Key Teaching Points.
-
•
We should always be ready to cope with coronary artery spasm (CAS), although CAS during pulmonary vein isolation is a rare complication.
-
•
Major possible mechanisms behind the incidence of CAS could be direct thermal damage from radiofrequency (RF) energy or an imbalance in autonomic nervous system activity.
-
•
An imbalance in autonomic nervous system activity was the more possible mechanism, because catheter manipulation after the application of RF energy in the left atrium induced severe CAS repeatedly.
Introduction
Radiofrequency (RF) ablation is commonly accepted as treatment of atrial fibrillation. Although this treatment carries a low risk of coronary artery spasm (CAS), precautions to treat this rare but serious side effect should be taken to allow for rapid response. In previous reports, CAS occurred in cases of cavotricuspid isthmus linear ablation and left atrium (LA) catheter ablation.1, 2 The mechanism for provoking CAS may be direct thermal trauma from RF energy applications near the coronary artery, or impairment of the autonomic nerve system.
We here report a case of severe CAS that was repeatedly induced by an LA ablation procedure in a patient with paroxysmal atrial fibrillation (PAF), likely owing to an imbalance in autonomic nerve activity.
Case report
The patient was a 65-year-old man with PAF and atrial flutter. He had no known allergies, no history of smoking, and no family history of sudden death or cardiac disease. Six months before the session, he underwent percutaneous coronary stenting of the distal portion of segment 3 in the right coronary artery after an acute inferior myocardial infarction.
He was admitted to our hospital for RF ablation of symptomatic PAF and atrial flutter. The CHA2DS2-VASc score was 3 owing to hypertension, old myocardial infarction, and his age, and transthoracic echocardiography revealed inferior left ventricular asynergy (the left ventricular ejection fraction was 50%) but no enlarged LA (diameter was 28 mm). Coronary computed tomography angiography revealed no significant stenotic lesions, including the in-stent segment of the right coronary artery. We administered general anesthesia with dexmedetomidine hydrochloride and fentanyl for the procedure. After transseptal access was obtained, the 4 pulmonary veins (PVs) were identified by LA angiography and we began pulmonary vein isolation (PVI) from the left PV using an ablation catheter (FlexAbility Ablation Catheter, St. Jude Medical, Plymouth, MN) under the navigation of the EnSite NavX system (St. Jude Medical, Saint Paul, MN). When we completed left PV encircling and confirmed the residual left superior PV potential using a ring catheter (Optima, St. Jude Medical, Irvine, CA), the heart rate plummeted to 30 beats per minute owing to complete atrioventricular block with ST-segment elevation in II, III, and aVF accompanied by reciprocal ST-segment depression in I and aVL (Figure 1A). The patient’s blood pressure fell to 60 mm Hg, so we immediately administered an isotonic sodium chloride load, and initiated ventricular pacing to support the heart rate. We manipulated intracardiac echocardiography to confirm pericardial effusion and ruled out cardiac tamponade. We then performed coronary angiography via the right femoral artery to confirm bilateral coronary artery flow, and coronary obstruction owing to severe spasm was observed from segment 2 to segment 4 in the right coronary artery (Figure 1B). Also, diffuse coronary slow flow was observed in the left coronary artery (Figure 1C). After an intracoronary injection of nicorandil (1–2 mg), the CAS gradually improved with normalization of the ST segment (Figure 2A–C). Because the patient recovered sinus rhythm and normal blood pressure, we inserted a ring catheter to the right PV to prepare for right PVI under peripheral intravenous infusion of nicorandil (4 mg/h). However, ST-segment elevation in II, III, and aVF, and complete atrioventricular block with severe hypotension reoccurred (Figure 3A). Once again we performed coronary artery angiography to confirm bilateral coronary artery flow, and the reoccurrence of severe CAS was confirmed (Figure 3B and C). Under right ventricular pacing, we injected nicorandil (2 mg) into the coronary artery repeatedly in order to terminate the CAS. The injection was successful, but since severe CAS reoccurred even under systemic nicorandil infusion, we determined it was difficult to continue catheter ablation and we aborted the procedure.
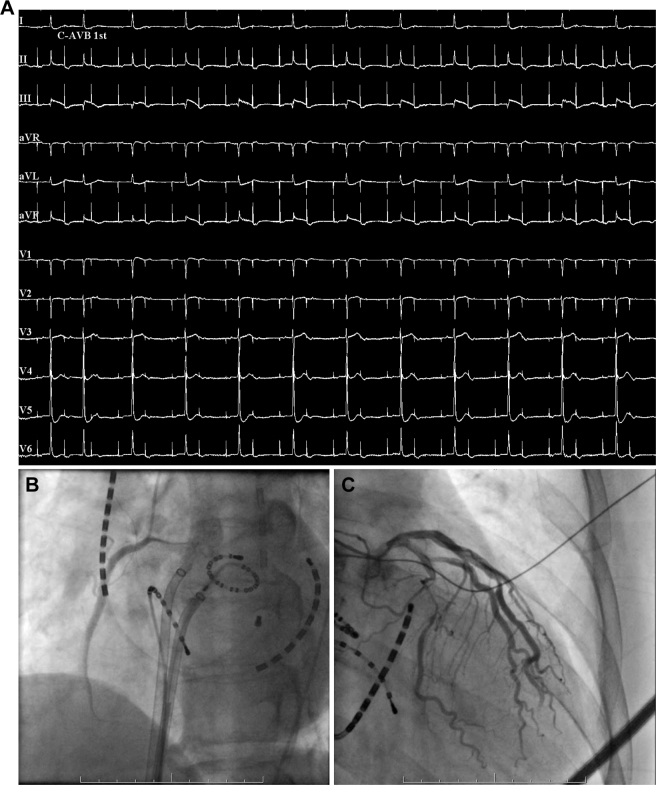
Figure 1.
A: Twelve-lead electrocardiography with ST-segment elevation in II, III aVF; reciprocal ST-segment depression in I, aVL; and complete atrioventricular block. B: Coronary angiogram showing an obstruction in midportion of right coronary artery. C: Coronary angiogram showing a shrinkage of and diffuse slow flow in left coronary artery.
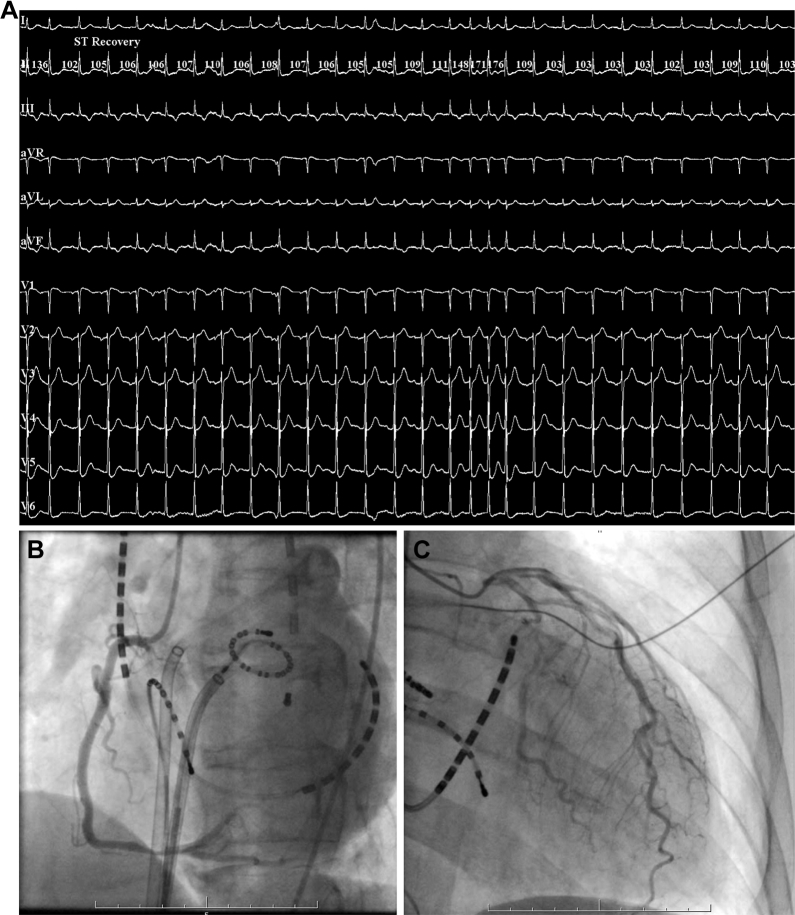
Figure 2.
A: Twelve-lead electrocardiography with improved ST-segment elevation in II, III, and aVF; reciprocal ST-segment depression in I, aVL; and complete atrioventricular block. B: Coronary angiogram showing an improvement in right coronary artery obstruction. C: Coronary angiogram showing an improvement in diffuse slow flow of left coronary artery and a remained shrinkage.
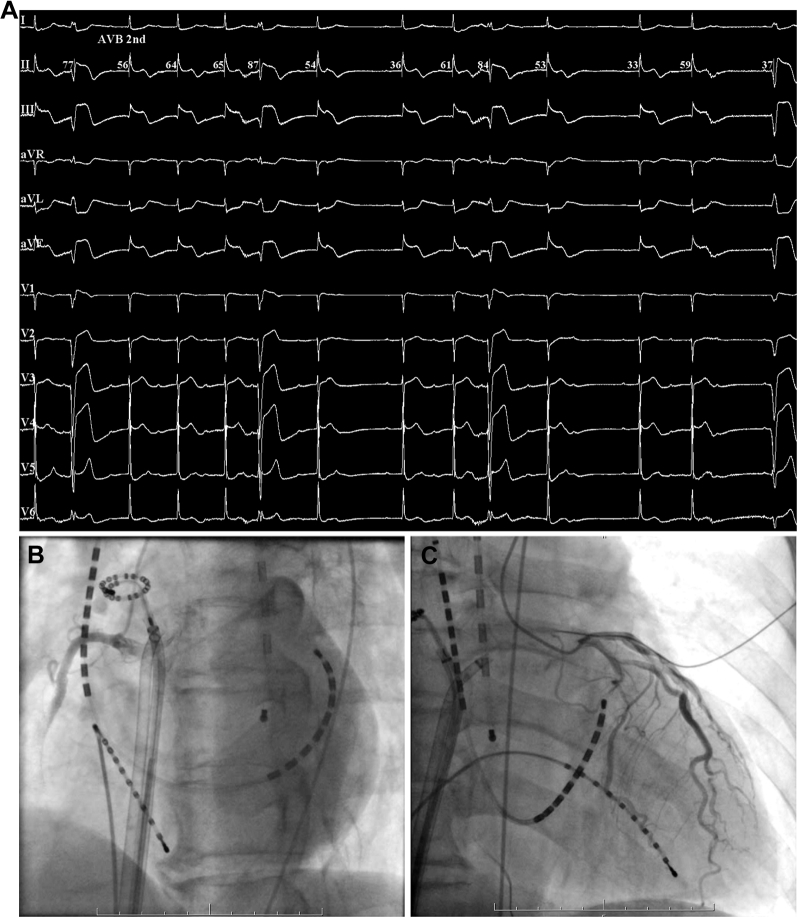
Figure 3.
A: Twelve-lead electrocardiography with recurrent ST-segment elevation in II, III, and aVF; reciprocal ST-segment depression in I, aVL; and complete atrioventricular block. B: Coronary angiogram showing the recurrent obstruction of right coronary artery. C: Coronary angiogram showing the recurrent shrinkage of and diffuse slow flow in left coronary artery.
CAS did not reoccur during the night under systemic infusion of nicorandil or the day after the procedure. Troponin I increased to 2.45 ng/dL but creatine kinase remained unchanged. We also added a calcium channel blocker to the patient’s regimen, but CAS never reoccurred during the 6 months of follow-up. We performed 6-month follow-up coronary angiography and the pharmacologic spasm provocation test after the cessation of vasodilators, but the patient did not experience a reoccurrence of CAS in any of the coronary arteries.
Discussion
RF ablation is an established treatment for atrial fibrillation in terms of efficacy and safety. In the present case, CAS—considered a rare complication during PVI—occurred repeatedly even under systemic infusion of nicorandil. Major possible mechanisms behind the incidence of CAS could be direct thermal damage from RF energy or an imbalance in autonomic nervous system activity.
Several papers have reported that ablation near the coronary artery causes CAS.3, 4 However, RF energy could not have injured the coronary artery through direct thermal trauma in our patient because there was sufficient space between the left PV and the right coronary artery. Some papers also have reported that an air embolism or thrombosis can cause obstruction of the coronary artery.5 However, we neither flushed the sheaths nor exchanged catheters before the occurrence of ST-segment elevation, and repeated coronary angiography showed no evidence of either phenomenon; furthermore, intracoronary infusion of nicorandil improved the coronary obstruction, making these unlikely mechanisms for the patient’s CAS.
Human histologic studies show that the atrium contains epicardial ganglionated plexuses (GP) that consist of different patterns of sympathetic and vagus nerves.6 Endocardial RF ablation could thus affect the epicardial GP through a thermal injury, which may have caused an imbalance in autonomic nervous system activity through stimulation of the bilateral left atrial GP.7, 8 Earlier reports also suggest that LA manipulation causes a similar imbalance, which results in CAS.9 Although contact to the LA and bilateral PVs did not cause CAS before left PVI, similar contact caused CAS repeatedly after the procedure. We reasoned that the imbalance in autonomic nervous system activity caused by left PVI and ring catheter manipulation in the LA is the most likely mechanism for causing CAS in this case.
The present patient suffered from an old myocardial infarction and underwent coronary stenting 6 months prior to the ablation procedure. It is well known that coronary atherosclerosis and implanted drug-eluting stents can lead to CAS owing to endothelial dysfunction.10 In addition, several previous reports suggest that autonomic nervous system activity can play an important role in provoking CAS.11, 12 A coronary spasm was not induced by pharmacologic provocation 6 months after the ablation procedure. This result indicated that this patient did not originally have variant angina. Thus, we consider that the imbalance in autonomic nervous system activity caused by the contact of the ring catheter in LA after left GP ablation could be mainly responsible for this phenomenon.
Also of note is that during the patient’s ablation procedure we administered dexmedetomidine, an alpha-2 adrenoceptor agonist that possesses sedative, analgesic, and anxiolytic properties. Dexmedetomidine stimulates the central alpha-2 adrenoceptor and suppresses sympathetic nervous system activity, making it a possible contributor to CAS in this case as a result of parasympathetic nerve hyperactivity relative to that of the sympathetic nervous system.13
Limitation
We could not directly confirm activity of the cardiac sympathetic/parasympathetic nervous systems during the ablation procedure. Therefore, we cannot completely ensure a cause-and-effect relationship in this case.
Conclusion
We reported a clinical case of severe CAS that occurred repeatedly after left PVI. The intracoronary infusion of nicorandil alleviated CAS, which we believe primarily resulted from an imbalance in autonomic nervous system activity caused by the application of RF energy and catheterization in the LA.
References
- 1.Tada H., Naito S., Oshima S., Taniguchi K. Vasospastic angina shortly after left atrial catheter ablation for atrial fibrillation. Heart Rhythm. 2005;2:867–870. doi: 10.1016/j.hrthm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita E., Tada H., Tadokoro K., Hashimoto T., Kaseno K., Miyaji K., Naito S., Oshima S., Taniguchi K. Left atrial catheter ablation promotes vasoconstriction of the right coronary artery. Pacing Clin Electrophysiol. 2007;30:S98–S102. doi: 10.1111/j.1540-8159.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi Y., Jaïs P., Hocini M., Sanders P., Rotter M., Rostock T., Sacher F., Jaïs C., Clémenty J., Haïssaguerre M. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol. 2005;6:1104–1107. doi: 10.1111/j.1540-8167.2005.50124.x. [DOI] [PubMed] [Google Scholar]
- 4.Wong K.C., Lim C., Sadarmin P.P., Jones M., Qureshi N., De Bono J., Rajappan K., Bashir Y., Betts T.R. High incidence of acute sub-clinical circumflex artery ‘injury’ following mitral isthmus ablation. Eur Heart J. 2011;32:1881–1890. doi: 10.1093/eurheartj/ehr117. [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara T., Takahashi A., Takahashi Y. Clinical characteristics of massive air embolism complicating left atrial ablation of atrial fibrillation: lessons from five cases. Europace. 2012;14:204–208. doi: 10.1093/europace/eur314. [DOI] [PubMed] [Google Scholar]
- 6.Kuwano H., Okada R., Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 7.Armour J.A., Murphy D.A., Yuan B.X., Macdonald S., Hopkins D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa H., Scherlaq B.J., Patterson E., Ikeda A., Lockwood D., Jackman W.M. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6 doi: 10.1016/j.hrthm.2009.07.029. S26-34. [DOI] [PubMed] [Google Scholar]
- 9.Le B.H., Black J.N., Huang S.K. Transient ST-segment elevation during transseptal catheterization for atrial fibrillation ablation. Tex Heart Inst J. 2010;37:717–721. [PMC free article] [PubMed] [Google Scholar]
- 10.Joner M., Nakazawa G., Finn A.V. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Kusama Y., Kodani E., Nakagomi A., Otsuka T., Atarashi H., Kishida H., Mizuho K. Variant angina and coronary artery spasm: the clinical spectrum, pathophysiology, and management. J Nippon Med Sch. 2011;78:4–12. doi: 10.1272/jnms.78.4. [DOI] [PubMed] [Google Scholar]
- 12.Lanza G.A., Careri G., Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G., Baumqart D., Camici P., Chilian W., Gregorini L., Hess O., Indolfi C., Rimoldi O. Alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–694. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]