Abstract
Comorbidity of personality disorders (PDs) and substance use disorders (SUDs) is common in clinical practice. Borderline PD and antisocial PD are particularly found to be associated with SUDs. Our review suggests that the overall prevalence of PD ranges from 10% to 14.8% in the normal population and from 34.8% to 73.0% in patients treated for addictions. Even though the types of PD seen in patients with drug and alcohol use disorder are similar, the prevalence of any PD is higher among patients with drug use disorder than alcohol use disorder. The higher comorbidity between these two conditions has been explained by a primary personality pathology followed by a secondary development of a SUD. The comorbidity with PD positively correlates with the severity of the SUD. Comorbid PD among patients with SUDs is a predictor of poor prognosis in terms of poorer treatment response and outcome. Psychotherapy is the mainstay of treatment in comorbid condition with dialectical behavioral therapy, dynamic deconstructive psychotherapy, and dual-focused schema therapy having the most evidence base. Pharmacotherapy is primarily indicated for the acute crisis management or for the treatment of other comorbid conditions such as psychosis and depression. However, the evidence is insufficient as of now to suggest one treatment over the other. Further research is required to identify more efficacious treatment approaches for this comorbidity.
Keywords: Drug use, personality disorders, substance dependence, substance use disorders
INTRODUCTION
Personality disorders (PDs) are defined as enduring patterns of inner experiences and behaviors that markedly deviate from the expectations of the individual culture.[1] As per the American Psychiatric Association, these disorders and associated traits are inflexible and pervasive in nature, with their onset in adolescence and early adulthood. These traits are stable over time and lead to significant impairment to the individual and others.
The Diagnostic and Statistical Manual of Mental Disorders – 5th edition (DSM-5) describes a total of 10 PDs divided into 3 clusters: cluster A includes paranoid, schizoid, and schizotypal PD; cluster B includes antisocial, borderline, histrionic, and narcissistic PD; and cluster C includes avoidant, dependent, and obsessive-compulsive PD.[1] ICD-10 also provides a classification of PDs and contains a total of 10 specific PDs.[2] DSM-5 also describes a more hybrid classification of PD in Section III. This alternative model of PD characterizes them by criterion A, that is, disturbances in self and interpersonal functioning and criterion B, that is, pathological personality traits. The personality traits are divided into five domains, which include negative affectivity, detachment, psychoticism, disinhibition, and antagonism.
In this narrative review, we tried to answer four questions regarding the comorbidity of the substance use disorders (SUDs) with PD: (1) What is the evidence that PD and SUDs commonly coexist? (2) What is the available literature on the etiopathogenesis of this comorbidity? (3) How does the comorbidity impact the course and prognosis of the SUDs? (4) What is the evidence base about the best available treatment for this group? This narrative review aims to provide an overview of the available literature on the selected topic.
We searched multiple scientific search engines including PubMed, PubMed Central, and Google Scholar. The search terms used include, but are not limited to, “personality,” “personality disorder,” “substance use disorder,” “drug use disorder,” “substance abuse,” “substance use,” “addiction,” “dependence,” “substance dependence,” “drug dependence,” “drug addiction,” and various combinations of these terms. We only included English language studies in this review. Studies published till October 2017 were retrieved and included in this review.
EPIDEMIOLOGY OF COMORBID PD AND SUDS
Prevalence of PD
The epidemiology of PD is less well understood as compared to the other mental disorders because of the difficulty in their assessment in surveys. However, the rates of PD vary between 4% and 15% depending on the study setting and population under survey.[3,4,5] A study conducted in seven countries spread over five continents reported a point prevalence of 6.1%, with the highest rates in North and South America and the lowest rates in Europe.[6] The prevalence of PD is especially higher among patients seeking treatment than in those not in contact with a health facility, and among people coming in contact with the criminal justice system.[7] Almost 50% of people coming in contact with the psychiatric setting and more than two-thirds of people coming in contact with the criminal justice system suffer from one or other PDs.[8,9,10]
Prevalence of PD among patients with SUDs
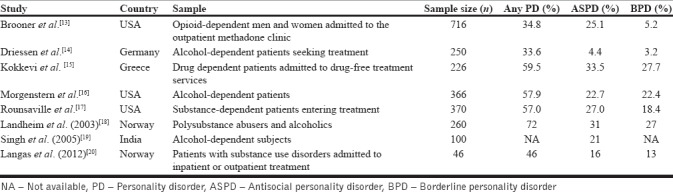
A great number of studies done till date suggest that the prevalence of PD is higher among patients with SUDs as compared to the general population [Table 1].[11,12,13,14,15,16,17,18] This is especially true for antisocial, borderline, avoidant, and paranoid PD. The overall prevalence of PD ranges from 10% to 14.8% in the normal population and from 34.8% to 73.0% in patients treated for addictions, with a median of 56.5%.[11] Similar findings have also been found among patients with SUDs who are currently not seeking treatment, suggesting that the apparent higher prevalence of PD cannot be only contributed to Berkson's bias.[19] In a more recent study, comorbidity of PD was found in a total of 46% of patients with SUDs, with two most common PDs being antisocial PD (ASPD) (16%) and borderline PD (BPD) (13%).[20] The comorbidity with PD positively correlates with the severity of the SUD.[21] These studies suggest that patients with SUDs commonly suffer from one or other kind of PD.
Table 1.
Prevalence rates of PD among patients with substance use disorders
Prevalence of SUDs among patients with PD
SUDs are also commonly found in patients with PD, especially BPD and ASPD.[22,23,24] Among patients with a PD, the risk of comorbid alcohol use disorder is increased by fivefold while the risk of drug use disorder is increased by 12-fold.[23] The comorbidity also depends on the type of PD. For example, studies suggest SUDs to be one of the most common psychiatric comorbidities among patients with BPD, with a lifetime prevalence of around 78%.[25] Another study reported that almost half of the BPD patients also exhibited SUDs.[26] A study reported rates of 47% and 22% for alcohol and drug dependence, respectively, among patients with BPD.[23] Overall, the odds of substance use, including tobacco, alcohol, and illicit drugs, are higher among patients with the BPD as compared to the general population.[25]
ETIOPATHOGENESIS OF COMORBID PD AND SUD
PD and SUDs cooccur at rates that far exceed their individual prevalence rates among the general population, suggesting that both these conditions are interlinked causally. Various hypotheses have been postulated to explain their relationship, including primary PD leading to secondary substance abuse and SUDs, trauma related to SUD causing personality changes, and common biological factors causing impulsivity and impulse control problems leading to PD and SUD.[27] Symptomatic model of SUDs, which suggested that substance use among the patients with PD was a symptom of underlying personality problems and that substance use was a part of “pre-addictive” personality, is now largely discarded.[28] Current understanding of this comorbidity suggests a presence of a primary personality pathology leading to the development of secondary SUD.[27] This has been proven by a plethora of evidence, including longitudinal studies, reporting prediction of later onset of SUD by personality factors during adolescence and early adulthood as well as retrospective studies suggesting precedence of personality psychopathology in a large number of patients with SUDs.[11] However, it is important to note here that personality pathology is neither exclusive nor essential for all the cases of SUDs.
Various causal or developmental pathways have been hypothesized till date which may explain the development of SUDs among PD patients, suggesting personality problems to be an important etiological factor. Among them, three developmental pathways may explain the observed high comorbidity between PD and SUDs. These pathways include (1) the behavioral disinhibition pathway, (2) the stress-reduction pathway, and (3) the reward-sensitivity pathway.[11,29]
Among these three, the behavioral disinhibition pathway has been the best documented in the literature and might account for the observed high comorbidity between PDs such as ASPD and BPD and substance use.[11,29] The pathway suggests that traits such as high anti-sociality and impulsivity, along with low harm avoidance, are associated with a higher risk of drug and alcohol use. Similarly, stress-reduction pathway suggests that individuals with high scores on neuroticism traits, anxiety, and stress reactivity are more prone to use substances during stressful life events as a part of self-medication.[11] This self-medication pathway has been extensively studied in relation to alcohol use and accounts for mainly later onset of alcohol use disorder, especially among females. The third pathway, the reward-sensitivity pathway, suggests higher use of substances (especially cocaine and other stimulants) among individuals with high novelty-seeking, reward-seeking, or extraversion.
Biological aspects of the comorbidity
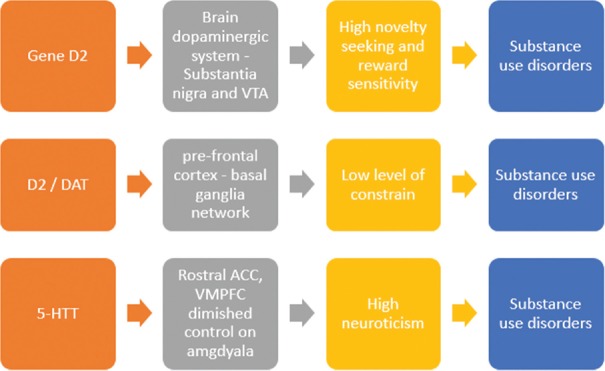
The developmental pathways from PD to substance use can also be explained in terms of biological aspects [Figure 1].[30] For example, the dimension of constraint includes the tendencies toward behavioral restrain and impulsivity.[30] Multiple neuroimaging and neuropsychological studies suggest the role of prefrontal cortex–basal ganglia network in the (dis) inhibition and impulsivity domain.[31,32,33] One of the most important brain regions involved in the higher order dimension of constrain is the right lateral inferior frontal gyrus.[30,31,32,33] Low level of constrain (i.e., disinhibition) is commonly found in patients with SUDs. Even the non-affected biological siblings of patients with SUDs also show similar deficits in response inhibition on neuropsychological testing, along with similar abnormalities in prefrontal-striatal circuitry (including reduced right lateral inferior frontal gyrus fiber tracts integrity).[34,35] Impaired control of negative emotions correlates with the reduction in gray matter in the inferior frontal gyrus.[34] The role of dopamine in the behavioral disinhibition has also gained a lot of research attention over the years.[36,37,38,39]
Figure 1.
The genes and brain circuits associated with personality traits (endophenotypes) leading to substance use disorders VTA: Ventral Tegmental Area, DAT: Dopamine Transporter, 5-HTT: Serotonin Transporter, ACC: Anterior Cingulate Cortex, VMPFC: Ventromedial Prefrontal Cortex
Similarly, the trait of neuroticism represents the sensitivity to punishment signals. Persons with high neuroticism scores are more likely to be affected by negative emotional states (such as anxiety, depressed mood, shame, etc.) as they respond more poorly to the stressors.[30] Various brain regions have been identified playing a major role in the expression of these affective states. These include anterior cingulate cortex (especially rostral part), prefrontal cortex (especially the ventromedial part), insula, and amygdala.[40,41] Diminished control of anterior cingulate and prefrontal cortices over amygdala is associated with diminished control over negative affective states seen in individuals with high neuroticism.[30] Many individuals with SUDs as well as their healthy relatives show higher stress sensitivity. Interestingly, patients with SUDs also have higher comorbidity with illnesses like depression and anxiety (both of which are associated with higher neuroticism scores).[42,43] This high neuroticism has also been linked to 5-HTT (human serotonin transporter) polymorphism, with some studies suggesting a link with the elevated amygdala activity.[44,45,46,47] This may imply that 5-HTT moderates the possible role of neuroticism as an endophenotype for SUDs.[30]
The third pathway involves the trait of high novelty-seeking and reward sensitivity, which is characterized by a state of strong motivation, positive affect, wanting, as well as desires. The dopamine system originating from substantia nigra and the ventral tegmental area and innervating the frontal cortex, striatum, and hippocampus has been the best studied in relation to this trait.[48,49] Studies suggest that reward sensitivity depends on a general sensitivity to D2 receptor agonists.[50,51] Substances themselves cause an increase in the dopaminergic transmission in the brain, suggesting the role of higher reward-seeking or sensation-seeking trait as a vulnerability or risk factor for SUDs.[52] However, several studies have suggested a positive or a protective role of this trait on SUDs, especially among adolescents, and indicated that SUD patients have a less sensitive dopaminergic system in the brain.[30,52,53,54]
IMPACT OF COMORBIDITY ON CLINICAL COURSE AND PROGNOSIS OF SUD
Generally, the clinical course and prognosis of SUDs differ when a comorbid PD is also present. Several studies suggest that comorbid PD among patients with SUDs is a predictor of poor prognosis in terms of poorer treatment response and outcome.[11,27,55] This also includes various problems in the therapeutic relationship between the client and the therapist, nonadherence issues, poor motivation to change, and more dropouts. In general, patients with comorbid personality and SUDs have an earlier onset of substance use problems, more severe problems of dependence (including more frequent relapses and shorter abstinence periods), increased psychopathological burden, more frequent use of other (including illegal) drugs, poorer social functioning, increased risk of suicide, and more frequent dropouts from treatment (both patient and center-initiated).[16,55,56,57,58,59,60] However, the evidence to the contrary also exists. Some studies do suggest that patients of SUDs with comorbid PD benefit from the treatment at least as much as those without comorbid personality problems.[61,62,63,64,65] It is important to note here that the type of comorbid PD also has a bearing on the course of SUD. For example, antisocial, borderline, and schizotypal PDs were more consistently associated with persistent alcohol, cannabis, and nicotine use disorders at 3-year follow-up as compared to other PD in a large nationally representative sample taken from the National Epidemiologic Survey on Alcohol and Related Conditions.[66] Thus, the comorbid PD exerts a negative impact on the course and prognosis of the SUDs. It has also been noted that the management of SUDs does not lead to the remission of the PD, suggesting that the treatment of SUDs alone has little impact on the course of the comorbid PD.[29] Hence, focus is required also on the management of the comorbid PD, and it should be incorporated into the drug use treatment services. The impact of PD on the stigma of SUD and the treatment seeking is unexplored till date.[67]
MANAGEMENT ASPECTS OF COMORBID PD AND SUDs
Psychotherapeutic interventions
Psychotherapy
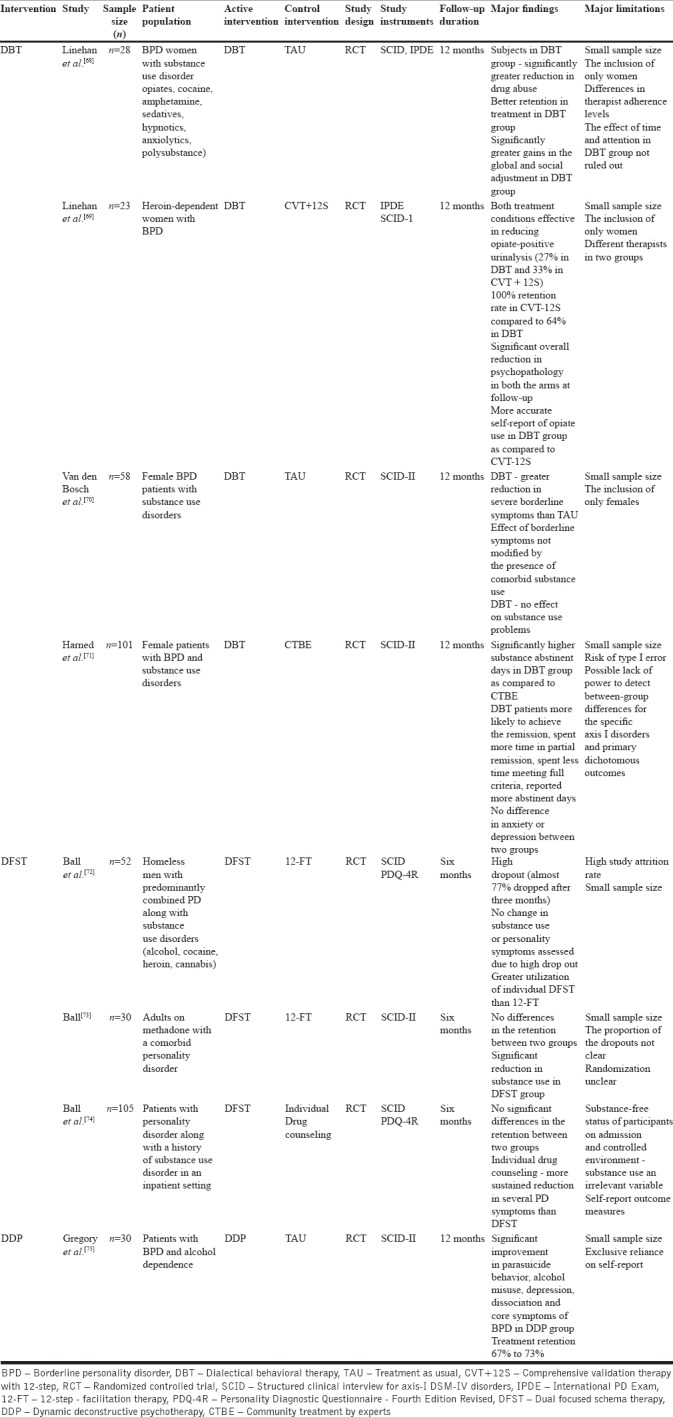
Psychotherapy is the mainstay of the treatment for patients with PD. Although not much literature is available on psychotherapy of comorbid personality and SUDs, previous literature suggests the use of disorder-specific psychotherapies for which some randomized controlled trials (RCTs) are available. Three therapies have been studied using a randomized controlled design till date: dialectical behavioral therapy (DBT), dual focused schema therapy (DFST), and dynamic deconstructive therapy (DDP). Table 1 provides an overview of the RCTs done till date among patients with comorbid personality and SUDs.
As shown in Table 2, DBT has been generally found to be effective compared to other treatment conditions in a number of good quality studies. However, the results need to be interpreted with caution considering the small sample sizes across the studies. This is also true for the studies involving DDP as a psychotherapy, the treatment which is shown to be an effective option for a range of symptoms (including substance use and suicidal behavior) across the studies. DFST does not appear to be an effective approach for such patients and requires further exploration. Based on this available literature and considering the fact that DBT is an evidence-based treatment option for female patients with BPD, DBT can be considered an effective approach for female BPD patients with comorbid SUDs. Overall, the studies included here do provide evidence for some gains in terms of treatment outcomes (both parameters, i.e., substance use and PD psychopathology), but the evidence by far is too less to provide specific clinical recommendations for their use in practice. In addition, there is very less evidence to support the superiority of one treatment over the other. All the therapies used for the comorbid conditions are of at least 6-month duration. This is an important barrier for the widespread use of these therapies in clinical practice, considering that the focus of most substance use treatment facilities on pharmacotherapy and the psychotherapy is usually time-limited. Moreover, as discussed previously, the comorbidity between the two conditions is rampant, and hence, it would be difficult to implement these therapies in resource-limited settings.
Table 2.
Studies on psychotherapy in patients with comorbid substance use disorder with PD
Apart from the disorder-specific psychotherapies, there are studies that explored the role of cognitive behavioral therapy (CBT) and other therapies such as coping skills training. Some of them, as they were not specifically designed for use among comorbid patients, did not report PD-related outcomes.[76,77,78,79,80,81] Some studies suggest a modest effect when CBT is tailored to the specific underlying personality traits. Other authors have developed an approach of integrating cognitive therapy with strategic interventions targeting maladaptive personality features, for example, personality-guided treatment for alcohol dependence, and reported their usefulness in substance use reduction.[81] The effects of psychotherapeutic interventions such as brief intervention, which are proven to be effective in patients with various SUDs, are yet to be studied in this population.[82]
Psychoeducation
Another way to help a patient with PD is through psychoeducation directed at the patients’ personality problems. If provided sensitively, it may help increase the awareness of an individual toward his/her behavioral issues and its impact on him/herself and others. This may, in turn, help the individual make an informed decision about treatment seeking.
The role of psychoeducation has been studied in patients with ASPD comorbid with substance use in at least one RCT.[83,84,85] In this, a total of 176 patients were randomized into two groups: treatment as usual (n = 80) or treatment as usual plus a psychoeducation program (i.e., impulsive lifestyle counseling) (n = 96). The diagnosis of ASPD was made using the Mini International Neuropsychiatric Interview [MINI] ASPD module while addiction severity index was used for assessing the severity of substance dependence. The results suggested a modest engagement in treatment sessions, with only 21% of the participants attending all six sessions, with the median number of sessions being two. There was a significant difference in terms of reduced drug and alcohol use favoring the treatment condition. However, the effect sizes were small. There was a significant difference in mean drug composite scores between the two groups. However, no significant differences in terms of change in aggression scores were noted between the two groups. The intervention was also found to be effective in decreasing the treatment dropout rates (hazard ratio = 0.63; P = 0.03).[83] A post hoc analysis of the data also reported increased perceived help for anti-social PD among participants.[85] At the 3-month follow-up, the perceived help was associated with more abstinent days, higher treatment satisfaction rates, and reduced rate of dropping out of treatment. Overall, from this study, it may be concluded that psychoeducation may add beneficially to the treatment compliance and retention in patients with ASPD and substance use.
Pharmacotherapy
As a diagnosis of comorbid dependence is usually considered an exclusion criterion for pharmacotherapy studies, available literature about management of the comorbid PD is scarce. Importantly, pharmacotherapy usually is indicated in PD only when there is a comorbid psychiatric condition such as depression or anxiety or for emergency indications like agitation and psychotic episodes.[27] Antidepressants and antipsychotics can be considered for this purpose.[86]
Only one RCT suggests about the effectiveness of pharmcotherapy in patients of alcohol dependence with a comorbid PD.[87] In this study, a total of 254 patients with alcohol dependence were included, and a comparison was made between patients with comorbid BPD, comorbid ASPD, and none of the two. The treatment arms included placebo, naltrexone alone, naltrexone plus disulfiram, and disulfiram plus placebo. In this 12-week trial, it was found that comorbid PD diagnosis had no impact on alcohol outcomes. There are no RCTs assessing the effectiveness of medications related to other substances of use and with other comorbid PD (as per authors’ knowledge). Hence, the use of evidence-based medicines in the form of acamprosate and naltrexone is recommended when there is alcohol use disorder comorbid with PD.[27,88] Similarly, when there is comorbidity of opioid dependence with PD, use of opioid substitution therapy is advisable which may lead to psychosocial rehabilitation in patients with severe dependence comorbid with a PD.[27] There is some evidence to suggest the use of anticonvulsants and mood stabilizers in patients with BPD comorbid with alcohol dependence. Both of them may also reduce alcohol consumption and craving.[89]
Complementary and alternative therapies
Some literature is also available about the effectiveness of acupuncture in comorbid BPD and substance abuse. A study conducted at a 90-day inpatient dual-diagnosis program reported the effectiveness of ear acupuncture on a sample of 231 patients (88% with nicotine dependence and 79% with a PD).[90] A total of 49 patients (21%) had no comorbid PD. ASPD (n = 37; 20%) and BPD (n = 78; 43%) were the most common PDs in the sample. The use of ear acupuncture was shown to be positively correlated with successful completion of the program for those with BPD diagnosis and was also positively correlated with the successful tobacco cessation. Similarly, interventions such as yoga are increasingly being studied in patients with SUDs and are found to be effective for at least some substance-related parameters.[91] However, they are yet to be studied in patients with comorbid PD.
METHODOLOGICAL ISSUES ACROSS STUDIES OF COMORBID PD AND SUDs
A few but important methodological issues need to be considered while assessing the studies of comorbid personality and SUDs, especially those on the treatment-related aspects. A wide range of prevalence rates is observed across the studies performed over the period of time based on the methodology adopted. The assessment of PD is especially difficult in the backdrop of a comorbid SUD. The use of a variety of instruments (e.g., clinical interview, IPDE, SCID-II, etc.) for the assessment of PD adds to the difficulty in the interpretation of the rates. One of the most important issues, especially for the studies on the management aspects of comorbidity, is the small sample sizes.[68,69] Use of different inclusion and exclusion criteria adds to the difficulties in making inferences. Moreover, majority of the studies has been conducted on patients with BPD and a few on ASPD. The management aspects of SUDs comorbid with other PD are largely untouched till now. Selective inclusion of gender (e.g., inclusion of only females in the studies assessing DBT efficacy) makes generalizing difficult. Another major concern is the high rates of treatment dropouts, with one RCT reporting almost 77% drop-out rate.[72] Heterogeneity in the outcome measures is another factor that makes the interpretations difficult. Implementation of various psychotherapies (e.g., DBT, DFST, DDP, etc.) might be difficult, especially in resource-poor settings. The excessive reliance on self-report measures for the substance use-related outcomes is also a major issue. Finally, most of the studies have been conducted in western settings or in developed countries, and hence, the generalizability to other settings and countries is questionable.
CONCLUSION AND FUTURE DIRECTIONS
The comorbidity between PD and SUDs is rampant. BPD and ASPD are amongst the most common PDs to cooccur with SUDs. The comorbid PD negatively impacts the course and outcome of SUDs. Although psychopharmacological approaches have been scarcely studied in this population, some good quality evidence in the form of well-conducted RCTs exists for the psychotherapeutic approaches. Of these, three PD-specific approaches appear to be effective for comorbid personality and SUD patients: (1) DBT, (2) DDP, and (3) DFST. However, there are only a limited number of studies with small sample sizes for these, and hence, further RCTs are necessary to make firm conclusions. However, in the absence of a strong evidence base as of now, disorder-specific psychotherapy, especially DBT, can be considered as a treatment of choice for female patients with comorbid BPD and SUD. Further studies with larger sample sizes that include patients with different PD diagnosis (and not just ASPD or BPD) are required. There is a need for studies assessing the role of various pharmacological interventions, especially mood stabilizers and second-generation antipsychotics, in this population. Lastly, there is a need for studies assessing different population groups from different countries and cultures for making generalizable recommendations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5R) American Psychiatric Pub; 2013. [Google Scholar]
- 2.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Vol. 1. World Health Organization; 1992. [Google Scholar]
- 3.Weissman MM. The epidemiology of PD: A 1990 update. J Pers Disord. 1993;7:44–62. [Google Scholar]
- 4.Torgersen S, Kringlen E, Cramer V. The prevalence of PD in a community sample. Arch Gen Psychiatry. 2001;58:590–6. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 5.Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Br J Psychiatry. 2006;188:423–31. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- 6.Huang B, Grant BF, Dawson DA, Stinson FS, Chou SP, Saha TD, et al. Race-ethnicity and the prevalence and co-occurrence of Diagnostic and Statistical Manual of Mental Disorders, alcohol and drug use disorders and Axis I and II disorders: United States, 2001 to 2002. Compr Psychiatry. 2006;47:252–7. doi: 10.1016/j.comppsych.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385:717–26. doi: 10.1016/S0140-6736(14)61995-4. [DOI] [PubMed] [Google Scholar]
- 8.Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: A systematic literature review. Personal Ment Health. 2014;8:91–101. doi: 10.1002/pmh.1252. [DOI] [PubMed] [Google Scholar]
- 9.Fazel S, Danesh J. Serious mental disorder in 23 000 prisoners: A systematic review of 62 surveys. Lancet. 2002;359:545–50. doi: 10.1016/S0140-6736(02)07740-1. [DOI] [PubMed] [Google Scholar]
- 10.Moran P, Jenkins R, Tylee A, Blizard R, Mann A. The prevalence of personality disorder among UK primary care attenders. Act Psychiatr Scand. 2000;102:52–7. doi: 10.1034/j.1600-0447.2000.102001052.x. [DOI] [PubMed] [Google Scholar]
- 11.Verheul R. Co-morbidity of PD in individuals with substance use disorders. Eur Psychiatr. 2001;16:274–82. doi: 10.1016/s0924-9338(01)00578-8. [DOI] [PubMed] [Google Scholar]
- 12.Walter M, Gunderson JG, Zanarini MC, Sanislow C, Grilo CM, McGlashan TH, et al. New onsets of substance use disorders in borderline personality disorder over seven years of follow-ups. Addiction. 2009;204:97–103. doi: 10.1111/j.1360-0443.2008.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. Psychiatric and substance use comorbidity among treatment seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 14.Driessen M, Veltrup C, Wetterling T, John U, Dilling H. Axis I and Axis II comorbidity in alcohol dependence and the two types of alcoholism. Alcohol Clin Exp Res. 1998;22:77–86. [PubMed] [Google Scholar]
- 15.Kokkevi A, Stefanis N, Anastasopoulou E, Kostogianni C. PD in drug abusers: Prevalence and their association with Axis I disorders as predictors of treatment retention. Addict Behav. 1998;23:841–53. [PubMed] [Google Scholar]
- 16.Morgenstern J, Langenbucher J, Labouvie E, Miller KJ. The comorbidity of alcoholism and PD in a clinical population: Prevalence rates and relation to alcohol typology variables. J Abnorm Psychol. 1997;106:74–84. doi: 10.1037//0021-843x.106.1.74. [DOI] [PubMed] [Google Scholar]
- 17.Rounsaville BJ, Kranzler HR, Ball SA, Tennen H, Poling J, Triffleman E. PD in substance abusers: Relation to substance use. J Nerv Ment Dis. 1998;186:87–95. doi: 10.1097/00005053-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Landheim AS, Bakken K, Vaglum P. Gender differences in the prevalence of symptom disorders and personality disorders among poly-substance abusers and pure alcoholics. Eur Addict Res. 2003;9:8–17. doi: 10.1159/000067732. [DOI] [PubMed] [Google Scholar]
- 19.Singh NH, Sharma SG, Pasweth AM. Psychiatric co-morbidity among alcohol dependants. Indian J Psychiatry. 2005;47:222–4. doi: 10.4103/0019-5545.43058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langås AM, Malt UF, Opjordsmoen S. In-depth study of personality disorders in first-admission patients with substance use disorders. BMC Psychiatry. 2012;12:180. doi: 10.1186/1471-244X-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preuss UW, Johann M, Fehr C, Koller G, Wodarz N, Hesselbrock V, et al. PD in alcohol-dependent individuals: Relationship with alcohol dependence severity. Eur Addict Res. 2009;15:188–95. doi: 10.1159/000228929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sher KJ, Trull TJ. Substance use disorder and personality disorder. Curr Psychiatry Rep. 2002;4:25–9. doi: 10.1007/s11920-002-0008-7. [DOI] [PubMed] [Google Scholar]
- 23.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord. 2010;24:412–26. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clin Psyschol Rev. 2000;20:235–53. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 25.Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: Comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734–50. doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGlashan TH, Grilo CM, Skodol AE, Gunderson JG, Shea MT, Morey LC, et al. The collaborative longitudinal PD study: Baseline axis I/II and II/II diagnostic co-occurrence. Acta Psychiatr Scand. 2000;102:256–64. doi: 10.1034/j.1600-0447.2000.102004256.x. [DOI] [PubMed] [Google Scholar]
- 27.Dom G, Moggi F. Co-occurring Addictive and Psychiatric Disorders. Berlin, Heidelberg: Springer; 2016. [Google Scholar]
- 28.Barnes GE. The alcoholic personality: A reanalysis of the literature. J Stud Alcohol. 1979;40:571–634. doi: 10.15288/jsa.1979.40.571. [DOI] [PubMed] [Google Scholar]
- 29.Verheul R, van den Brink W. The role of personality pathology in the etiology and treatment of substance use disorders. Curr Opin Psychiatry. 2000;13:163–9. [Google Scholar]
- 30.Belcher AM, Volkow ND, Moeller FG, Ferré S. Personality traits and vulnerability or resilience to substance use disorders. Trends Cogn Sci. 2014;18:211–7. doi: 10.1016/j.tics.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–52. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59:2860–70. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–10. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–4. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 36.Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- 37.Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–98. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- 38.González S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, et al. Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry. 2012;17:650–62. doi: 10.1038/mp.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3’-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46:151–60. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 40.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terracciano A, Löckenhoff CE, Bienvenu OJ, Costa PT, Crum RM. Five-factor model personality profiles of drug users. BMC Psychiatry. 2008;8:22. doi: 10.1186/1471-244X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol Bull. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- 44.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 45.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 46.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 47.Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2010;169:926–36. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 49.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baik SH, Yoon HS, Kim SE, Kim SH. Extraversion and striatal dopaminergic receptor availability in young adults: An [18F] fallypride PET study. Neuroreport. 2012;23:251–4. doi: 10.1097/WNR.0b013e3283507533. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: Possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 52.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–42. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wills TA, DuHamel K, Vaccaro D. Activity and mood temperament as predictors of adolescent substance use: Test of a self-regulation mediational model. J Pers Soc Psychol. 1995;68:901–16. doi: 10.1037//0022-3514.68.5.901. [DOI] [PubMed] [Google Scholar]
- 54.Wills TA, Sandy JM, Yaeger A, Shinar O. Family risk factors and adolescent substance use: Moderation effects for temperament dimensions. Dev Psychol. 2001;37:283–97. [PubMed] [Google Scholar]
- 55.Kienast T, Stoffers J, Bermpohl F, Lieb K. Borderline personality disorder and comorbid addiction: Epidemiology and treatment. Dtsch Arztebl Int. 2014;111:280–6. doi: 10.3238/arztebl.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preuss UW, Koller G, Barnow S, Eikmeier M, Soyka M. Suicidal behavior in alcohol-dependent subjects: The role of PD. Alcohol Clin Exp Res. 2006;30:866–77. doi: 10.1111/j.1530-0277.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- 57.Verheul R, Van den Brink W, Hartgers C. PD predict relapse in alcoholic patients. Addict Behav. 1998;23:869–82. doi: 10.1016/s0306-4603(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 58.Wilson ST, Fertuck EA, Kwitel A, Stanley MC, Stanley B. Impulsivity, suicidality and alcohol use disorders in adolescents and young adults with borderline personality disorder. Int J Adolesc Med Health. 2006;18:189–96. doi: 10.1515/ijamh.2006.18.1.189. [DOI] [PubMed] [Google Scholar]
- 59.Tull MT, Gratz KL. The impact of borderline personality disorder on residential substance abuse treatment dropout among men. Drug Alcohol Depend. 2012;121:97–102. doi: 10.1016/j.drugalcdep.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krampe H, Wagner T, Stawicki S, Bartels C, Aust C, Kroener-Herwig B, et al. Personality disorder and chronicity of addiction as independent outcome predictors in alcoholism treatment. Psychiatr Serv. 2006;57:708–12. doi: 10.1176/ps.2006.57.5.708. [DOI] [PubMed] [Google Scholar]
- 61.Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Boardman CR. Prediction of 7 months methadone maintenance treatment response by four measures of antisociality. Drug Alcohol Depend. 1998;49:217–23. doi: 10.1016/s0376-8716(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 62.Cacciola JS, Alterman AI, Rutherford MJ, Alterman AI, McKay JR, Snider EC. PD and treatment outcome in methadone maintenance patients. J Nerv Ment Dis. 1996;184:234–9. doi: 10.1097/00005053-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Cacciola JS, Alterman AI, Rutherford MJ, Snider EC. Treatment response of antisocial substance abusers. J Nerv Ment Dis. 1995;183:166–71. doi: 10.1097/00005053-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Cecero JJ, Ball SA, Tennen H, Kranzler HR, Rounsaville BJ. Concurrent and predictive validity of ASPD subtyping among substance abusers. J Nerv Ment Dis. 1999;187:478–86. doi: 10.1097/00005053-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Crits-Cristoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: NIDA collaborative cocaine treatment study. Arch Gen Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- 66.Fenton MC, Keyes K, Geier T, Greenstein E, Skodol A, Krueger B, et al. Psychiatric comorbidity and the persistence of drug use disorders in the United States. Addiction. 2012;107:599–609. doi: 10.1111/j.1360-0443.2011.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balhara YP, Parmar A, Sarkar S, Verma R. Stigma in dual diagnosis: A narrative review. Indian J Soc Psychiatr. 2016;32:128–133. [Google Scholar]
- 68.Linehan MM, Schmidt H, Dimeff LA, Craft JC, Kanter J, Comtois KA. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addict. 1999;8:279–92. doi: 10.1080/105504999305686. [DOI] [PubMed] [Google Scholar]
- 69.Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67:13–26. doi: 10.1016/s0376-8716(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 70.van den Bosch LM, Verheul R, Schippers GM, van den Brink W. Dialectical behavior therapy of borderline patients with and without substance use problems: Implementation and long-term effects. Addict Behav. 2002;27:911–23. doi: 10.1016/s0306-4603(02)00293-9. [DOI] [PubMed] [Google Scholar]
- 71.Harned MS, Chapman AL, Dexter-Mazza ET, Murray A, Comtois KA, Linehan MM. Treating co-occurring Axis I disorders in recurrently suicidal women with borderline personality disorder: A 2-year randomized trial of dialectical behavior therapy versus community treatment by experts. J Consult Clin Psychol. 2008;76:1068–75. doi: 10.1037/a0014044. [DOI] [PubMed] [Google Scholar]
- 72.Ball SA, Cobb-Richardson P, Connolly AJ, Bujosa CT, O’Neall TW. Substance abuse and PD in homeless drop-in center clients: Symptom severity and psychotherapy retention in a randomized clinical trial. Compr Psychiatry. 2005;46:371–9. doi: 10.1016/j.comppsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Ball SA. Comparing individual therapies for personality disordered opioid dependent patients. J Pers Disord. 2007;21:305–21. doi: 10.1521/pedi.2007.21.3.305. [DOI] [PubMed] [Google Scholar]
- 74.Ball SA, Maccarelli LM, LaPaglia DM, Ostrowski MJ. Randomized trial of dual-focused versus single-focused individual therapy for PD and substance dependence. J Nerv Ment Dis. 2011;199:319–28. doi: 10.1097/NMD.0b013e3182174e6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregory RJ, Chlebowski S, Kang D, Remen AL, Soderberg MG, Stepkovitch J, et al. A controlled trial of psychodynamic psychotherapy for co-occurring borderline personality disorder and alcohol use disorder. Psychotherapy (Chic) 2008;45:28–41. doi: 10.1037/0033-3204.45.1.28. [DOI] [PubMed] [Google Scholar]
- 76.Conrod PJ, Castellanos-Ryan N, Strang J. Brief, personality-targeted coping skills interventions and survival as a non-drug user over a 2-year period during adolescence. Arch Gen Psychiatry. 2010;67:85–93. doi: 10.1001/archgenpsychiatry.2009.173. [DOI] [PubMed] [Google Scholar]
- 77.Conrod PJ, Stewart SH, Comeau N, Maclean AM. Efficacy of cognitive-behavioral interventions targeting personality risk factors for youth alcohol misuse. J Clin Child Adolesc Psychol. 2006;35:550–63. doi: 10.1207/s15374424jccp3504_6. [DOI] [PubMed] [Google Scholar]
- 78.Conrod PJ, Stewart SH, Pihl RO, Côté S, Fontaine V, Dongier M. Efficacy of brief coping skills interventions that match different personality profiles of female substance abusers. Psychol Addict Behav. 2000;14:231–42. doi: 10.1037//0893-164x.14.3.231. [DOI] [PubMed] [Google Scholar]
- 79.Nordholm D, Nielsen B. PD among Danish alcoholics attending outpatient treatment. Eur Addict Res. 2007;13:222–9. doi: 10.1159/000104885. [DOI] [PubMed] [Google Scholar]
- 80.Watt M, Stewart S, Birch C, Bernier D. Brief CBT for high anxiety sensitivity decreases drinking problems, relief alcohol outcome expectancies, and conformity drinking motives: Evidence from a randomized controlled trial. J Ment Health. 2006;15:683–95. [Google Scholar]
- 81.Nielsen P, Røjskjær S, Hesse M. Personality-guided treatment for alcohol dependence: A quasi-randomized experiment. Am J Addict. 2007;16:357–64. doi: 10.1080/10550490701525640. [DOI] [PubMed] [Google Scholar]
- 82.Parmar A, Sarkar S. Brief interventions for cannabis use disorders: A review. Addict Disord Their Treat. 2017;16:80–93. [Google Scholar]
- 83.Thylstrup B, Hesse M. Impulsive lifestyle counseling to prevent dropout from treatment for substance use disorders in people with ASPD: A randomized study. Addict Behave. 2007;57:48–54. doi: 10.1016/j.addbeh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Thylstrup B, Schrøder S, Hesse M. Psycho-education for substance use and ASPD: A randomized trial. BMC Psychiatry. 2015;15:283. doi: 10.1186/s12888-015-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thylstrup B, Schrøder S, Fridell M, Hesse M. Did you get any help? A post-hoc secondary analysis of a randomized controlled trial of psychoeducation for patients with ASPD in outpatient substance abuse treatment programs. BMC Psychiatry. 2017;17:7. doi: 10.1186/s12888-016-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herpertz SC, Zanarini M, Schulz CS, Siever L, Lieb K, Möller HJ. WFSBP task force on PD. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of PD. World J Biol Psychiatry. 2007;8:212–44. doi: 10.1080/15622970701685224. [DOI] [PubMed] [Google Scholar]
- 87.Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of PD on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16:443–9. doi: 10.1080/10550490701643336. [DOI] [PubMed] [Google Scholar]
- 88.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: A double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–9. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 89.Gianoli MO, Jane JS, O’brien E, Ralevski E. Treatment for comorbid borderline personality disorder and alcohol use disorders: A review of the evidence and future recommendations. Exp Clin Psychopharmacol. 2012;20:333–44. doi: 10.1037/a0027999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stuyt EB. Ear acupuncture for co-occurring substance abuse and borderline personality disorder: An aid to encourage treatment retention and tobacco cessation. Acupunct Med. 2014;32:318–24. doi: 10.1136/acupmed-2014-010540. [DOI] [PubMed] [Google Scholar]
- 91.Kuppili PP, Parmar A, Gupta A, Balhara YP. Role of yoga in management of substance-use disorders: A narrative review. J Neurosci Rural Pract. 2018;9:117–122. doi: 10.4103/jnrp.jnrp_243_17. [DOI] [PMC free article] [PubMed] [Google Scholar]