Abstract
Massive, irreparable rotator cuff tears (MIRCTs) provide a significant dilemma for orthopaedic surgeons. One treatment option for MIRCTs is reverse total shoulder arthroplasty. However, other methods of treating these massive tears have been developed. A search of the current literature on nonoperative management, arthroscopic debridement, partial repair, superior capsular reconstruction (SCR), graft interposition, balloon spacer arthroplasty, trapezius transfer, and latissimus dorsi transfer for MIRCTs was performed. Studies that described each surgical technique and reported on clinical outcomes were included in this review. Arthroscopic debridement may provide pain relief by removing damaged rotator cuff tissue, but no functional repair is performed. Partial repair has been suggested as a technique to restore shoulder functionality by repairing as much of the rotator cuff tendon as possible. This technique has demonstrated improved clinical outcomes but also fails at a significantly high rate. SCR has recently gained interest as a method to prohibit superior humeral head translation and has been met with encouraging early clinical outcomes. Graft interposition bridges the gap between the retracted tendon and humerus. Balloon spacer arthroplasty has also been recently proposed and acts to prohibit humeral head migration by placing a biodegradable saline-filled spacer between the humeral head and acromion; it has been shown to provide good clinical outcomes. Both trapezius and latissimus dorsi transfer techniques involve transferring the tendon of these respective muscles to the greater tuberosity of the humerus; these 2 techniques have shown promising restoration in shoulder function, especially in a younger, active population. Arthroscopic debridement, partial repair, SCR, graft interposition, balloon spacer arthroplasty, trapezius transfer, and latissimus dorsi transfer have all been shown to improve clinical outcomes for patients presenting with MIRCTs. Randomized controlled trials are necessary for confirming the efficacy of these procedures and to determine when each is indicated based on specific patient and anatomic factors.
Keywords: massive, irreparable rotator cuff tear; superior capsular reconstruction; graft interposition; balloon spacer arthroplasty; tendon transfer
Massive, irreparable rotator cuff tears (MIRCTs) provide a significant dilemma for orthopaedic surgeons. These lesions are characterized by rotator cuff tears >3 cm with advanced fatty infiltration of the rotator cuff tendons, a reduced acromiohumeral interval, significant tendon retraction, and the presence of poor-quality tissue.36,62 Historically, limited reliable options have existed for the treatment of these tears, and although multiple options have been described, many have been insufficient to meet patients’ demands (eg, isolated arthroscopic debridement), are technically demanding and result in difficult rehabilitation (eg, tendon transfers), and have generally been met with underwhelming clinical success as a whole. One of the recent popular options for MIRCTs has been reverse total shoulder arthroplasty (rTSA), as it allows the deltoid muscle to take over the function of the irreparable rotator cuff muscles and has shown promising clinical results in multiple studies when employed for this indication.23,41,62,63 This technique has become the treatment of choice among surgeons for patients with MIRCTs complicated by significant glenohumeral arthritis, anterosuperior escape, and/or pseudoparalysis.62 However, complications after rTSA, although improving, have been reported to occur at a rate of 20% to 50% in both the recent and past literature.23,41,63 Additionally, there are concerns regarding the longevity of these implants, with a revision rate of 10% to 33% and increasing complication rates with each revision.3,4,41,63 Therefore, surgeons have attempted to develop novel surgical treatment methods for massive tears. It should be noted that inferior results of rTSA have been demonstrated in patients who have undergone previous shoulder surgery.20 Therefore, while nonarthroplasty procedures may be useful in select patients, they may jeopardize the results of rTSA if they fail.
The purpose of this review was to provide an overview of these surgical techniques for the treatment of MIRCTs, including arthroscopic debridement, partial repair, superior capsular reconstruction (SCR), graft interposition, balloon spacer arthroplasty, trapezius transfer, and latissimus dorsi transfer, as well as to highlight the current literature on clinical outcomes after these procedures.
Search Strategy
A search through May 2018 was performed using various combinations of the following keywords/phrases: “massive,” “irreparable,” “rotator cuff tear,” “surgical,” “operative,” “non-operative,” “arthroscopic debridement,” “partial repair,” “superior capsular reconstruction,” “graft interposition,” “balloon spacer arthroplasty,” “trapezius transfer,” and “latissimus dorsi transfer.” Surgical technique descriptions and clinical studies that described and reported on the clinical outcomes of arthroscopic debridement, partial repair, SCR, graft interposition, balloon spacer arthroplasty, trapezius transfer, and latissimus dorsi transfer for the treatment of MIRCTs were included in this review.
Nonoperative Management
Because of the challenges that an MIRCT presents surgically, it is recommended to have patients attempt nonoperative treatment before surgical intervention. Nonoperative management options typically include activity modification, steroid injections, and physical therapy.44 Studies have shown that nonoperative management and physical therapy with an emphasis on rehabilitation of the anterior deltoid can improve clinical outcomes in patients with MIRCTs (Table 1).44,64 Dunn et al15 examined a group of patients who underwent nonoperative treatment for MIRCTs to define specific patient factors as predictors of treatment failure and the need for future surgery. Predictors of surgical intervention included low expectations of physical therapy, a high activity level, and not smoking. Whereas some studies44 have suggested that patients with chronic MIRCTs or significant pain due to MIRCTs tend to have inferior outcomes with nonoperative management, Dunn et al15 found no correlation between patient symptoms or anatomic features of the rotator cuff tear and the need for future surgery. However, additional studies have suggested that nonoperative management can cause significant degenerative structural joint changes and progression of the tear, potentially complicating any future surgical intervention if indicated.64
TABLE 1.
Summary of Clinical Studies on Massive, Irreparable Rotator Cuff Tearsa
| Study | No. of Patients | Mean Follow-up, mo | Clinical Outcomes | ||
|---|---|---|---|---|---|
| Preoperative | Postoperative | P Value | |||
| Nonoperative management | |||||
| Zingg et al64 (2007) | 19 | 48 | CM: NR SSV: NR FF: 115° AB: 118° IR: 76° ER: 44° |
CM: 69 SSV: 68% FF: 139° AB: 139° IR: 67° ER: 43° |
CM: NR SSV: NR FF: .047 AB: .070 IR: .054 ER: .864 |
| Arthroscopic debridement | |||||
| Berth et al3 (2010) | 21 | 16.8 | CM: 29.9 DASH: 69.5 AB: 93.5° ER: 40.5° IR: 49.5° |
CM: 40.7 DASH: 35.3 AB: 103.5° ER: 42.7° IR: 71.6° |
CM: <.01 DASH: <.01 AB: .074 ER: .157 IR: <.01 |
| Franceschi et al21 (2015) | 34 | 93.6 | UCLA: 7.6 VAS: 6.7 FF: 104.1° ER: 42.9° IR: 37.8° |
UCLA: 21.4 VAS: 1.2 FF: 132.0° ER: 48.0° IR: 46.7° |
UCLA: <.0001 VAS: <.0001 FF: <.001 ER: <.001 IR: <.001 |
| Heuberer et al32 (2016) | 23 | 45.0 | CM: 34 SSV: 35% DASH: 62 |
CM: 65 SSV: 72% DASH: 23 |
CM: <.001 SSV: <.001 DASH: <.001 |
| Liem et al38 (2008) | 31 | 47.0 | ASES: 24.0 VAS: 7.8 |
ASES: 69.8 VAS: 2.9 |
ASES: <.001 VAS: <.001 |
| Veado and Rodrigues60 (2015) | 27 | 27.0 | UCLA: 15 | UCLA: 31 | UCLA: NR |
| Partial repair | |||||
| Chen et al11 (2017) | 37 | 29.6 | ASES: 46.0 VAS: 5.22 |
ASES: 78.6 VAS: 1.51 |
ASES: <.001 VAS: <.001 |
| Cuff et al12 (2016) | 28 | 71.1 | ASES: 46.6 SST: 5.6 VAS: 6.9 FF: 168° ER: 38° IR: 84% |
ASES: 79.3 SST: 9.1 VAS: 1.9 FF: 154° ER: 39° IR: 80% |
ASES: <.001 SST: <.001 VAS: <.001 FF: .074 ER: >.99 IR: >.99 |
| Duralde and Bair16 (2005) | 68 | 43.0 | ASES: 41.0 FF: 114° ER: 44° |
ASES: 80.1 FF: 154° ER: 54° |
ASES: <.001 FF: NR ER: NR |
| Galasso et al22 (2017) | 90 | 84.0 | CM: 39.1 SST: NR FFb: 171° ABb: 167° ERb: 28° IRb: T7 |
CM: 76.3 SST: 9.1 FFc: 174° ABc: 177° ERc: 31° IRc: T7 |
CM: <.001 SST: NR FF: .062 AB: <.001 ER: .022 IR: <.001 |
| Kim et al36 (2012) | 27 | 41.3 | SST: 5.1 CM: 43.6 UCLA: 10.5 |
SST: 8.8 CM: 74.1 UCLA: 25.9 |
SST: <.001 CM: <.001 UCLA: <.001 |
| Pandey et al47 (2017) | 13 | 24.0 | OSS: 17.8 CM: 43.1 |
OSS: 37.1 CM: 70.8 |
OSS: .009 CM: .01 |
| Shon et al59 (2015) | 31 | 40.5 | VAS: 5.13 ASES: 41.97 SST: 3.61 |
VAS: 2.13 (1 y f/u) ASES: 73.78 SST: 6.07 |
VAS: .001 ASES: <.001 SST: .003 |
| Superior capsular reconstruction | |||||
| Denard et al13 (2018) | 59 | 17.7 | ASES: 43.6 VAS: 5.8 SSV: 35.0 FF: 130° ER: 36° IR: L3 |
ASES: 77.5 VAS: 1.7 SSV: 76.3 FF: 158° ER: 45° IR: L1 |
ASES: <.001 VAS: <.001 SSV: <.001 FF: <.001 ER: .008 IR: <.001 |
| Lee and Min37 (2018) | 36 | 24.8 | ASES: 50.3 CM: 56.3 |
ASES: 84.0 CM: 82.8 |
ASES: <.01 CM: .02 |
| Mihata et al39 (2013) | 23 | 34.1 | JOA: 48.3 ASES: 23.5 UCLA: 9.9 FF: 84° ER: 26° |
JOA: 92.6 ASES: 92.9 UCLA: 32.4 FF: 148° ER: 40° |
JOA: <.00001 ASES: <.00001 UCLA: <.00001 FF: <.001 ER: <.01 |
| Pennington et al48 (2018) | 88 | 12 | VAS: 4.0 ASES: 52 FF: 120° AB: 103° |
VAS: 1.5 ASES: 82 FF: 160° AB: 159° |
VAS: .005 ASES: .005 FF: .007 AB: .02 |
| Graft interposition | |||||
| Audenart et al2 (2006) | 41 | 43 | CM: 25.7 | CM: 72.1 | CM: <.001 |
| Gupta et al30 (2012) | 24 | 36 | ASES: 66.6 SF-12: 48.8 VAS: 5.4 FF: 111.7° ER: 46.2° AB: 105.0° |
ASES: 88.7 SF-12: 56.8 VAS: 0.9 FF: 157.3° ER: 65.1° AB: 151.7° |
ASES: .0003 SF-12: .03 VAS: .0002 FF: .0002 ER: .001 AB: .002 |
| Neumann et al45 (2017) | 60 | 50.3 | VAS: 4.0 ASES: NR FF: 140.7° ER: 55.6° IR: 52.0° |
VAS: 1.0 ASES: 87.8 FF: 160.4° ER: 70.1° IR: 76.2° |
VAS: <.001 ASES: NR FF: <.001 ER: .001 IR: .001 |
| Ranebo et al54 (2018) | 13 | 216 | CM: NR WORC: NR |
CM: 46 WORC: 59 |
CM: NR WORC: NR |
| Venouziou et al61 (2013) | 14 | 30.2 | VAS: 7.4 ASES: 23.8 FF: 73.6° AB: 67.5° ER: 7.9° |
VAS: 1.7 ASES: 72.3 FF: 129.3° AB: 117.9° ER: 43.2° |
VAS: .001 ASES: .001 FF: .002 AB: .002 ER: .001 |
| Balloon spacer arthroplasty | |||||
| Deranlot et al14 (2017) | 37 | 32.8 | CM: 44.8 FF: 130° AB: 100° ER: 30° |
CM: 76.0 FF: 160° AB: 160° ER: 45° |
CM: <.001 FF: .02 AB: .01 ER: .0001 |
| Gervasi et al27 (2016) | 15 | 12.0 | CM: 31.9 ASES: 24.5 |
CM: 69.8 ASES: 76.0 |
CM: <.0001 ASES: <.0001 |
| Piekaar et al51 (2017) | 44 | 12.0 | OSS: 21.8 CM: 37.1 |
OSS: 32.4 CM: 60.2 |
OSS: <.001 CM: <.001 |
| Prat et al53 (2018) | 22 | 14.4 | UCLA: 10.9 FF: 90° ER: 34.1° IR: L5 |
UCLA: 15.9 FF: 106.5° ER: 37.5° IR: L4 |
UCLA: .001 FF: .17 ER: .48 IR: .37 |
| Senekovic et al57 (2017) | 24 | 60.0 | CM: 34.2 | CM: 67.4 | CM: <.0001 |
| Senekovic et al58 (2013) | 20 | 36.0 | CM: 33.4 | CM: 65.4 | CM: <.0001 |
| Trapezius transfer | |||||
| Elhassan et al19 (2016) | 33 | 47.0 | SSV: 54% DASH: 52 FF: 70° AB: 40° ER: 20° |
SSV: 78% DASH: 18 FF: 120° AB: 90° ER: 50° |
SSV: <.01 DASH: <.01 FF: <.01 AB: <.01 ER: <.01 |
| Latissimus dorsi transfer | |||||
| Castricini et al8 (2016) | 86 | 36.4 | CM: 35.5 | CM: 69.5 | CM: <.001 |
| Castricini et al10 (2014) | 27 | 27 | CM: 36.0 ER: 23° |
CM: 74.0 ER: 38° |
CM: <.05 ER: <.05 |
| El-Azab et al17 (2015) | 108 | 111.6 | CM: 36.1 ASES: 30.1 VAS: 7.8 FF: 86.0° AB: 88.7° ER: 17.6° |
CM: 62.0 ASES: 70.2 VAS: 2.4 FF: 133.5° AB: 127.4° ER: 29.2° |
CM: <.0001 ASES: <.0001 VAS: <.0001 FF: <.0001 AB: <.0001 ER: <.0001 |
| Gerber et al24 (2013) | 44 | 146.6 | SSV: 29.0% CM: 47.3 FF: 118.0° AB: 112.1° ER: 17.9° |
SSV: 70.1% CM: 63.5 FF: 132.4° AB: 122.6° ER: 32.5° |
SSV: .0001 CM: <.0001 FF: .029 AB: .089 ER: .0001 |
| Grimberg et al29 (2015) | 55 | 29.0 | SSV: 26% CM: 37.0 FF: 134° AB: 67° ER: 29° |
SSV: 71.1% CM: 65.4 FF: 157° AB: 92.5° ER: 41.5° |
SSV: <.001 CM: <.001 FF: <.001 AB: <.001 ER: <.001 |
| Kanatli et al34 (2017) | 15 | 26.4 | UCLA: 6.53 CM: 21.0 VAS: 7.47 FF: 58° AB: 51° ER: 13.3° |
UCLA: 27.47 CM: 59.73 VAS: 2.47 FF: 130° AB: 129.7° ER: 32° |
UCLA: <.001 CM: <.001 VAS: <.001 FF: <.001 AB: <.001 ER: <.001 |
| Mun et al42 (2018) | 24 | 12 | CM: 46 ASES: 40 VAS: 6 FF: 135° IR: L5 ER: 51° |
CM: 69 ASES: 70 VAS: 2 FF: 166° IR: L1 ER: 68° |
CM: <.001 ASES: <.001 VAS: .006 FF: .016 IR: .010 ER: .062 |
| Petricciolo et al50 (2016) | 33 | 35.7 | CM: 34.6 VAS: 5 DASH: 49.7 FF: 138° ER: 7° |
CM: 64.9 VAS: 1.4 DASH: 22.6 FF: 168° ER: 34° |
CM: <.05 VAS: <.0001 DASH: <.001 FF: <.05 ER: <.05 |
aAB, abduction; ASES, American Shoulder and Elbow Surgeons; CM, Constant-Murley; DASH, Disabilities of the Arm, Shoulder and Hand; ER, external rotation; FF, forward flexion; f/u, follow-up; IR, internal rotation; JOA, Japanese Orthopaedic Association; NR, not reported; OSS, Oxford Shoulder Score; SF-12, 12-Item Short Form Health Survey; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale for pain; WORC, Western Ontario Rotator Cuff Index.
bValues are presented for surgically repaired shoulder.
cValues are presented for contralateral shoulder.
Arthroscopic Debridement
Arthroscopic debridement has been described as a surgical treatment option for patients with MIRCTs.31,55 In 1995, Rockwood et al55 described the use of this procedure in a group of patients treated from 1976 to 1988. The technique involves debridement of the torn rotator cuff by removing avascular or unstable tissue that could be caught or impinged between the humeral head and acromion during shoulder flexion.31,55 Additionally, surgeons may elect to perform bursectomy of the subacromial bursa, scar tissue removal, acromioplasty, subacromial decompression, and biceps tenotomy or tenodesis in conjunction with rotator cuff debridement.31 The goal of this intervention is to provide pain relief by removing the sources of mechanical irritation or inflammation. Some believe that this procedure should only be used as a salvage option for patients who seek pain relief, as no functional repair is performed.31 Therefore, arthroscopic debridement is indicated in patients with an irreparable rotator cuff tear who have minimal to mild osteoarthritis with a chief complaint of pain after the failure of conservative treatment.31
Multiple studies have reported promising clinical outcomes after arthroscopic debridement of MIRCTs (Table 1).3,21,32,38,55,60 Berth et al3 evaluated a group of 21 patients who underwent arthroscopic debridement with subacromial bursectomy and decompression for the treatment of MIRCTs. At a mean follow-up of 16.8 months, there was a significant improvement in the mean Constant-Murley (CM) score (29.9 to 40.7; P < .01) and the mean Disabilities of the Arm, Shoulder and Hand (DASH) score (69.5 to 35.3; P < .01). However, this study demonstrated no significant change in abduction (93.5° to 103.5°; P = .074) or external rotation (40.5° to 42.7°; P = .157) from preoperatively to final follow-up, despite a significant increase in internal rotation (49.5° to 71.6°; P < .01). Franceschi et al21 performed a similar study with a group of 34 patients undergoing arthroscopic debridement, subacromial bursectomy, acromioplasty, and in select cases biceps tenotomy. The authors found a significant improvement in the mean University of California, Los Angeles (UCLA) score (7.6 to 21.4; P < .0001) and visual analog scale (VAS) score for pain (6.7 to 1.2; P < .0001) at a mean follow-up of 93.6 months. Range of motion also significantly improved over the study period in terms of forward flexion (104.1° to 132.0°; P < .001), external rotation (42.9° to 48.0°; P < .001), and internal rotation (37.8° to 46.7°; P < .001).
Many of the studies examining the clinical outcomes of arthroscopic debridement of MIRCTs also assessed preoperative factors and their influence on clinical outcomes.3,21,32,38,60 Liem et al38 found male patients to have significantly improved clinical outcomes relative to those of female patients (P = .008), although no significant effect was found based on age, tear size, or preoperative osteoarthritis. Franceschi et al21 found that patients with a reduced preoperative acromiohumeral distance fared worse with regard to the UCLA score at final follow-up. The authors also found that high-demand manual laborers reported significantly worse clinical outcomes compared with nonmanual workers (P < .001). In contrast, 3 other studies3,32,60 found no relation between age, sex, profession, or fatty infiltration status of the rotator cuff tendons and clinical outcomes.
Because functional repair is not performed with arthroscopic debridement of MIRCTs, revision rates are low. One study32 reported a single case of revision to rTSA because of persistent pain and functional limitation. Berth et al3 also reported a single case of revision to hemiarthroplasty because of the development of severe glenohumeral osteoarthritis. No other cases of failure or revision were reported. Additionally, there was no incidence of intraoperative complications reported.
Partial Repair
Partial repair of massive rotator cuff tears was first described by Burkhart et al7 in 1994. This technique involves repairing as much of the rotator cuff tissue as possible to partially restore shoulder functionality. Burkhart et al7 described repairing the inferior portion of the rotator cuff by reattaching the infraspinatus and subscapularis tendons to their anatomic insertions while leaving the irreparable supraspinatus unrepaired. This creates force coupling of the deltoid and repaired rotator cuff tendons to allow for effective elevation of the arm. Although the supraspinatus is left unrepaired, complete coverage of the humeral head is unnecessary because the biomechanics of the shoulder are restored with repair of the infraspinatus and subscapularis. This technique was originally described as an open procedure, although several recent advancements5,9,12,16 using the same principles have allowed surgeons to arthroscopically perform partial tendon repair as well as to repair different combinations of tendon tears. This procedure is typically indicated in patients with an irreparable supraspinatus and a reparable infraspinatus and subscapularis who lack glenohumeral arthritis and who continue to have pain and dysfunction after conservative management.9,12,35
Several studies have reported on the clinical outcomes after partial repair of MIRCTs (Table 1).11,12,16,22,36,47,59 All studies found a statistically significant improvement in functional outcome scores compared with preoperatively. One study59 evaluated patients at 1 year and again at >2 years postoperatively. The mean VAS, American Shoulder and Elbow Surgeons (ASES), and Simple Shoulder Test scores significantly improved (P ≤ .003) from preoperatively to 1-year follow-up. However, despite this initial improvement, the number of patients reporting that they were dissatisfied with the procedure increased from 1-year follow-up (6%) to >2-year follow-up (32%). Additionally, the VAS score was significantly worse at >2-year follow-up (3.16) compared with 1-year follow-up (2.13) (P = .039).
Three studies evaluated range of motion in patients undergoing partial repair of MIRCTs (Table 1).12,16,22 Duralde and Bair16 found an average increase in both forward flexion (114° to 154°) and external rotation (44° to 54°) over an average follow-up of 43 months. Cuff et al12 found no significant changes in forward flexion (–14°; P = .07), external rotation (+1°; P > .99), or internal rotation (–4%; P > .99) from preoperatively to postoperatively. However, only patients with preoperative forward flexion >120° were included in this study. Galasso et al22 compared forward flexion, abduction, external rotation, and internal rotation of patients’ affected shoulder with those of the contralateral shoulder postoperatively. No significant difference was found between the 2 shoulders with regard to forward flexion or external rotation, although patients did have an average 10° less in abduction (P < .001) and significantly less internal rotation (P < .001) in the affected shoulder compared with the contralateral shoulder at an average follow-up of 7 years.
Several studies have attempted to examine preoperative factors and their effects on clinical outcomes after partial repair of MIRCTs.16,22,59 Shon et al59 investigated a variety of factors such as patient demographics, tear size, and fatty infiltration of the rotator cuff tendons. The authors found that fatty infiltration of the teres minor was the only preoperative factor associated with poor outcomes. Galasso et al22 found male patients to have significantly greater postoperative strength in abduction, external rotation, and internal rotation (all P < .001) compared with female patients, while younger patients displayed greater postoperative range of motion in abduction (P = .019) and external rotation (P < .03) compared with older patients. However, Duralde and Bair16 also examined similar preoperative factors and found no correlation between clinical outcomes and sex, age, or preoperative duration of symptoms. Additionally, Chen et al11 found only a lower preoperative ASES score, a higher preoperative VAS score, and night pain to be associated with a greater degree of functional improvement, while age, sex, diabetes status, smoking status, acromiohumeral distance, and preoperative duration of symptoms had no effect on clinical outcomes.
Although partial repair of MIRCTs has shown promising clinical outcomes, studies have revealed a relatively high failure rate after this procedure.11,12,16,22,59 Chen et al11 found the rate of repair failure to be 41.6%. Failed procedures are often revised to subsequent partial repair16 or rTSA.12,22 The rate of complications other than failure or the need for revision is low with partial repair of MIRCTs and is reported to be 4%.16
Superior Capsular Reconstruction
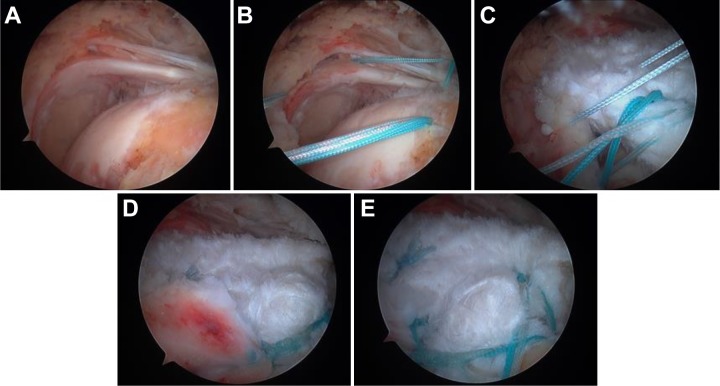
MIRCTs are often associated with superior migration of the humeral head in relation to the glenoid.6,40,49 SCR is a technique first described by Mihata et al40 to reconstruct the superior glenohumeral joint capsule and to prohibit superior migration of the humerus. This is achieved by arthroscopically attaching a fascia lata autograft medially to the superior glenoid and laterally to the greater tuberosity while simultaneously adding sutures between the graft and residual torn rotator cuff tendons (Figure 1).39 This in turn prevents superior migration of the humeral head, optimizing the force coupling necessary for arm elevation. In addition to a fascia lata autograft, recent studies have described the use of an acellular dermal allograft for this procedure.6,13,39 Indications for this procedure include failed conservative management in a patient with an irreparable rotator cuff tear, lack of significant osteoarthritis, superior migration of the humeral head, and subjective shoulder dysfunction.39
Figure 1.
Arthroscopic images of superior capsular reconstruction (SCR). (A) Massive rotator cuff tear. (B) Anchor placement. (C) Graft passage and coupling the graft to the posterior rotator cuff. (D) Coupling sutures tied. (E) Completed SCR.
Studies13,37,39,48 have reported on SCR in human participants and compared clinical outcomes preoperatively and postoperatively (Table 1). Mihata et al39 analyzed a group of 24 shoulders in 23 consecutive patients undergoing SCR with a fascia lata autograft. Significant improvements were demonstrated in the mean Japanese Orthopaedic Association score (48.3 to 92.6; P < .00001), ASES score (23.5 to 92.9; P < .00001), and UCLA score (9.9 to 32.4; P < .00001) at an average follow-up of 34.1 months. Additionally, average active elevation and external rotation both increased significantly by 64° (P < .001) and 14° (P < .01), respectively. The acromiohumeral distance also increased significantly from 4.6 mm to 8.7 mm (P < .001) over the study period, and no progression of osteoarthritis or rotator cuff muscle atrophy occurred in any patient.
Denard et al13 evaluated a group of 59 patients over a minimum 1-year follow-up who underwent SCR with a human dermal allograft for the treatment of MIRCTs. Compared with preoperatively, the ASES score improved from 43.6 to 77.5 (P < .001), the VAS score decreased from 5.8 to 1.7 (P < .001), and the Subjective Shoulder Value (SSV) improved from 35.0 to 76.3 (P < .001). This same study also analyzed range of motion and found significant improvements in forward flexion (130° to 158°; P < .001), external rotation (36° to 45°; P = .008), and internal rotation (L3 to L1; P < .001). The acromiohumeral distance also improved from a mean of 6.6 mm to 7.6 mm after just 2 weeks postoperatively. However, this outcome was not maintained at >1-year follow-up, and the acromiohumeral distance actually decreased to a mean of 6.7 mm (P = .89). Postoperative magnetic resonance imaging results revealed only 45% of the grafts to have healed at final follow-up. Graft healing did correlate with better outcomes, as 100% of the patients with complete graft healing had successful outcomes as well as a significantly better mean ASES score (P = .027) and mean VAS score (P = .038) when compared with the nonhealed group. There was also a significantly greater prevalence of preoperative subscapularis atrophy (P = .006) in the nonhealed group.
An additional study48 analyzed a group of 88 consecutive shoulders undergoing arthroscopic SCR using an acellular dermal allograft for MIRCTs with a minimum 12-month follow-up. The mean VAS and ASES scores improved from 4.0 to 1.5 (P = .005) and from 52 to 82 (P = .005), respectively. Mean range of motion values at a minimum 1-year follow-up also improved with regard to active forward flexion (120° to 160°; P = .007) and active abduction (103° to 159°; P = .02). Radiographic analysis from this study showed that the acromiohumeral interval improved from a mean of 7.1 mm preoperatively to 9.7 mm at 1-year follow-up (P = .049). Additionally, the superior capsular distance improved from a mean of 52.9 mm preoperatively to 46.2 mm at 1-year follow-up (P = .011). Lee and Min37 investigated similar clinical outcomes after SCR for MIRCTs but also included in their analysis predictive factors for a retear. Although the authors found promising results in a group of 36 shoulders at a mean follow-up of 24.8 months (ASES: 50.3 to 84.0 [P < .01]; CM: 56.3 to 82.8 [P = .02]), they also found poor posterior remnant tissue and inadequate acromiohumeral interval improvement in the immediate postoperative phase to be predictive of failure and graft retears.
Failure rates after SCR for MIRCTs are moderate. Mihata et al39 reported that 16.7% of patients undergoing SCR sustained a retear of either the graft or the repaired rotator cuff tendons. However, these patients either had severe fatty degeneration of the infraspinatus tendon or a history of rotator cuff surgery. Lee and Min37 reported that 36.1% of patients sustained a graft retear. Denard et al13 reported 25.4% of cases to be failures, defined as a final ASES score of <50, a <17-point improvement in the ASES score over the study period, or revision to repeat SCR or rTSA. The revision rate after SCR has been reported as 18.6%, with the majority of revisions being conversion to rTSA.13 Intraoperative and postoperative complications other than failure or the need for revision occurred at a rate of 6.8% in 1 study and included an infection requiring debridement and placement of an antibiotic spacer and persistent biceps pain requiring biceps tenodesis.13
Graft Interposition
The inability of the torn rotator cuff tendon to reach the anatomic footprint on the proximal humerus is a key characteristic of an MIRCT. In 1985, Post52 described using a graft to bridge the gap between the torn rotator cuff tendon and the anatomic footprint of the tendon. More recently, this has been accomplished by first arthroscopically mobilizing the torn and retracted native rotator cuff tendon, followed by a mini-open approach in which the graft is passed into the shoulder, sutured to the native rotator cuff tendon, and then anchored to the anatomic footprint on the greater tuberosity.30,45 The purpose of this is to re-create the biomechanical action of the torn rotator cuff by filling the void between the retracted tendon and its insertion. Graft options for this procedure vary, and studies have reported on the use of synthetic grafts, allografts, and xenografts.30,45,52 Graft interposition is indicated in patients with symptomatic massive and irreparable tears of the supraspinatus and infraspinatus who have failed conservative treatment and lack glenohumeral arthritis.45 Some authors45 have suggested that patients with severe atrophy and fatty infiltration of the torn tendon should not be considered for graft interposition, as these conditions are irreversible and reconstructing the tendon would likely not restore strength and function.
Neumann et al45 reported on the clinical outcomes of 60 patients who underwent graft interposition with a porcine acellular dermal matrix xenograft for the treatment of MIRCTs (Table 1). The mean VAS score decreased from 4.0 preoperatively to 1.0 at a mean follow-up of 50.3 months (P < .001). The mean modified ASES score at final follow-up was 87.8, although the authors did not state whether this was a significant change from preoperatively. Mean range of motion measurements improved significantly over the study period in terms of active forward flexion (140.7° to 160.4°; P < .001), active external rotation at 0° of abduction (55.6° to 70.1°; P = .001), and active internal rotation at 90° of abduction (52.0° to 76.2°; P = .001). Strength was defined on a 10-point scale,45 and supraspinatus strength improved from 7.7 preoperatively to 8.8 postoperatively (P < .001), while infraspinatus strength improved from 7.7 preoperatively to 9.3 postoperatively (P < .001). Postoperative ultrasonography revealed 91.8% of grafts to be intact, indicating a failure rate of 8.2%.
Two studies30,61 have described the use of a human dermal allograft for graft interposition in the setting of MIRCTs (Table 1). Gupta et al30 observed a group of 24 patients over a mean 36-month follow-up period and found significant improvements in both the ASES score (66.6 to 88.7; P = .0003) and 12-Item Short Form Health Survey (SF-12) score (48.8 to 56.8; P = .03). Additionally, the VAS score significantly decreased from 5.4 preoperatively to 0.9 at follow-up (P = .0002). Mean active forward flexion (111.7° to 157.3°; P = .0002), external rotation (46.2° to 65.1°; P = .001), and abduction (105.0° to 151.7°; P = .002) all significantly improved as well. Postoperative ultrasonography showed 76% of repairs to be intact, while all other repairs were found to be at least partially intact. Venouziou et al61 demonstrated similar results in a group of 14 patients with a mean follow-up of 30.2 months. The mean VAS score improved from 7.4 preoperatively to 1.7 at 18-month follow-up (P = .001) and was maintained until final follow-up. The mean ASES score also improved significantly from 23.8 to 72.3 postoperatively (P = .001). Range of motion improved in terms of forward flexion (73.6° to 129.3°; P = .002), abduction (67.5° to 117.9°; P = .002), and external rotation (7.9° to 43.2°; P = .001). The authors found that a smaller gap size between the retracted tendon and greater tuberosity correlated with a significantly improved postoperative VAS score, ASES score, and range of motion. However, there was no significant correlation between postoperative outcomes and age, sex, duration of symptoms, type of acromion, presence of acromioclavicular joint arthritis, muscle atrophy, or fatty infiltration.
Synthetic grafts have also been used for graft interposition in the treatment of MIRCTs.2,54 Among a cohort of 41 patients, Audenart et al2 found a significant improvement in the CM score from 25.7 to 72.1 (P < .001) at a mean follow-up of 43 months. Ranebo et al54 conducted a long-term study on 13 consecutive patients treated with graft interposition for MIRCTs using a synthetic graft made from Dacron (Table 1). Ten patients were reached at a mean follow-up of 18 years, with a mean CM score of 46 and a mean Western Ontario Rotator Cuff Index (WORC) score of 59. Ultrasonography at follow-up revealed that 7 of the 10 (70%) patients’ grafts were not intact and that 9 of 10 (90%) patients also had full- or partial-thickness tears of the subscapularis. Therefore, the authors of this study concluded that graft interposition with a synthetic graft could not preserve rotator cuff integrity or prevent rotator cuff tear arthropathy.
Balloon Spacer Arthroplasty
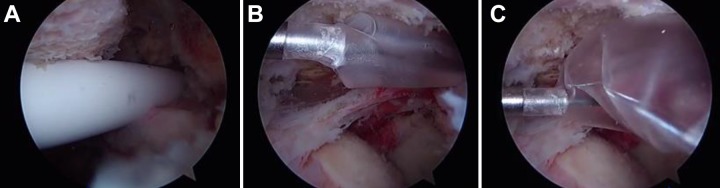
Balloon spacer arthroplasty is a procedure that was first described by Savarese and Romeo56 in 2012. As mentioned previously, because of the severe disruption to the rotator cuff musculature in patients with MIRCTs, the humeral head is prone to superior migration, which can disrupt shoulder function. Balloon spacer arthroplasty was designed to prevent this superior migration by inserting a biodegradable saline-filled balloon spacer between the humerus and acromion.56 This is accomplished arthroscopically using a cylindrical insertion device to introduce the spacer to the subacromial space and then inflating the spacer to its maximum volume with saline solution (Figure 2). The patient is then subjected to a period of rehabilitation, and the balloon dissolves over a period of 12 to 18 months. It is hypothesized that this restores shoulder biomechanics by permitting smooth, frictionless gliding within the joint as well as by allowing for effective action of the deltoid muscle.26,56 Gervasi et al26 recently described a fluoroscopy-guided technique for inserting the balloon under local anesthesia. Balloon spacer arthroplasty is contraindicated in patients with a known allergy to the device material, patients having an active or latent infectious process, or patients with signs of tissue necrosis in the subacromial space but otherwise is indicated in patients who continue to have pain or dysfunction due to MIRCTs after failure of conservative management.56 However, balloon spacer arthroplasty is only available in Europe or the United States through a currently ongoing US Food and Drug Administration trial.
Figure 2.
Arthroscopic images of balloon spacer arthroplasty. (A) Cylindrical insertion device entering the subacromial space. (B) Deflated spacer within the subacromial space. (C) Spacer inflating with saline solution.
Senekovic et al58 first reported on the clinical outcomes of arthroscopic balloon spacer arthroplasty for MIRCTs in a group of 20 consecutive patients (see Table 1). The authors found a significant increase in the CM score as early as 6 weeks postoperatively (42.8) compared with preoperatively (33.4) (P = .010), with an average CM score increase of 31.5 points (P < .0001) at a final follow-up of 3 years. Senekovic et al57 performed a similar study in 24 patients and found that the CM score significantly increased from 34.2 preoperatively to 67.4 at 5-year follow-up (P < .0001) (Table 1). In a group of 44 consecutive patients undergoing arthroscopic balloon spacer arthroplasty, Piekaar et al51 found the mean Oxford Shoulder Score and CM score to increase from 21.8 to 32.4 (P < .001) and from 37.1 to 60.2 (P < .001), respectively, at 12-month follow-up (Table 1). This same study demonstrated a significant decrease in pain on a simple 1-to-10 numeric scale (6.84 to 3.27; P < .001). Gervasi et al27 found significant increases in the CM and ASES scores of 37.9 points (P < .0001) and 51.5 points (P < .0001), respectively, at 1-year follow-up (Table 1).
Deranlot et al14 evaluated a group of 37 consecutive patients (39 shoulders) undergoing arthroscopic balloon spacer arthroplasty for MIRCTs (Table 1). At a mean follow-up of 32.8 months, the average adjusted CM score increased significantly from 44.8 preoperatively to 76 at final follow-up (P < .001). Additionally, the mean adjusted CM score at final follow-up was significantly greater than at 1-year follow-up (P = .02). This study found significantly increased range of motion at final follow-up compared with preoperatively in terms of forward flexion (130° to 160°; P = .02), abduction (100° to 160°; P = .01), and external rotation (30° to 45°; P = .0001). Despite these promising clinical results, radiographic evidence from this study showed that the mean acromiohumeral distance actually decreased from 8.2 mm preoperatively to 6.2 mm at final follow-up (P = .002). Similarly, Prat et al53 found no significant difference in the acromiohumeral distance at a mean follow-up of 14.4 months in a group of 22 patients treated with arthroscopic balloon spacer arthroplasty for MIRCTs. The same study found a significant increase in the mean UCLA score at follow-up (10.9 to 15.9; P = .001) but no significant differences in preoperative and postoperative range of motion values for active forward flexion (90° to 106.5°; P = .17), active external rotation (34.1° to 37.5°; P = .48), and active internal rotation (L5 to L4; P = .37). However, there was a moderate-strong correlation (r = 0.64) between preoperative range of motion and subjective general satisfaction after the procedure.
The failure rate after balloon spacer arthroplasty for MIRCTs is reported to be 3% to 8.3%.14,27,53,57,58 One study14 reported a single case of failure due to migration of the spacer anteriorly, which was later revised to a new spacer with satisfactory clinical outcomes. Most other cases of failure in the literature were revised to rTSA.27,57,58 The complication rate is also low after balloon spacer arthroplasty but was reported to be 16.7% in 1 study.53 Synovitis was reported to occur in 10% of patients in 1 study,57 while another study reported the incidence of transient neural damage and both superficial and deep wound infections.53
Trapezius Transfer
Tendon transfer is an available treatment option for patients presenting with MIRCTs. Lower trapezius transfer is one such procedure, in which the lower trapezius tendon is transferred to the humeral head to take the place of an irreparable posterior-superior rotator cuff tear.18,19 With this type of tear, the humeral rotational position, joint reaction forces, and kinematics of the shoulder are altered compared with an intact shoulder.46 Repair with lower trapezius transfer restores these biomechanical measures to the level of an intact shoulder.46 Although first reported as an open procedure, Elhassan et al19 described an arthroscopic approach in which the lower trapezius muscle is dissected and its tendinous insertion at the medial aspect of the scapular spine is detached. This is then augmented with an Achilles tendon allograft, which is attached to the supraspinatus footprint on the greater tuberosity. Lower trapezius transfer is typically indicated in younger and active patients with a posterior-superior irreparable rotator cuff tear who have minimal to mild glenohumeral arthritis.18 Another study28 described and attempted to determine the efficacy of superior trapezius transfer for the treatment of an irreparable subscapularis tear. However, this study was met with poor clinical outcomes, and the authors do not recommend it as a viable treatment option for MIRCTs.
Only 1 study has reported on clinical outcomes after lower trapezius transfer for the treatment of MIRCTs (see Table 1).19 At a mean follow-up of 47 months, the mean SSV significantly increased from 54% preoperatively to 78% postoperatively (P < .01). The mean DASH score significantly improved from 52 to 18 (P < .01). Preoperative range of motion included 70° of forward flexion, 40° of abduction, and 20° of external rotation, which all significantly increased (all P < .01) to mean values of 120°, 90°, and 50°, respectively, while mean internal rotation was maintained at the L3 spinous process level. Interestingly, patients with >60° of forward flexion and abduction preoperatively demonstrated a significantly greater improvement in range of motion compared with patients with less preoperative range of motion. The radiographic evaluation from this study demonstrated that 26 of 33 (79%) patients had proximal migration of the humeral head preoperatively, with a mean acromiohumeral distance of 2.3 mm, which increased to 8 mm at final follow-up. Failure occurred in 1 of 33 patients (3%) because of a postoperative infection that required debridement and later revision to shoulder fusion. Seroma formation was reported in 4 of 33 patients (12%), but no other complications were reported in this study.
Latissimus Dorsi Transfer
Latissimus dorsi transfer was first described by Gerber et al25 in 1988 as a surgical technique used for patients with MIRCTs, specifically of the supraspinatus and infraspinatus tendons. The original technique involves harvesting the latissimus dorsi tendon from its insertion on the floor of the intertubercular groove of the humerus and fixing it to the superolateral humeral head. This allows the latissimus dorsi to function as an external rotator as well as a direct countering force to superior migration of the humeral head on attempted flexion and abduction of the shoulder. The original technique was performed as an open procedure, although an arthroscopically assisted technique has been described in multiple studies.8,10,29,33–35,42,50 Indications for this procedure include younger patients who are suffering from severe functional disabilities caused by an irreparable posterior-superior rotator cuff tear and patients with minimal to no glenohumeral arthritis.1,34,43
Numerous studies have discussed functional and clinical outcomes after latissimus dorsi transfer for MIRCTs (see Table 1).§ One study24 evaluated the functional outcome scores of 46 cases in which open transfer was performed. At a minimum 10-year follow-up, the mean SSV increased from 29.0% preoperatively to 70.1%, and the mean CM score improved from 47.3 to 63.5 (both P < .0001). Additionally, mean forward flexion increased from 118.0° to 132.4° (P = .029), mean abduction increased from 112.1° to 122.6° (P = .089), and mean external rotation increased from 17.9° to 32.5° (P = .0001).
Another study17 evaluated 108 patients undergoing open latissimus dorsi transfer for MIRCTs. At a mean follow-up of 9.3 years, the CM score improved to 62.0 from 36.1 preoperatively (P < .0001), excluding the 10% of patients in whom the procedure failed. The mean ASES score improved from 30.1 to 70.2 (P < .0001), and the mean VAS score decreased from 7.8 to 2.4 (P < .0001). However, an increase in rotator cuff arthropathy and a decrease in the acromiohumeral distance (5.9 mm to 4.9 mm; P < .0001) were also noted.
Eight studies have examined clinical outcomes after arthroscopically assisted latissimus dorsi transfer.8,10,17,24,29,34,42,50 Grimberg et al29 evaluated 55 cases clinically and radiographically at a mean follow-up of 29 months. The authors noted that the CM score improved significantly from 37.0 preoperatively to 65.4 postoperatively (P < .001). Additionally, the SSV improved from a mean of 26% preoperatively to 71.1% postoperatively (P < .001). Active forward flexion increased from 134° to 157° (P < .001), mean active abduction increased from 67° to 92.5° (P < .001), and mean active external rotation increased from 29° to 41.5° (P < .001). Mean abduction strength increased from 1.4 kg to 4.8 kg, and the mean CM pain subscore improved from 1.7 preoperatively to 12.6 postoperatively. The authors noted that there was no statistical difference between preoperative and follow-up acromiohumeral distance and that there was also no increase in osteoarthritic stage with this procedure in contrast to the open technique.17,24 Kanatli et al34 evaluated patients undergoing the same technique after a mean of 26.4 months and noted similar improvements in functional outcome scores. They also found that active forward flexion improved from 58° to 130° (P < .001), active abduction increased from 51° to 129.7° (P < .001), and active external rotation increased from 13.3° to 32° (P < .001). The mean acromiohumeral distance significantly improved from 3.13 mm preoperatively to 5.67 mm postoperatively (P < .001) in this study.
In another study,33 9 patients who underwent arthroscopic-assisted latissimus dorsi transfer were analyzed to determine changes in maximum shoulder flexion/extension, abduction/adduction, and internal/external rotation. The authors found a significant increase in shoulder range of motion in all movements after 6 months compared with preoperatively (P < .001). Similarly, Castricini et al10 noted a significant improvement in external rotation at a mean follow-up of 27 months (P < .05). The authors also noted a significant improvement in the mean CM score and pain score (P < .05) and no significant osteoarthritis progression or proximal migration of the humeral head after surgery. Petricciolo et al50 also noted significant improvements in shoulder range of motion after latissimus dorsi transfer. Of the 33 patients included in their retrospective study, it was noted that forward flexion improved from an average of 138° preoperatively to 168° (P < .05) and that active external rotation increased from an average of 7° to 34° (P < .05) at an average follow-up of 35.7 months.
One study42 evaluated a group of 24 patients undergoing arthroscopic latissimus dorsi transfer for irreparable subscapularis tendon tears. At a follow-up of >1 year, the mean CM score improved from 46 to 69 (P < .001), the ASES score improved from 40 to 70 (P < .001), and the VAS score improved from 6 to 2 (P = .006). This study also found significant improvements in active forward flexion (135° to 166°; P = .016) and internal rotation (L5 to L1; P = .010), while active external rotation trended toward a significant improvement (51° to 68°; P = .062). Postoperative magnetic resonance imaging studies of these patients showed adequate healing of the transferred latissimus dorsi tendon to the humeral head in all patients.
Several studies have investigated preoperative factors that affect the clinical outcomes of latissimus dorsi transfer for MIRCTs.1,8,17,24,29,50 Anastasopoulos et al1 suggested that patients with irreparable posterior-superior rotator cuff tears with associated atrophy or fatty degeneration of the subscapularis and deltoid, as well as a large critical shoulder angle, are more likely to have poor clinical outcomes. Successful outcomes were associated with a preoperative critical shoulder angle of ≤36°. El-Azab et al17 noted that damage to the deltoid had an adverse effect on revision latissimus dorsi transfer. Gerber et al24 noted that patients had a higher risk of being dissatisfied if they had teres minor atrophy, preoperative forward flexion <90°, superior fixation of latissimus dorsi transfer, and workers’ compensation status. Grimberg et al29 suggested that patients had a higher chance of being satisfied if they were men, were younger than 65 years, had no history of shoulder surgery, and underwent antegrade-type fixation consisting of a round button applied on the anterior humeral cortex at the distal end of the humeral tunnel using 2 knotted sutures for fixation on the button.
Latissimus dorsi transfer has shown promising clinical results in select patients, with a moderate rate of failure.17,24,29,34,35,43 The highest reported failure rate for this procedure is 38%35 within the first 2 years postoperatively. However, other studies17,24,34,43 have reported much lower rates of failure, with 1 study reporting an 86% satisfaction rate at 10-year follow-up.17 Kany et al35 found that patients with failure of the repair had significantly worse clinical outcomes than patients with adequate tendon healing. Complications include stiffness, traumatic failure of the transfer, hematoma, subscapularis retears, resolving nerve dysesthesia, and deltoid reattachment failure, although appropriate patient selection is of paramount importance, and the procedure requires a high level of surgical skill and experience.24,35
Limitations
The limitations of this review should be noted. There is a lack of randomized controlled trials comparing these procedures with each other or to rTSA. Many of the studies included in this review present a short-term follow-up. In contrast, Gerber et al23 have reported on the 15-year follow-up of rTSA with a relatively high failure rate, which may be related to older prosthetic designs and a long duration of follow-up, making these techniques difficult to compare. Several of the studies included in this review lack postoperative imaging results to confirm the integrity of repairs or grafts. Many of these studies also have an inconsistent definition of a successful outcome. For example, 1 study34 reporting on the outcomes of arthroscopic latissimus dorsi transfer considered an average postoperative CM score of 59.73 to be a successful result.
Conclusion
Reverse total shoulder arthroplasty has gained favor as a surgical treatment option for patients presenting with MIRCTs. However, complications after rTSA occur at a rate of 20% to 50%, and there are concerns regarding the longevity of these implants, as revision is necessary in 10% to 33% of cases. Arthroscopic debridement provides pain relief and improved clinical outcomes but does not provide functional benefits for patients with MIRCTs. Partial repair is an alternative treatment option available for patients with an irreparable supraspinatus tendon and reparable infraspinatus and subscapularis tendons; this technique partially restores shoulder biomechanics and has shown promising clinical outcomes but high failure rates as well. SCR reduces superior translation of the glenohumeral joint for patients with MIRCTs, and while preliminary outcomes are promising, additional investigation is necessary to confirm these results. Graft interposition has shown success with dermal allografts and xenografts in short-term follow-up studies. However, synthetic grafts, especially at long-term follow-up, have demonstrated a high risk of retears. Balloon spacer arthroplasty is a simple and minimally invasive technique that improves clinical outcomes but does not appear to have an effect on the acromiohumeral distance. Both latissimus dorsi and trapezius transfer provide significant functional improvement and are typically indicated in younger, active patients who present with MIRCTs. Future studies involving randomized controlled trials are necessary for confirming the efficacy of these procedures as well as to determine when each is indicated based on specific patient and anatomic factors.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: J.T.B. is a consultant for DJ Orthopaedics, Shukla Medical, Smith & Nephew, and Encore Medical; receives royalties from Shukla Medical; receives research support from Stryker; and has received fellowship funding from Mitek, Smith & Nephew, and Stryker. E.C.M. receives royalties from Zimmer Biomet and Elsevier; is a consultant for Zimmer Biomet; and receives research support from Zimmer Biomet, Mitek, Smith & Nephew, and Stryker. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Anastasopoulos PP, Alexiadis G, Spyridonos S, Fandridis E. Latissimus dorsi transfer in posterior irreparable rotator cuff tears. Open Orthop J. 2017;11:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Audenart E, Van Nuffel J, Schepens A, Verhelst M, Verdonk R. Reconstruction of massive irreparable rotator cuff lesions with synthetic interposition graft: a prospective study of 41 patients. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):360–364. [DOI] [PubMed] [Google Scholar]
- 3. Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):1359–1370. [DOI] [PubMed] [Google Scholar]
- 5. Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clin Orthop Relat Res. 2001;(390):107–118. [DOI] [PubMed] [Google Scholar]
- 6. Burkhart SS, Denard PJ, Adams CR, Brady PC, Hartzler RU. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech. 2016;5(6):e1407–e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363–370. [DOI] [PubMed] [Google Scholar]
- 8. Castricini R, De Benedetto M, Familiari F, et al. Functional status and failed rotator cuff repair predict outcomes after arthroscopic-assisted latissimus dorsi transfer for irreparable massive rotator cuff tears. J Shoulder Elbow Surg. 2016;25(4):658–665. [DOI] [PubMed] [Google Scholar]
- 9. Castricini R, Galasso O, Riccelli DA, et al. Arthroscopic partial repair of irreparable, massive rotator cuff tears. Arthrosc Tech. 2017;6(1):e143–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castricini R, Longo UG, De Benedetto M, et al. Arthroscopic-assisted latissimus dorsi transfer for the management of irreparable rotator cuff tears: short-term results. J Bone Joint Surg Am. 2014;96(14):e119. [DOI] [PubMed] [Google Scholar]
- 11. Chen KH, Chiang ER, Wang HY, Ma HL. Arthroscopic partial repair of irreparable rotator cuff tears: factors related to greater degree of clinical improvement at 2 years of follow-up. Arthroscopy. 2017;33(11):1949–1955. [DOI] [PubMed] [Google Scholar]
- 12. Cuff DJ, Pupello DR, Santoni BG. Partial rotator cuff repair and biceps tenotomy for the treatment of patients with massive cuff tears and retained overhead elevation: midterm outcomes with a minimum 5 years of follow-up. J Shoulder Elbow Surg. 2016;25(11):1803–1809. [DOI] [PubMed] [Google Scholar]
- 13. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93–99. [DOI] [PubMed] [Google Scholar]
- 14. Deranlot J, Herisson O, Nourissat G, et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospective cases. Arthroscopy. 2017;33(9):1639–1644. [DOI] [PubMed] [Google Scholar]
- 15. Dunn WR, Kuhn JE, Sanders R, et al. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25(8):1303–1311. [DOI] [PubMed] [Google Scholar]
- 16. Duralde XA, Bair B. Massive rotator cuff tears: the result of partial rotator cuff repair. J Shoulder Elbow Surg. 2005;14(2):121–127. [DOI] [PubMed] [Google Scholar]
- 17. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462–469. [DOI] [PubMed] [Google Scholar]
- 18. Elhassan BT, Alentorn-Geli E, Assenmacher AT, Wagner ER. Arthroscopic-assisted lower trapezius tendon transfer for massive irreparable posterior-superior rotator cuff tears: surgical technique. Arthrosc Tech. 2016;5(5):e981–e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elhassan BT, Wagner ER, Werthel JD. Outcome of lower trapezius transfer to reconstruct massive irreparable posterior-superior rotator cuff tear. J Shoulder Elbow Surg. 2016;25(8):1346–1353. [DOI] [PubMed] [Google Scholar]
- 20. Familiari F, Rojas J, Nedim Doral M, Huri G, McFarland EG. Reverse total shoulder arthroplasty. EFFORT Open Rev. 2018;3(2):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franceschi F, Papalia R, Vasta S, Leonardi F, Maffulli N, Denaro V. Surgical management of irreparable rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):494–501. [DOI] [PubMed] [Google Scholar]
- 22. Galasso O, Riccelli DA, De Gori M, et al. Quality of life and functional results of arthroscopic partial repair of irreparable rotator cuff tears. Arthroscopy. 2017;33(2):261–268. [DOI] [PubMed] [Google Scholar]
- 23. Gerber C, Canonica S, Catanzaro S, Ernstbrunner L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: results after 15 years. J Shoulder Elbow Surg. 2018;27(5):831–838. [DOI] [PubMed] [Google Scholar]
- 24. Gerber C, Rahm SA, Cantanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920–1926. [DOI] [PubMed] [Google Scholar]
- 25. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff: a preliminary report. Clin Orthop Relat Res. 1988;(232):51–61. [PubMed] [Google Scholar]
- 26. Gervasi E, Cautero E, Dekel A. Fluoroscopy-guided implantation of subacromial “biodegradable spacer” using local anesthesia in patients with irreparable rotator cuff tear. Arthrosc Tech. 2014;3(4):e455–e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gervasi E, Maman E, Dekel A, Cautero E. Fluoroscopy-guided biodegradable spacer implantation using local anesthesia: safety and efficacy study in patient with massive rotator cuff tears. Musculoskelet Surg. 2016;100(suppl 1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goutallier D, De Abreu L, Postel JM, Le Guilloux P, Radier C, Ziber S. Is the trapezius transfer a useful treatment option for irreparable tears of the subscapularis? Orthop Traumatol Surg Res. 2011;97(7):719–725. [DOI] [PubMed] [Google Scholar]
- 29. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599–607. [DOI] [PubMed] [Google Scholar]
- 30. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. [DOI] [PubMed] [Google Scholar]
- 31. Hawi N, Schmiddem U, Omar M, et al. Arthroscopic debridement of irreparable rotator cuff tears. Open Orthop J. 2016;10:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heuberer PR, Kolblinger R, Buchleitner S, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3828–3837. [DOI] [PubMed] [Google Scholar]
- 33. Ippolito G, Serrao M, Napoli F, et al. Three-dimensional analysis of the shoulder motion in patients with massive irreparable cuff tears after latissimus dorsi tendon transfer (LDT). Arch Orthop Trauma Surg. 2016;136(10):1363–1370. [DOI] [PubMed] [Google Scholar]
- 34. Kanatli U, Ozer M, Ataoglu MB, et al. Arthroscopic-assisted latissimus dorsi tendon transfer for massive, irreparable rotator cuff tears: technique and short-term follow-up of patients with pseudoparalysis. Arthroscopy. 2017;33(5):929–937. [DOI] [PubMed] [Google Scholar]
- 35. Kany J, Grimberg J, Amaravathi RS, Sekaran P, Scorpie D, Werthel JD. Arthroscopically-assisted latissimus dorsi transfer for irreparable rotator cuff insufficiency: modes of failure and clinical correlation. Arthroscopy. 2018;34(4):1139–1150. [DOI] [PubMed] [Google Scholar]
- 36. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761–768. [DOI] [PubMed] [Google Scholar]
- 37. Lee SJ, Min YK. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2205–2213. [DOI] [PubMed] [Google Scholar]
- 38. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Maquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743–748. [DOI] [PubMed] [Google Scholar]
- 39. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. [DOI] [PubMed] [Google Scholar]
- 40. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248–2255. [DOI] [PubMed] [Google Scholar]
- 41. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544–2556. [DOI] [PubMed] [Google Scholar]
- 42. Mun SW, Kim JY, Yi SH, Baek CH. Latissimus dorsi transfer for irreparable subscapularis tendon tears. J Shoulder Elbow Surg. 2018;27(6):1057–1064. [DOI] [PubMed] [Google Scholar]
- 43. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891–898. [DOI] [PubMed] [Google Scholar]
- 44. Neri BR, Chan KW, Kwon YW. Management of massive irreparable rotator cuff tears. J Shoulder Elbow Surg. 2009;18(5):808–818. [DOI] [PubMed] [Google Scholar]
- 45. Neumann JA, Zgonis MH, Rickert KD, et al. Interposition dermal matrix xenografts: a successful alternative to traditional treatment of massive rotator cuff tears. Am J Sports Med. 2017;45(6):1261–1268. [DOI] [PubMed] [Google Scholar]
- 46. Omid R, Heckmann N, Wang L, McGarry MH, Vangsness CT, Jr, Lee TQ. Biomechanical comparison between the trapezius transfer and latissimus transfer for irreparable posterosuperior rotator cuff tears. J Shoulder Elbow Surg. 2015;24(10):1635–1643. [DOI] [PubMed] [Google Scholar]
- 47. Pandey R, Tafazal S, Shyamsundar S, Modi A, Singh HP. Outcome of partial repair of massive rotator cuff tears with and without human tissue allograft bridging repair. Shoulder Elbow. 2017;9(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34(6):1764–1773. [DOI] [PubMed] [Google Scholar]
- 49. Petri M, Greenspoon JA, Millett PJ. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e751–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petricciolo D, Bertone C, Marchi G. Recovery of active external rotation and elevation in young active men with irreparable posterosuperior rotator cuff tear using arthroscopically assisted latissimus dorsi transfer. J Shoulder Elbow Surg. 2016;25(9):e265–e275. [DOI] [PubMed] [Google Scholar]
- 51. Piekaar RSM, Bouman ICE, van Kampen PM, van Eijk F, Hujismans PE. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear [published online November 18, 2017]. Musculoskelet Surg. doi:10.1007/s12306-017-0525-5 [DOI] [PubMed] [Google Scholar]
- 52. Post M. Rotator cuff repair with carbon filament: a preliminary report of five cases. Clin Orthop Relat Res. 1985;(196):154–158. [PubMed] [Google Scholar]
- 53. Prat D, Tenenbaum S, Pritsch M, Oran A, Vogel G. Sub-acromial balloon spacer for irreparable rotator cuff tears: is it an appropriate salvage procedure? J Orthop Surg (Hong Kong). 2018;26(2):2309499018770887. [DOI] [PubMed] [Google Scholar]
- 54. Ranebo MC, Bjornsson Hallgren HC, Norlin R, Adolfsson LE. Long-term clinical and radiographic outcome of rotator cuff repair with synthetic interposition graft: a consecutive case series with 17 to 20 years follow-up. J Shoulder Elbow Surg. 2018;27(9):1622–1628. [DOI] [PubMed] [Google Scholar]
- 55. Rockwood CA, Jr, Williams GR, Jr, Burkhead WZ., Jr Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77(6):857–866. [DOI] [PubMed] [Google Scholar]
- 56. Savarese E, Romeo R. New solution for massive, irreparable rotator cuff tears: the subacromial “biodegradable spacer.” Arthrosc Tech. 2012;1(1):e69–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with a 5-year follow-up. Arch Orthop Trauma Surg. 2017;137(1):95–103. [DOI] [PubMed] [Google Scholar]
- 58. Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol. 2013;23(3):311–316. [DOI] [PubMed] [Google Scholar]
- 59. Shon MS, Koh KH, Lim TK, Kim WJ, Kim KC, Yoo JC. Arthroscopic partial repair of irreparable rotator cuff tears: preoperative factors associated with outcome deterioration over 2 years. Am J Sports Med. 2015;43(8):1965–1975. [DOI] [PubMed] [Google Scholar]
- 60. Veado MA, Rodrigues AU. Functional evaluation of patients who have undergone arthroscopic debridement to treat massive and irreparable tears of the rotator cuff. Rev Bras Ortop. 2015;45(5):426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop (Belle Mead NJ). 2013;42(2):63–70. [PubMed] [Google Scholar]
- 62. Virk MS, Nicholson GP, Romeo AA. Irreparable rotator cuff tears without arthritis treated with reverse total shoulder arthroplasty. Open Orthop J. 2016;10:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–1486. [DOI] [PubMed] [Google Scholar]
- 64. Zingg PO, Jost B, Sukthankar A, Buhler M, Pfirrmann CW, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am. 2007;89(9):1928–1934. [DOI] [PubMed] [Google Scholar]