Introduction
Patients with congenital heart disease (CHD) and significant postoperative atrioventricular (AV) nodal injury frequently require lifelong backup pacing. However, lifelong pacing in this population is complicated primarily by lead-related issues. Pacing leads in pediatric patients with CHD fail earlier and at higher rates than leads in adults with structurally normal hearts for both epicardial and endocardial leads.1 Somatic growth and the vigorous activity of pediatric patients lead to higher rates of fracture and dislodgment. Surgical repair requiring exogenous materials, including bioprosthetic valves and patches, places transvenous pacing leads in patients with CHD at higher risk for infection; this necessitates extraction procedures for lead removal, which carry up to 2% risk of serious complications, including death.2, 3 Transvenous leads carry significant risk of venous thrombosis and occlusion over the life of pediatric patients and thus have a profound effect in CHD patients who may require frequent venous access for interventional cardiac catheterization procedures, including balloon angioplasty of pulmonary arteries (PAs) and transcatheter pulmonary valve implantation. For these reasons, leadless pacing can be a valuable intervention to minimize the lifelong complications of epicardial or transvenous pacing in pediatric patients with CHD.
Case report
A 12-year-old, 37-kg girl with a history of repaired tetralogy of Fallot with pulmonary atresia and postoperative high-grade heart block status post epicardial pacemaker placement at 5 months was referred to the pediatric electrophysiology department for epicardial pacemaker lead fracture. The patient was asymptomatic, with first-degree heart block and right bundle branch block at baseline and documented second-degree type II heart block at night. Based on the unpredictability of recurrence of postoperative heart block in patients with CHD4, 5 and the continued occasional high-grade heart block, implantation of a new VVI pacing system was recommended.
Because the patient had previously undergone 4 sternotomies for surgical repairs, including central shunt placement and unifocalization of the PAs at age 3 weeks, complete repair with right ventricle to pulmonary artery (RV-PA) conduit and closure of ventricular septal defect at age 5 months, which was complicated by complete heart block requiring placement of an epicardial dual-chamber pacemaker 5 days after the repair, and pulmonary valve replacement at age 18 months, placement of a new epicardial pacing system was not recommended by the cardiac surgery team. The patient was referred for a transvenous pacing system. After discussion with the referring cardiologist and the patient’s parents, and after careful consideration of the risks of lifelong transvenous pacing compared to the risks of a large-bore (23F) delivery sheath in the femoral vein, possible cardiac perforation and/or pericardial effusion, and the many potential unknown or unreported risks of leadless pacing (eg, multiple abandoned devices within the ventricle, possible need for surgical retrieval or extraction), transcatheter leadless pacing (Micra Transcatheter Pacing System, Medtronic, Minneapolis, MN) was offered to the patient.
While under general anesthesia, the patient underwent a diagnostic right and left heart catheterization to confirm normal hemodynamics, minimal stenosis of the RV-PA conduit, and normal PA pressures. She then underwent electrophysiological study to confirm the absence of sustained ventricular arrhythmias and supraventricular tachycardia.
After a right femoral venogram was performed to rule out significant stenosis of the common femoral vein, iliac vein, and inferior vena cava, serial dilations of the right femoral vein were performed with 12F, 16F, 18F, 20F, and 22F hydrophilic dilators. The proprietary 23F sheath was then placed over a wire and advanced to the middle of the right atrium. The delivery catheter was advanced through the sheath, and the sheath was withdrawn into the inferior vena cava below the level of the diaphragm. The delivery catheter was deflected across the tricuspid valve into the RV. Contrast injection through the catheter under biplane fluoroscopy confirmed the septal and apical location of the catheter.
The pacing system was exposed from the catheter and implanted onto the RV septum. The catheter was withdrawn, and control was maintained on the pacemaker using the suture adhered to the distal ring. Initial implant parameters were suboptimal, with pacing threshold >2.0 V at 0.24 ms, sensed R waves <5 mV, and pacing impedance <500 Ω. The pacing system was recaptured with the delivery catheter, and the delivery system was repositioned. Again, suboptimal implant parameters were achieved, and the device was recaptured and repositioned. Ultimately, the device required repositioning 7 times before acceptable implant parameters were achieved: pacing threshold of 1.25 V at 0.24 ms, sensed R wave of 4.4 mV, and pacing impedance of 480 Ω. The delivery sheath was removed, and intracardiac echocardiography was used to visualize the pacing system in the RV (Figure 1). The delivery sheath was removed, and hemostasis was maintained with 2 vascular closure devices (PerClose Proglide, Abbott Vascular, Abbott Park, IL) placed with the assistance of interventional cardiology. The existing pacemaker generator in the right upper abdomen was removed, and the epicardial leads were capped with the assistance of cardiothoracic surgery.
Figure 1.
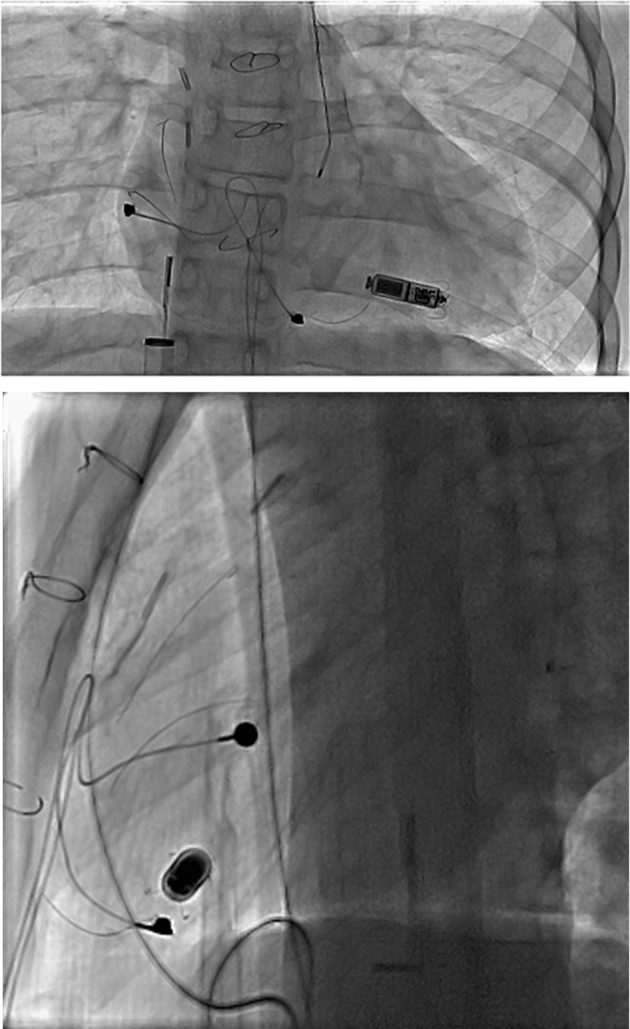
Leadless pacemaker implant final position. Biplane fluoroscopy of leadless pacemaker in the right ventricle with fractured atrial and ventricular epicardial leads, surgical clips, and sternal wires. The intracardiac echocardiogram catheter is seen in the right atrium advanced through the proprietary 23F delivery sheath from the right femoral venous approach.
The patient was admitted overnight. A pacemaker interrogation performed the following day revealed a mild increase in pacing threshold to 2.6 V at 0.24 ms and 2.0 V at 0.4 ms, with mild decrease in R-wave sensing to 4.2 mV but stable pacing impedance of 480 Ω. Chest radiograph and transthoracic echocardiogram confirmed stable device position. After discussion with the device manufacturer, the decision was made to not reposition the device but to follow the threshold closely on an outpatient basis, and the patient was discharged home.
The pacing threshold continued to rise during outpatient follow-up, reaching a maximum pacing threshold of 3.25 V at 0.24 ms 2 weeks after implant despite stable appearance on chest radiograph, and stable R-wave sensing and impedance. Over the next 10 weeks, the pacing threshold steadily decreased. Three months after implant, the pacing threshold was 2.0 V at 0.24 ms, with R-wave sensing of 6.0 mV and pacing impedance of 490 Ω. At 9-month follow-up, pacing parameters remain stable, and the patient is paced approximately 1% of the time.
Discussion
Leadless transcatheter pacing systems have been studied extensively in adults with structurally normal hearts in both Europe and the United States.6, 7 These systems have demonstrated low complication rates at both implantation and throughout short- to mid-term follow-up.8, 9 However, implantation of a leadless transcatheter pacing system in a pediatric patient or a patient with significant CHD had not previously been described before this case of implantation of the Micra Transcatheter Pacing System in a 12-year-old girl with tetralogy of Fallot.
The decision to implant this device rather than a transvenous pacing system or an epicardial pacing system was based on long-term complications. Because the life expectancy of patients with tetralogy of Fallot and other significant CHDs continues to increase, approaching that of people without CHD, the expectation of lifelong pacing in such individuals means that our 12-year-old patient potentially could require rare, intermittent pacing for 60–70 years. Thus, considering the lifelong complications of pacing becomes crucial to extending the patient’s lifespan.
Pacing in pediatric patients and patients with CHD is associated with higher complication rates than pacing in adults with structurally normal hearts.10, 11 Lead-related complications remain the most significant, with lead failure rates as high as 15% over mid- to long-term follow-up.1 Venous thrombosis and endovascular infection are other long-term risks of transvenous pacing systems. Even in ideal situations without immediate complications, lead performance deteriorates over time. Therefore, a young patient with transvenous leads is likely to experience deterioration in lead performance and possibly lead failure at some point over the life course, and the physician caring for the patient must decide between lead extraction and abandonment.12 The rate of major complications from lead extraction procedures, including major vascular injury, remains high in pediatric and CHD patients.13 By eliminating the primary instrument of complications, leadless pacemakers are well suited to meet the pacing demands of many pediatric patients with CHD who require intermittent pacing because of surgically created conduction system disease.
As part of the preprocedural discussion with the family and informed consent to perform this procedure for the first time at our institution, we acknowledged that data on the long-term performance of the device and its potential long-term complications are lacking compared to traditional approaches, and that complications may present that have not previously been described. We also discussed future pacing strategies for the patient, which may include transvenous pacing to provide DDD pacing or cardiac resynchronization therapy if the patient were to develop indications for either treatment. Regarding the abandonment of this leadless pacemaker and/or multiple leadless systems in the RV, we discussed with our patient that she is likely to require at least 1 other cardiac surgery for RV-PA conduit replacement in adolescence or young adulthood before transvenous pulmonary valve replacement, at which time the abandoned device(s) could be retrieved by a cardiothoracic surgeon.
Regarding the implant technique, an RV angiogram performed before implantation as part of planning the cardiac catheterization was helpful in determining the true ventricular dimensions (Figure 2). In addition, intracardiac echocardiography, which was used as part of the electrophysiological study, was helpful in evaluating the position of the device in relation to the takeoff of the RV-PA conduit (Figure 3).
Figure 2.
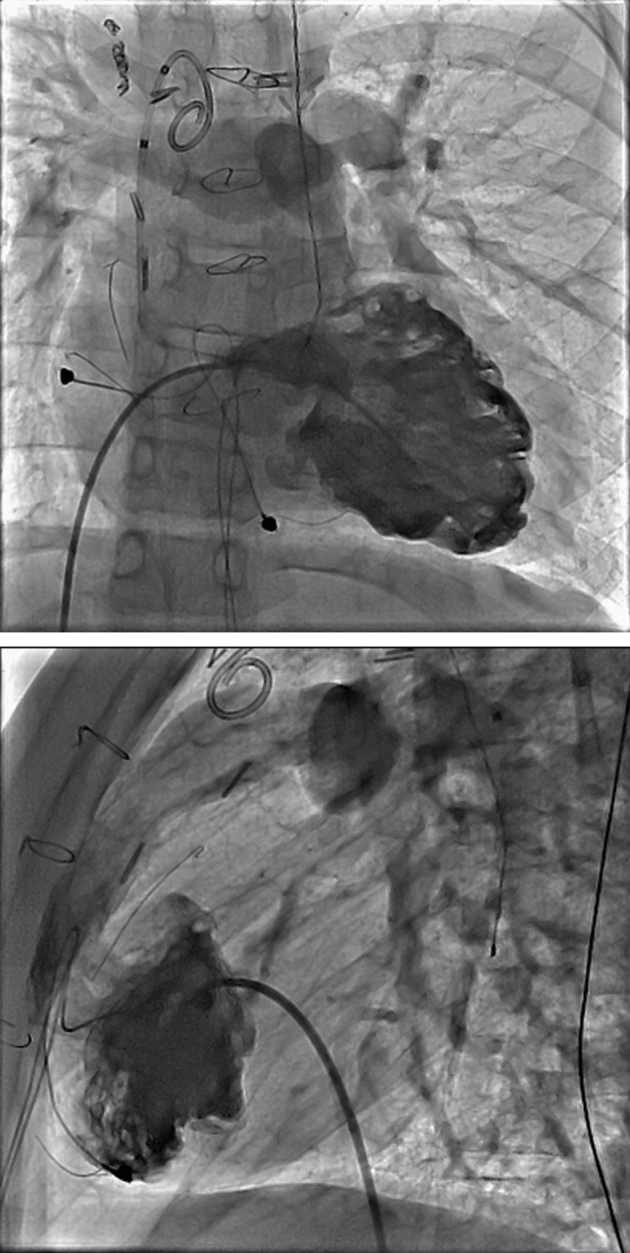
Biplane right ventriculogram showing heavily trabeculated right ventricle with right ventricle to pulmonary artery conduit extending from the mid-anterior right ventricle.
Figure 3.
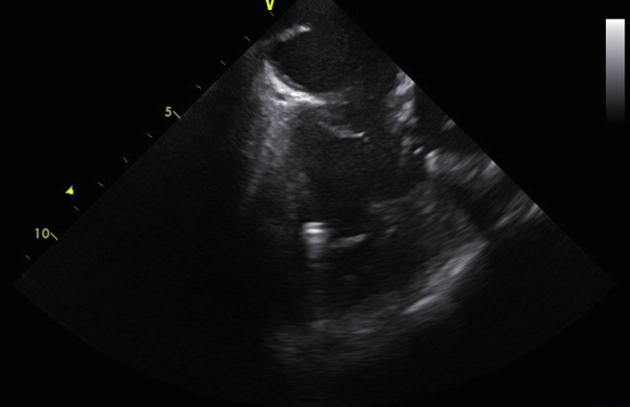
Postimplant intracardiac echocardiogram showing the leadless pacemaker in an apical location inferior to the takeoff of the right ventricle to pulmonary artery conduit.
Many of the sites sampled within the RV had suboptimal lead parameters despite multiple deployments. Similarly, the parameters worsened over the first several weeks before they stabilized. However, knowing that improvement in suboptimal pacing thresholds in patients with the Micra system had been studied and described, our implant was not repositioned after the initial procedure. Fortunately, our experience is similar to that of Piccini and colleagues,14 showing improvement of the threshold by 1 month and 6 months. Although the exact cause of the suboptimal parameters is not known, the patient had undergone cardiopulmonary bypass 3 times during her staged repair of tetralogy of Fallot, all of which had the potential for endocardial ischemia. RV hypertrophy with thick trabeculations defines tetralogy of Fallot, and contact of the distal electrode may have been altered by its positioning within trabeculations. Fortunately, the parameters returned to an acceptable range, and the device did not need to be retrieved or repositioned.
Conclusion
This case demonstrates the feasibility of implanting a leadless pacemaker in a 12-year-old, 37-kg patient with tetralogy of Fallot. To our knowledge, this is among the first transcatheter pacemaker implants reported in a pediatric patient with significant CHD. Despite challenges in finding an optimal implant site possibly because of factors specific to repaired tetralogy of Fallot, such as a history of endocardial ischemia from bypass and thick trabeculations of the RV, the implant was successful, without procedural complications and with adequate pacing parameters. By eliminating the lead-related complications of endovascular pacing, leadless pacing offers an excellent option for young patients with CHD in need of lifelong pacing.
Key Teaching Points.
-
•
Postoperative atrioventricular (AV) nodal injury frequently necessitates lifelong backup pacing in patients with repaired congenital heart disease because of the unpredictability of late recurrence of AV nodal block.
-
•
Permanent pacing in pediatric and congenital heart disease patients requires careful consideration of the cumulative risks of pacemaker implant techniques over the entire course of the patient’s life.
-
•
Leadless pacing via a transcatheter pacing system is feasible in pediatric patients with repaired congenital heart disease and minimizes the lifelong burden of pacing by eliminating lead-related complications.
References
- 1.Fortescue E.B., Berul C.I., Cecchin F., Walsh E.P., Triedman J.K., Alexander M.E. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004;1:150–159. doi: 10.1016/j.hrthm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Maytin M., Jones S.O., Epstein L.M. Long-term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol. 2012;5:252–257. doi: 10.1161/CIRCEP.111.965277. [DOI] [PubMed] [Google Scholar]
- 3.Brunner M.P., Cronin E.M., Wazni O., Baranowski B., Saliba W.I., Sabik J.F., Lindsay B.D., Wilkoff B.L., Tarakji K.G. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm. 2014;11:419–425. doi: 10.1016/j.hrthm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Aziz P.F., Serwer G.A., Bradley D.J., LaPage M.J., Hirsch J.C., Bove E.L., Ohye R.G., Dick M., 2nd Pattern of recovery for transient complete heart block after open heart surgery for congenital heart disease: duration alone predicts risk of late complete heart block. Pediatr Cardiol. 2013;34:999–1005. doi: 10.1007/s00246-012-0595-y. [DOI] [PubMed] [Google Scholar]
- 5.Gross G.J., Chiu C.C., Hamilton R.M., Kirsh J.A., Stephenson E.A. Natural history of postoperative heart block in congenital heart disease: implications for pacing intervention. Heart Rhythm. 2006;3:601–604. doi: 10.1016/j.hrthm.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds D., Duray G.Z., Omar R. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 7.Ritter P., Duray G.Z., Zhang S., Narasimhan C., Soejima K., Omar R., Laager V., Stromberg K., Williams E., Reynolds D. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace. 2015;17:807–813. doi: 10.1093/europace/euv026. [DOI] [PubMed] [Google Scholar]
- 8.Roberts P.R., Clementy N., Al Samadi F. A leadless pacemaker in the real-world setting: the Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm. 2017;14:1375–1379. doi: 10.1016/j.hrthm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Soejima K., Asano T., Ishikawa T. Performance of leadless pacemaker in Japanese patients vs. rest of the world. Circ J. 2017;81:1589–1595. doi: 10.1253/circj.CJ-17-0259. [DOI] [PubMed] [Google Scholar]
- 10.Chaouki A.S., Spar D.S., Khoury P.R., Anderson J.B., Knilans T.K., Morales D.L., Czosek R.J. Risk factors for complications in the implantation of epicardial pacemakers in neonates and infants. Heart Rhythm. 2017;14:206–210. doi: 10.1016/j.hrthm.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Sachweh J.S., Vazquez-Jimenez J.F., Schondube F.A., Daebrtiz S.H., Dorge H., Muhler E.G., Messmer B.J. Twenty years experience with pediatric pacing: epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17:455–461. doi: 10.1016/s1010-7940(00)00364-x. [DOI] [PubMed] [Google Scholar]
- 12.McCanta A.C., Schaffer M.S., Collins K.K. Pediatric and Adult Congenital Endocardial Lead Extraction or Abandonment Decision (PACELEAD) survey of lead management. Pacing Clin Electrophysiol. 2011;34:1621–1627. doi: 10.1111/j.1540-8159.2011.03226.x. [DOI] [PubMed] [Google Scholar]
- 13.Cecchin F., Atallah J., Walsh E.P., Triedman J.K., Alexander M.E., Berul C.I. Lead extraction in pediatric and congenital heart disease patients. Circ Arrhythm Electrophysiol. 2010;3:437–444. doi: 10.1161/CIRCEP.110.957324. [DOI] [PubMed] [Google Scholar]
- 14.Piccini J.P., Stromberg K., Jackson K.P. Long-term outcome in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: results from the Micra Transcatheter Pacing System Global Clinical Trial. Heart Rhythm. 2017;14:685–691. doi: 10.1016/j.hrthm.2017.01.026. [DOI] [PubMed] [Google Scholar]


