profiling of mucinous tumors of the ovary identifies genes of clinicopathologic importance. Clin Cancer Res 2006; 12: 690–700. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima N, Sato N, Prasad N, et al. Characterization of gene expression in mucinous cystic neoplasms of the pancreas using oligonucleotide microarrays. Oncogene 2004; 23: 9042–9051. [DOI] [PubMed] [Google Scholar]
- 14.Tone AA, Begley H, Sharma M, et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res 2008; 14: 4067–4078. [DOI] [PubMed] [Google Scholar]
- 15.Yeung TL, Leung CS, Wong KK, et al. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res 2013; 73: 5016–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009; 16: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: a language and environment for statistical computing R Foundation for Statistical Computing: Vienna, Austria, 2013. Available from: www.r-project.org [Google Scholar]
- 18.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4: 249–264. [DOI] [PubMed] [Google Scholar]
- 19.Gautier L, Cope L, Bolstad BM, et al. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004; 20: 307–315. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg E, Levanon EY. Human housekeeping genes are compact. Trends Genet 2003; 19: 362–365. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon-Bartsch JA, Speed TP. Using control genes to correct for unwanted variation in microarray data. Biostatistics+ 2012; 13: 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang da W, Sherman BT, Stephens R, et al. DAVID gene ID conversion tool. Bioinformation 2008; 2: 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A 2011; 108: 7547–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias KM, Labidi-Galy SI, Vitonis AF, et al. Prior appendectomy does not protect against subsequent development of malignant or borderline mucinous ovarian neoplasms. Gynecol Oncol 2014; 132: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perets R, Wyant GA, Muto KW, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013; 24: 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 2017; 8: 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep 2014; 16: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthaei H, Schulick RD, Hruban RH, et al. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol 2011; 8: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu PG, Chung L, Weiss LM, et al. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol 2011; 35: 1830–1836. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Sugiyama M, Abe N, et al. Immunohistochemical similarities between pancreatic mucinous cystic tumor and ovarian mucinous cystic tumor. Pancreas 2008; 36: e40–46. [DOI] [PubMed] [Google Scholar]
- 33.Jones RE. Chapter 5: Sexual differentiation and development In: Human reproductive biology. Academic press: San Diego, California, USA, 1991. [Google Scholar]
- 34.Wang Y, Shwartz LE, Anderson D, et al. Molecular analysis of ovarian mucinous carcinoma reveals different cell of origins. Oncotarget 2015; 6: 22949–22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosman F, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive System. (Fourth Edition ed). 2010. [Google Scholar]
- 36.Matsubara T, Sato Y, Sasaki M, et al. Immunohistochemical characteristics and malignant progression of hepatic cystic neoplasms in comparison with pancreatic counterparts. Hum Pathol 2012; 43: 2177–2186. [DOI] [PubMed] [Google Scholar]
- 37.Adsay NV, Eble JN, Srigley JR, et al. Mixed epithelial and stromal tumor of the kidney. Am J Surg Pathol 2000; 24: 958–970. [DOI] [PubMed] [Google Scholar]
- 38.Calio A, Eble JN, Grignon DJ, et al. Mixed Epithelial and Stromal Tumor of the Kidney: A Clinicopathologic Study of 53 Cases. Am J Surg Pathol 2016; 40: 1538–1549. [DOI] [PubMed] [Google Scholar]
- 39.Prendergast GC. Actin’ up: RhoB in cancer and apoptosis. Nat Rev Cancer 2001; 1: 162–168. [DOI] [PubMed] [Google Scholar]
- 40.Maris P, Blomme A, Palacios AP, et al. Asporin Is a fibroblast-derived TGF-beta1 inhibitor and a tumor suppressor associated with good prognosis in breast cancer. PLoS Med 2015; 12:e1001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoyoshi R, Kuriyama S, Aiba N, et al. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 2015; 34: 650–660. [DOI] [PubMed] [Google Scholar]
- 42.Rochette A, Boufaied N, Scarlata E, et al. Asporin is a stromally expressed marker associated with prostate cancer progression. Br J Cancer 2017; 116: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Felici M Chapter 2: Origin, migration, and proliferation of human primordial germ cells In: Oogenesis. Springer: London, 2013; 19–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.