Introduction
Key Teaching Points.
-
•
The left atrial appendage can have fibers connecting to the left ventricle that can be involved in sustaining a reentrant arrhythmia.
-
•
Ablation of these fibers inside of the left atrial appendage can be achieved with the use of irrigated catheters.
-
•
The use of intracardiac echocardiography can assist in mapping and to avoid complications in difficult cases of Wolff-Parkinson-White syndrome.
A left atrial appendage (LAA) connection to the left ventricle (LV) via an accessory pathway (AP) is a rare phenomenon with a potential for sudden cardiac death. Conventional catheter ablation can be challenging inside of the LAA. We report a case of an adolescent with Wolff-Parkinson-White (WPW) syndrome owing to an LAA-to-LV connection, who was successfully ablated using an irrigated ablation catheter and intracardiac echocardiography to assist the mapping process.
Case report
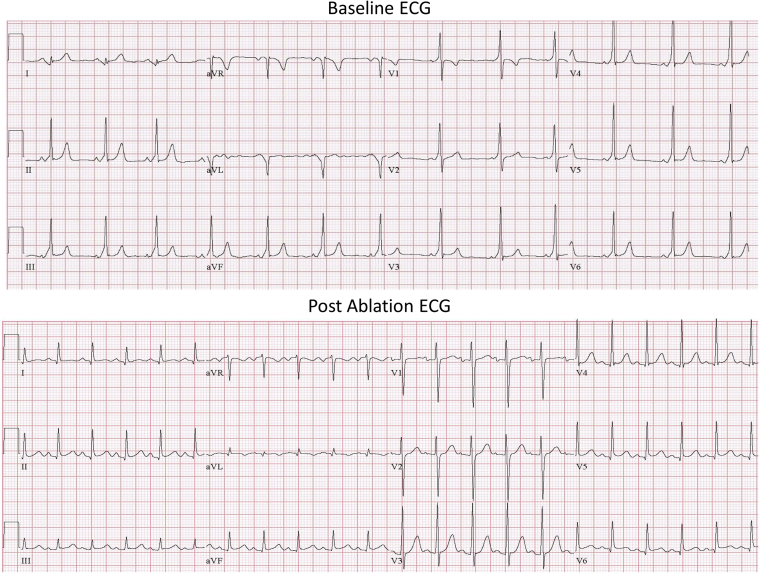
We present a 12-year-old adolescent with recurrent palpitations since the age of 7. He was found to have a short PR interval and preexcitation on the baseline electrocardiogram (ECG) (Figure 1), and was diagnosed as having WPW syndrome. Based on the conventional criteria the pathway was assumed to be a left lateral AP.1 He was then referred for an electrophysiology study and ablation.
Figure 1.
A 12-lead electrocardiogram (ECG) showing a short PR interval with a delta wave positive in V1 and negative in lead I, consistent with a left side accessory pathway. Postablation ECG is shown at the bottom.
The electrophysiology study noted 2 APs: AP1 was mapped to 3 o’clock on the mitral annulus and had the ability to only conduct retrograde, and AP2 was mapped to the base of the LAA and had bidirectional conduction properties.
At the beginning of the case, the local preexcitation pattern was consistent with a coronary sinus (CS) proximal-to-distal pattern, while the observed retrograde activation pattern with ventricular pacing had a distal-to-proximal pattern, already suggesting the presence of 2 distinct APs.
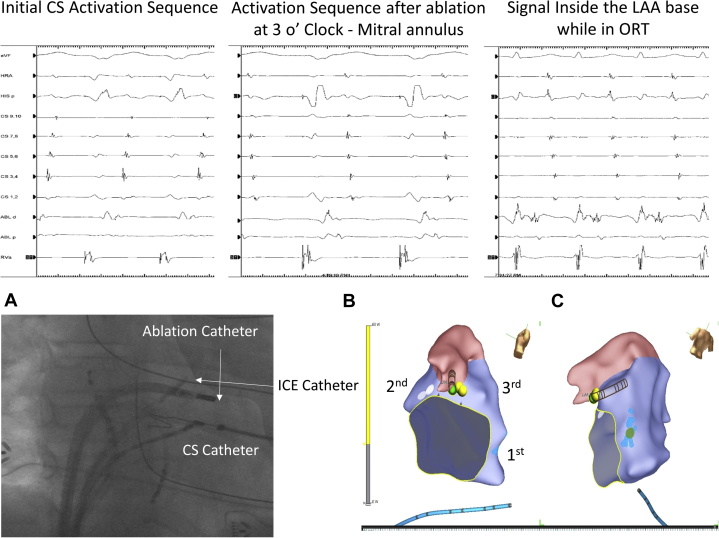
The antegrade AP had a short effective refractory period (ERP) of 250 ms when assessed with programmed atrial stimulation (Stim). At this point the retrograde conduction was nondecremental, with a retrograde ERP of <280 ms (limited by inducing tachycardia). There was an easily inducible orthodromic reentrant tachycardia (ORT) at a cycle length of 260 ms, with a distal-to-proximal CS activation sequence. The earliest retrograde atrial activation during the tachycardia was at the left lateral mitral valve annulus at its 3 o’clock site (in tachycardia VALL: 80 ms; in ventricular pacing from Stim to Atrial SALL: 140 ms; see Figure 2A). Ablation with a regular 4-mm-tip radiofrequency (RF) ablation catheter (MC Marinr, Medtronic, Minneapolis, MN) during ventricular pacing was performed via a transseptal approach. Following this ablation the ventriculoatrial (VA) conduction sequence changed, as assessed via ventricular pacing, to a more proximal-to-distal sequence, ORT was still easily inducible with a similar atrial activation sequence as compared to ventricular pacing, and the preexcitation pattern was the same as noted at baseline (CS proximal-to-distal pattern) (Figure 2B and C).
Figure 2.
A: Left lateral accessory pathway (AP) ablation site with a distal-to-proximal coronary sinus (CS) retrograde atrial activation sequence during ventricular pacing. B: Activation sequence during ventricular pacing after the first ablation at 3 o’clock on the mitral annulus. With the ablation catheter at the left coronary cusp, the VA signal and a different CS atrial activation sequence are shown. C: Ablation catheter signal at the left atrial appendage (LAA) base during orthodromic reentrant tachycardia (ORT). The local electrogram shows a wide and fragmented signal, starting with the ventricular signal, followed by the pathway potential and the near field atrial signal. The lower left panel shows a right anterior oblique fluoroscopy image with the intracardiac echocardiogram (ICE) catheter sitting in the right ventricular (RV) outflow tract and pointed toward the aortic valve–left atrium–LAA. Also present is the CS catheter, ablation catheter at the time of mitral annulus (MA) ablation, and right atrial and RV catheter. On the right bottom panel is the 3-dimensional map with the sites where the lesions were delivered and marked in order (as 1st, 2nd, and 3rd): 1st corresponds to the first AP ablated at 3 o’clock on the MA; 2nd, to ablation of the second AP on the MA; 3rd, to ablation inside of the LAA base.
After elimination of AP1, the AP2 conduction properties were again studied by program stimulation and were found to have an antegrade AP ERP of 250 ms and a retrograde ERP of <210 ms. The retrograde conduction was noted to have some decremental properties (Stim to Atrial lengthened 40 ms before reaching the ventricular ERP). Atrial fibrillation was not induced to study the shortest preexcited R-R interval. After the annulus was carefully mapped and we could not localize the AP, the coronary cusps were mapped using a retrograde aortic approach to better evaluate the area. Using the cusp as a vantage point to reach the aortomitral continuity region, the earliest atrial activation was noted at the left coronary cusp (SALCC: 150 ms). Intracardiac echocardiogram (ICE) showed that at this site, the tip of the ablation catheter was in close proximity to the left main coronary artery orifice; therefore further mapping was done from the endocardial left atrium and LV aspect, and ablation was not attempted.
Using an irrigated-tip RF ablation catheter (FlexAbility, DF curve, St. Jude Medical, Saint Paul, MN) and a deflectable long sheath (Agilis NxT Steerable Introducer, St. Jude Medical), mapping of the LAA was done via a transseptal approach. The earliest retrograde atrial deflection was inside the LAA close to the base (Figure 2C). Owing to the concern that the left circumflex artery may be near this region, as it was noted on ICE, the ablation was started at the mitral annulus, opposite to the area of earliest activation inside of the LAA (SAma: 150 ms). During ablation (30 watts for 1 minute) and ventricular pacing, the Stim-to-Atrial interval was gradually prolonged from 150 to 190 ms without changing the retrograde atrial conduction sequence. After additional insurance lesions, the antegrade AP conduction was also eliminated.
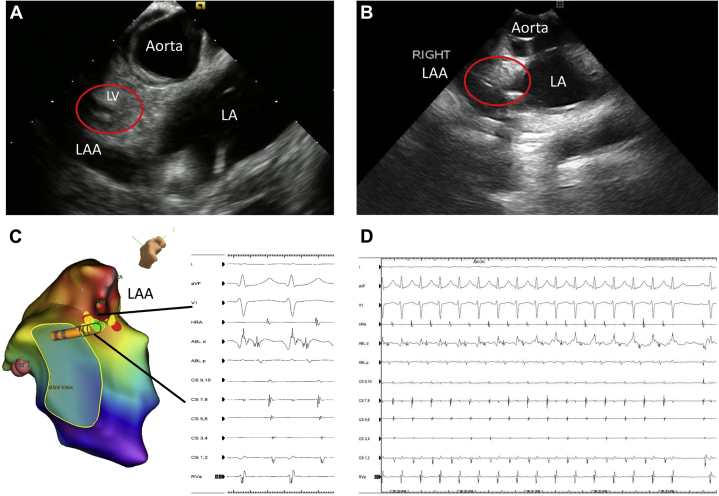
Following ablation on the mitral annulus, ventricular burst pacing under isoproterenol challenge again induced the tachycardia. At this point and with the catheter inside of the LAA, as documented by ICE, careful mapping for the site of earliest atrial activation was performed. Ablation at 20 watts for 1 minute during ORT at the LAA base (VALAA: 89 ms) terminated the tachycardia 3.4 seconds into the lesion (Figure 3). This last lesion was delivered under direct ICE visualization of the LV as well as ECG monitoring, to ensure that no changes in LV function or ECG changes suggestive of coronary injury would be seen. As no changes were noted, an angiogram to visualize the left circumflex artery was not deemed necessary.
Figure 3.
A: Intracardiac echocardiogram (ICE) example of the left atrial appendage (LAA) base relationship to the left ventricle (LV; red circle) and the left coronary cusp region. B: ICE image of the catheter located inside of the LAA base (red circle) where the accessory pathway was successfully eliminated. C: Image of the electroanatomic mapping system reconstruction of the LAA anatomy, and the site of ablation with the recorded signals highlighted. D: Intracardiac recording at the time of ablation at the successful site depicting the orthodromic reentrant tachycardia terminating 3.4 seconds into the ablation. LA = left atrium.
Postablation ventricular pacing showed VA dissociation with and without isoproterenol challenge. Isoproterenol challenge did not evoke any wall motion abnormalities or ECG changes suggestive of ischemia. With adenosine at 0.2 mg/kg intravenous bolus, there was still no evidence of return of VA conduction while pacing the LV (no dormant AP conduction), and no further antegrade conduction via the AP was noted (ECG in Figure 1).
On follow-up 6 months postablation there was no preexcitation on the ECG and the patient remained arrhythmia free, with no clinical evidence of coronary injury.
Discussion
LAA APs have been rarely reported before. Servatius and colleagues2 reported a patient with a split AP connecting a funnel-shaped bilobular LAA with the LV free wall, which was successfully ablated with a conventional RF catheter. Di Biase and colleagues3 reported 2 adult cases of LAA AP that were successfully ablated using an irrigated-tip catheter. Mah and colleagues4 reported 3 children with LAA APs: 1 had a very short antegrade AP ERP < 140 ms; 2 also had right auricular appendage AP and both had a history of ventricular fibrillation. They all required surgical ablation after aggressive catheter ablation with regular 4-mm and 8-mm RF ablation or cryoablation had failed. During surgery, their auricular appendages were all found diffusely adherent to the overlying LV. After dissection of the appendages the A-to-V abnormal conduction disappeared.
In our case, 2 pathways were identified: The preablation sinus beat had an antegrade proximal-to-distal CS activation pattern, which was different from the retrograde distal-to-proximal CS activation pattern, suggesting the existence of 2 different APs. AP1 was successfully ablated along the left lateral aspect of the mitral annulus with retrograde conduction only. AP2, with bidirectional conduction, had the same antegrade preexcitation and retrograde proximal-to-distal CS activation pattern. AP2 was located at the higher mitral annulus and base of the LAA. Ablating of AP2 from the mitral annulus resulted in gradual Stim-to-Atrial prolongation and elimination of antegrade conduction but still with persistent VA conduction with ventricular pacing and arrhythmia inducibility.
Since the LAA can be in close proximity to the left circumflex artery, ablation inside the LAA may injure the coronary artery in young patients. Therefore we started ablating from the endocardial annular site under the LAA. The antegrade AP conduction was eliminated with this approach; however, ablation inside the LAA was still required in order to eliminate the retrograde conduction and render the patient not inducible. This suggests that this LAA AP had a broad band, possibly caused by a diffusely adherent LAA base to the overlying LV, similar to Mah’s reported cases.4 We chose an irrigated catheter in order to overcome the difficulties posed by the presence of a fat pad underneath the LAA and over the epicardial LV.3
During ablation inside of the LAA, continuous ECG monitoring showed no ST-segment changes and continued ICE visualization of the LV function was performed, to ensure no LV wall motion abnormalities suggestive of coronary injury were caused by the lesion. Having said that, we recommend the use of a low power inside of the LAA in young patients.
Conclusion
WPW syndrome caused by an LAA-to-LV connection is rare. These pathways can be successfully ablated but may require lesions both in the LAA and on the mitral valve annulus owing to a broad band of AP tissue from adherence of the LAA to the LV. Ablation of the LAA base proved to be successful in our case, but careful energy titration is suggested, especially in young individuals, to avoid injury to the left circumflex coronary artery.
References
- 1.Arruda M.S., McClelland J.H., Wang X., Beckman K.J., Widman L.E., Gonzalez M.D., Nakagawa H., Lazzara R., Jackman W.M. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 1998;9:2–12. doi: 10.1111/j.1540-8167.1998.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 2.Servatius H., Rostock T., Hoffmann B.A., Willems S. Catheter ablation of an atrioventricular bypass tract connecting a funnel-shaped bilobular left atrial appendage with the ventricular free wall. Heart Rhythm. 2009;6:1075–1076. doi: 10.1016/j.hrthm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L., Schweikert R.A., Saliba W.I., Horton R., Hongo R., Beheiry S., Burkhardt D.J., Natale A. Left atrial appendage tip: an unusual site of successful ablation after failed endocardial and epicardial mapping and ablation. J Cardiovasc Electrophysiol. 2010;21:203–206. doi: 10.1111/j.1540-8167.2009.01561.x. [DOI] [PubMed] [Google Scholar]
- 4.Mah D., Miyake C., Clegg R., Collins K.K., Cecchin F., Triedman J.K., Mayer J., Walsh E.P. Epicardial left atrial appendage and biatrial appendage accessory pathways. Heart Rhythm. 2010;7:1740–1745. doi: 10.1016/j.hrthm.2010.08.013. [DOI] [PubMed] [Google Scholar]