Abstract
The present study deals with the coproduction of laccase and pectinase enzymes through solid state fermentation using mixed fungal culture of Trametes hirsuta and Phanerochaete sp., to minimize the cost and time of the process. Substrates selected for the enzyme production were wheat bran, pulse husk and mustard peel. To get optimum yield of laccase and pectinase in a single fermenter, EVOP factorial design technique with factors like pH, incubation temperature and substrates ratio have been explored. At search level of EVOP, outcomes of the one factor at a time has been considered as incubation temperature 30 °C for 7 days at pH 5 with wheat bran: pulse husk: mustard peel ratio of 2:2:1 (w/w/w) and yield of laccase and pectinase was found as 78.1 U/gds and 105.0 U/gds respectively. In first setup of EVOP factorial both laccase and pectinase activities were not found optimum in same set of experiments; therefore, on the basis of its decision making steps, second set of experiment was performed by taking decisions of first set of EVOP as search level. Optimum yield of laccase and pectinase was achieved about 250 U/gds and 247 U/gds respectively at 34 °C, pH 4.5 with 1.75:1.75:1.5 (w/w/w) wheatbran: pulse husk: mustard peel as substrate, which was 2–3 times higher than the outcomes of one factor at a time method.
Keywords: Pectinase, Laccase, Production, Optimization, EVOP, Co-culture
Introduction
Laccases and pectinases, have leading position among the commercially produced industrial enzymes in global market. Laccase (EC:1.10.3.2) belongs to oxidoreductase family, also known as blue copper family of oxidases, which is produced by various microorganisms either extracellularly or intracellularly. Laccase catalyses the oxidation of its substrate molecules, i.e., phenols and amines, by monoelectronic transfer process and converting the substrate into its corresponding free radical moieties (Agrawal et al. 2018). Laccase has extended application in synthetic chemistry, it can be used in various technological processes such as bioremediation, detoxify industrial pollutants, water detoxification and in nanobiotechnology applications (Couto and Herrera 2006).
Pectinase enzymes (EC no. 4.2.2.10) catalyses the breakdown of α-1,4-glycosidic linkages in pectin by trans-elimination reaction and resultants are unsaturated methyl galactouronates and galactouronates (Rebello et al. 2017). The blend of laccase and pectinase has important applications in wine, beer and fruit juice industries. On using the blend of both, laccase modifies the colour and eliminates the phenolic substances for browning, turbidity development and haze formation, whereas, pectinases facilitates the extraction and filtration process, to intensify the flavour, colour and maximise the juice yield (Chaudhari and Suneetha 2012; Bhat 2000; Jayani et al. 2005). Other industries such as textile, polymer and biofuels etc have also broaden the scope of the blend of these two enzymes such as in textile for bio-bleaching, dyeing, finishing, bio-scouring, wash-off treatment and printing, in biofuels for saccharification of agricultural waste (Pandey et al. 2016; Pusic et al. 2015; Maurya et al. 2015) and in polymer industries for the synthesis of biopolymers, generation of monomers and cross-linking of the monomers (Selinheimo et al. 2006; Minussi et al. 2002).
Studies have also been performed which describe the production of the single enzyme or mixture of enzymes using fungal co-cultivations processes, e.g., co-cultivation of Trichoderma reesei and Aspergillus niger for the production of cellulase enzyme (Maheshwari et al. 1994), Gupte and Madamwar (1997) has reported production of β-glucosidase and cellulase from coculture of A. fumigatus and A. Ellipticus. Similarly, for the higher yield of lignolytic enzymes, co-cultivation of Phanerochaete chrysosporium and Pleurotus ostreatus (Verma and Madamwar 2002) and Trametes and a Trichoderma strain (Zhang et al. 2006) were explored. While the production of laccase and pectinase enzymes from the same reactor using co-culture technique, it remained a bottle neck of the process to get common optimal physicochemical conditions for maximum yield of both the enzymes and to achieve it, scientists have approached various statistical and computational methods.
Statistical cum computational methods such as RSM, Factorial design technique, ANN have been used by Negi and Banerjee (2010) for optimization of amylase and protease production, Maurya et al. (2013) for enzymatic saccharification in biofuels. Blend of pectinase and laccase have been produced by Giese et al. (2008) with enzyme activities 46 U/ml of laccase and 32 U/ml of pectinase from the strain Botryosphaeria rhodina MAMB-05, however, the yield/activities of these two enzymes were not up to the demand. Therefore, in current study Trametes hirsuta and Phanerochaete sp. fungal strains have been explored for the production of the blend of laccase and pectinase and optimum physicochemical conditions were achieved using EVOP factorial design technique. The findings of this study would be promising to fulfil the current increase demand of the blend of these enzymes.
Materials and methods
Screening of pectinase and laccase producing strains
Samples were collected aseptically from the soil containing Pine decompost of Uttarakhand region and microbes capable of producing laccase and pectinase enzymes extracellularly were screened. Serial dilution was performed. After serial dilution, pour plate technique was used to screen out the microbes. For laccase producing strains selective media having composition (in g/l): Peptone (3.0), Glucose (10.0), KH2PO4 (0.6), ZnSO4 (0.001), K2HPO4 (0.4), FeSO4 (0.0005), MnSO4 (0.05), MgSO4 (0.5), Agar (20), pH-6, supplemented with 0.02% guaiacol was used and for pectinase producing microbes media containing (g/l) KCl (0.5), MgSO4.7H2O (0.5), FeSO4.7H2O (0.01), NaNO3 (2.0), KH2PO4 (4.5), Peptone (0.5), Beef extract (0.3), Agar (15), Pectin (4.0) was used. Laccase and pectinase producing microorganisms were confirmed by performing their respective quantitative assays. The microbial isolates found to be more efficient for the desired enzymes were characterized by 18S r-RNA sequencing using forward and reverse primers ITS1 and ITS4, respectively, from Bioserve Biotechnologies Pvt. Ltd., Hyderabad, India.
SSF for enzymes production
For the production of Laccase and pectinase enzymes through solid state fermentation (SSF), co-culture of Trametes hirsuta and Phanerochaete sp. strains were inoculated on substrate containing different ratio of wheat bran: pulse husk and mustard peel (WB:PH:MP). Substrate was taken into a 250 ml flasks and the minimal salt media (modified Chapex-Dox) having composition (in g/l): KCl (0.5), MgSO4·7H2O (0.5), FeSO4·7H2O (0.01), NaNO3 (2.0), KH2PO4 (4.5) was added to moistened the substrate (1:2 w/v ratio) and autoclaved. The inoculation was done with the spore suspension containing 1.8 × 108 spores/ml of the fungal culture. Spore count was measured using haemocytometer. After inoculation the flasks were kept for 7 days at 28 ± 2 °C under static condition. The flask containing substrate without inoculation was used as control.
Enzyme extraction
After one week of incubation, enzyme extraction was done by adding 50 ml of acetate buffer (pH 4.5, 50 mM) into the fermented flask and the material inside the flask were kept for soaking on orbital shaker at 30 ± 2 °C for 2 h at 100 rpm. The broth was filtered and solid material was separated using muslin cloth. After filtration, the filtrate was centrifuged at 10,000 rpm for 10 min at 4 °C to remove remaining solid residues and get a clear supernatant (Negi and Banerjee 2009). Supernatant obtained after centrifugation was used as crude enzyme. All the tests were performed in duplicate and the given data are the mean values of the results obtained.
Enzyme assay
Pectinase assay
Pectinase activity was determined using the reaction between thiobarbituric acid and end product of pectin degradation. Supernatant was added to a mixture of thiobarbituric acid, HCl and dist. water, heated in boiling water for 30 min. and absorbance was measured at 550 nm (Pitt 1988). One unit of activity is the amount of enzyme required to change in the absorbance of 0.01 under the assay conditions.
Laccase assay
Laccase activity was determined by observing the change in absorbance at 420 nm, change related to the rate of oxidation of 1 mM 2,2-azino-bis-[3-ethylbenzthiazoline-6-sulfonate] (ABTS) in acetate buffer (50 mM, pH 4.5). Assay was carried out at 25 ± 1 °C with 50 µl of diluted liquid media containing culture (Elisashvili et al. 2008). One unit of enzyme activity is the amount of enzyme required to transform 1 µmol of substrate per minute per ml in to product.
Optimization of the physico-chemical parameters by one factor at a time method
Different physico-chemical parameters for, e.g., substrate ratio (WB:PH:MP), size of the inoculum, pH, temperature, and inoculum age for the coproduction of the enzymes were studied. Ratio of the substrates (WB:PH:MP) were varied while keeping the final weight of the total substrate constant, i.e., 5 gm (3:1:1, 2:2:1, 1:3:1, 1:2:2, 1:1:3, 1.4:1.4:1.4, 5:0:0, 0:5:0, 0:0:5) w/w, effect of pH and temperature was studied in the range between 3 and 9 and 25–50 °C, respectively. Inoculum size was varied from 1 to 3 ml and incubation time was studied between 5 and 10 days. Optimization was done using ‘one factor at a time method’ in which only one factor at a time was changed while keeping other factors constant for seeking the optimum level (Negi and Banerjee 2009).
Optimization of variables by EVOP-factorial design technique
Evolutionary optimization factorial design (EVOP) is a method which depends upon two rules. (1) It looks for optimum conditions first for each and every parameter. (2) Depending upon optimum levels of different parameters, it set up new trials with in the upper and lower limit of the optimum conditions. If we want to determine the integrated effect of various parameters including the minor one also then we have to follow statistical approach. In case of three variable systems, the effects can be calculated using the calculation worksheet given in Table 1. Based on the size of the effects, changes in main effect value, and error limit values calculations, decision making process can be done so as to find the optimum conditions (Negi and Banerjee 2006). The experimental set up for three variables system contains, one upper (+ 1) limit and one lower (− 1) limit from its corresponding optimum conditions(0) in ten separate experiments The effects were calculated and compared with the error limit and change in mean effect values to get the final conclusion of the optimization process. As per the decision making step, if the calculated values for effects are smaller than the error limits and change in mean effect value is negative and large then only the optimum conditions for the process will be achieved.
Table 1.
Calculation table for three variables
| Exp. parameter | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (WB + PH + MP) | 0 | _ | _ | + | + | 0 | + | _ | + | _ |
| Temperature | 0 | _ | + | _ | + | 0 | + | _ | _ | + |
| pH | 0 | _ | + | + | _ | 0 | + | + | _ | _ |
| Response | a1 | a2 | a3 | a4 | a5 | a6 | a7 | a8 | a9 | a10 |
| Block1 | Block2 |
Total no. of experiments performed were ten in 2 cycles. (0) is the optimum search level, (+) is the upper limit and (−) represent the lower limit
WB wheat bran, PH pulse husk, MP Mustard peel
Decision making process
Decision making in EVOP-factorial design technique is a crucial step. During this process error limits and change in mean effect values are taken into consideration and compared with the magnitude of the effects. This step further provides us the direction to change the parameters so as to get improved response. If the calculated value of effects is found to be higher than the error limit value then a slight change in the parameters can achieve optimum level. In this study the parameters such as temperature, substrate ratio and pH were taken into account for optimization using Evolutionary operation factorial design (EVOP) technique.
Results and discussion
Screening of pectinase and laccase producing strains
Seven fungal isolates were screened for the laccase and pectinase enzymes. Pectinase producing fungus (F6) was isolated which shows cottony growth and had white colour spores and on molecular characterization has shown 96% sequence similarity with Phanerochaete sp. on NCBI data base analysis. The isolated strains for laccase production shows negligible laccase activity in comparison with the commercial strain Trametes hirsuta (TH). Therefore, for coproduction of pectinases and laccases, mixed culture of Phanerochaete sp. and Trametes hirsuta (TH) strain was used as inocculum. There are very few studies available which show coproduction of laccase and pectinase enzymes, Giese et al. (2008) reports coproduction of these enzymes using orange bagasse under solid state fermentation from Botryospheria rhodina MAMB-05. But the yield of Laccase and Pectinase was found lower (46 U/ml and 32 U/ml respectively) than the reported in present work. In this study, we are using the combination of the two fungal strains T. hirsuta and Phanerochaete sp. which has not been explored yet for the coproduction of these enzymes.
Optimization of the physico-chemical parameters by one factor at a time approach
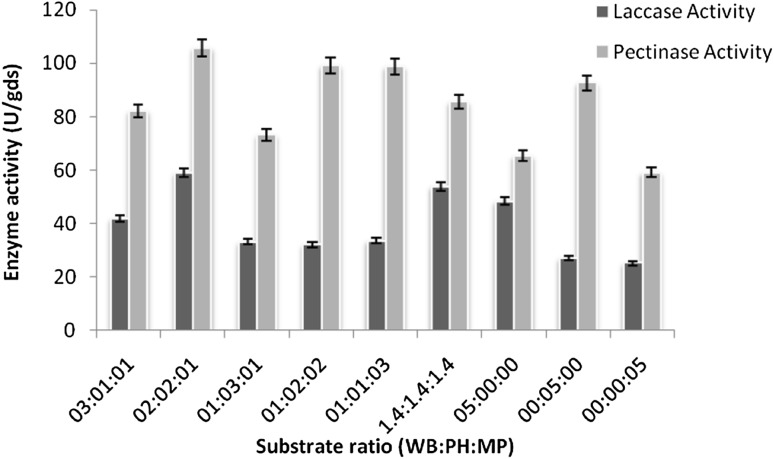
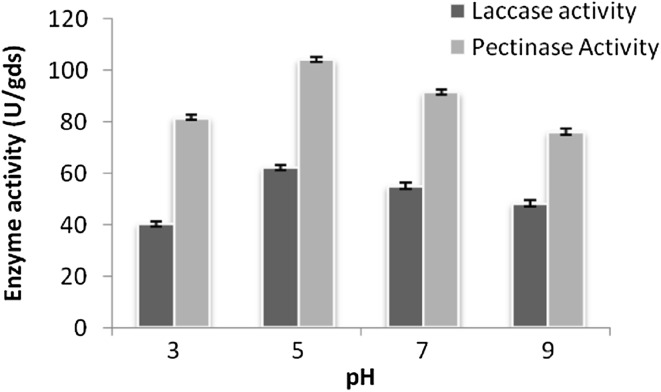
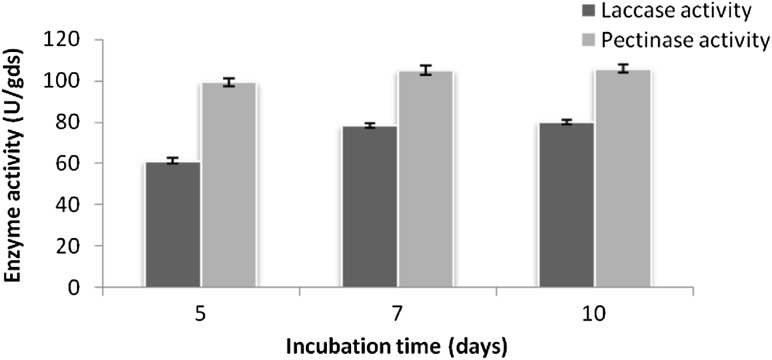
Different physico-chemical parameters such as substrate ratio, inoculum age, inoculum size, pH and temperature for coproduction of the enzymes were studied and optimized first by “one factor at a time” approach (Figs. 1, 2, 3). Maximum laccase and pectinase activity were found to be 78.25 U/gds and 105.31 U/gds, respectively with wheat bran: pulse husk: mustard peel (WB:PH:MP) in 2:2:1 ratio as substrate at pH 5.0 temperature 30 °C and incubation time of 7 days. Similar optimum conditions were reported for the laccase produced by T. hirsuta and T. versicolor cultured individually on the substrates grape seed and barley bran under solid state fermentation (Couto et al. 2006) and for pectinase enzyme from the source Penicillium viridicatum RFC3 on agro-industrial wastes and mixed culture of Aspergillus species on pine apple residues (Silva et al. 2002; Singh and Mandal 2006).
Fig. 1.
Optimization of substrates (Wheat bran: Pulse husk: Mustard peel) ratio by one factor at a time method
Fig. 2.
Optimization of pH of media by one factor at a time method
Fig. 3.
Optimization of incubation time period by one factor at a time method
EVOP Factorial design technique to achieve optimum conditions
To optimize three variable systems EVOP technique needs lesser number of experimental setups, which make it a cost effective and less time taking process (Negi and Banerjee 2006; Kumar et al. 2011; Kumari and Negi 2014; Pandey et al. 2016). It is a real time experiment based optimization process in which we perform different combinations of experiments and then calculate the combinatorial effect of different parameters on yield. The chances of error are less since each combination were performed manually and provides interfactorial impact on the yield of the product. The aim of this study was to increase the production of laccase and pectinase enzyme concomitantly which should be cost effective and having high yield in a single step using EVOP-factorial design. The parameters such as substrate ratio, pH and temperature shows significant effect on enzyme activities in “one at a time method” therefore these variables were further taken into consideration for further optimization using EVOP (Table 2). In this study the substrate ratio (S), Temperature (T) and pH (P) variables were considered (Table 3). In the first cycle of EVOP after performing the experiments on the basis of decision making process it was found that most of the effects are positive and larger than the error limits and change in mean effect is smaller (Table 4), which shows that to achieve optimum condition either we have to increase the range of variables or new variables and new EVOP should be performed. Hence a new EVOP was performed using a new range (Table 5) of the variables and set up new experimental design shown in Table 6. In the second round of EVOP experiments, all the calculated values for the effects of different variables had smaller magnitude than the error limit and the change in mean effect value were negative and higher than the effects (Table 7). Based on these observations and decision making procedure, it was concluded that the optimum conditions for the coproduction of laccase and pectinase enzymes in single bioreactor were found as: pH 4.5, incubation temperature 34 °C and with substrate containing 1.75:1.75:1.5 (w/w/w) wheat bran: Pulse husk: Mustard Peel ratio. With these optimum conditions, laccase and pectinase activities were enhanced up to 250 U/gds and 247 U/gds, respectively; whereas it was found 78.102 U/gds and 105.013 U/gds at 30 °C for 7 days with wheatbran:Pulsehusk:MustardPeel ratio 2:2:1 (w/w/w) by conventional method. On achieving two–threefold increased enzyme activities, it can be concluded that the Evolutionary operation factorial (EVOP) technique is useful tool to achieve the goal with very less time and efforts by taking in to consideration, the inter factorial impact on output. As the method involves the production of two different enzymes at the same time and in the single reactor system, therefore, it will reduce the cost and time duration of the production at industrial scale.
Table 2.
Experimental setup of optimization (EVOP1)
| Parameters | (+) | (0) | (−) |
|---|---|---|---|
| Substrate | 3:1:1 | 2:2:1 | 1:3:1 |
| pH | 6 | 5 | 4 |
| Temperature | 33 | 30 | 27 |
‘0’ optimum search level, ‘+’ upper limit, ‘−’ lower limit
Table 3.
Experimental design table (EVOP1)
| Exp. parameter | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (WB + PH + MP) | 2:2:1 | 1:3:1 | 1:3:1 | 3:1:1 | 3:1:1 | 2:2:1 | 3:1:1 | 1:3:1 | 3:1:1 | 1:3:1 |
| Temperature | 30 | 27 | 33 | 27 | 33 | 30 | 33 | 27 | 27 | 33 |
| pH | 5 | 4 | 6 | 6 | 4 | 5 | 6 | 6 | 4 | 4 |
| Response | a1 | a2 | a3 | a4 | a5 | a6 | a7 | a8 | a9 | a10 |
Table 4.
Effects and limits for experimental (EVOP1)
| Effect of variables | Laccase activity (U/gds) | Pectinase activity (U/gds) | |
|---|---|---|---|
| E1 = effect of (S-TP) | − 1082 | − 20.35 | |
| E2 = effect of (S-TP) | 1342 | 15.255 | |
| E3 = effect of (S-TP) | − 1106 | − 41.175 | |
| E4 = effect of (S-TP) | − 311.2 | 10.1712 | |
| E5 = effect of (S-TP) | 1462 | 33.05 | |
| E6 = effect of (S-TP) | 1084 | 58.48 | |
| E7 = effect of (S-TP) | 1062 | 33.05 | |
| E8 = effect of (S-TP) | 230.4 | − 13.234 | |
| Effect of S | Effect of substrate ratios (S) | 190 | 6.36 |
| Effect of T | Effect of temperature (T) | 1213 | 36.86 |
| Effect of P | Effect of pH (P) | − 22 | 37.112 |
| Effect of ST | 1084 | − 4.06 | |
| Effect of SP | − 129 | 21.612 | |
| Effect of TP | 1272 | 26.7 | |
| Changes in mean effect | − 40.4 | − 1.53 | |
| Standard Deviation | 405.39 | 22.887 | |
| Error Limits | |||
| For Averages | ± 573.39 | ± 32.362 | |
| For Effects | ± 406.60 | ± 22.95 | |
| For Change in mean | ± 361.12 | ± 20.388 |
Table represents the calculated value of effects for different variables
S substrate ratio, P pH, T Temperature in first round of EVOP method
Table 5.
Experimental setup for optimization using new range (EVOP2)
| Parameters | (+) | 0 | (−) |
|---|---|---|---|
| Substrate | 2.25:2.25:0.5 | 2:2:1 | 1.75:1.75:1.5 |
| pH | 6.5 | 5.5 | 4.5 |
| Temperature | 34 | 32 | 30 |
Experimental set up for second round of EVOP optimization with new range
Table 6.
Experimental design table (EVOP2)
| Exp. parameter | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (WB + PH + MP) | 2:2:1 | 1.75:1.75:1.5 | 1.75:1.75:1.5 | 2.25:2.25:0.5 | 2.25:2.25:0.5 | 2:2:1 | 2.25:2.25:0.5 | 1.75:1.75:1.5 | 2.25:2.25:0.5 | 1.75:1.75:1.5 |
| Temperature | 32 | 30 | 34 | 30 | 34 | 32 | 34 | 30 | 30 | 34 |
| pH | 5.5 | 4.5 | 6.5 | 6.5 | 4.5 | 5.5 | 6.5 | 6.5 | 4.5 | 4.5 |
| Response | a1 | a2 | a3 | a4 | a5 | a6 | a7 | a8 | a9 | a10 |
Table 7.
Effects and limits for experimental (EVOP-2)
| Effect of variables | Laccase activity (U/gds) | Pectinase activity (U/gds) | |
|---|---|---|---|
| E1 = effect of (S-TP) | − 40 | 97.12 | |
| E2 = effect of (S-TP) | − 168 | 36.99 | |
| E3 = effect of (S-TP) | − 51 | 159.55 | |
| E4 = effect of (S-TP) | − 168.4 | − 222.912 | |
| E5 = effect of (S-TP) | 193.5 | 315.635 | |
| E6 = effect of (S-TP) | 26.5 | − 54.335 | |
| E7 = effect of (S-TP) | − 76.5 | − 38.195 | |
| E8 = effect of (S-TP) | − 31 | − 167.874 | |
| Effect of S | Effect of substrate ratios (S) | 76.75 | 206.375 |
| Effect of T | Effect of temperature (T) | − 70.75 | 8.674 |
| Effect of P | Effect of pH (P) | − 12.75 | 98.8725 |
| Effect of ST | 63.75 | 60.6775 | |
| Effect of SP | 97 | 45.6625 | |
| Effect of TP | 116.75 | 109.257 | |
| Changes in mean effect | 168.5 | 295.391 | |
| Standard deviation | 73.35 | 51.3325 | |
| Error Limits | |||
| For averages | ± 103.74 | ± 72.586 | |
| For effects | ± 173.66 | ± 251.536 | |
| For change in mean | ± 65.341 | ± 45.729 |
Table represents the calculated value of effects for different variables
S substrate ratio, P pH, T Temperature in second round of EVOP method
Conclusion
Evolutionary operation optimization technique (EVOP) was found promising to achieve same range of physico-chemical conditions for optimum yield of both the enzymes. It also provides interfactorial impact of different parameters on yield. Coproduction of more than one enzyme concept would get more preference in industry where more than one enzymes are used simultaneously, for e.g., in wine industries and polymer industries. The results based on present study would be helpful in improving the cost effectiveness and minimize the time of the laccase and pectinase production in a single step for various industrial applications.
Acknowledgements
Authors are grateful to Department of Biotechnology, Government of India and Ministry of Human Resource Development, Government of India, for providing financial assistance and Motilal Nehru National Institute of Technology, Allahabad, India to provide facilities and space to carry out this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Agrawal K, Chaturvedi V, Verma P. Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess. 2018;5:4. doi: 10.1186/s40643-018-0190-z. [DOI] [Google Scholar]
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/S0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Chaudhari A, Suneetha V. Microbially derived pectinases: a Review. IOSR J Pharm Biol. 2012;2:1–5. [Google Scholar]
- Couto SR, Herrera JLT. Industrial and biotechnological applications of laccases: a review. Biotechnol Adv. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Couto SR, Lopez E, Sanroman MA. Utilisation of grape seeds for laccase production in solid-state fermentors. J Food Eng. 2006;74:263–267. doi: 10.1016/j.jfoodeng.2005.03.004. [DOI] [Google Scholar]
- Elisashvili V, Penninckx M, Kashlishvili E, Tsiklauri N, Metreveli E, Kharziani T, Kvesitadze G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of Lignocellulosic wastes of different composition. Bioresour Technol. 2008;99(3):457–462. doi: 10.1016/j.biortech.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Giese EC, Dekker RFH, Barbosa AM. Orange bagasse as a substrate for the production of pectinase and laccase by Botryosphaeria rhodina MAMB-05 in submerged and solid state fermentation. BioResour. 2008;3:335–345. [Google Scholar]
- Gupte A, Madamwar D. Production of cellulolytic enzymes by coculturing of Aspergillus ellipticus and Aspergillus fumigatus grown on bagasse under solid state fermentation. Appl Biochem Biotechnol. 1997;62:267–274. doi: 10.1007/BF02788002. [DOI] [Google Scholar]
- Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005;40:2931–2944. doi: 10.1016/j.procbio.2005.03.026. [DOI] [Google Scholar]
- Kumar S, Katiyar N, Ingle P, Negi S. Use of evolutionary operation (EVOP) factorial design technique to develop a bioprocess using grease waste as a substrate for lipase production. Bioresour Technol. 2011;102:4909–4912. doi: 10.1016/j.biortech.2010.12.114. [DOI] [PubMed] [Google Scholar]
- Kumari J, Negi S. Development of bioprocess for the production of laccase by Pleurotus ostreatus MTCC 1802 using evolutionary optimization technique. Indi J Exper Bio. 2014;52:1106–1111. [PubMed] [Google Scholar]
- Maheshwari DK, Gohade S, Paul J, Varma A. Paper-mill sludge as a potential source for cellulase production by Trichoderma-Reesei Qm-9123 and Aspergillus-Niger Using Mixed Cultivation. Carbohydr Polym. 1994;23:161–163. doi: 10.1016/0144-8617(94)90098-1. [DOI] [Google Scholar]
- Maurya DP, Vats S, Rai S, Negi S. Optimization of enzymatic saccharification of microwave pretreated sugarcane tops through response surface methodology for biofuel. Ind J Exp Biol. 2013;51:992–996. [PubMed] [Google Scholar]
- Maurya DP, Singla A, Negi S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 2015;5:597–609. doi: 10.1007/s13205-015-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minussi RC, Pastore GM, Duran N. Potential applications of laccase in the food industry. Trends Food Sci Technol. 2002;13:205–216. doi: 10.1016/S0924-2244(02)00155-3. [DOI] [Google Scholar]
- Negi S, Banerjee R. Optimization of amylase and protease production from Aspergillus awamori in single bioreactor through EVOP factorial design technique. Food Technol Biotechnol. 2006;44:257–261. [Google Scholar]
- Negi S, Banerjee R. Optimization of extraction and purification of glucoamylase produced by Aspergillus awamori in solid-state fermentation. Biotechnol Bioprocess Eng. 2009;14:60–66. doi: 10.1007/s12257-008-0107-3. [DOI] [Google Scholar]
- Negi S, Banerjee R. Optimization of culture parameters to enhance production of amylase and protease from Aspergillus awamori in a single fermentation system. Afr J Biochem Res. 2010;4:73–80. [Google Scholar]
- Pandey AK, Edgard G, Negi S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy. 2016;98:51–56. doi: 10.1016/j.renene.2016.05.071. [DOI] [Google Scholar]
- Pitt D. Pectin lyase from Phoma medicaginis var. Pinodella. Methods Enzymol. 1988;161:350–354. doi: 10.1016/0076-6879(88)61039-1. [DOI] [Google Scholar]
- Pusic T, Tarbuk A, Dekanić T. Bio-innovation in cotton fabric scouring- acid and neutral pectinases. Fibres Text East Eur. 2015;23:98–103. [Google Scholar]
- Rebello S, Mohandas A, Anees EM, Raveendran S, Parmeswaran B, Pandey A. Recent advancements in the production and applications of microbial pectinases—an overview. Rev Environ Sci Biotechnol. 2017 doi: 10.1007/s11157-017-9437-y. [DOI] [Google Scholar]
- Selinheimo E, Kruus K, Buchert J, Hopia A, Autio K. Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J Cereal Sci. 2006;43:152–159. doi: 10.1016/j.jcs.2005.08.007. [DOI] [Google Scholar]
- Silva D, Martins ES, Silva DR, Gomes E. Pectinase production by Penicillium viridicatum RFC3 by solid state fermentation using agricultural wastes and agro-industrial byproducts. Braz J Microbiol. 2002;33:318–324. [Google Scholar]
- Singh S, Mandal SK. Optimization of processing parameters for production of pectinolytic enzymes from fermented pineapple residue of mixed Aspergillus species. Jordan J Biol Sci. 2012;5:307–314. [Google Scholar]
- Verma P, Madamwar D. Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotus ostreatus and Phanerochaete chrysosporium under solid-state fermentation. Appl Biochem Biotechnol Part A Enzym Eng Biotechnol. 2002;102–103:109–118. doi: 10.1385/ABAB:102-103:1-6:109. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hong YZ, Xiao YZ, Yuan J, Tu XM, Zhang XQ. Efficient production of laccases by Trametes sp. AH28-2 in cocultivation with a Trichoderma strain. Appl Microbiol Biotechnol. 2006;73:89–94. doi: 10.1007/s00253-006-0430-6. [DOI] [PubMed] [Google Scholar]