Abstract
During the pulsed-electron beam direct grafting of neat styrene onto poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP) substrate, the radiolytically-produced styryl and carbon-centered FEP radicals undergo various desired and undesired competing reactions. In this study, a high-dose rate is used to impede the undesired free radical homopolymerization of styrene and ensure uniform covalent grafting through 125-µm FEP films. This outweighs the enhancement of the undesired crosslinking reactions of carbon-centered FEP radicals and the dimerization of the styryl radicals. The degree of uniform grafting through 125- µm FEP films increases from ≈8%, immediately after pulsed electron irradiation to 33% with the subsequent thermal treatment exceeding the glass transition temperature of FEP of 39°C. On the contrary, steady-state radiolysis using 60Co gamma radiolysis, shows that the undesired homopolymerization of the styrene has become the predominant reaction with a negligible degree of grafting. Time-resolved fast kinetic measurements on pulsed neat styrene show that the styryl radicals undergo fast decays via propagation homopolymerization and termination reactions at an observed reaction rate constant of 5 × 108 l mol−1 s−1. The proton conductivity of 25- µm film at 80°C is 0.29 ± 0.01 s cm−1 and 0.007 s cm−1 at relative humidity of 92% and 28%, respectively. The aims of this work are: 1. electrolyte membranes are prepared via grafting initiated by a pulsed electron beam; 2. postirradiation heat-treated membranes are uniformly grafted, ideal for industry; 3. High dose rate is the primary parameter to promote the desired reactions; 4. measurement of kinetics of undesired radiation-induced styrene homopolymerization; and 5. The conductivity of prepared membranes is on par or higher than industry standards.
INTRODUCTION
For a material to be a viable candidate for use as a proton exchange membrane within a fuel cell, it must have high proton conductivity (approximately 10 ms cm−1) and be impermeable to hydrogen and oxygen (1–4). Additionally, it must display high chemical and thermal stability, high mechanical strength and be compatible with all of the components in a fuel cell. The conventional means of producing such membranes are based on wet chemical processing techniques, which require initiator chemicals and are limited in terms of temperature and monomer choices. The production of membranes using radiation grafting removes the need for initiators, which can add residual impurities to the product, and offers the ability to employ a wide range of reaction temperatures and monomers (5–10). However, the existence of additional undesired degradation and crosslinking reactions must be taken into consideration when radiation techniques are employed for grafting reactions (11, 12).
Recently, there has been a great deal of interest in the optimization of radiation grafting of fluoropolymers and styrene derivatives for fuel cell membrane applications (13–15). Published reviews by Gubler, Nasef and Kabanov describe the application of radiation towards the grafting of styrene onto various fluorocarbon polymeric substrates (16–20). The radiation grafting of styrene onto the poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP) polymer substrate produces membranes with a high degree of homogeneity, although there is a sharp decrease in resistivity upon an increase in the degree of grafting (21–23). The decrease in resistivity is attributed to an increase in proton mobility, which is associated with an increase in the hydration of the sulfonic acid groups within the membrane (24, 25).
The published works of Lyons and Sheirs includes a detailed discussed on the radiation chemistry of FEP in the presence and absence of oxygen at various doses and dose rates (26, 27). The radiolytic chemical structures and the radiation chemical yields (G values) of irradiated FEP have been reviewed elsewhere (28, 29). Radiolysis of FEP yields various types of C-centered radicals. Along the backbone of the FEP chains, the scissions of the C-C bonds produce chain-end primary alkyl radicals, and the scissions of the C-F bonds produce secondary alkyl radicals within the chain. The cleavage of the C-C bonds directly decreases the average molecular weight and subsequently leads to a decrease in the mechanical properties of the FEP. The original published work of Florin and Wall showed that at radiation doses up to 160 kGy, FEP undergoes crosslinking rather than scission (30) due to the presence of the hexafluoropropylene group. Therefore, C-centered free radicals produced on the backbone of the chain through C-F scission is more probable than that of C-C ruptures, as is the case in tetrafluorpolyethylene. This makes FEP a very suitable material for the synthesis of membranes via ionizing radiation. This suggestion has been supported by the fact that the CF4 yield of irradiated FEP is 10 times higher than irradiated polytetrafluoroethylene (PTFE) (31). In general, at very high doses of 2,000 kGy and above, the G value was estimated for scission to be G(S) = 1 per 100 eV (32). Furthermore, there was only a 25% decrease in the FEP tensile strength at the high dose of 100 kGy (33). Therefore, it can be concluded FEP is a viable substrate for radiation grafting, especially when the doses are lower than 100 kGy. In our work, the maximum dose is approximately 80 kGy.
During the direct irradiation of styrene and FEP, as shown in Fig. 1, both species are expected to form C-centered free radicals. The high electronegativity of fluorine causes the C-F bonds in FEP to be sensitive to radiation-induced scission on the backbone of the FEP chains. As a result, FEP is expected to generate radicals much more rapidly and with higher yields than styrene.
FIG. 1.
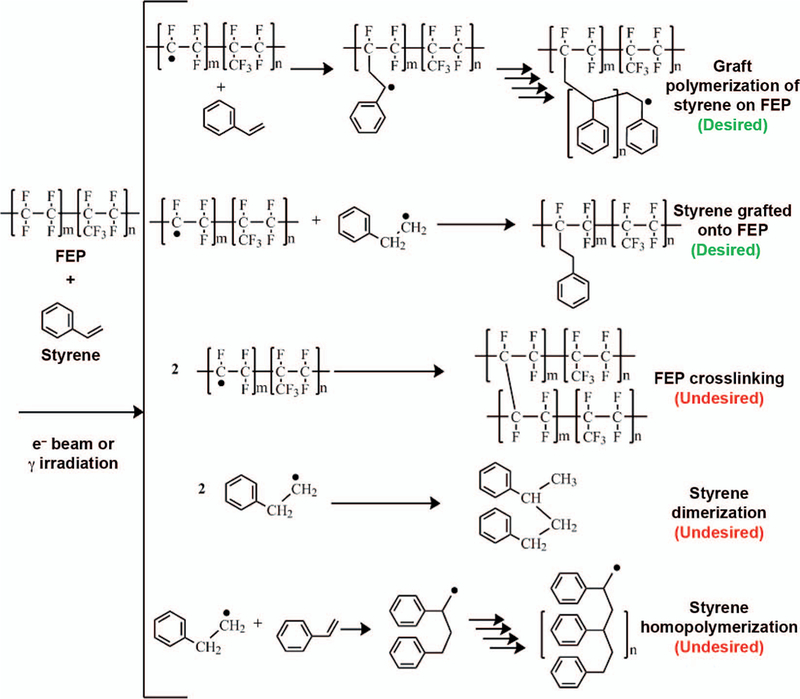
A selection of possible chemical reactions between FEP and styrene during direct and indirect irradiation. The high-energy electrons may interact with either FEP or styrene to produce an excited state species that will result in radical species. These radical species undergo propagation reactions that result in the polymerization of styrene, as shown in the desired polymerization of styrene on FEP and the undesired homopolymerization. There are also desired termination reactions such as the grafting of styrene, or the undesired FEP crosslinking.
In radiation grafting, the penetration of the monomer into polymer substrates is greatly enhanced at temperatures above the glass transition temperature (Tg) because the amorphous region of the polymer is converted from a rigid to a rubbery form. The transport of the grafting species has important implications for the uniformity of the grafted monomer throughout the film and it affects the final properties of the product. There are different techniques that may be employed to produce such grafted materials. These include: 1. direct irradiation of the substrate immersed in the monomer; 2. preirradiation of the substrate followed by introduction of the monomer; or 3. preirradiation of the substrate followed by introduction of the monomer and then an additional dose (34). These techniques are commonly referred to as direct, indirect and combined indirect and direct. The radiation parameters may be adjusted for an optimized chemical composition, uniformity and proton conductivity of the resulting styrene-grafted FEP material.
Competing reactions during electron-beam irradiation, as shown in Fig. 1, include desired reactions, which enhance the covalently bonded styrene onto FEP, and undesired reactions, which enhance the homopolymerization and crosslinking. Once the homopolymerization is removed by washing, the added mass is quantified as the degree of grafting (DOG). In the current study, the mechanism of the radiation grafting of styrene to FEP is further investigated. In particular, the effects of the radiation dose, dose rate and postirradiation heat treatment on the yield and homogeneity of the graft within the substrate are explored.
MATERIALS AND METHODS
Materials
FEP substrate sheets of 125 µm (CS Hyde Co., Lake Villa, IL) and 25 µm (DuPont, Wilmington, DE) thicknesses were cut into 2 × 4 cm rectangular segments. The density was reported to be 2,150 kg/m3 with approximately 35% crystallinity. While the 25- µm films are more representative of common membranes used in fuel cell applications, the thicker 125-µm films allow for a more detailed characterization of the progress of the grafting process. Styrene, xylene, chlorosulfonic acid and dichloromethane (Sigma-Aldrich® LLC, St. Louis, MO) were used as received.
Radiation Grafting
Samples where irradiated at the radiation facilities of the University of Maryland (College Park, MD). Two types of ionizing radiation were used in this work: 1. electron radiation from a pulsed electron linear accelerator (model no. 5V-7715, energy of 7 MeV, pulse length of 3 µs, pulse frequency of 60 Hz, dose of 2–4 Gy per pulse; Varian Medical Systems Inc., Palo Alto, CA); and 2. steady-state radiolysis using gamma radiation from a cobalt-60 (60Co) source (model no. 200364, 1.7 × 1013 Bq activity, 0.17 Gy/s dose rate in water; Neutron Products Inc., Dickerson, MD). The absorbed dose was calibrated using nine film dosimeters (Far West Technology Inc., Goleta, CA) placed in an array that covered the area of the FEP film.
For both electron-beam and 60Co-gamma irradiation, the FEP films were fully immersed in argon-saturated solutions of styrene in heat-sealed aluminized polyethylene bags. Immediately after irradiation, the sealed specimens were placed in an oven at 60°C for varying periods of time up to 25 h. After this postirradiation heat treatment, the films were removed from the aluminized polyethylene bags, sonicated in a xylene bath to remove residual styrene monomer and homopolymer not grafted to the substrate, and air-dried at room temperature. Dosimetry was performed using FWT-60 radiochromic films traceable to National Institute of Standards and Technology. All reported doses have an uncertainty of 3%.
Sulfonation
The styrene-grafted FEP films were sulfonated by immersing them in a boiling 0.2 M solution of chlorosulfonic acid in dichloromethane at 40°C for 8 h. After washing with water, the sulfonated films were hydrated in water at 80°C for 12 h. These samples were stored in deionized water at room temperature.
Characterization
Degree of grafting.
The DOG was determined through at least three measurements of the difference between the weight of the film before grafting, W0, and its weight after grafting, Wg, according to Eq. (1):
| (1) |
Morphology.
Analysis of changes in the morphology of the surface and cross-section of the grafted films as a function of various preparation conditions was performed using atomic force microscopy (AFM) and scanning electron microscopy (SEM). Samples were embedded in epoxy and sputtered with gold prior to SEM analysis. Energy dispersive X-ray spectroscopy (EDS) was employed to measure the chemical composition of the material along its cross section, thereby confirming the graft uniformity as a function of depth.
Chemical analysis.
The samples were characterized by Fourier transform infrared spectroscopy (FTIR) using a Nicolet Magna 550 FTIR spectrometer to verify successful formation of the grafted material. Each spectrum was determined by averaging the data obtained in 32 scans, each having a resolution of 4 cm−1. The transmittance spectra were collected for both the entire 125-µm thick film and for 10-µm microtome slices.
X-ray photoelectron spectroscopy (XPS) was performed using a Kratos Axis 165 X-ray photoelectron spectrometer operating in hybrid mode with monochromated aluminum Kα X rays (1,486.6 eV) at 300 W. Charge neutralization was used to minimize sample charging and the pressure of the system was maintained at 1 × 10−8 Torr (1.3 × 10−6 Pa) or lower throughout the measurement. Survey spectra and highresolution spectra were collected at pass energies of 160 eV and 20 eV, respectively. Some sample degradation was noted during XPS data collection, as reflected in a gradual decrease in the C/F ratio, accompanied by changes in the C 1s peak shape and yellowing of the sample with increased analysis time (35). Therefore, the XPS data presented here were collected from samples that were X-ray irradiated for a maximum of 20 min to ensure minimal changes to the C/F ratio.
Determination of Tg.
To determine the value of the Tg of the FEP film, differential scanning calorimetry (DSC) measurements were performed on a TA Instruments Q100 DSC (New Castle, DE). Approximately 5 mg of FEP film was placed in aluminum hermetic pans in small pieces. Each sample, under nitrogen purge, was heated up to 300°C at a rate of 10°C /min and cooled back to 0°C at a rate of 5°C /min and cycled through the temperatures three times.
Proton conductivity.
Proton conductivity measurement, performed by the 3M Company (Maplewood, MN), was performed using an alternating current impedance method using a standard, in-plane, fourpoint probe conductivity apparatus with platinum electrodes. The cell was electrically connected to a potentiostat (model no. 273; Princeton Applied Research/Ametek® Inc., Berwyn, PA) and an Impedance/ Gain-Phase Analyzer (SI 1260; Schlumberger/Solartron, Hampshire, UK). Alternating current impedance measurements were performed using ZPlot® and ZView® software (Scribner Assoc. Inc., Southern Pines, NC). Temperature and relative humidity were controlled using a temperature/humidity chamber (model no. 1007H; TestEquity LLC, Moorpark, CA) (36). Measurements of conductivity were taken at 80°C with 10% intervals where the relative humidity cycled from an initial relative humidity of 70% to 20%, then to 90%. The results of the styrene-grafted films were compared with those obtained with a 25-µm perfluoro sulfonic acid (PFSA) membrane Nafion® 112 and the PFSA-type 3M ionomer with an equivalent weight (EW) of 825 (825EW) (37, 38), typical of membranes used in fuel cell applications.
Pulse Radiolysis
The pulse radiolysis of styrene samples with optical detection was performed at room temperature using a 2-MeV Febetron with an absorbed dose of 300 Gy per 50 ns pulse width at the National Institute of Standards and Technology (NIST; Gaithersburg, MD). The setup has been described in detail elsewhere (39).
RESULTS
Direct Grafting of Styrene onto FEP Using Pulsed-Electron Beam
The degree of grafting of 125-µm-thick FEP films grafted simultaneously with styrene using 50 kGy electron-beam irradiation (applied over a time period of approximately 200 s to 400 s in pulses of 2–4 Gy at a frequency of 60 Hz) is shown as a function of postirradiation heat treatment temperature and time in Fig. 2. The Tg of the FEP film was determined to be 39 ± 0.5°C via three independent DSC measurements. Therefore, the FEP film is in a glassy form at room temperature and not glassy at 60°C. The DOG of the FEP film at room temperature (glassy form) does not increase over time. However, there is an increase of DOG over time for the FEP film that received a heat treatment above the Tg. This is due to an enhancement of the diffusivity of the styrene monomer through the non-glassy FEP film, causing the rates of the initiation step of the grafting copolymerization process to increase. In an earlier study, the initial rapid increase in DOG was attributed to the predominance of the initiation and propagation steps of the reaction, while the observation of the slower rate of grafting at longer times was attributed to the significant increase of the termination reaction. A similar pattern of change in the rate of grafting was reported earlier by Gupta et al. (40).
FIG. 2.
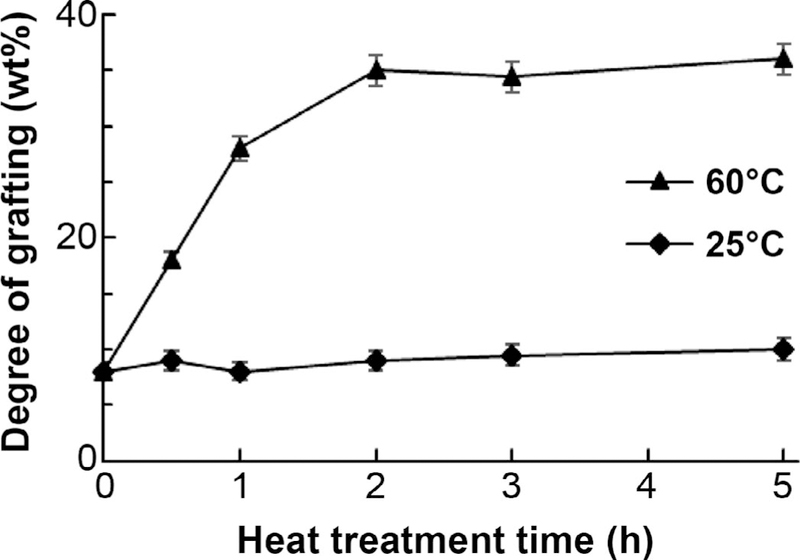
The average degree of grafting (wt%) of at least three samples as a function of heat treatment time (h) after irradiation at 25 and 60°C. FEP film (125 µm) and styrene monomer were simultaneously irradiated with pulsed electron beam to a dose of 50 kGy at 2.8 Gy/pulse.
As shown in Fig. 2, a heat treatment of at least 1 h at 60°C generated a degree of grafting four times larger than that obtained without heat treatment. This enhanced grafting is attributed to the increase in the diffusivity of the styrene monomer through the nonglassy FEP film, as discussed above. These diffused styrene molecules then react with the trapped radiolytically-produced FEP C-centered radicals, initiating the grafting copolymerization process. Figure 2 also shows that the DOG reaches a plateau after approximately 2 h at 60°C. This may be due to the temperature-induced enhancement of the termination reactions of both styryl and FEP C-centered radicals.
Uniformity of grafted films.
Figure 3 shows the SEM image and EDS line scans of a 125-µm-thick FEP film, which has been grafted with styrene by means of electron-beam irradiation followed by heat treatment at 45°C for 3 h. The cross-section appears to consist of three distinct regions, and the atomic composition of each of these regions has been analyzed by EDS. In the central region of the film, which is approximately 70 µm in thickness, fluorine is the predominant element. The outer regions of the film located on either side of this central region, which are each approximately 50 µm in thickness, both contain carbon as the predominant element. This indicates that under the grafting conditions employed in preparing this material, the styrene grafting front has penetrated to a depth of 50 µm on either side of the FEP film. Of course, a thicker film requires a longer treatment time for grafting to take place throughout its entire thickness. Additionally, it appears that postirradiation thermal treatment at a temperature close to the Tg is not sufficient to achieve uniformity.
FIG. 3.
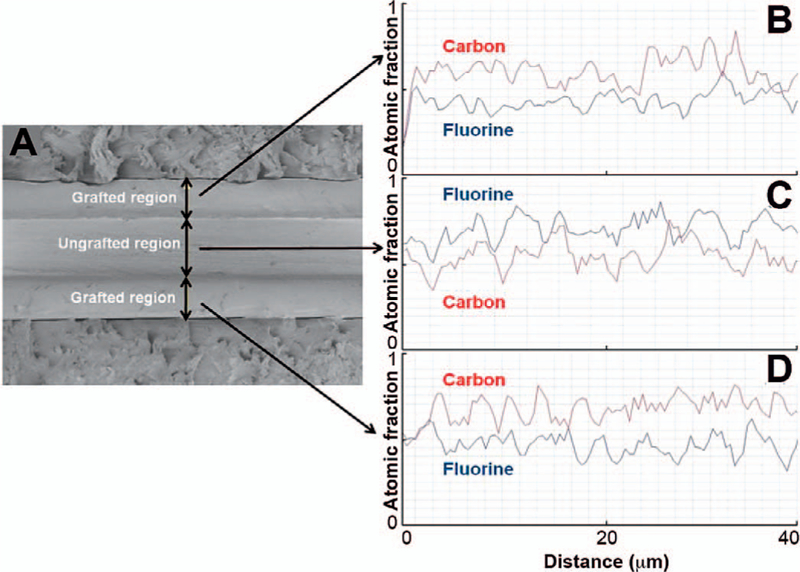
SEM cross-section image (panel A) and EDS line scans of styrene-grafted (panels B and D) and nongrafted (panel C) regions of FEP film (125 µm). Grafting was performed through direct irradiation of FEP and styrene with electron beam (50 kGy, 2.8 Gy/pulse), followed by heat treatment at 45°C for 3 h. Degree of grafting: 24.3 wt%.
Grafting uniformity was indeed achieved with a thermal treatment of 60°C for 5 h. Figure 4 shows the SEM crosssection micrographs of a 125-µm FEP film before and after electron-beam grafting of styrene with heat treatments. The substrate and monomer were simultaneously irradiated with 50 kGy, and then subjected to heat treatment. The uniformity of the morphology of the cross section appears to increase with heat treatment time, as can be seen by comparing Fig. 4E with shorter heat treatment times. During the first hour of heat treatment (Fig. 4B and C), the cross section of the film appears to be divided into three distinct regions. The outer regions of the film are grafted with styrene, while the central region contains nongrafted FEP substrate material. After 2 h of heat treatment (see Fig. 4D and E), the grafted regions meet each other at the center of the film, and the morphology of the cross-section becomes homogeneous. These images show that styrene grafting is initiated at the surfaces of the FEP film, and then proceeds toward the center of the film to produce a uniform grafting, which extends through the entire depth of the film.
FIG. 4.
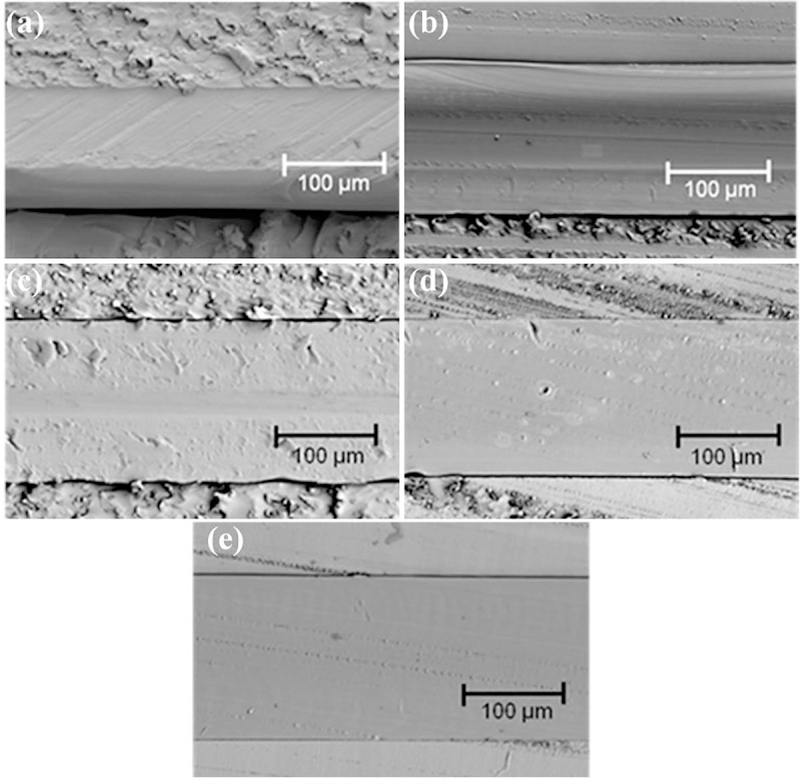
SEM cross-section micrographs of (panel A) nonirradiated FEP (125 µm) and electron-beam irradiated FEP/styrene (50 kGy, 2.8 Gy/pulse) with postirradiation heat treatment at 60°C for (panels B–E) 0.5, 1, 2 and 5 h, respectively.
Figure 5 shows the EDS line scans for carbon and fluorine of FEP after electron-beam grafting with styrene followed by heat treatment for different times. This analysis serves as an additional way to observe the uniformity of the depth of the grafting by monitoring the elemental composition along a cross-section of the material. The nongrafted FEP substrate contains a higher concentration of fluorine than the concentration of carbon throughout the entire depth of the material. All data shown in Fig. 5 are from samples that have been irradiated with 50 kGy and stored in an oven at 60°C for various lengths of time. The sample that was only heat treated for 30 min exhibits a concentration of carbon exceeding that of fluorine up to 50 µm from either edge into the film depth. These regions of higher carbon content are regions that are grafted with styrene. The depth of these grafted regions increases with heat treatment time. At heat treatment times of at least 2 h, the carbon concentration exceeds that of fluorine throughout the entire depth of the film. At even greater heat treatment times, the profiles of both carbon and fluorine become more uniform. This conclusion is also supported by the observation that increasing the heat treatment time up to 2 h results in an increase in the weight of the grafted film.
FIG. 5.
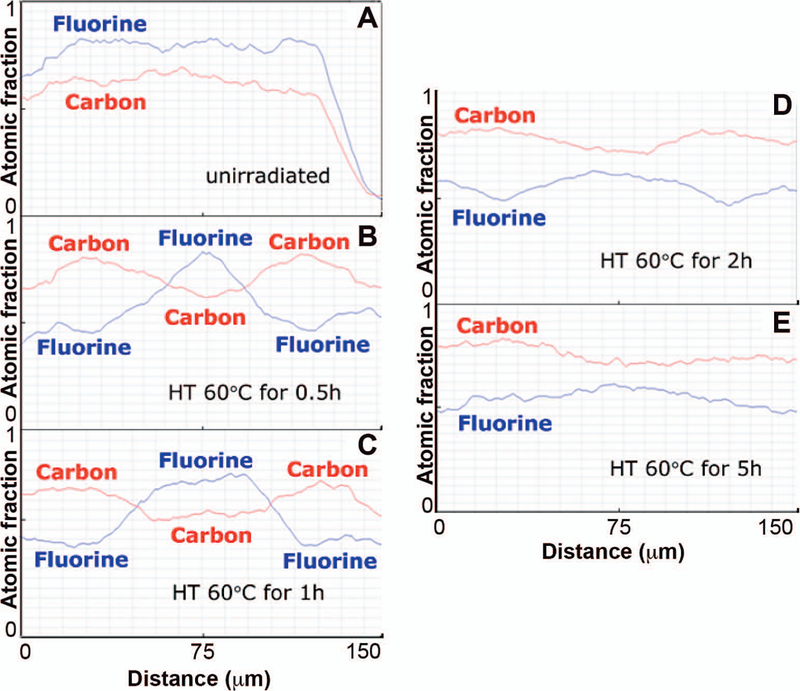
EDS line scans of fluorine and carbon across the cross-sections of (panel A) nonirradiated FEP (125 µm) and electron-beam-irradiated FEP/styrene (50 kGy, 2.8 Gy/pulse) with postirradiation heat treatment at 60°C for (panels B–E) 0.5, 1, 2 and 5 h, respectively.
Chemical structure of grafted films.
Figure 6 shows the comparison between the FTIR spectrum of nongrafted FEP film and the FTIR spectra of 10-µm microtomed sections of a 125-µm thick styrene-grafted FEP film. The absorption bands near 1,265 cm−1 to 1,110 cm−1 and 975 cm−1 correspond to the CF2 and CF3 stretching vibrations, respectively, of the FEP substrate (41). New bands arise in the spectrum, which are attributed to styrene, and are present in the spectrum of every slice analyzed. This verifies that grafting has taken place throughout the entire depth of the FEP film. The bands near 3,200 to 3,000 cm−1 are associated with the stretching vibration of aromatic CH groups of the grafted styrene. The bands at 2,910 and 2,840 cm−1 are associated with the asymmetric and symmetric stretching vibrations of CH2, respectively. Bands associated with the grafting of styrene are observed at 1,597 cm−1, 1490 cm−1 and 1,444 cm−1 (41, 42). In addition, the grafting with styrene onto FEP gives rise to the appearance of bands around 758 cm−1 and 698 cm−1, which may be attributed to – C-H bending and the ring-puckering effect (42). FTIR analysis of each slice of the grafted film offers clear evidence of the production of a homogeneous styrene graft within the FEP film. Thus, slice-by-slice measurements of the FTIR spectrum, in contrast to a single measurement on the entire film, confirms styrene has been grafted uniformly.
FIG. 6.
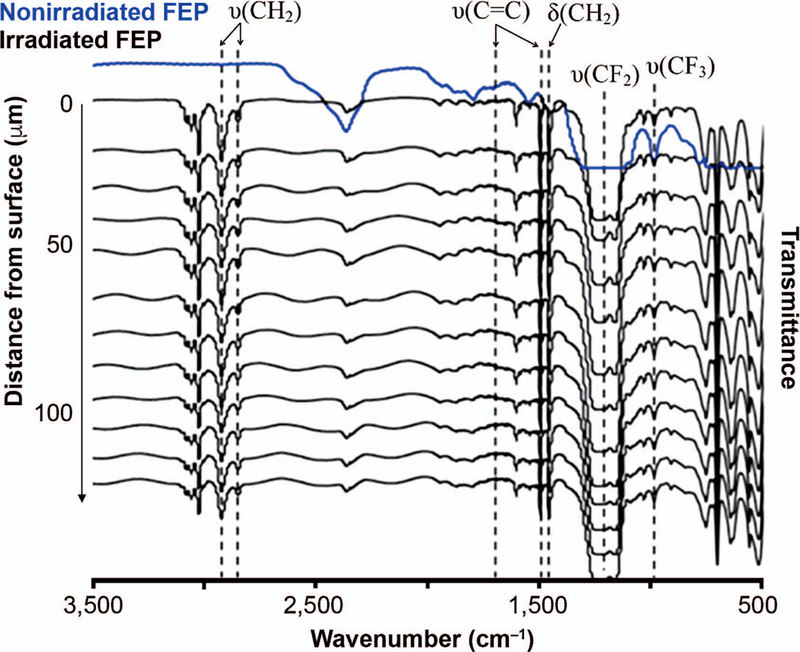
FTIR spectra of nonirradiated FEP film (125 µm) and FEP/styrene-grafted film microtomed into 10-µm sections parallel to film thickness. Grafting was performed through direct irradiation of FEP and styrene with electron beam (50 kGy, 2.8 Gy/pulse), followed by heat treatment at 60°C for 3 h. Degree of grafting: 38.5 wt%.
Surface morphology and composition.
XPS spectra were analyzed using CasasXPS software and quantification was based on relative sensitivity factors obtained from the Kratos Vision library, 1.000 for fluorine and 0.278 for carbon. The C 1s spectra were fitted with peaks of equal full width at half maximum, with a 50/50% Gaussian/Lorentzian product function peak shape after removal of a Shirley background. Data were collected for two samples: a nonirradiated FEP substrate (Fig. 7A) and an electron-beam styrene-grafted FEP (Fig. 7B). The degree of grafting shown in Fig. 7B was 38.5 wt.%.
FIG. 7.
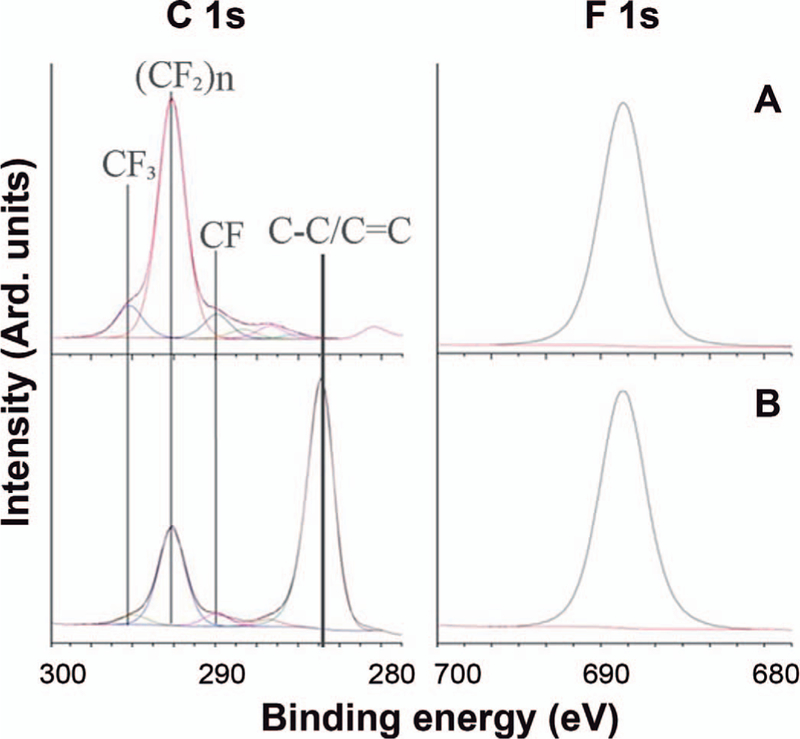
C 1s and F 1s XPS of (panel A) nonirradiated FEP (125 µm) and (panel B) FEP/styrene-grafted by electron-beam irradiation, 50 kGy at 2.8 Gy/pulse and HT at 60°C for 3 h, DOG: 38.5 wt%.
Figure 7A shows the C 1s and F 1s regions for the nonirradiated FEP substrate, for which the calculated F/C ratio is 2.2. The observed spectrum in the C 1s region can be fitted to a combination of five peaks and, assuming minimal sample degradation and hydrocarbon contamination from the air, this is consistent with the expected structure. The first two peaks on the high-energy side are probably due to sample degradation and a small amount of oxidized hydrocarbon contamination, which can be expected to occur to some extent on the surface of all samples exposed to the atmosphere (43).
Figure 7B shows the C 1s and F 1s spectra after electron-beam grafting of styrene. The calculated F/C ratio was 0.69; this marked decrease in fluorine concentration is expected with increased carbon content due to significant styrene incorporation into the film. The C 1s spectrum shows an almost complete disappearance of the peak due to CF at ≈290.0 eV, while there is still a significant peak due to CF2 at ≈292.2 eV (44, 45). This indicates that the styrene bonds preferentially at the CF groups. Additional evidence of styrene incorporation is provided by the large increase in the number of C-C and C-H bonds. Angle resolved spectra (not shown here) demonstrate that the grafting of styrene is homogeneous over the entire depth at which XPS is sensitive, ≈10 nm.
The XPS technique only generates atomic bonding information from the top surface, to a maximum depth of 10 nm, and since the results show almost no fluorine and only C-C bonding, it is likely that only styrene species (polymers or oligomers) are present on the top surface. Due to the low-dose rate of the gamma radiation, it is possible that homopolymerization of styrene occurs instead of a rapid production of free radicals on both styrene monomer and FEP. For instance, the surface region may consist of a brush-like structure of polymerized styrene, containing many carbon atoms, which is bound to the FEP membrane through a single C-F bond at a considerable distance from the outer surface.
Direct Grafting Using Steady-State Radiolysis
Attempts were made to directly graft neat styrene onto FEP using steady-state radiolysis via 60Co-gamma irradiation, however negligible increases in gravimetric measurements were observed. A gamma radiation dose of 50 kGy resulted in a substrate with a sticky polystyrene or styrene oligomer layer. Prior to characterization, these samples were treated using a xylene solvent and sonication to remove, at least partially, the sticky layer. The SEM and EDS analysis of the steady-state radiolysis grafted styrene onto FEP reveal that the film produced with a dose of 50 kGy was not uniform. The composition profile (not shown) indicates that carbon is the predominant element in the outer 10 µm of the film, while fluorine predominates within the innermost regions of the film.
This may very well be attributed to the predominance of the undesired homopolymerization of the styrene under steady-state radiolysis conditions. The formation of the polystyrene on the surface and underneath the surface impedes the diffusion of the styrene monomers leading to the inhomogeneous grafting.
SEM micrographs of FEP films after styrene grafting using gamma radiation at an accumulated dose of 50 kGy are shown in Fig. 8. The striated distributions of material on the surface of the grafted films are more pronounced after gamma irradiation than after electron-beam irradiation. An increase in surface roughness involving the formation of a bubble morphology on polyethylene after radiation grafting with styrene has also been reported (46). Under the lower dose-rate conditions, the primary reaction is the styrene homopolymerization. The produced homopolymer layer prevents the styrene monomers from diffusing into the FEP.
FIG. 8.
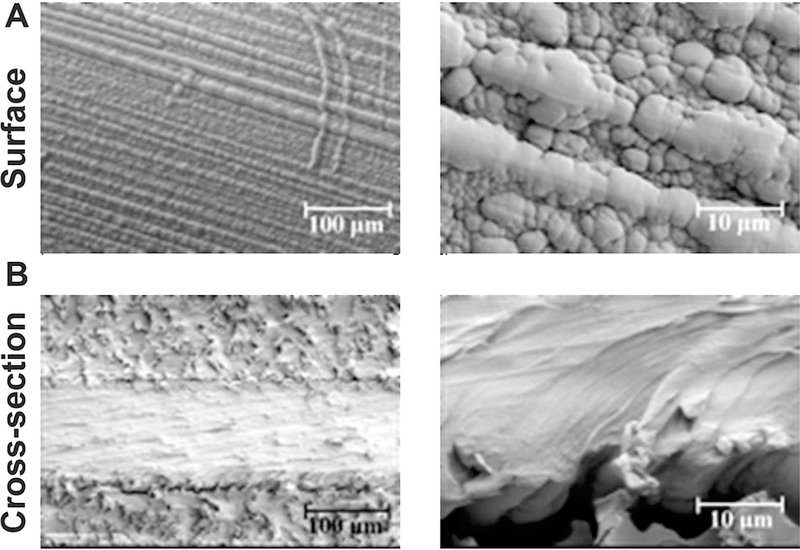
SEM micrographs of the surface and cross-sections of FEP (125 µm) simultaneously 60Co gamma-irradiated in argon with styrene monomer to a dose of 50 kGy at 0.17 Gy/s.
Indirect Grafting
To eliminate the undesired homopolymerization reaction of the styrene, attempts were made to achieve a reasonable DOG of styrene onto FEP with the indirect grafting approach. This is where the FEP is irradiated under anaerobic conditions with a dose of ≈80 kGy followed by the addition of argon-saturated neat styrene. However, the DOG values for these indirect grafting experiments were very low, ,5%, compared to the direct-grafting DOG results. This can be attributed to the fast crosslinking of the FEP C-centered free radicals during the postirradiation heat treatment at 60°C, which is higher than the Tg of FEP. As a result, there are no FEP C- centered free radicals available to the diffused styrene monomer to initiate the grafting reactions.
Pulse Radiolysis of Neat Styrene
Pulse radiolysis was performed to elucidate the radiation-induced grafting mechanism of the styryl free radicals and to evaluate the competition between the undesired homo-polymerization reaction and the grafting reaction.
Figure 9 shows the transient spectrum of the styryl C-centered radical 3 µs after the pulse in pulse-irradiated samples of neat styrene saturated with N2. The main spectral feature observed is an absorption band with a maximum of approximately 315 nm to 320 nm. This observation generally agrees with results reported elsewhere (47, 48). The capture of the solvated electrons by styrene to produce styrene anion and cation radicals is extremely fast (49). It is expected and reported to be completed within a couple of nanoseconds (50). Therefore, it is impossible to follow this reaction with our pulse radiolytic setup, which has a pulse width of 3 µs. The fast buildup in Fig. 9, which is completed within 2 µs, represents the protonation reaction of the styryl radical anion to form the neutral C-centered radical (Sty•). The decay of the Sty•, which is the basis of the radical polymerization, was monitored at 315 nm. The kinetics of this decay are determined by a combination of the propagation and dimerization-termination reactions. The inset in Fig. 9 also shows that the absorption does not decay to zero even 2 ms after the pulse. The transient spectrum at 3 µs represents the Sty•. The Sty• would then undergo the following two competing decay reactions: the dimerization reaction between two Sty• producing Sty-Sty and the addition of Sty• onto the double bond of Sty (propagation reaction) producing another free radical Sty-Sty•. This is a clear indication that this transient is the spectrum of the growing free radicals. Based on a G value of 0.0155 µmol J−1 (0.15 radical per 100 eV) of absorbed energy (49), we calculated the molar absorptivity of the styryl radical at 315 nm to be ε315 = (2.2 ± 0.1) 103 l mol−1 cm−1. Since the kinetics fit a second-order rate constant, it is concluded that the predominant reaction is dimerization reaction (Sty-Sty) instead of the propagation reaction, which would be expected to be pseudo-first order. The observed second-order rate constant for the decay is 5 108 L mol−1 s−1. This fast reaction decay of styryl radicals clearly demonstrates that the radiolytically produced styryl radicals undergo fast reactions that can compete with the grafting reaction on FEP, resulting in the formation of a styrene homopolymer and dimers.
FIG. 9.
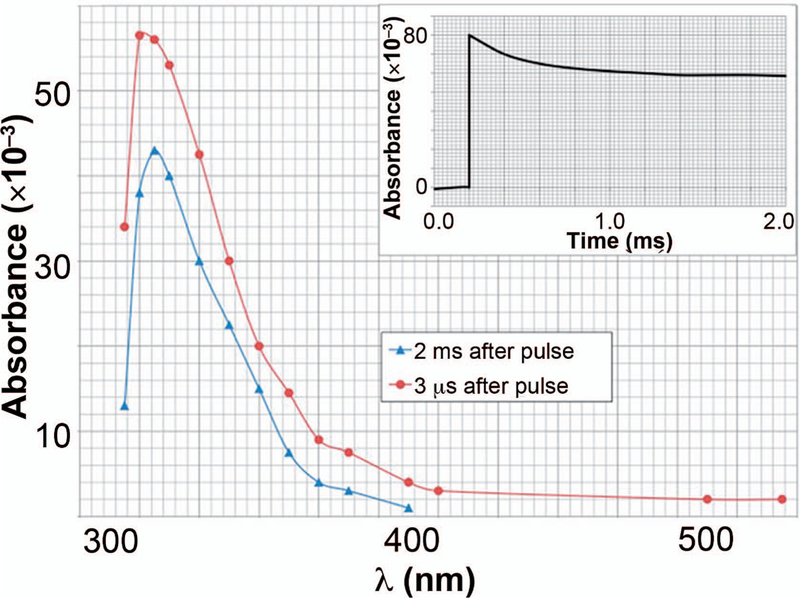
The transient spectrum of the styryl free radical measured at 2 µs after the pulse in N2-saturated neat styrene. Insert: The observed bimolecular decay of the styryl radical at 315 nm.
Proton Conductivity
Because the 125-µm FEP films were shown to be uniformly grafted under the experimental conditions described above, it is expected that the same is true for the thinner 25-µm films. Therefore, all conductivity measurements were performed on uniformly grafted 25-µm FEP films similar to those used in fuel cell applications. Table 1 shows the DOG averages of three or more films that were directly grafted with styrene using electron-beam irradiation under various total doses and heat treatment times at 60°C. This indicates that heat treatment times longer than 2 h and a total dose of at least 60 kGy do not generate significant increases in the grafting yields.
TABLE 1.
Degree of Grafting of Styrene on 25-µm FEP Films as a Function of Electron-Beam Radiation Dose followed by Heat Treatment at 60°C
| Grafting dose (kGy) | Heat treatment time (h) | Degree of grafting |
|---|---|---|
| 81.2 | 24 | 24.1 ± 2.4 |
| 86.2 | 5 | 22.8 ± 2.2 |
| 63.0 | 2 | 20.6 ± 2.0 |
| 62.0 | 2 | 21.8 ± 2.2 |
The results of proton conductivity measurements using the alternating current impedance method on the 25-µm films in Table 1 are shown in Fig. 10, together with results obtained for a 25-µm film of 3M 825EW ionomer and the Nafion 112. The results show that at low humidity the proton conductivity of the FEP films grafted with styrene sulfonate prepared in the current study is lower than that of the PFSA film. However, the proton conductivity of the styrene-grafted films described above becomes at least as large as that of the PFSA film at relative humidity of 60% or higher.
FIG. 10.
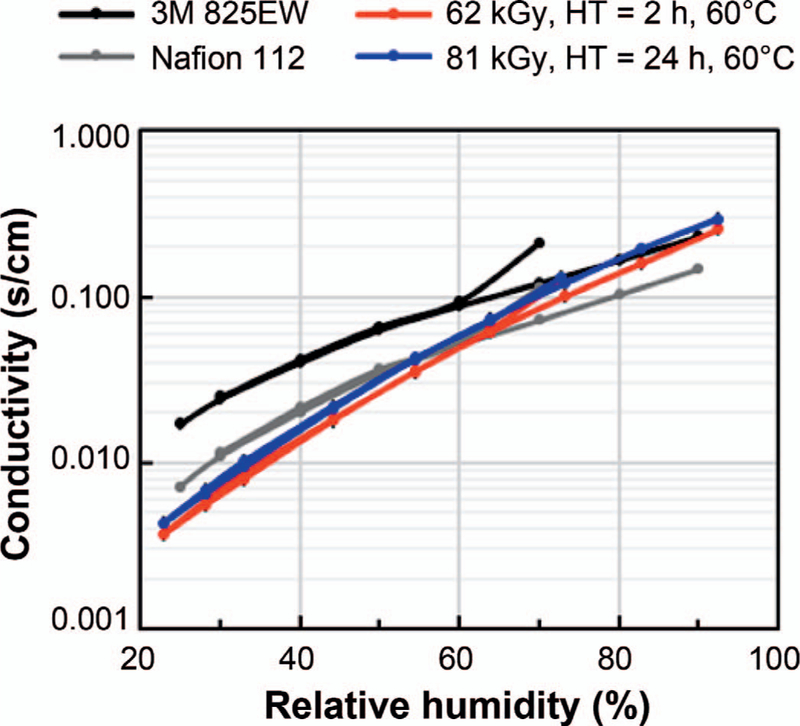
Proton conductivity was measured at 80°C at a fixed humidity using an alternating current impendence method. Two electron-beam-grafted FEP-styrene films (25 µm) with varying heat treatments (HT) were compared to commercial substrates, Nafion 112 and 3M 825EW. Two independent measurements were performed on each sample.
DISCUSSION
The effects of the postirradiation temperature on the degree of grafting can be related to the Tg of FEP. For the post-heat treatment at 60°C, FEP is no longer in the glassy form and results in the enhancement of the diffusivity of the styrene monomer into the FEP film. During the direct radiation grafting, C-centered free radicals of styrene (Sty•) (Sty•) and FEP (•FEP (–H)) are produced.
In the absence of oxygen, these free radicals decay through the following competing undesired and desired grafting reactions:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The first three chemical reactions represent undesired outcomes, specifically the initiation homopolymerization reaction [Eq. (1)], the dimerization reaction [Eq. (2)], and the crosslinking reaction [Eq. (3)]. The last two chemical reactions listed represent the desired grafting [Eqs. (4) and (5)].
| (6) |
| (7) |
The kinetics of the grafting of styrene onto FEP are described by Eqs. (6) and (7). The undesired reactions, Eqs. (2)and (3), would become the predominant reactions at high-dose rates, and in pulsed irradiation, Eq. (1) would be dominant at very high concentrations of styrene. In addition to the above reactions, H-transfer reaction also takes place, enhancing the undesired homopolymerization reaction. Pulse radiolysis results show that the undesired reactions, Eqs. (1) and (2), are very fast with combined observed reaction rate constant of 5 × 108 l mol−1 s−1.
The most effective technique of styrene grafting to FEP, investigated in this study, involved direct electron-beam irradiation followed by a postirradiation heat treatment. This approach produced a styrene graft throughout the entire thickness of a 125-µm FEP film, with a degree of grafting of over 30 wt% and a smooth-surface morphology. The uniformity of grafting as a function of depth in the FEP substrate was observed by EDS to be highly dependent on the length of time that the irradiated samples were exposed to heat treatment. The results, including data from the FTIR spectra, SEM micrographs and EDS line scans, indicate that after 50 kGy electron irradiation and a postirradiation heat treatment of 60°C for 2 h, a uniform graft forms within the FEP film. Shorter heat treatment times produce ‘‘grafting fronts,’’ which indicate that the styrene has only diffused partially through the film.
In general, the proton conductivity of hydrophilic FEP-based membranes exhibits a large increase with rising temperature in the range of 110–120°C, where the conductivity levels off (51). Sulfonated membranes prepared by radiation-induced grafting of styrene onto FEP films have high thermal stability (except for a loss of the part of the water content that is weakly bound) up to temperatures of approximately 290°C (14).
The conductivities of the prepared membranes in this study are slightly higher than those of typical membranes developed for applications such as fuel cells under conditions of high humidity, and lower than those of such membranes at low levels of humidity. The lower proton conductivity of the electrolyte membranes made of poly(vinylbenzyl sulfonic acid)-grafted FEP in this study, compared to that of perfluorosulfonic acid (PFSA) membranes such as Nafion 212 and the 3M 825EW, can be attributed to the fact that in general, the acid strength of the PFSA SO3H is stronger than that of an aromatic SO3H (pKa ≈ –6 vs. ≈ –3). In addition, the PFSA may undergo phase separation into very distinct ionic channels and fluoropolymer matrix, while the sulfonated hydrocarbons have less tendency to undergo phase separation (52, 53).
CONCLUSION
Pulsed-electron beam provides a high-dose rate that can impede the undesired homopolymerization reaction of the styrene, but also enhances the undesired crosslinking of the carbon-centered FEP radicals and the dimerization of the styryl radicals, as evidenced by relatively low-DOG values of 34.4 ± 3.0%. The desired grafting copolymerization reactions occur when irradiation reaches a DOG of 8.4 ± 0.8% and additional grafting occurs subsequently during the postirradiation heat treatment resulting in a DOG of 34.4 ± 3.0%. During the first step (irradiation), the desired grafting occurs mainly on the surface of the glassy FEP through the carbon-centered FEP radical and the styryl to produce FEP-styrene structures. The second step of the grafting takes place during the subsequent thermal heating where the diffusivity of the styrene monomer increases tremendously. This is evident in the comparison of the increasing DOG values of the heat-treated samples, in contrast to that of room temperature samples, which exhibit constant DOG values as a function of time. Our results show that, to enhance the diffusivity of the styrene monomer, even through relatively large-thickness (125-µm) FEP film, it is essential that the FEP is not in a glassy state. Therefore, a post-thermal treatment higher than Tg, 39 ± 0.5°C, is essential to achieve a reasonable grafting density so that the styrene monomers are able to diffuse into the bulk of the FEP film. Importantly however, at the thermal treatment temperature of 60°C, a competition state is established between the crosslinking reaction of the carbon-centered FEP radicals and the diffusion of the styrene through the FEP bulk with subsequent addition reaction of the double bond of the styrene monomer with the carbon-centered FEP radical. As a result of the crosslinking reactions of the FEP radicals, there are few carbon-centered FEP radicals available to react with styrene monomers and initiate the grafting polymerization reaction. This explains why, even at the highest styrene concentration (neat styrene), the DOG is still relatively low at a value of 34.4 ± 3.0%.
On the other hand, steady-state radiolysis enhances the undesired styrene homopolymerization, leading to negligible DOG values. This is because under these radiation conditions the homopolymerization predominates, leading to the formation of nongrafted styrene oligomers and polymers on the surface. As a result, these products of homopolymerization impede the diffusion of the styrene into the bulk of the substrate.
Despite the fact that utilization of indirect grafting tremendously impedes the undesired homopolymerization reaction, our results demonstrate negligible DOG values for indirect grafting of FEP with neat styrene using pulsed-electron-beam irradiation. This finding is in agreement with the fact that FEP undergoes crosslinking rather than scission at doses up to 160 kGy in the absence of oxygen. Therefore, at the time the styrene is added, postirradiation, there is a low concentration of carbon-centered FEP free radicals available to react with the styrene monomers.
Overall, our approach shows that direct high-dose-rate radiation grafting with subsequent heat treatment above the Tg of the 25 µm-thick FEP produces proton conductivity membranes. At high humidity, the prepared membranes in this work are on par with the 3M 825EW membrane and higher than the Nafion membrane with a measured proton conductivity of 0.29 s cm−1 at 92% humidity. However, the proton conductivity is 0.007 s cm−1 at low (28%) humidity, which is lower than both industry standard PFSA membranes tested here. This is due to the pKa value of the sulfonated styrene being higher than the values of the PFSA membrane. In addition, due to the low tendency of the sulfonated styrene to undergo phase separation, fewer distinct ionic channels are formed in comparison with the PFSA membranes.
ACKNOWLEDGMENTS
Certain commercial equipment or materials are identified in this article to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
REFERENCES
- 1.Liu C, Li L, Liu Z, Guo M, Jing L, Liu B, et al. Sulfonated naphthalenic polyimides containing ether and ketone linkages as polymer electrolyte membranes. J Memb Sci 2011; 366:73–81. [Google Scholar]
- 2.Kim YS, Kim DS, Guiver MD, Pivovar BS. Interpretation of direct methanol fuel cell electrolyte properties using non-traditional length-scale parameters. J Memb Sci 2011; 374:49–58. [Google Scholar]
- 3.Liu B, Hu W, Kim YS, Zou H, Robertson GP, Jiang Z, et al. Preparation and DMFC performance of a sulfophenylated poly(arylene ether ketone) polymer electrolyte membrane. Electrochim Acta 2010; 55:3817–23. [Google Scholar]
- 4.Sherazi TA, Guiver MD, Kingston D, Ahmad S, Kashmiri MA, Xue X. Radiation-grafted membranes based on polyethylene for direct methanol fuel cells. J Power Sources 2010; 195:21–9. [Google Scholar]
- 5.Chen JH, Asano M, Yamaki T, Yoshida M. Preparation of sulfonated crosslinked PTFE- graft-poly(alkyl vinyl ether) membranes for polymer electrolyte membrane fuel cells by radiation processing. J Memb Sci 2005; 256:38–45. [Google Scholar]
- 6.Chen JH, Asano M, Yamaki T, Yoshida M. Preparation and characterization of chemically stable polymer electrolyte membranes by radiation-induced graft copolymerization of four monomers into ETFE films. J Memb Sci 2006; 269:194–204. [Google Scholar]
- 7.Herman H, Slade RCT, Varcoe JR. The radiation-grafting of vinylbenzyl chloride onto poly (hexafluoropropylene-co-tetrafluoroethylene) films with subsequent conversion to alkaline anion-exchange membranes: optimisation of the experimental conditions and characterisation. J Memb Sci 2003; 218:147–63. [Google Scholar]
- 8.Nasef MM, Saidi H. Preparation of crosslinked cation exchange membranes by radiation grafting of styrene/divinylbenzene mixtures onto PFA films. J Memb Sci 2003; 216:27–38. [Google Scholar]
- 9.Scott K, Taama WM, Argyropoulos P. Performance of the direct methanol fuel cell with radiation-grafted polymer membranes. J Memb Sci 2000; 171:119–30. [Google Scholar]
- 10.Shen M, Roy S, Kuhlmann JW, Scott K, Lovell K, Horsfall JA. Grafted polymer electrolyte membrane for direct methanol fuel cells. J Memb Sci 2005; 251:121–30. [Google Scholar]
- 11.Rosiak JM. Radiation polymerization in solution. Advances in radiation chemistry of polymers. Vienna: International Atomic Energy Agency; 2004. [Google Scholar]
- 12.Nasef MM, Hegazy ESA. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog Polym Sci 2004; 29:499–561. [Google Scholar]
- 13.Kim BN, Lee DH, Han DH. Characteristics of fuel cell membranes prepared by EB radiation grafting onto FEP with styrene derivatives, styrene and 2-methylstyrene. J Electrochem Soc 2008; 155:B680–B85. [Google Scholar]
- 14.Nasef MM, Saidi H, Nor HM. Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto poly(tetrafluoroethylene-co-hexafluoropropylene) films. I. Effect of grafting conditions. J Appl Polym Sci 2000; 76:220–7. [Google Scholar]
- 15.Becker W, Bothe M, Schmidt-Naake G. Grafting of poly(styreneco-acrylonitrile) onto pre-irradiated FEP and ETFE films. Macromol Mater Eng 1999; 273:57–62. [Google Scholar]
- 16.Gubler L Polymer design strategies for radiation-grafted fuel cell membranes. Adv Energy Mater 2014; 4:1300827. [Google Scholar]
- 17.Kabanov VY. Preparation of polymer membranes for fuel cells by radiation graft polymerization. High Energy Chem 2004; 38:57– 65. [Google Scholar]
- 18.Nasef MM. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem Rev 2014; 114:12278–329. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Lee CH, Guiver MD, Lee YM. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog Polym Sci 2011; 36:1443–98. [Google Scholar]
- 20.Gubler L, Gursel SA, Scherer GG. Radiation grafted membranes for polymer electrolyte fuel cells. Fuel Cells (Weinh) 2005; 5:317– 35. [Google Scholar]
- 21.Chen JH, Asano M, Maekawa Y, Yoshida M. Suitability of some fluoropolymers used as base films for preparation of polymer electrolyte fuel cell membranes. J Memb Sci 2006; 277:249–57. [Google Scholar]
- 22.Huslage J, Rager T, Schnyder B, Tsukada A. Radiation-grafted membrane/electrode assemblies with improved interface. Electrochim Acta 2002; 48:247–54. [Google Scholar]
- 23.Kim B-N, Lee D-H, Lee S-W, Han D-H. Improvement of polymer electrolyte membrane by radiation-induced grafting of styrene onto FEP film with subsequent sulfonation. Korean J Chem Eng 2008; 25:1212–20. [Google Scholar]
- 24.Rouilly MV, Kotz ER, Haas O, Scherer GG, Chapiro A. Proton-exchange membranes prepared by simultaneous radiation grafting of styrene onto teflon-fep films: Synthesis and characterization. J Memb Sci 1993; 81:89–95. [Google Scholar]
- 25.Gupta B, Buchi FN, Scherer GG, Chapiro A. Crosslinked ion exchange membranes by radiation grafting of styrene/divinylbenzene into FEP films. J Memb Sci 1996; 118:231–38. [Google Scholar]
- 26.Lyons BJ. Radiation cross-linking of fluoropolymers: A review. Radiat Phys Chem 1995; 45:159–74. [Google Scholar]
- 27.Sheirs J (editor). Modern fluoropolymers: High performance polymers for diverse applications Hoboken, NJ: John Wiley & Sons Inc.; 1997. [Google Scholar]
- 28.Forsythe JS, Hill DJT. The radiation chemistry of fluoropolymers. Prog Polym Sci 2000; 25:101–36. [Google Scholar]
- 29.Hill D Whittaker JT, Andrew K NMR studies of the radiation modification of polymers. Annual Reports on NMR Spectroscopy 2002; 46:1–35. [Google Scholar]
- 30.Florin RE, Wall LA. Gamma irradiation of fluorocarbon polymers. J Res Natl Bur Stand 1961; 65A:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovejoy ER, Bro MI, Bowers GH. Chemistry of radiation crosslinking of branched fluorocarbon resins. J Appl Polym Sci 1965; 9:401–10. [Google Scholar]
- 32.Hill DJT, Mohajerani S, Whittaker AK, Scheler U. Chain scission and branching in irradiated poly(tetrafluoroethylene-co-hexafluoropropylene). Polym Int 2003; 52:1725–33. [Google Scholar]
- 33.Kim BN, Lee DH, Han DH. Thermal, mechanical and electrical properties on the styrene-grafted and subsequently sulfonated FEP film induced by electron beam. Polym Degrad Stab 2008; 93:1214–21. [Google Scholar]
- 34.Yatender Bhardwaj MT, Young-Chang Nho, Mohamed Nasef, Olgun Guven. Harmonized protocol for radiation-induced grafting. IAEA Workshop on Harmonized Radiation Graft Protocol. Report No. RAS1014/9002/01 Vienna: International Atomic Energy Agency; 2014. (https://bit.ly/2IFr4zy) [Google Scholar]
- 35.Ferraria AM, da Silva JDL, do Rego AMB. XPS studies of directly fluorinated HDPE: problems and solutions. Polymer 2003; 44:7241–49. [Google Scholar]
- 36.Maalouf M, Pyle B, Sun CN, Wu DS, Paddison SJ, Schaberg M, et al. Proton exchange membranes for high temperature fuel cells: Equivalent weight and end group effects on conductivity. ECS Trans 2009; 25:1473–81. [Google Scholar]
- 37.Emery M, Frey M, Guerra M, Haugen G, Hintzer K, Lochhaas KH, et al. The development of new membranes for proton exchange membrane fuel cells. ECS Trans 2007; 11:3–14. [Google Scholar]
- 38.Hamrock SJ, Rivard LM, Moore GGI, Freemyer HT. Polymer electrolyte membrane. U.S. Patent No 7,348,088 B2; 2008.
- 39.Al-Sheikhly M, Silverman J, Neta P, Karam L. Mechanisms of ionizing radiation-induced destruction of 2,6-dichlorobiphenyl in aqueous solutions. Environ Sci Technol 1997; 31:2473–77. [Google Scholar]
- 40.Gupta B, Buchi FN, Scherer GG. Cation-exchange membranes by preirradation grafting of styrene into FEP films. 1. Influence of synthesis conditions. J Polym Sci A Polym Chem 1994; 32:1931– 38. [Google Scholar]
- 41.Li J, Sato K, Ichizuri S, Asano S, Ikeda S, Iida M, et al. Pre-irradiation induced grafting of styrene into crosslinked and non-crosslinked polytetrafluoroethylene films for polymer electrolyte fuel cell applications. II: Characterization of the styrene grafted films. Eur Polym J 2005; 41:547–55. [Google Scholar]
- 42.Sherazi TA, Ahmad S, Kashmiri MA, Kim DS, Guiver MD. Radiation-induced grafting of styrene onto ultra-high molecular weight polyethylene powder for polymer electrolyte fuel cell application II. Sulfonation and characterization. J Memb Sci 2009; 333:59–67. [Google Scholar]
- 43.Briggs DS, Seah MP Practical surface analysis, Vol. 1: Auger and X-ray photoelectron spectroscopy New York: John Wiley and Sons; 1994. [Google Scholar]
- 44.Brundle CR, Baker AD. Electron spectroscopy: Theory, techniques, and applications Vol. 4 Cambridge: Academic Press; 1981. [Google Scholar]
- 45.Castner DG, Lewis KB, Fischer DA, Ratner BD, Gland JL. Determination of surface-structure and orientation of polymerized tetrafluoroethylene films by near-edge X-ray absorption fine-structure, X-ray photoelectron-spectroscopy, and static secondary ion mass-spectrometry. Langmuir 1993; 9:537–42. [Google Scholar]
- 46.Machi S, Silverma J. Bubble formation in radiation-induced grafting of styrene to polyethylene. J Polym Sci A1 1969; 7, 2737– 40. [Google Scholar]
- 47.Harayma H, Al-Sheikhly M, Silverman J. Oligomer formation in the radiation-induced polymerization of styrene. Radiat Phys Chem 2003; 68:1023–29. [Google Scholar]
- 48.Silverman J, Tagawa S, Kobayashi H, Katsumura Y, Washio M, abata Y. Pulse-radiolysis studies on short-lived intermediates in radiation-induced polymerization of bulk styrene and methonal styrene solutions. Radiat Phys Chem 1983; 22:1039–42. [Google Scholar]
- 49.Metz DJ, Potter RC. Thomas JK Pulse radiolysis studies of styrene. J Polym Sci A1 1967; 5:877–90. [Google Scholar]
- 50.Tang FW, Alsheikhly M, Silverman J. The effects of small concentrations of methanol on the radiation polymerization of styrene. In memoriam Gyula Hardy. In: Dobo J, Nyikos L, Schiller R, editors. Proceedings of the Seventh Tihany Symposium on Radiation Chemistry Budapest: Hungarian Chemical Society 1991; p. 219–25. [Google Scholar]
- 51.Schmidt C, Schmidt-Naake G. Proton conducting membranes obtained by doping radiation-grafted basic membrane matrices with phosphoric acid. Macromol Mater Eng 2007; 292:1164–75. [Google Scholar]
- 52.Roy A, Hickner MA, Yu X, Li Y, Glass TE, McGrath JE. Influence of chemical composition and sequence length on the transport properties of proton exchange membranes. J Polym Sci B Polym Phys 2006; 44:2226–39. [Google Scholar]
- 53.Hamrock SJ, Herring AM. Proton exchange membrane fuel cells: high-temperature, low-humidity operation. In: Kreuer K-D, editor. Fuel cells: Selected entries from the Encyclopedia of Sustainability Science and Technology New York: Springer; 2013. [Google Scholar]


