Abstract
Introduction:
Intensive behavioral counseling is effective in preventing type 2 diabetes, and insurance coverage for such interventions is increasing. Although primary care provider referrals are not required for entry to the Centers for Disease Control and Prevention (CDC)–recognized National Diabetes Prevention Program lifestyle change program, referral rates remain suboptimal. This study aims to assess the association between primary care provider behaviors regarding prediabetes screening, testing, and referral and awareness of the CDC-recognized lifestyle change program and the Prevent Diabetes STAT: Screen, Test, and Act Today™ toolkit. Awareness of the lifestyle change program and the STAT toolkit, use of electronic health records, and the ratio of lifestyle change program classes to primary care physicians were hypothesized to be positively associated with primary care provider prediabetes screening, testing, and referral behaviors.
Methods:
Responses from primary care providers (n= 1,256) who completed the 2016 DocStyles cross-sectional web-based survey were analyzed in 2017 to measure self-reported prediabetes screening, testing, and referral behaviors. Multivariate logistic regression was used to estimate the effects of primary care provider awareness and practice characteristics on these behaviors, controlling for provider characteristics.
Results:
Overall, 38% of primary care providers were aware of the CDC-recognized lifestyle change program, and 19% were aware of the STAT toolkit; 27% screened patients for prediabetes using a risk test; 97% ordered recommended blood tests; and 23% made referrals. Awareness of the lifestyle change program and the STAT toolkit was positively associated with screening and referring patients. Primary care providers who used electronic health records were more likely to screen, test, and refer. Referring was more likely in areas with more lifestyle change program classes.
Conclusions:
This study highlights the importance of increasing primary care provider awareness of and referrals to the CDC-recognized lifestyle change program.
INTRODUCTION
Diabetes is common, costly, and responsible for significant morbidity and mortality in the U.S. The total economic cost of diagnosed diabetes in 2017 was estimated to be $327 billion,1 with more than 200,000 people dying each year, making it the seventh leading cause of death.2 Nationally, an estimated 30.3 million people have diabetes, and an additional 84.1 million (33.9%) adults have prediabetes.2 Prediabetes is a serious health condition characterized by blood glucose levels that are elevated, but not high enough to be classified as diabetes.3 People with prediabetes are at increased risk of developing type 2 diabetes, heart disease, and stroke, and tend to have higher healthcare utilization and expenditures.4–6
Individuals with prediabetes can mitigate their clinical and economic risks by participating in a structured, evidenced-based lifestyle change program (LCP), such as the one offered through the Centers for Disease Control and Prevention (CDC)–led National Diabetes Prevention Program (n=ational DPP). The National DPP is a partnership of public and private organizations working to reduce the burden of type 2 diabetes by building the infrastructure for nationwide delivery of a yearlong LCP.7 The program is modeled after the DPP research study,8 and subsequent translational studies,9–16 to prevent or delay onset of type 2 diabetes. DPP participants lost on average 5%–7% of their body weight and reduced their risk of developing type 2 diabetes by 58% for adults aged 25 years or older and 71% for those aged 60 years or older.8 Early results from CDC’s National DPP showed an average weight loss of 4.2%, with 35.5% of participants achieving 5% or more weight loss.17 The Community Preventive Services Task Force and the U.S. Preventive Services Task Force issued recommendations for clinicians, specifying screening and testing guidelines, and suggesting that all patients with laboratory results in the prediabetes range be referred to an evidence-based type 2 diabetes prevention program18 to reduce type 2 diabetes risk19 and improve cardiometabolic risk factors.20,21
This evidence led the American Medical Association (AMA) to partner with CDC to launch the Prevent Diabetes STAT™ initiative in 2015 aimed at increasing awareness of the National DPP. The collaboration developed audience-specific messages around three key steps: (1) Screen patients for prediabetes using available risk tests; (2) Test for prediabetes among patients at risk using either a hemoglobin A1c, 2-hour glucose tolerance, or fasting plasma glucose test; and (3) Act Today by referring patients with prediabetes to CDC-recognized LCP classes. The Prevent Diabetes STAT website provides a toolkit with materials to engage and aid healthcare providers in incorporating the screening, testing, and referral processes into their practices and workflows. In January 2016, the first national campaign was launched in partnership with the Ad Council, CDC, AMA, and the American Diabetes Association (ADA) to raise awareness of prediabetes and the importance of assessing risk.
Despite considerable evidence of the benefits associated with participation in a structured LCP and increasing insurance/employer coverage, healthcare provider referral rates remain suboptimal.22,23 There is limited research documenting primary care provider (PCP) awareness of the CDC-recognized LCP and the STAT toolkit, and the relationship between their awareness and behaviors regarding screening, testing, and referral to the CDC-recognized LCP. Based on their experiences working with healthcare providers, AMA and CDC developed a conceptual model (Appendix Figure 1) and used this to design survey questions to assess these associations and identify factors that may contribute to low rates of referrals to the CDC-recognized LCP. Awareness of the CDC-recognized LCP and the STAT toolkit, use of electronic health records (EHRs) to manage patients, and practicing in areas with a high ratio of LCP classes to primary care physicians were hypothesized to be positively associated with PCPs’ self-reported behaviors on prediabetes screening, testing, and referral.
METHODS
Study Sample
Three data sources were used for this study. The primary source was the 2016 DocStylesa cross-sectional web-based survey administered by Porter Novelli, a public relations firm with a specialty practice in health and social marketing, between June and July, including a main sample of primary care physicians and additional samples of other specialties drawn from SERMO’s Global Medical Panel.b Physicians and nurse practitioners (NPs) were included if they had been practicing for ≥3 years in the U.S., actively seeing patients, and working in an individual/group outpatient or inpatient practice. Of the 3,110 health professionals invited to participate, 2,006 completed the entire survey for a 64.5% response rate (Figure 1).
Figure 1.
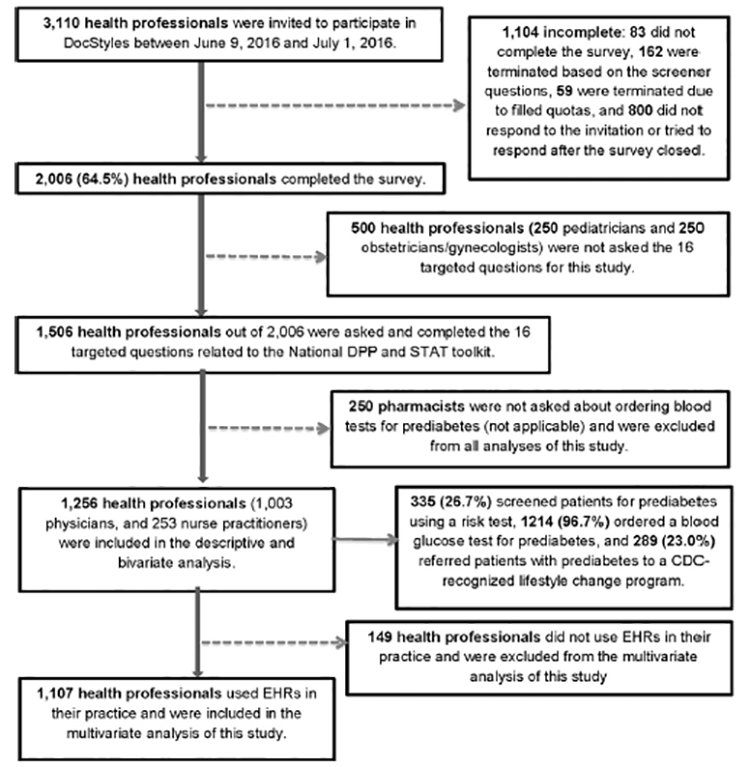
Flow chart for survey sample.
CDC, Centers for Disease Control and Prevention; National DPP, National Diabetes Prevention Program; EHRs, electronic health records; STAT, Screen patients for prediabetes using a risk test, Test patients for prediabetes using a blood glucose test, and Act Today by referring patients with prediabetes to the CDC-recognized lifestyle change program.
Only primary care physicians, NPs, and pharmacists (n= 1,506) were asked the 16 diabetes-related questions. Pharmacists (n= 250) were excluded from the current analysis because a question about ordering blood tests for prediabetes was not applicable to their profession. Data from 1,256 PCPs (1,003 physicians and 253 NPs) were included in descriptive and bivariate analysis; 1,107 PCPs who reported using EHRs in their practice were included in multivariate analysis (Figure 1). No individual identifiers were included in the data set, and this study was deemed exempt from CDC’s IRB approval.
A second data source, the CDC Diabetes Prevention Recognition Programc (DPRP) registry, contains information on the locations of CDC-recognized LCP classes as of December 2016. A third data set, the AMA Physician Masterfile (a registry of all licensed physicians in the U.S.), was used to construct ZIP code– level counts of primary care physicians that were matched to the ZIP codes for publicly available LCP classes and the practice ZIP codes of the DocStyles respondents. There were 38,095 primary care physicians in the AMA Masterfile and 1,039 LCP classes in the DPRP that were matched with the ZIP code of the 1,256 DocStyles respondents.
Measures
The 2016 DocStyles survey contained 144 questions addressing provider age, gender, race/ethnicity, specialties, years of practice, work settings/locations, patients’ average household incomes, and attitudes and counseling behaviors on a variety of health issues. After defining the CDC-recognized LCP and STAT toolkit, the following questions were asked: Have you heard of the CDC-recognized LCP to prevent or delay type 2 diabetes? and Have you heard of the AMA/CDC Prevent Diabetes STAT toolkit? PCPs’ self-reported behaviors regarding screening, testing, and referral were measured with these questions: Have you screened your patients for prediabetes using the CDC Prediabetes Screening Test or ADA Type 2 Diabetes Risk Test? Which of the following recommended blood tests (fasting plasma glucose, hemoglobin A1c, or 2-hour glucose tolerance test) do you most commonly order to test your patients for prediabetes? and Have you referred your patients to an in-person or online CDC-recognized LCP class to prevent or delay type 2 diabetes like the one described previously? PCPs who reported using EHRs were then asked if EHR systems were used to identify and manage patients with prediabetes.
The number of LCP classes available for a physician to refer to was measured as the ratio of publicly available LCP classes to primary care physicians at the practice ZIP code level for DocStyles respondents, which captures the potential supply induced demand for the LCP, similar to a physician population density ratio.24 These ratios were categorized as high (Q2-Q3) or low (Q1) based on proportion of LCP classes in each tertile.
Statistical Analysis
Pearson chi-square tests were used to assess bivariate associations between PCPs’ self-reported behaviors regarding prediabetes screening, testing, and referral and their awareness of the CDC-recognized LCP and the STAT toolkit. Multivariate logistic regressions were used to estimate the effects of these associations conditional on other factors, including PCP demographics, practice characteristics, and the ratio of LCP classes to primary care physicians. AORs in relation to a reference category were reported with their respective 95% CIs. Results with p< 0.05 were considered statistically significant. All analyses were conducted in 2017 using SAS, version 9.3.
RESULTS
Table 1 displays univariate and bivariate analyses of survey respondents by prediabetes screening, testing, and referral practices. Overall, 27% of PCPs screened patients for prediabetes using the CDC or ADA risk test, 97% ordered recommended blood tests, and 23% referred patients to CDC-recognized LCP classes. Mean PCP age was 47 years, with most being aged ≥40 years (72%) and having practiced medicine for ≥15 years (52%). The majority were male (60%) and non-Hispanic white (64%) and worked in group outpatient settings (64%).
Table 1.
Characteristics and Behaviors of PCPs Regarding Prediabetes Screening, Testing, and Referring to the CDC-Recognized LCP
| PCP characteristics | Total PCPs, % (n=1,256) |
Screen for prediabetes, % (n=335) |
PCP behaviors Test for prediabetes, % (n=1,214) |
Refer to LCP, % (n=289) |
|---|---|---|---|---|
| Percentage of total respondents |
— | 26.7 | 96.7 | 23.0 |
| Mean age (Q1, Q3), years | 47 (38, 56) | 47 (39, 55) | 47 (38, 55) | 48 (39, 56) |
| Age, years | ||||
| <40 | 28.0*** | 26.1 | 95.7 | 21.6 |
| 40–49 | 33.1 | 27.4 | 97.6 | 22.4 |
| ≥50 | 38.9 | 26.4 | 96.5 | 24.6 |
| Gender | ||||
| Male | 60.1*** | 27.2 | 97.0 | 21.9 |
| Female | 39.9 | 26.0 | 96.2 | 24.8 |
| Race/ethnicity | ||||
| Hispanic | 4.1*** | 36.5*** | 98.1 | 25.0 |
| Non-Hispanic black | 3.3 | 39.0 | 97.6 | 26.8 |
| Non-Hispanic othera | 29.0 | 32.4 | 97.0 | 25.3 |
| Non-Hispanic white | 63.6 | 22.8 | 96.4 | 21.7 |
| Region | ||||
| Northeast | 27.0*** | 25.4 | 96.8 | 20.1* |
| Midwest | 19.8 | 26.5 | 95.2 | 22.9 |
| South | 33.2 | 26.6 | 97.4 | 21.6 |
| West | 20.0 | 28.7 | 96.8 | 29.5 |
| Provider type | ||||
| Primary care physician | 79.9*** | 27.6 | 97.6*** | 22.9 |
| Nurse practitioner | 20.1 | 22.9 | 92.9 | 23.3 |
| Years practicing medicine | ||||
| < 15 years | 47.9 | 26.1 | 96.4 | 21.1 |
| ≥ 15 years | 52.1 | 27.2 | 96.9 | 24.8 |
| Work setting | ||||
| Individual outpatient practice |
20.0*** | 27.5 | 97.6* | 19.9 |
| Group outpatient practice |
64.0 | 25.9 | 97.1 | 24.4 |
| Inpatient practice | 16.0 | 28.9 | 93.5 | 21.4 |
| Patient household income | ||||
| ≤$49,999 | 31.9 | 26.7 | 96.8 | 21.7 |
| $50,000–$99,999 | 34.3 | 30.2 | 96.3 | 22.1 |
| ≥ $100,000 | 33.8 | 23.1 | 96.9 | 25.2 |
| Ratio of CDC-recognized LCP classes to total primary care physicians at practice ZIP code | ||||
| High | 83.7*** | 24.9 | 96.6 | 28.8* |
| Low | 16.3 | 27.0 | 96.7 | 21.9 |
| Used EHRs to manage patients with prediabetes | ||||
| Yes | 40.4* | 38.8*** | 99.0*** | 35.8*** |
| No/don’t know | 59.6 | 17.4 | 95.0 | 13.7 |
Boldface indicates statistical significance (*p< 0.05; **p< 0.01; ***p< 0.001) using Pearson χ2 test of difference between each category of independent variables among total PCPs, PCPs who screened for prediabetes versus PCPs who did not screen, PCPs who tested for prediabetes versus PCPs who did not test, and PCPs who made referrals versus PCPs who did not refer.
Non-Hispanic other race/ethnicity includes multiracial, non-Hispanic Asian, non-Hispanic Native Hawaiian or other Pacific Islander, non-Hispanic American Indian or Alaska Native, and non-Hispanic other race.
CDC, Centers for Disease Control and Prevention; EHRs, electronic health records; LCP, lifestyle change program; PCPs, primary care providers (family practitioners, internists, and nurse practitioners).
There were significant differences in the proportion of PCPs who screened using a risk test by race/ethnicity (22.8% of non-Hispanic whites, 39.0% of non-Hispanic blacks, p< 0.001) and use of EHRs (17.4% of those not using EHRs vs 38.8% of those using them, p< 0.001). Differences in the proportion who ordered blood tests for prediabetes were identified between NPs and physicians (92.9% vs 97.6%, p< 0.001); by work setting (93.5% inpatient practice, 97.6% individual outpatient settings, p= 0.025); and by use of EHRs (95.0% vs 99.0%, p< 0.001). Differences in the proportion of PCPs who referred patients to the LCP were found by region of practice (ranging from 20.1% in the Northeast to 29.5% in the West, p= 0.044); ratio of CDC-recognized LCP classes to total primary care physicians (21.9% in areas with low ratios vs 28.8% in areas with high ratios, p= 0.032); and use of EHRs (13.7% vs 35.8%, p< 0.001).
Table 2 compares awareness and self-reported behaviors regarding prediabetes screening, testing, and referral between NPs and physicians. There were no significant differences in awareness of the CDC-recognized LCP or the STAT toolkit, or for screening or referral of patients. However, a significantly lower percentage of NPs ordered blood tests for prediabetes compared with physicians (92.9% vs 97.6%, p< 0.001). Among PCPs who had heard of the CDC-recognized LCP, 40.9% (vs 18.0% of those who had not, p< 0.001) screened for prediabetes using a risk test; 97.3% (vs 96.3%, p 0.34) ordered blood tests; and 39.8% (vs 12.7%, p< 0.001) referred to an LCP class. Of those who had heard of the STAT toolkit, 65.8% (vs 17.6%, p< 0.001) screened; 99.2% (vs 96.1%, p= 0.018) tested; and 56.5% (vs 15.2%, p< 0.001) referred patients. PCPs who screened patients for prediabetes were more likely to test (99.7% vs 95.6%, p< 0.001) and refer (47.5% vs 14.1%, p< 0.001) than those who did not screen. Compared with PCPs who did not test, those who tested were more likely to screen (27.5% vs 2.4%, p< 0.001) and refer (23.7% vs 2.4%, p= 0.001). Finally, PCPs who referred patients to an LCP class were more likely to screen (55.0% vs 18.2%, p< 0.001) and test (99.7% vs 95.8%, p= 0.001) than those not referring.
Table 2.
PCP Awareness and Behaviors Toward Screening, Testing, and Referring Patients to the CDC-Recognized LCP
| PCP awareness and behaviors |
Provider type | PCP behaviors | |||
|---|---|---|---|---|---|
| Nurse practitioners, % (n=253) |
Physicians (family physicians and internists), % (n=1,003) |
Screen for prediabetes, % (n=335) | Test for prediabetes, % (n=1,214) |
Refer to LCP, % (n=289) |
|
| PCP awareness | |||||
| Heard of CDC-recognized LCP | |||||
| Yes | 38.3 | 37.9 | 40.9*** | 97.3 | 39.8*** |
| No/don’t know | 61.7 | 62.1 | 18.0 | 96.3 | 12.7 |
| Heard of AMA/CDC Prevent Diabetes STAT toolkit | |||||
| Yes | 22.9 | 17.9 | 65.8*** | 99.2* | 56.5*** |
| No/don’t know | 77.1 | 82.1 | 17.6 | 96.1 | 15.2 |
| PCP behaviors | |||||
| Screen patients for prediabetes | |||||
| Yes | 22.9 | 27.6 | — | 99.7*** | 47.5*** |
| No/don’t know | 77.1 | 72.4 | - | 95.6 | 14.1 |
| Test patients for prediabetes | |||||
| Yes | 92.9*** | 97.6*** | 27.5*** | — | 23.7** |
| No/don’t know | 7.1 | 2.4 | 2.4 | — | 2.4 |
| Refer patients with prediabetes to the CDC-recognized LCP | |||||
| Yes | 23.3 | 22.9 | 55.0*** | 99.7** | — |
| No/don’t know | 76.7 | 77.1 | 18.2 | 95.8 | — |
Boldface indicates statistical significance (*p< 0.05; **p< 0.01; ***p< 0.001) using Pearson χ2 test of differences between each category of independent variables among nurse practitioners versus physicians, PCPs who screened for prediabetes versus PCPs who did not screen, PCPs who tested for prediabetes versus PCPs who did not test, and PCPs who made referrals versus PCPs who did not refer.
AMA, American Medical Association; CDC, Centers for Disease Control and Prevention; LCP, lifestyle change program; PCPs, primary care providers (family practitioners, internists, and nurse practitioners); STAT, Screen patients for prediabetes using a risk test, Test patients for prediabetes using a blood glucose test, and Act Today by referring patients with prediabetes to the CDC-recognized lifestyle change program.
Table 3 shows the results from multivariate analyses with AORs and 95% CIs for PCP behaviors among those who use EHRs. Screening patients for prediabetes using a risk test was significantly higher among PCPs who had heard of the CDC-recognized LCP (AOR= 1.43, 95% CI= 1.03, 1.98) and the STAT toolkit (AOR= 7.30, 95% CI= 5.04, 10.58), compared with those who had not, after controlling for other factors. The odds of screening was significantly higher among those who used EHRs to manage patients with prediabetes (AOR= 2.23, 95% CI= 1.62, 3.06) compared with those who did not, and among PCPs who saw mostly patients with household incomes of $50,000–$99,999 (AOR= 1.93, 95% CI= 1.33, 2.81) compared with those who saw patients with incomes ≥$100,000. PCPs who screened patients for prediabetes (AOR= 8.02, 95% CI= 1.04, 61.64) and who used EHRs for prediabetes management (AOR= 4.09, 95% CI= 1.52, 11.01), compared with those who did not, were more likely to test for prediabetes. NPs were less likely than physicians to test for prediabetes (AOR= 0.21, 95% CI= 0.08, 0.56). The odds of referring patients with prediabetes to a CDC-recognized LCP class were significantly higher among PCPs who had heard of the CDC-recognized LCP (AOR= 2.21, 95% CI= 1.57, 3.11) and the STAT toolkit (AOR= 2.96, 95% CI= 1.99, 4.38), among those who screened for prediabetes (AOR= 2.99, 95% CI= 2.09, 4.26) or used EHRs for prediabetes management (AOR= 2.07, 95% CI= 1.48, 2.92), and among PCPs who practiced in areas with a high ratio of CDC-recognized LCP classes to total primary care physicians (AOR= 1.85, 95% CI= 1.22, 2.81). PCPs who practiced in the Northeast region (AOR= 0.57, 95% CI= 0.35, 0.92) were less likely to make referrals, compared with those in the West. There were no significant differences in the odds of screening, testing, and referring based on PCPs’ gender, years practicing medicine, or work setting.
Table 3.
AOR for Prediabetes Screening, Testing, and Referral Behaviors Among PCPs Using EHRs (n=1,107)
| Predictors | Screen for prediabetes, AOR (95% CI) |
Test for prediabetes, AOR (95% CI) |
Refer to LCP, AOR (95% CI) |
|---|---|---|---|
| PCP awareness | |||
| Heard of CDC-recognized LCP | |||
| Yes | 1.43 (1.03, 1.98)* | 0.74 (0.34, 1.60) | 2.21 (1.57, 3.11)*** |
| No/don’t know (ref) | 1.00 | 1.00 | 1.00 |
| Heard of AMA/CDC Prevent Diabetes STAT toolkit | |||
| Yes | 7.30 (5.04, 10.58)*** | 2.08 (0.45, 9.58) | 2.96 (1.99, 4.38)*** |
| No/don’t know (ref) | 1.00 | 1.00 | 1.00 |
| PCP behaviors | |||
| Screen patients for prediabetes | |||
| Yes | - | 8.02 (1.04, 61.64)* | 2.99 (2.09, 4.26)*** |
| No/don’t know (ref) | - | 1.00 | 1.00 |
| Test patients for prediabetes | |||
| Yes | - | - | 6.12 (0.78, 48.08) |
| No/don’t know (ref) | - | - | 1.00 |
| Practice and provider characteristics | |||
| Ratio of CDC-recognized LCP classes to total primary care physicians at practice ZIP code | |||
| High (Q2-Q3) | 0.90 (0.60, 1.37) | 0.73 (0.30, 1.79) | 1.85 (1.22, 2.81)** |
| Low (Q1) (ref) | 1.00 | 1.00 | 1.00 |
| Used EHR to manage patients with prediabetes | |||
| Yes | 2.23 (1.62, 3.06)*** | 4.09 (1.52, 11.01)** | 2.07 (1.48, 2.92)*** |
| No/don’t know (ref) | 1.00 | 1.00 | 1.00 |
| Gender | |||
| Male (ref) | 1.00 | 1.00 | 1.00 |
| Female | 1.07 (0.75, 1.52) | 1.32 (0.53, 3.28) | 1.19 (0.82, 1.73) |
| Race/ethnicity | |||
| Hispanic | 1.22 (0.57, 2.61) | 0.91 (0.11, 7.37) | 0.54 (0.23, 1.26) |
| Non-Hispanic black | 2.35 (0.99, 5.61) | 1.04 (0.12, 8.78) | 0.69 (0.26, 1.81) |
| Non-Hispanic othera | 1.44 (1.02, 2.03)* | 0.79 (0.32, 1.99) | 0.94 (0.65, 1.36) |
| Non-Hispanic white (ref) | 1.00 | 1.00 | 1.00 |
| Region | |||
| Northeast | 1.04 (0.66, 1.64) | 0.99 (0.31, 3.12) | 0.57 (0.35, 0.92)* |
| Midwest | 0.95 (0.59, 1.54) | 0.45 (0.15, 1.38) | 0.81 (0.50, 1.32) |
| South | 0.93 (0.60, 1.44) | 1.46 (0.44, 4.82) | 0.72 (0.46, 1.13) |
| West (ref) | 1.00 | 1.00 | 1.00 |
| Provider type | |||
| Primary care physician (ref) | 1.00 | 1.00 | 1.00 |
| Nurse practitioner | 0.72 (0.45, 1.13) | 0.21 (0.08, 0.56)** | 0.94 (0.58, 1.50) |
| Years practicing medicine | |||
| <15 years (ref) | 1.00 | 1.00 | 1.00 |
| ≥15 years | 1.26 (0.92, 1.74) | 1.03 (0.50, 2.14) | 1.39 (1.00, 1.95) |
| Work setting | |||
| Individual outpatient practice | 0.77 (0.45, 1.33) | 2.77 (0.72, 10.67) | 0.95 (0.52, 1.74) |
| Group outpatient practice | 0.69 (0.45, 1.06) | 1.82 (0.81, 4.10) | 1.01 (0.63, 1.62) |
| Inpatient practice (ref) | 1.00 | 1.00 | 1.00 |
| Patient household income | |||
| ≤$49,999 | 1.40 (0.95, 2.08) | 1.14 (0.47, 2.79) | 0.89 (0.59, 1.34) |
| $50,000–$99,999 | 1.93 (1.33, 2.81)*** | 0.82 (0.35, 1.92) | 0.87 (0.59, 1.29) |
| ≥$100,000 (ref) | 1.00 | 1.00 | 1.00 |
Boldface indicates statistical significance (*p< 0.05; **p< 0.01; ***p< 0.001). Data are presented as AOR (95% CI).
Non-Hispanic Other race/ethnicity includes multiracial, non-Hispanic Asian, non-Hispanic Native Hawaiian or other Pacific Islander, non-Hispanic American Indian or Alaska Native, or non-Hispanic other race.
AMA, American Medical Association; CDC, Centers for Disease Control and Prevention; EHRs, electronic health records; LCP, lifestyle change program; PCPs, primary care providers (family practitioners, internists, and nurse practitioners); STAT, Screen patients for prediabetes using a risk test, Test patients for prediabetes using a blood glucose test, and Act Today by referring patients with prediabetes to the CDC-recognized lifestyle change program.
DISCUSSION
This study utilized the 2016 DocStyles survey to examine patterns in PCPs’ self-reported rates of screening, testing, and referring patients with prediabetes to a CDC-recognized LCP class. Overall, 97% of PCPs tested for prediabetes by ordering one of the three recommended blood tests, whereas fewer than one third (27%) screened patients for prediabetes using the CDC Prediabetes Screening Test or the ADA Type 2 Diabetes Risk Test, and consistent with prior research,23 fewer than one quarter (23%) referred patients to CDC-recognized LCP classes.
PCPs who had heard of the CDC-recognized LCP and STAT toolkit were more likely to screen patients for prediabetes, consistent with a study showing that physicians who had a positive attitude toward prediabetes as a clinical construct were more likely to follow national guidelines for screening.25 There was no significant difference in testing behavior based on PCPs’ reported awareness of the CDC-recognized LCP and the STAT toolkit, perhaps because of near universal testing behavior (97%) in the study sample. However, NPs were less likely than physicians to test for prediabetes, consistent with prior research on rates of hemoglobin A1c testing for patients with diabetes.26 Similar to another study,27 39.8% and 56.5% of PCPs who had heard of the CDC-recognized LCP and the STAT toolkit, respectively, made referrals. Moreover, referral was associated with a practice being located in an area with a high ratio of CDC-recognized LCP classes to primary care physicians. The finding that PCPs who practiced in the Northeast (versus West) region were less likely to refer may be because of greater availability of CDC-recognized LCP classes28and focused AMA–CDC stakeholder engagements in the West.29
To the authors’ knowledge, this is the first study to assess PCP awareness and self-reported behaviors regarding prediabetes screening, testing, and referral to the CDC-recognized LCP. The overall results suggest there is an opportunity to increase PCP awareness of the CDC-recognized LCP and the STAT toolkit. More targeted efforts by the AMA–CDC STAT initiative to reach the physician market by collaborating with state medical societies and health departments may help increase awareness and referrals,30 given that this study shows PCPs who had heard of the CDC-recognized LCP and the STAT toolkit were two to three times more likely to refer patients with prediabetes to LCP classes. The low percentage (26.7%) of PCPs who reported screening patients for prediabetes using a risk test may be attributed to their preference for laboratory testing, as 97% reported ordering blood tests for prediabetes, although those who screened were more likely to order blood tests and make referrals than those who did not. This was consistent with AMA’s observation that the risk screener is primarily intended to be used in the community setting as opposed to clinical practice. Finally, this study also found that PCPs who saw patients with incomes of $50,000–$99,999 (versus ≥$100,000) were more likely to screen patients for prediabetes, which may suggest increased screening practices for people with lower incomes, where there is higher risk for type 2 diabetes.31,32
In addition, 88% of 1,256 PCPs reported using EHRs at their practices, and 40.4% of those PCPs used them to manage patients with prediabetes. These PCPs were more likely to screen, test, and refer, suggesting that access to referral systems that support prediabetes management may facilitate these practices. This finding is consistent with a recent study of lifestyle intervention in primary care, which found that intervention centers struggled to implement in-house referral structures for lifestyle promotion despite some effectiveness in increasing positive attitudes and competency among staff.33 Another study designed to increase referrals from federally qualified health centers to a YMCA-based LCP found that modifying the electronic referral system and implementing provider education significantly increased patient referrals.34 This is also consistent with AMA’s experience in working directly with more than 20 healthcare delivery organizations to implement systematic prediabetes screening, testing, and referral initiatives. Challenges in adapting an EHR to support screening, testing, and referring are a key barrier. Other barriers such as patients’ limited economic resources,25 incomplete insurance coverage for the CDC-recognized LCP, and lack of referral loops may also contribute to the overall low referral rate. To address these issues, the Centers for Medicare & Medicaid Services began accepting applications from qualified CDC-recognized organizations providing the LCP in January 2018 to implement the Medicare DPP, and in April 2018 expanded coverage for eligible Medicare beneficiaries.35 Lessons learned from Medicare DPP expansion may provide opportunities to tackle financial barriers in other segments of the population.
Limitations
There are several limitations in this study. First, PCP awareness of the CDC-recognized LCP and the STAT toolkit, and behaviors toward screening, testing, and referring, were self-reported; thus, results may be subject to social desirability bias in favor of what is recommended in medical practice. Second, survey results reported here may not be generalizable to all PCPs in the U.S. because DocStyles sampling methodology uses quotas per specialty to limit the number of completed responses. Finally, limitations exist in web-based survey platforms; however, primary care physicians sampled were similar demographically to those in the 2016 AMA Masterfile (data not shown) and survey response rates were high.
CONCLUSIONS
This study highlights the importance of increasing PCP awareness of and referrals to the CDC-recognized LCP. As the nation continues to expand its efforts toward type 2 diabetes prevention, PCPs will be called on to play an ever-growing critical role in prediabetes screening and testing, and referral to the CDC-recognized LCP.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the members of the Centers for Disease Control and Prevention (CDC)/the American Medical Association (AMA) Strategic Collaboration Workgroup, and the CDC’s National Diabetes Prevention Program Team who contributed to the design of the 2016 DocStyles survey items and validation and aggregation of the National Diabetes Prevention Program data used in this study. In addition, the authors would like to acknowledge the contributions of Annalynn Skipper from AMA and Deanne Weber from Porter Novelli Public Services for their review, as well as the CDC-recognized organizations for collecting and submitting the program data used in this study.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC or the AMA.
No financial disclosures were reported by the authors of this paper.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j. amepre.2018.04.017.
The DocStyles survey instrument was developed by Porter Novelli with technical guidance provided by federal public health agencies and other non-profit and for-profit clients.
SERMO is a global market research company Porter Novelli contracted with to verify their active panelists by telephone confirmation at place of work, and send them invitations with a link to the web-based survey. Quotas were set to reach ≅1,000 PCPs, 250 pediatricians, 250 obstetricians/gynecologists, 250 NPs, 150 retail pharmacists, and 100 hospital pharmacists.
The Diabetes Prevention Recognition Program is the quality assurance arm of the National DPP, through which CDC awards recognition to organizations that are able to meet the National Standards and achieve quality outcomes. www.cdc.gov/diabetes/prevention/lifestyle-program/requirements.html.
REFERENCES
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. https://doi.org/10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report. 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed August 14, 2017.
- 3.Centers for Disease Control and Prevention (CDC). Diabetes Report Card 2014. www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Accessed August 14, 2017.
- 4.Nichols GA, Brown JB. Higher medical care costs accompany impaired fasting glucose. Diabetes Care. 2005;28(9):2223–2229. https://doi.org/10.2337/diacare.28.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Dall TM, Chen Y, et al. Medical cost associated with prediabetes. Popul Health Manag. 2009;12(3):157–163. https://doi.org/10.1089/pop.2009.12302. [DOI] [PubMed] [Google Scholar]
- 6.Khan T, Tsipas S, Wozniak G. Medical care expenditures for individuals with prediabetes: the potential cost savings in reducing the risk of developing diabetes. Popul Health Manag. 2017;20(5):389–396. https://doi.org/10.1089/pop.2016.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med. 2013;44(4 suppl 4):S346–S351. https://doi.org/10.1016/j.amepre.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. https://doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Name MA, Camp AW, Magenheimer EA, et al. Effective translation of an intensive lifestyle intervention for Hispanic women with prediabetes in a community health center setting. Diabetes Care. 2016;39(4):525–531. https://doi.org/10.2337/dc15-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattin RW, Williams LB, Dias J, et al. Community trial of a faith-based lifestyle intervention to prevent diabetes among African-Americans. J Community Health. 2016;41(1):87–96. https://doi.org/10.1007/s109 00-015-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall DL, Lattie EG, McCalla JR, Saab PG. Translation of the Diabetes Prevention Program to ethnic communities in the United States. J Immigr Minor Health. 2016;18(2):479–489. https://doi.org/10.1007/s10903-015-0209-x. [DOI] [PubMed] [Google Scholar]
- 12.Fianu A, Bourse L, Naty N, et al. Long-term effectiveness of a lifestyle intervention for the primary prevention of type 2 diabetes in a low socio-economic community—an intervention follow-up study on Reunion Island. PLoS One. 2016;11(1):e0146095 https://doi.org/10.1371/journal.pone.0146095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiBenedetto JC, Blum NM, O’Brian CA, Kolb LE, Lipman RD. Achievement of weight loss and other requirements of the Diabetes Prevention and Recognition Program: a National Diabetes Prevention Program Network based on nationally certified diabetes self-management education programs. Diabetes Educ. 2016;42(6):678–685. https://doi.org/10.1177/0145721716668415. [DOI] [PubMed] [Google Scholar]
- 14.Gong QH, Kang JF, Ying YY, et al. Lifestyle interventions for adults with impaired glucose tolerance: a systematic review and meta-analysis of the effects on glycemic control. Intern Med. 2015;54(3):303–310. https://doi.org/10.2169/internalmedicine.54.2745. [DOI] [PubMed] [Google Scholar]
- 15.Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci 2015;10:172 https://doi.org/10.1186/s13012-015-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75. https://doi.org/10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 17.Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–1341. https://doi.org/10.2337/dc16-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siu AL. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(11):861–868. https://doi.org/10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 19.Pronk NP, Remington PL. Combined diet and physical activity promotion programs for prevention of diabetes: Community Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(6):465–468. https://doi.org/10.7326/M15-1029. [DOI] [PubMed] [Google Scholar]
- 20.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–451. https://doi.org/10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, Groom HCUS Preventive Services Task Force evidence syntheses, formerly systematic evidence reviews. Behavioral Counseling to Promote a Healthy Lifestyle for Cardiovascular Disease Prevention in Persons With Cardiovascular Risk Factors: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 22.Schmittdiel JA, Adams SR, Segal J, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care. 2014;37(2):565–568. https://doi.org/10.2337/dc13-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mainous Tanner RJ 3rd, Baker R Prediabetes diagnosis and treatment in primary care. J Am Board Fam Med. 2016;29(2):283–285. https://doi.org/10.3122/jabfm.2016.02.150252. [DOI] [PubMed] [Google Scholar]
- 24.Leonard C, Stordeur S, Roberfroid D. Association between physician density and health care consumption: a systematic review of the evidence. Health Policy. 2009;91(2):121–134. https://doi.org/10.1016/j.healthpol.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Mainous Tanner RJ 3rd, Scuderi CB, Porter M, Carek PJ. Prediabetes screening and treatment in diabetes prevention: the impact of physician attitudes. J Am Board Fam Med. 2016;29(6):663–671. https://doi.org/10.3122/jabfm.2016.06.160138. [DOI] [PubMed] [Google Scholar]
- 26.Kuo YF, Goodwin JS, Chen NW, Lwin KK, Baillargeon J, Raji MA. Diabetes mellitus care provided by nurse practitioners vs primary care physicians. J Am Geriatr Soc. 2015;63(10):1980–1988. https://doi.org/10.1111/jgs.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafez D, Nelson DB, Martin EG, Cohen AJ, Northway R, Kullgren JT. Understanding type 2 diabetes mellitus screening practices among primary care physicians: a qualitative chart-stimulated recall study. BMC Fam Pract. 2017;18(1):50 https://doi.org/10.1186/s12875-017-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). Diabetes Prevention Recognition Program—Registry of Recognized Organizations. https://nccd.cdc.gov/DDT_DPRP/Registry.aspx. Accessed January 28, 2018.
- 29.California Department of Public Health. Diabetes prevention in California: promoting awareness and scaling-up the National Diabetes Prevention Program. https://rightcare.berkeley.edu/wp-content/uploads/2016/06/RightCareInitiativeUBP-DiabetesPrev-6-13-16.pdf. Accessed January 28, 2018.
- 30.American Medical Association. AMA launches multi-state effort to prevent type 2 diabetes. Press release. www.ama-assn.org/ama-launches-multi-state-effort-prevent-type-2-diabetes. Published 2017. Accessed January 28, 2018.
- 31.Fisher-Hoch SP, Vatcheva KP, Rahbar MH, McCormick JB. Undiag-nosed diabetes and pre-diabetes in health disparities. PLoS One. 2015;10(7):e0133135 https://doi.org/10.1371/journal.pone.0133135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moody A, Cowley G, Ng Fat L, Mindell JS. Social inequalities in prevalence of diagnosed and undiagnosed diabetes and impaired glucose regulation in participants in the Health Surveys for England series. BMJ Open. 2016;6(2): e010155 https://doi.org/10.1136/bmjopen-2015-010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas K, Krevers B, Bendtsen P. Implementing healthy lifestyle promotion in primary care: a quasi-experimental cross-sectional study evaluating a team initiative. BMC Health Serv Res. 2015;15:31 https://doi.org/10.1186/s12913-015-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers EC, Wylie-Rosett J, Blank AE, et al. Increasing referrals to a YMCA-based diabetes prevention program: effects of electronic referral system modification and provider education in federally qualified health centers. Prev Chronic Dis. 2015;12:E189 https://doi.org/10.5888/pcd12.150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Medicare and Medicaid Services. Revisions to payment policies under the Physician Fee Schedule and other revisions to Part B for CY 2018; Medicare Shared Savings Program Requirements; and Medicare Diabetes Prevention Program. www.gpo.gov/fdsys/pkg/FR-2017-11-15/pdf/2017-23953.pdf. Accessed November 20, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


