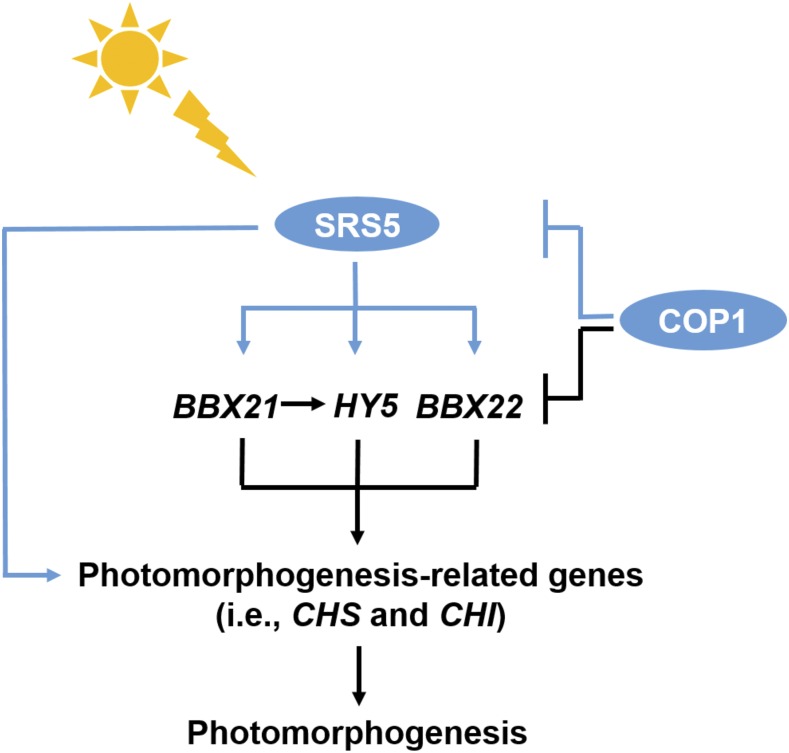
Blue light-induced SRS5 promotes photomorphogenesis by directly activating photomorphogenesis-promoting genes such as HY5, BBX21 and BBX22, but COP1 directly targets SRS5 for degradation in the dark.
Abstract
Plant seedlings undergo distinct developmental processes in the dark and in the light. Several genes, including ELONGATED HYPOCOTYL5 (HY5), B-BOX PROTEIN21 (BBX21), and BBX22, have been identified as photomorphogenesis-promoting factors in Arabidopsis thaliana; however, the overexpression of these genes does not induce photomorphogenesis in the dark. Using an activation-tagging approach, we identified SRS5ox, which overexpresses SHI-RELATED SEQUENCE5 (SRS5) following induction with estradiol. SRS5 overexpression in SRS5ox and Pro35S:SRS5-GFP seedlings results in a constitutive photomorphogenesis phenotype in the dark, whereas SRS5 loss of function in the srs5-2 mutant results in long hypocotyls in the light. This indicates that SRS5 is a positive regulator of photomorphogenesis. Furthermore, SRS5 promotes photomorphogenesis by directly binding to the promoters of photomorphogenesis-promoting genes, such as HY5, BBX21, and BBX22, and activating their expression, thus affecting the expression of downstream light-signaling genes. These data indicate that SRS5 acts in the upregulation of photomorphogenesis-promoting genes. In addition, CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1), which plays a central repressive role in seedling photomorphogenesis, directly ubiquitinates SRS5, promoting its degradation in the dark. Taken together, our results demonstrate that SRS5 directly activates the expression of downstream genes HY5, BBX21, and BBX22 and is a target of COP1-mediated degradation in Arabidopsis.
INTRODUCTION
In addition to providing biological energy via photosynthesis, light is an informative environmental signal that influences growth and development in plants (Chen et al., 2004; Kami et al., 2010). In the light, seedlings undergo photomorphogenesis, having compact hypocotyls and open cotyledons with mature chloroplasts. By contrast, seedlings grown in the dark undergo skotomorphogenesis, having etiolated hypocotyls and developing etioplasts in closed cotyledons protected by the apical hook.
Plants perceive light via intracellular photoreceptors, which govern molecular signaling pathways that ultimately modulate the transcriptome and cause changes in growth and development. Microarray analysis has revealed that up to a third of Arabidopsis thaliana genes exhibit altered expression in seedlings grown in the dark, compared with those grown in the light (Ma et al., 2001). One such gene is ELONGATED HYPOCOTYL5 (HY5), which is a positive regulator of light-regulated processes, based on the partially etiolated phenotype of light-grown hy5 seedlings; HY5 also plays a key signaling role in the developmental transition from dark to light (Osterlund et al., 2000b; Lee et al., 2007; Zhang et al., 2011). In the light, HY5 expression increases and the resulting increased abundance of HY5 facilitates the expression of a large number of photomorphogenesis-promoting genes (Osterlund et al., 2000a; Lee et al., 2007; Zhang et al., 2011).
Multiple factors regulate HY5 expression and HY5 protein levels. For example, CALMODULIN7, a unique member of the calmodulin family, functions as a transcription regulator to modulate HY5 expression by directly binding to the HY5 promoter besides its interaction with HY5 (Abbas et al., 2014). In addition, HY5 and its homolog HYH also bind to the HY5 promoter to activate HY5 transcription in response to UV-B (Binkert et al., 2014). Recent work showed that the transcription factor B-BOX PROTEIN21 (BBX21) can bind the T/G box in the HY5 promoter and thus was also implicated in HY5 transcriptional regulation (Xu et al., 2016). In the dark, skotomorphogenesis is promoted through HY5 ubiquitination by the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1), which leads to HY5 degradation via the 26S proteasome (Lau and Deng, 2012; Huang et al., 2014). Thus, COP1 activity directly affects seedling photomorphogenesis (Lau and Deng, 2012; Huang et al., 2014).
COP1 functions as a central repressor of seedling photomorphogenesis and cop1 mutant seedlings that produce an impaired version of COP1 exhibit constitutive photomorphogenesis when grown in the dark (Lau and Deng, 2010). In wild-type seedlings grown in the dark, nuclear-localized COP1 ubiquitinates HY5, promoting its degradation (Osterlund et al., 2000b; Wang et al., 2001; Lau and Deng, 2010), whereas light exposure reduces nuclear COP1 to a level that permits the accumulation of HY5 (von Arnim and Deng, 1994; von Arnim et al., 1997). Light also represses COP1 activity by activating the photoreceptors phytochrome A (phyA) and phyA/B and cryptochrome 1 (CRY1) and CRY2 to modulate the complex between COP1 and SUPPRESSOR OF PHYA-105, ultimately leading to HY5 accumulation (Yang et al., 2000, 2001; Hoecker and Quail, 2001; Wang et al., 2001; Saijo et al., 2003; Sang et al., 2005).
HY5 was the first protein shown to be regulated by COP1; however, HY5 does not act alone in the promotion of light responses (Ang et al., 1998; Datta et al., 2008; Chang et al., 2011). Subsequent studies have revealed that a series of B-box-containing proteins, including BBX21 and BBX22, are also direct targets of COP1 (Datta et al., 2008; Chang et al., 2011). BBX-containing proteins share many roles with HY5 in response to light, such as the regulation of anthocyanin accumulation and the inhibition of seedling hypocotyl elongation (Holm et al., 2002; Datta et al., 2008; Chang et al., 2011; Binkert et al., 2014; Xu et al., 2016). It is also known that BBX22 can interact with HY5 and that BBX21 modulates HY5 expression though its binding to the promoter of HY5 (Holm et al., 2002; Datta et al., 2008; Chang et al., 2011; Binkert et al., 2014; Xu et al., 2016). However, no corresponding overexpression lines (35S:HY5, 35S:BBX21, and 35S:BBX22) exhibit constitutive photomorphogenesis comparable to cop1 seedlings in the dark (Ang et al., 1998; Holm et al., 2002; Chang et al., 2011; Xu et al., 2016). Furthermore, it remains unclear whether and how additional unidentified regulator(s) play a role in photomorphogenesis.
In this study, we describe the identification and characterization of SRS5 as a positive regulator of photomorphogenesis. SRS5 belongs to SHORT-INTERNODES (SHI) gene family, members of which act as transcription factors involved in regulating the development of diverse plant organs (Fridborg et al., 1999; Kuusk et al., 2002; Baylis et al., 2013). We demonstrate that SRS5 overexpression seedlings exhibit photomorphogenesis in the dark, whereas light-grown seedlings of the srs5-2 mutant line display greater hypocotyl elongation compared with wild-type seedlings. Furthermore, light-induced SRS5 accumulation promotes seedling photomorphogenesis via direct activation of a number of other photomorphogenesis-promoting genes such as HY5, BBX21, and BBX22. In the dark, SRS5 is targeted by COP1 for 26S proteasome-mediated degradation.
RESULTS
SRS5 Functions as a Positive Regulator of Photomorphogenesis
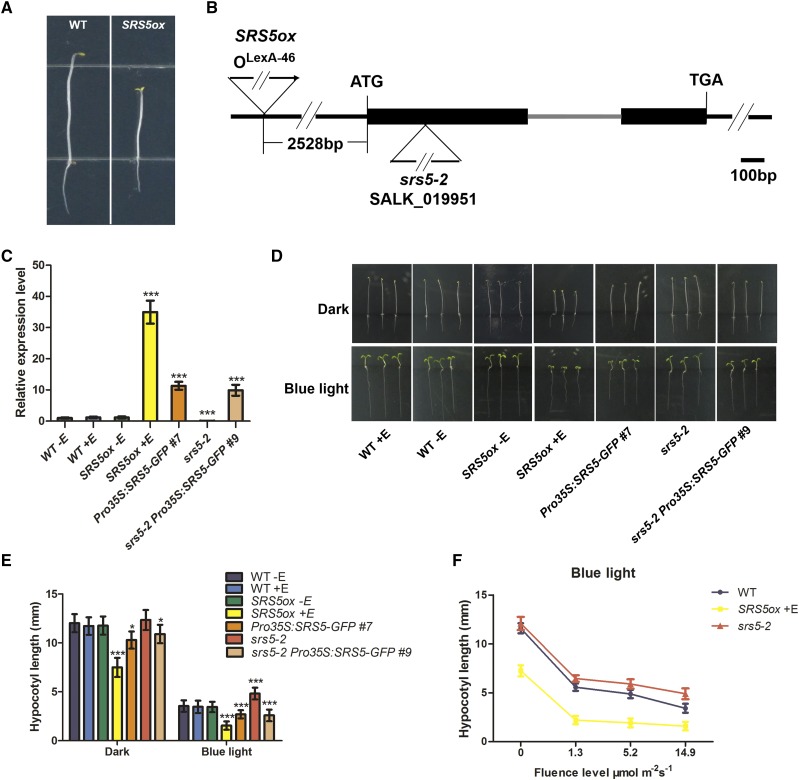
To investigate the regulatory mechanisms behind photomorphogenesis, we employed a chemically inducible activation tagging approach to identify genes that play a role in this process, in particular to identify those genes with redundant functions or lethal mutant phenotypes (Marsch-Martínez and Pereira, 2011). Arabidopsis T-DNA insertion lines generated with an estradiol-inducible expression system driven by the chimeric transcription activator XVE (Zuo et al., 2000) were screened for photomorphogenesis in the dark in the presence of estradiol. This led to the isolation of a mutant line displaying short hypocotyls and open cotyledons, which we designated SRS5ox based on our subsequent characterization (Figure 1A).
Figure 1.
Phenotypes of SRS5ox and srs5-2 Seedlings.
(A) Photographs show the dark-grown phenotypes of wild-type seedlings and SRS5ox seedlings treated with 5 µM estradiol.
(B) Schematic representation of the T-DNA insertion sites in SRS5 (At1g75520) in the SRS5ox and srs5-2 mutant lines. Black boxes represent exons, the gray line represents an intron, and the black lines represent regions upstream and downstream of the gene.
(C) SRS5 expression level assayed by RT-qPCR in seedlings of the wild type (± estradiol treatment), SRS5ox (± estradiol treatment), Pro35S:SRS5-GFP, srs5-2, and srs5-2 Pro35S:SRS5-GFP. SRS5 expression in all lines was normalized to that in nontreated wild-type seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(D) Phenotypes of 5-d-old seedlings grown with (+E) or without (−E) 5 µM estradiol in the dark or in blue light (14.9 µmol m−2 s−1). Bar = 5 mm.
(E) Hypocotyl lengths of 5-d-old seedlings grown with (+E) or without (−E) 5 µM estradiol in the dark or in blue light (14.9 µmol m−2 s−1). Data are means ± sd; n ≥ 30. Asterisks indicate significant differences with respect to each control (Student’s t test): *P < 0.05 and ***P < 0.001. Data are shown from Pro35S:SRS5-GFP line #7 and srs5-2 Pro35S:SRS5-GFP line #9, which are representative of three independent transgenic lines for each genotype.
(F) Hypocotyl lengths of wild-type, srs5-2, and estradiol-treated SRS5ox seedlings grown under various fluence rates of blue light. Data are means ± sd; n ≥ 30.
To characterize the genomic sequence flanking the XVE T-DNA in SRS5ox, we performed thermal asymmetric interlaced PCR. This identified the T-DNA insertion site as 2528 bp upstream of the predicted translation start site of SRS5 (Figure 1B), a SHI family gene encoding a protein with a RING finger-like zinc finger motif (Kuusk et al., 2006). Subsequent reverse transcription-quantitative PCR (RT-qPCR) analyses revealed that SRS5 expression was significantly induced in estradiol-treated SRS5ox seedlings (Figure 1C).
To further examine the SRS5ox phenotype, wild-type and SRS5ox seedlings were grown on medium with or without estradiol in the dark for 5 d. Whereas wild-type seedlings displayed skotomorphogenesis under these conditions, SRS5ox seedlings displayed photomorphogenesis with shorter hypocotyls and open cotyledons (Figures 1D and 1E). Furthermore, seedlings expressing SRS5-GFP driven by the cauliflower mosaic virus 35S promoter (Pro35S:SRS5-GFP) also exhibited shorter hypocotyls when grown in the dark, compared with the wild-type seedlings; however, these transgenic seedlings maintained closed cotyledons in the dark, which may be attributed to a lower SRS5 expression level compared with that in SRS5ox (Figures 1C to 1E). Combined, these results indicate that SRS5 overexpression leads to photomorphogenesis in the dark.
Following this, we examined light responsiveness in SRS5ox seedlings and observed that, compared with the wild type, the hypocotyls of estradiol-treated SRS5ox seedlings were significantly shorter when grown in various fluence rates of different light (Figures 1D to 1F; Supplemental Figure 1). In agreement, seedlings of the loss-of-function homozygous mutant srs5-2 (Salk_019951) had hypocotyls that were longer than wild-type hypocotyls when grown in various fluence rates of different light, though hypocotyl length was comparable between srs5-2 and wild-type seedlings grown in the dark (Figures 1D to 1F; Supplemental Figure 1). Moreover, the Pro35S:SRS5-GFP transgenic lines displayed shorter hypocotyls when grown in the different light conditions, compared with the wild-type seedlings. The srs5-2 mutant phenotype was rescued by SRS5 overexpression, as demonstrated by the comparable hypocotyl elongation in srs5-2 Pro35S:SRS5-GFP and Pro35S:SRS5-GFP seedlings grown either in the different light conditions or in the dark (Figures 1D and 1E; Supplemental Figures 1A and 1B). Collectively, our data indicate that SRS5 functions as a positive regulator of photomorphogenesis.
Blue Light Promotes SRS5 Expression
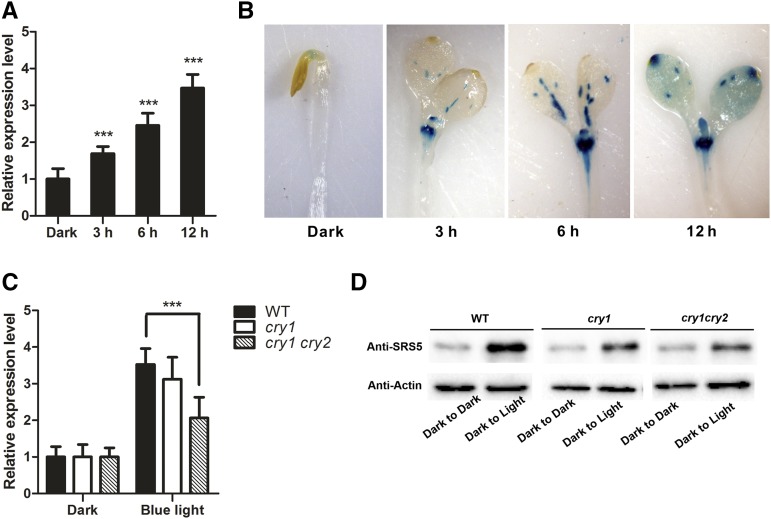
We examined whether SRS5 is regulated by blue light by conducting a time-course analysis of SRS5 expression. For this purpose, 5-d-old Arabidopsis seedlings grown in the dark were treated with blue, red, or far-red light for 0, 3, 6, or 12 h, and SRS5 expression was measured by RT-qPCR. We observed a significant increase in SRS5 expression following blue light exposure (Figure 2A). Then, we examined the SRS5 expression in cry1 and cry1 cry2 mutants. Our results showed that blue light-induced accumulation of SRS5 transcripts was repressed in the mutants (Figure 2C). Consistently, higher SRS5 protein accumulation by blue light was also reduced in the mutants (Figure 2D). However, while our phenotype analysis showed that SRS5 was needed for photomorphogenesis under red and far-red light (Supplemental Figure 1), similar levels of SRS5 transcript were detected after treating with red or far-red light compared with dark-treated control (Supplemental Figure 2).
Figure 2.
SRS5 Expression Is Significantly Induced by Blue Light.
(A) The SRS5 expression level as measured by RT-qPCR in 5-d-old wild-type seedlings grown in the dark and treated with blue light (14.9 µmol m−2 s−1) for either 0, 3, 6, or 12 h. Expression levels were normalized against that in continuous dark-treated seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(B) GUS staining in 5-d-old ProSRS5:GUS transgenic seedlings treated with blue light for either 0, 3, 6, or 12 h.
(C) SRS5 expression as measured by RT-qPCR in 5-d-old wild-type, cry1, and cry1 cry2 seedlings grown in the dark and treated with blue light (14.9 µmol m−2 s−1) for 12 h. Expression levels were normalized against that in continuous dark-treated wild-type seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test).
(D) Immunodetection of SRS5 in 5-d-old wild-type, cry1, and cry1 cry2 seedlings grown in the dark and treated with blue light (14.9 µmol m−2 s−1) for 12 h. Anti-actin served as a loading control.
To verify this result, we generated ProSRS5:GUS transgenic plants and monitored SRS5 expression via GUS staining of dark-grown ProSRS5:GUS seedlings following blue light treatment for 0, 3, 6, or 12 h. This revealed SRS5 expression in dark-grown etiolated seedlings in the apical hook, whereas SRS5 expression was significantly induced in the cotyledons and hypocotyl following blue light treatment (Figure 2B).
Therefore, in combination with the role of SRS5 in blue light-dependent photomorphogenesis described above, these data suggest that blue light modulates photomorphogenesis by stimulating SRS5 expression.
SRS5 Promotes Photomorphogenesis by Directly Activating HY5 Expression
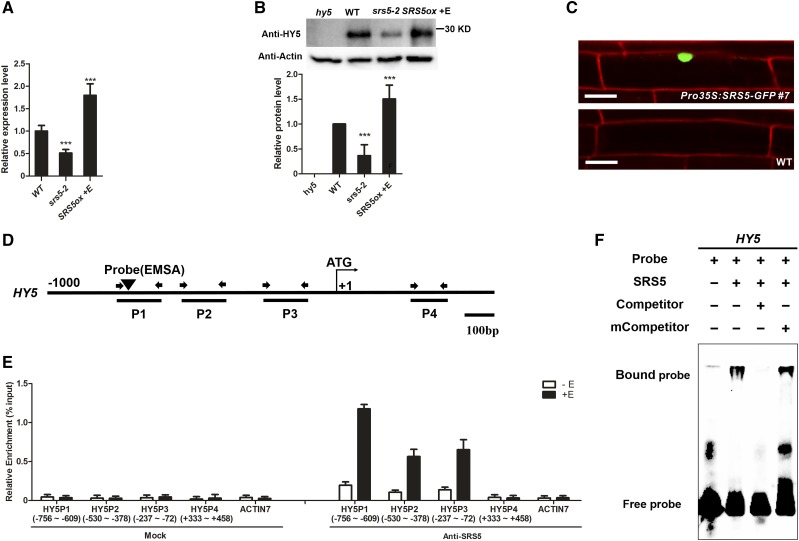
HY5, a positive regulator of photomorphogenesis, is also induced by blue light (Osterlund et al., 2000b; Lee et al., 2007; Zhang et al., 2011). Therefore, to investigate the potential crosstalk between HY5 and SRS5, we analyzed HY5 expression in srs5-2 and estradiol-treated SRS5ox. More HY5 transcript accumulated in estradiol-treated SRS5ox seedlings than in the wild type, whereas HY5 expression was lower in srs5-2 seedlings than in wild-type seedlings, which suggests that the function of SRS5 in photomorphogenesis involves induction of HY5 expression (Figure 3A). This hypothesis was further supported by the significant increase in HY5 abundance observed in estradiol-treated SRS5ox seedlings, which was attributed to SRS5 overexpression, and the decreased HY5 abundance in srs5-2 mutants (Figure 3B).
Figure 3.
SRS5 Activates HY5 Expression by Directly Binding to Its Promoter.
(A) HY5 expression as measured by RT-qPCR in wild-type, srs5-2, and estradiol-treated SRS5ox seedlings grown under white light. Expression levels were normalized against that in wild-type seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(B) Immunodetection of HY5 in wild type, srs5-2, and estradiol-treated SRS5ox seedlings grown under white light (top). Relative HY5 band intensities of the immunoblot analysis (bottom panel). HY5 protein level in wild-type seedlings was set to 1. The error bars indicate the sd from triplicate experiments. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(C) Nuclear localization of SRS5-GFP. The wild-type and Pro35S:SRS5-GFP seedlings were stained with propidium iodide. Fluorescence microscopy images show SRS5-GFP fusion protein in the nucleus in Pro35S:SRS5-GFP plants. Bar = 20 µm.
(D) Schematic diagram of the DNA fragments used for ChIP and the probes used for EMSA. The sequences 1 kb upstream of the start sites and parts of the coding sequences of HY5 are shown. The translational start site (ATG) is shown at position +1.
(E) Enrichment of the indicated DNA fragments following ChIP using anti-SRS5 antibodies. Chromatin from estradiol-treated or nontreated SRS5ox plants was immunoprecipitated using anti-SRS5 antibodies, and the presence of the indicated DNA in the immune complex was determined by RT-qPCR. The numbers of the analyzed DNA fragments indicate the positions relative to the translation start site (referred to as position +1). The ACTIN7 promoter fragment was used as a negative control. The ChIP values were normalized to their respective DNA inputs. The experiments were repeated three times with similar results. Data shown are representative of three independent experiments. Error bars indicate se of three technical replicates.
(F) EMSA of SRS5 binding to HY5 in vitro. Biotin-labeled probes were incubated with SRS5, and free and bound DNAs were separated in an acrylamide gel. As indicated, unlabeled probes were used as competitors.
Like STYLISH1 (STY1), another SHI family member that is present in the nucleus (Eklund et al., 2010), SRS5 was also found to be nuclear localized (Figure 3C), suggesting that SRS5 may act as a transcription factor to promote HY5 expression. Therefore, we examined whether SRS5 associates with the HY5 promoter region via chromatin immunoprecipitation (ChIP) assays using anti-SRS5 antibody prepared with SRS5 purified from Escherichia coli (Supplemental Figure 3). We found that the P1 region of the HY5 promoter was strongly enriched among the immunoprecipitated chromatin of estradiol-treated SRS5ox plants compared with that of nontreated SRS5ox plants (Figures 3D and 3E), suggesting that SRS5 binds to this HY5 promoter region.
To verify this result, we conducted DNA electrophoretic mobility shift assays (EMSAs) using SRS5 purified as described above. SRS5 bound to the DNA probe corresponding to the P1 region, and an unlabeled version of this DNA probe (competitor) could compete for SRS5 binding (Figure 3F). Analysis of the DNA probe revealed the nucleotides ACTCTAC (−742 to −735 bp) in the antisense sequence corresponding to the STY1 binding site consensus sequence (Eklund et al., 2010), which suggests that this promoter element could be the SRS5 target. This hypothesis was supported by the finding that a similar unlabeled DNA probe with a mutated element in the consensus sequence (mCompetitor) did not affect the binding of SRS5 to the P1 region of the HY5 promoter (Figure 3F). Together, these results indicate that SRS5 regulates HY5 expression via direct association with its promoter.
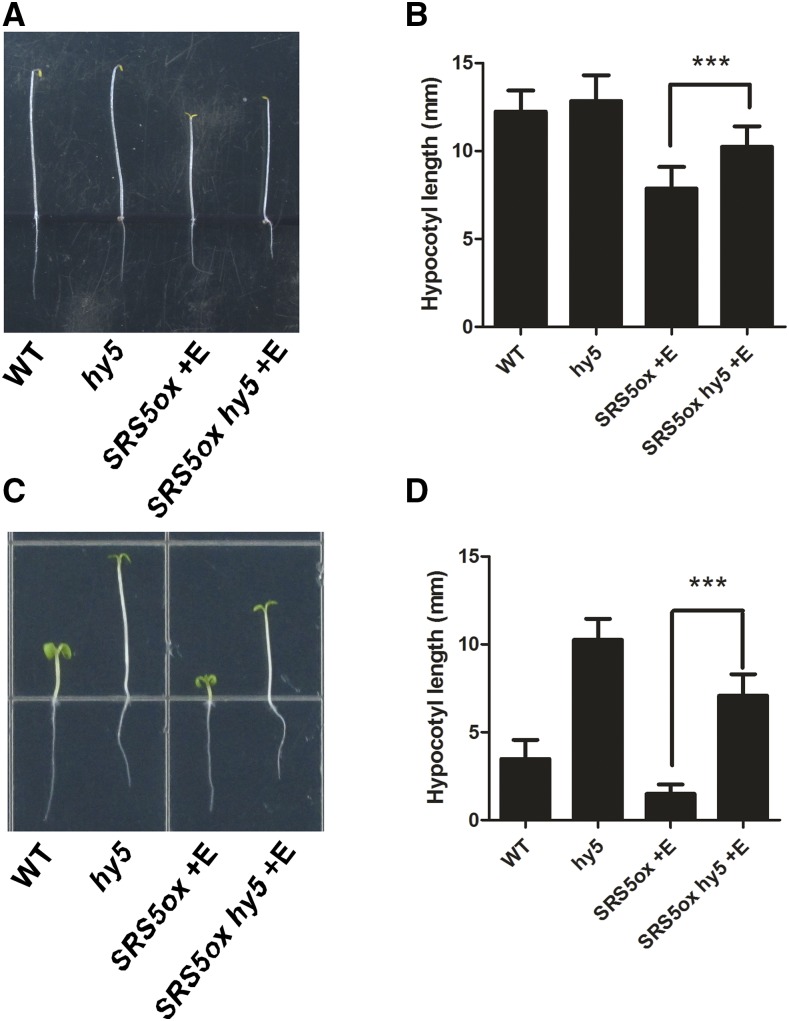
Finally, we tested whether a mutation in HY5 can rescue the SRS5ox phenotype. For this, a SRS5ox hy5 line was obtained by crossing. Our data showed that the short-hypocotyl phenotype of SRS5ox seedlings was partially reversed in SRS5ox hy5 seedlings grown in either the light or the dark in the presence of estradiol (Figure 4). Thus, combined with the above data that SRS5 stimulates HY5 expression, these results indicate that SRS5 promotes photomorphogenesis via direct modulation of HY5 expression.
Figure 4.
The Short Hypocotyl of SRS5ox Seedlings Is Partially Rescued by hy5.
(A) and (C) Five-day-old Arabidopsis seedlings grown in the dark (A) or in blue light (14.9 µmol m−2 s−1) (C).
(B) and (D) Hypocotyl lengths of wild-type, hy5, estradiol-treated SRS5ox, and estradiol-treated hy5 SRS5ox seedlings grown in the dark (B) or in blue light (14.9 µmol m−2 s−1) (D).
Data are means ± sd; n ≥ 30. Asterisks indicate significant differences between SRS5ox and hy5 SRS5ox (Student’s t test; Supplemental File 1). ***P < 0.001.
SRS5 Degradation Mediated by COP1 Ensures Proper Seedling Development
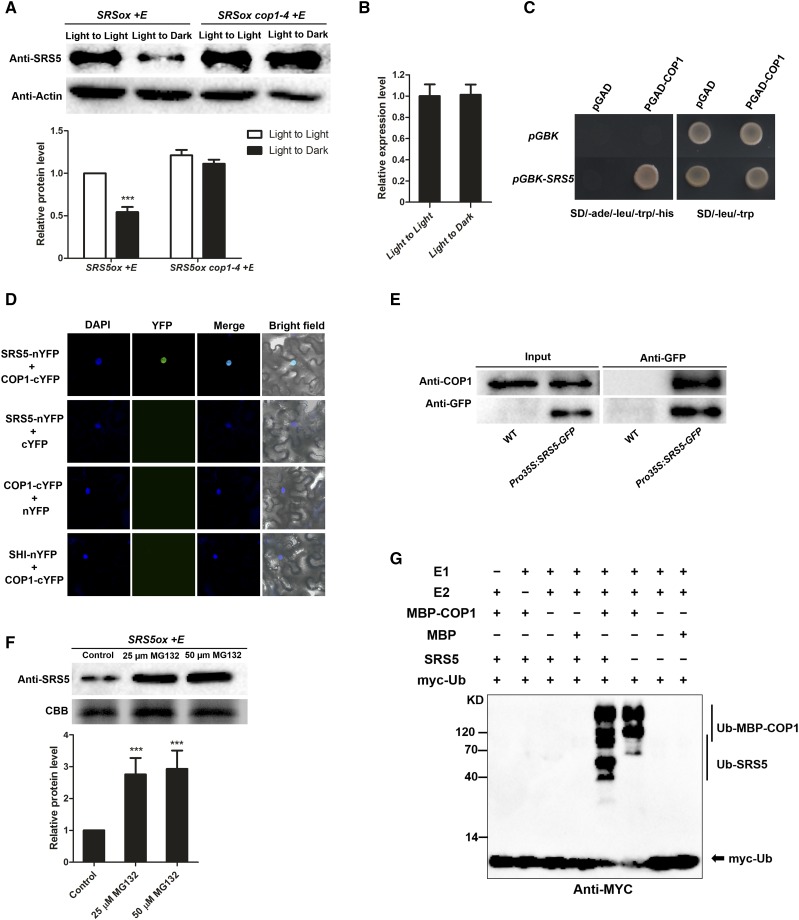
Since posttranslational regulation plays essential roles in the accumulation of several transcriptional factors that are involved in photomorphogenesis, including HY5, BBX21, and BBX22 (Holm et al., 2002; Datta et al., 2008; Chang et al., 2011; Xu et al., 2016), we assessed the effect of posttranslational regulation on SRS5 accumulation. We found that SRS5 accumulation in estradiol-treated SRS5ox seedlings significantly decreased following a transition from the light to the dark in spite of similar SRS5 transcript levels observed in seedlings either maintained in the light or moved to the dark (Figures 5A and 5B). Also, SRS5 accumulation in various fluence rates of different light was examined. We found that the levels of SRS5 proteins were higher in SRS5ox seedlings exposed to blue, red, and far-red light, compared with in continuous dark (Supplemental Figure 4). These results indicate that posttranslational regulation affects SRS5 abundance.
Figure 5.
SRS5 Abundance Is Controlled by COP1-Mediated Degradation.
(A) Immunodetection of SRS5 in estradiol-treated SRS5ox and SRS5ox cop1-4 seedlings which were grown under white light for 5 d and then transferred to darkness for 12 h compared with that in the seedlings under continuous white light (top). Anti-actin served as a loading control. Relative SRS5 band intensities of the immunoblot analysis (bottom panel). SRS5 protein level in estradiol-treated SRS5ox under continuous light was set to 1. The error bars indicate the sd from triplicate experiments. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(B) SRS5 expression assayed by RT-qPCR in estradiol-treated SRS5ox after dark treatment for 12 h compared with that in seedlings under continuous light. Expression levels were normalized against that in seedlings under continuous light, which was set to 1. Data are means ± sd of three independent biological replicates.
(C) Yeast two-hybrid interaction assay showing the interaction of SRS5 and COP1.
(D) BiFC assay showing the interaction of SRS5 and COP1 in N. benthamiana leaves. Coexpressing SHI-nYFP and COP1-cYFP and unfused YFP C-terminal (cYFP) or N -terminal (nYFP) fragments served as negative controls, as indicated. DAPI staining marked nuclei. Merge: Merged images of YFP channel and DAPI.
(E) Co-IP analysis showing that SRS5 interacts with COP1 in vivo. Wild-type and Pro35S:SRS5-GFP seedlings were used in a co-IP assay using anti-GFP antibodies, and the immunoprecipitated proteins were analyzed by immunoblot using anti-COP1 and anti-GFP, respectively.
(F) Immunodetection of SRS5 abundance in estradiol-treated SRS5ox seedlings in the presence of various concentrations of MG132 (25 or 50 μM) for 3 h (top). Relative SRS5 band intensities of the immunoblot analysis (bottom panel). SRS5 protein level in estradiol-treated SRS5ox without MG132 treatment was set to 1. The error bars indicate the sd from triplicate experiments. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(G) COP1 ubiquitinates SRS5 in vitro. In vitro ubiquitination assays were performed in a reaction mix containing UBE1 (E1), UbcH5b (E2), and myc-tagged ubiquitin (myc-Ub). Ubiquitinated MBP-COP1 and SRS5 were detected by anti-myc. The “+” and “−” indicate presence and absence, respectively.
COP1 functions as a central repressor of seedling photomorphogenesis and ubiquitinates a number of targets, including HY5 and BBX21, promoting their degradation in the dark. Therefore, we hypothesized that COP1 also plays a role in the posttranslational regulation of SRS5. Protein interaction assays using the yeast two-hybrid system showed that SRS5 can physically interact with COP1 (Figure 5C). This COP1-SRS5 protein interaction was further verified via bimolecular fluorescence complementation (BiFC) by transient expression of SRS5-nYFP and COP1-cYFP in Nicotiana benthamiana leaves. In contrast to the lack of YFP fluorescence in the negative control, N. benthamiana leaf epidermal cells coexpressing SRS5-nYFP and COP1-cYFP displayed a reconstituted YFP signal (Figure 5D), indicating that SRS5 directly interacts with COP1. In addition, coimmunoprecipitation (co-IP) assays revealed that COP1 was pulled down using anti-GFP in Pro35S:SRS5-GFP seedlings, whereas no COP1 signal resulted from the same co-IP in wild-type seedlings, demonstrating that SRS5 associates with COP1 in vivo (Figure 5E). Furthermore, the involvement of the 26S proteasome in SRS5 degradation was verified by our observation that treatment with MG132, a 26S proteasome inhibitor, resulted in the increased accumulation of SRS5 proteins in SRS5ox grown in the dark (Figure 5F). While SRS5 accumulation in estradiol-treated SRS5ox seedlings significantly decreased, SRS5 accumulation in estradiol-treated SRS5ox cop1-4 seedlings was maintained following a transition from the light to the dark (Figure 5A). Consistent with this, after transfer to the dark, SRS5 accumulation was significantly decreased in wild-type seedlings, but SRS5 accumulation was unchanged in cop1-4 seedlings (Supplemental Figure 5). These data demonstrated that COP1 promotes SRS5 degradation.
We further tested whether COP1 is able to ubiquitinate SRS5 with in vitro ubiquitination assays. The full-length COP1 with an N-terminal MBP tag was expressed and purified in E. coli, and its self-ubiquitination activity was tested by incubation with E1 (UBE1), E2(UbcH5b), and myc-tagged ubiquitin (Figure 5G). While in the absence of E1 or E2, no polyubiquitination conjugates was observed, polyubiquitinated MBP-COP1 conjugates were observed when all reagents were present (Figure 5G). When SRS5 was added together with MBP-COP1 in the reaction, both ubiquitinated SRS5 and MBP-COP1 were detected (Figure 5G). However, when MBP was added together with SRS5, no polyubiquitination conjugates were observed. Taken together, these results reveal that COP1 is able to ubiquitinate SRS5 in vitro.
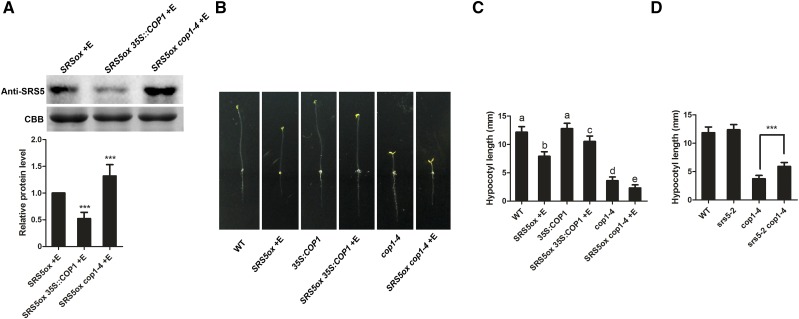
Lastly, we explored whether the role of COP1 in photomorphogenesis includes promoting SRS5 degradation. Whereas SRS5 was less abundant in SRS5ox 35S:COP1 than in SRS5ox plants, we observed higher SRS5 accumulation in SRS5ox cop1-4 compared with that in SRS5ox (Figure 6A). Our assays also showed that SRS5ox cop1-4 had more SRS5 protein than cop1-4, and cop1-4 accumulated more SRS5 protein than the wild type (Supplemental Figure 6). Together, these data indicated that COP1 promotes the selective degradation of SRS5. Subsequent examination of SRS5ox 35S:COP1 and SRS5ox cop1-4 plant phenotypes in the dark showed that estradiol-treated SRS5ox 35S:COP1 had shorter hypocotyls than 35S:COP1 seedlings (Figures 6B and 6C). Likewise, the constitutive photomorphogenesis phenotype of cop1-4 seedlings was more severe when accompanied by SRS5 overexpression in SRS5ox cop1-4 (Figures 6B and 6C). These phenotypes are consistent with the observed SRS5 accumulation in SRS5ox 35S:COP1 and SRS5ox cop1-4 seedlings described above. Furthermore, we generated srs5-2 cop1-4 plants by crossing srs5-2 with cop1-4 and found that the srs5-2 cop1-4 double mutant had longer hypocotyls than the cop1-4 mutant in the dark (Figure 6D). These results support the hypothesis that COP1-mediated degradation of SRS5 is essential for COP1 repression of seedling photomorphogenesis.
Figure 6.
COP1 Controls SRS5 Abundance in Plant Photomorphogenesis.
(A) Immunodetection of SRS5 in estradiol-treated SRS5ox, 35S:COP1 SRS5ox and cop1-4 SRS5ox grown in darkness (top). Relative SRS5 band intensities of the immunoblot analysis (bottom panel). SRS5 protein level in estradiol-treated SRS5ox was set to 1. The error bars indicate the sd from triplicate experiments. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(B) The dark-grown phenotypes of 5-d-old seedlings of the wild type, estradiol-treated SRS5ox, 35S:COP1, estradiol-treated SRS5ox 35S:COP1, cop1-4, and estradiol-treated SRS5ox cop1-4.
(C) Quantitation of hypocotyl lengths in seedlings from (B). Data are means ± sd; n ≥ 30. Different letters indicate significant differences between the annotated columns (P < 0.05 by Tukey’s test).
(D) Hypocotyl lengths of wild-type, srs5-2, cop1-4, and srs5-2 cop1-4 seedlings grown in the dark. Data are means ± sd; n ≥ 30. Asterisks indicate significant differences between cop1-4 and srs5-2 cop1-4 (Student’s t test; Supplemental File 1). ***P < 0.001.
SRS5 Directly Activates Expression of the Photomorphogenesis-Promoting Factors BBX21 and BBX22
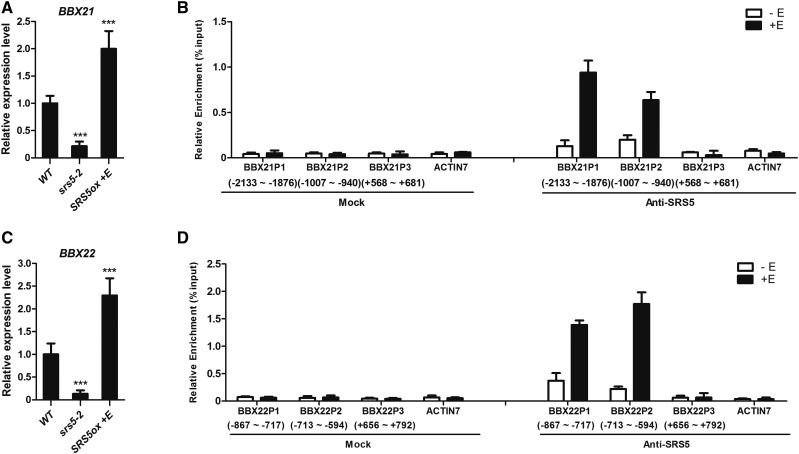
BBX proteins, including BBX21 and BBX22, positively regulate photomorphogenesis in the light and undergo COP1-mediated degradation in the dark, similar to HY5 (Datta et al., 2008; Chang et al., 2011). Thus, we tested whether SRS5 modulates expression of BBX21 and BBX22 in addition to HY5. We found that the transcripts of BBX21 and BBX22 were upregulated in estradiol-treated SRS5ox but reduced in srs5-2 compared with that in the wild type (Figures 7A and 7C).
Figure 7.
SRS5 Activates Photomorphogenesis-Promoting Genes BBX21 and BBX22 by Directly Binding to Their Promoters.
(A) and (C) The expression levels of BBX21 (A) and BBX22 (C) in wild-type, srs5-2, and estradiol-treated SRS5ox seedlings grown under white light were assayed by RT-qPCR. Expression levels were normalized against that in wild-type seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
(B) and (D) ChIP-qPCR was performed for the BBX21 (B) and BBX22 (D) promoters. The numbers of the analyzed DNA fragments indicate the positions relative to the translation start site (referred to as position +1). The ACTIN7 promoter fragment was used as a negative control. The ChIP values were normalized to their respective DNA inputs. The experiments were repeated three times with similar results. Data shown are representative of three independent experiments. Error bars represent se of three technical replicates.
As a transcription factor, SRS5 may modulate the expression of BBX21 and BBX22 by binding to their promoter sequences, as was demonstrated for HY5. In support of this notion, the AATCTAC sequence was identified once in the BBX21 promoter (−1934 to −1941 bp) and the ATTCTAC sequence was identified twice in the BBX22 promoter (−687 to −694 bp and −677 to −684 bp). These sequences are similar to the consensus sequence ACTCTAC (Eklund et al., 2010). Furthermore, ChIP-qPCR assays indicated that BX21 and BBX22 promoter regions were strongly enriched in anti-SRS5 immunoprecipitated chromatin from estradiol-treated SRS5ox plants compared with that from nontreated SRS5ox plants (Figures 7B and 7D). These results indicate that SRS5 directly activates BBX21/22 expression by directly binding to their promoters, which underlies the function of SRS5 in photomorphogenesis.
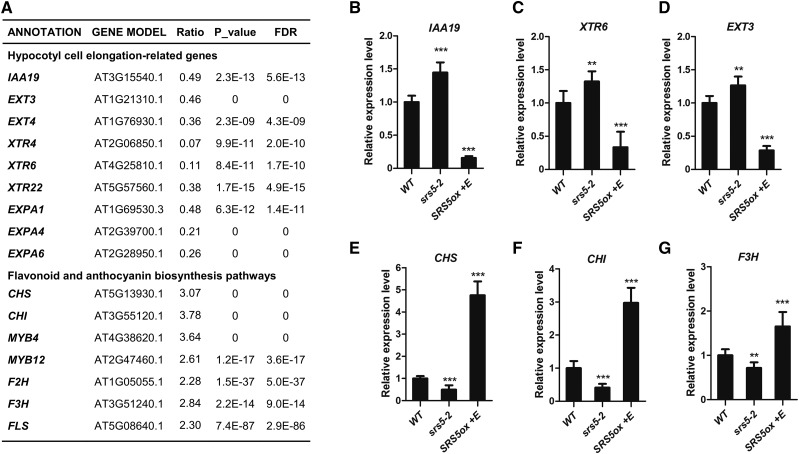
Previous reports have shown that HY5, BBX21, and BBX22 can modulate the expression of many downstream genes involved in plant photomorphogenesis (Lee et al., 2007; Shin et al., 2007; Chang et al., 2011; Jing et al., 2013). Therefore, we explored whether SRS5 regulates the expression of genes affected by the activity of HY5 or BBX21 and BBX22. RNA sequencing data revealed that many genes related to photomorphogenesis, including hypocotyl cell elongation-related and flavonoid/anthocyanin biosynthetic genes, were affected in estradiol-treated SRS5ox plants (Figure 8A; Supplemental Data Set 1). For example, in these SRS5 overexpression plants, the expression of hypocotyl cell elongation-related genes such as IAA19, XTR6, EXT3, and EXP3 was downregulated and the expression of genes involved in flavonoid/anthocyanin biosynthesis such as CHS, CHI, F3H, F2H, FLS, MYB4, and MYB12 was upregulated (Figure 8A). Furthermore, we verified the expression of several genes among them by RT-qPCR and indicated that while IAA19, XTR6, and EXT3 were downregulated in estradiol-treated SRS5ox but upregulated in srs5-2 compared with that in the wild type (Figures 8B to 8D), the transcripts level of CHS, CHI, and F3H was higher in estradiol-treated SRS5ox but lower in srs5-2 than in the wild type (Figures 8E to 8G). These expression changes are reminiscent of those induced by HY5 or BBX21 and BBX22 activity (Lee et al., 2007; Shin et al., 2007; Chang et al., 2011; Jing et al., 2013). Therefore, these data further support the idea that SRS5 modulates plant photomorphogenesis by regulating the expression of other photomorphogenesis-promoting genes including HY5, BBX21, and BBX22.
Figure 8.
Genes Related to Photomorphogenesis Differentially Regulated in Estradiol-Treated SRS5ox Plants.
(A) The expression levels of genes related to photomorphogenesis assayed by RNA-seq in 5-d-old SRS5ox seedlings grown under white light treated with or without estradiol for 2 h, respectively. The expression ratios were calculated using the formula: ratio = SRS5ox +E/ SRS5ox −E. FDR, false discovery rate.
(B) to (G) The expression levels of IAA19 (B), XTR6 (C), EXT3 (D), CHS (E), CHI (F), and F3H (G) in wild-type, srs5-2, and estradiol-treated SRS5ox seedlings grown under white light were assayed by RT-qPCR. Expression levels were normalized against that in wild-type seedlings, which was set to 1. Data are means ± sd of three independent biological replicates. Asterisks indicate significant difference at ***P < 0.001 (Student’s t test; Supplemental File 1).
DISCUSSION
Light is one of the most influential environmental signals that affect plant development. The activation of different photoreceptors by varying light qualities modulates core signaling networks and induces several plant photomorphogenesis-promoting factors, including HY5, BBX21, and BBX22 (Jiao et al., 2007; Gangappa and Botto, 2014). In this study, we identified the transcription factor SRS5 as a positive player in signaling that promotes photomorphogenesis. This role for SRS5 is exemplified by longer hypocotyl phenotype of light-grown srs5-2 seedlings compared with that of the wild type. Whereas overexpression of other well-known photomorphogenesis-promoting factors such as HY5, BBX21, and BBX22 does not result in photomorphogenesis in dark-grown seedlings (Ang et al., 1998; Holm et al., 2002; Chang et al., 2011; Xu et al., 2016), SRS5 overexpression leads to constitutive photomorphogenesis that is characterized by shorter hypocotyls and more open cotyledons compared with those in wild-type seedlings grown in the dark. This SRS5 overexpression phenotype is likely due to SRS5 binding to promoter regions and upregulating the expression of the photomorphogenesis-promoting genes HY5, BBX21, and BBX22. Thus, SRS5 acts as a positive regulator of photomorphogenesis that upregulates the expression of photomorphogenesis-promoting genes to modulate plant light responses.
While HY5, BBX21, and BBX22 play key roles in light signaling, SRS5 also functions in photomorphogenesis by modulating the expression of HY5, but the short-hypocotyl phenotype of SRS5ox seedlings is partially reversed in SRS5ox hy5 seedlings grown in either the light or the dark in the presence of estradiol, implying that besides HY5, other factors may be also involved in SRS5-promoted photomorphogenesis. Indeed, SRS5 also binds to the promoters of BBX21 and BBX22 to activate their expression. In addition, our ChIP-qPCR assays showed that the promoter regions of both CHS and CHI genes were enriched in anti-SRS5 immunoprecipitated chromatin from estradiol-treated SRS5ox plants compared with that from untreated control (Supplemental Figure 7), suggesting that SRS5 could bind to CHS and CHI promoters to modulate their expression.
SRS5 is a member of the SHI gene family, which is characterized by the presence of two functional domains: a 43-amino acid RING-like zinc finger domain and a more C-terminal, unique IGGH domain (Kuusk et al., 2002; Eklund et al., 2010). This RING-like zinc finger domain consists of two fingers that may form a cross-brace arrangement, resembling a DNA binding domain. The IGGH domain carries several acidic residues is involved in mediating the homo- and heterodimerization between SHI family proteins (Fridborg et al., 2001; Eklund et al., 2010). STY1, a member of the SHI family, directly binds to the YUCCA4 promoter to regulate auxin biosynthesis (Eklund et al., 2010). Several family members regulate leaf vein development in Arabidopsis, which is related to their functions in auxin biosynthesis (Baylis et al., 2013). To further explore the potential function of other SHI family genes in photomorphogenesis, we examined the phenotypes of loss-of-function mutants of SHI family genes SHI, SHI-RELATED SEQUENCE7 (SRS7), and LATERAL ROOT PRIMORDIUM1 (LRP1) and found that these mutants have similar hypocotyl length as the wild type grown either in the light or in darkness. We also obtained double mutants srs5-2 shi, srs5-2 srs7, and srs5-2 lrp1 by crossing srs5-2 with shi, srs7, and lrp1, respectively. The hypocotyl lengths of these double mutants were comparable to those of srs5-2 seedlings grown either in the light or in darkness (Supplemental Figure 8).
COP1, a central player in light signaling pathways, represses photomorphogenesis in dark-grown seedlings by ubiquitinating photomorphogenesis-promoting factors, including HY5, BBX21, and BBX22, thus promoting their degradation (Osterlund et al., 2000b; Wang et al., 2001; Lau and Deng, 2010). Our results revealed that SRS5 physically interacts with COP1 and that SRS5 accumulation is reduced following COP1 overexpression, whereas SRS5 accumulation is further increased in the cop1-4 mutant, which lacks COP1 function. Furthermore, SRS5 overexpression enhanced the cop1-4 constitutively photomorphogenic phenotype in dark-grown seedlings, and the short hypocotyl phenotype that accompanied SRS5 overexpression was reversed by COP1 overexpression. Therefore, comparable to other photomorphogenesis-promoting factors, such as HY5, BBX21, and BBX22, COP1-mediated SRS5 degradation is also involved in seedling photomorphogenesis.
In conclusion, our study demonstrates that SRS5 acts as a positive regulator of photomorphogenesis. Whereas SRS5 degradation mediated by COP1 is essential for skotomorphogenesis in the dark, increased SRS5 expression in the light leads to the positive modulation of photomorphogenesis via SRS5-mediated activation of other photomorphogenesis-promoting genes through direct binding of their promoters (Figure 9).
Figure 9.
Model for the Role of SRS5 in Photomorphogenesis.
Blue light-induced SRS5 promotes seedling photomorphogenesis by directly activating several other photomorphogenesis-promoting genes such as HY5, BBX21, and BBX22, but COP1 directly targets SRS5 for 26S proteasome-mediated degradation in the dark.
METHODS
Plant Materials
The Arabidopsis thaliana lines srs5-2 (Salk_019951), hy5 (SALK_096651), cry1 (SALK_069292), srs7 (salk_151552), shi (N126329), and lrp1 (SAIL_70_H09) were obtained from the ABRC (http://www.arabidopsis.org/abrc). All of the mutant lines used in this study were verified by PCR and RT-PCR. Double mutant lines were obtained by crossing and were confirmed by PCR. All PCR primers used for genotyping are listed in Supplemental Table 1. The published transgenic lines used in this study are cop1-4 (Saijo et al., 2003), 35S:COP1 (Wang et al., 2001), and cry1 cry2 (Mao et al., 2005).
Plant Growth
Arabidopsis seeds were surface sterilized with 5% (w/v) bleach for 5 min, washed three times with sterile water, placed at 4°C for 3 d, and then planted on medium containing 0.5× MS, 1% sucrose, and 1% (m/v) agar at pH 5.8 (adjusted using 1 M KOH). Seedlings were grown at 23°C under a long-day photoperiod (16-h white light [100 μmol m−2 s−1]/8-h dark), under blue light (14.9 µmol m−2 s−1), under red light (25.3 µmol m−2 s−1), under far-red light (41.7 µmol m−2 s−1), or in darkness for 5 d. The biotron (model LH-100SP-LED; NK Systems) with LED lighting unit was used. Blue, red, and far-red light were generated at 470, 655, and 730 nm, respectively. For estradiol treatment, β-estradiol (Sigma-Aldrich) was added to the culture medium where indicated.
Genetic Screen
To screen for mutants that exhibit photomorphogenesis in the dark, Arabidopsis T-DNA insertion lines were generated with an estradiol-inducible expression system driven by the chimeric transcription activator XVE (Zuo et al., 2000). Over 12,000 XVE tagging T-DNA insertion lines were obtained and the seeds per each line were harvested individually. Then, 20 seeds of one independent line were planted on the medium with 5 μM estradiol and grown in the dark. The seedlings after their growth for 1 week were examined for phenotypic analysis, and a mutant SRS5ox was obtained as it exhibits short hypocotyl and open cotyledons compared with wild-type seedlings. The genomic sequence surrounding the XVE T-DNA in SRS5ox was identified by thermal asymmetric interlaced PCR (Liu et al., 1995).
Transgene Construction
Agrobacterium tumefaciens (strain C58C1) containing plasmid constructs was used to transform plants by the floral-dip method (Wang et al., 2013). The full-length SRS5 cDNA was amplified using SRS5-forward and SRS5-reverse primers and cloned into the BamHI site of pBI121-GFP vector (Yuan et al., 2014) where expression was driven by the CaMV 35S promoter, and then the resulting Pro35S:SRS5-GFP plasmid was transformed into Col-0 and srs5-2 plants.
To construct the ProSRS5:GUS plasmid, a 6-kb fragment upstream of the ATG translation initiation codon of SRS5 was amplified using primers SRS5 pro-Forward and pro-Reverse and then cloned into the SalI site of pBI101 vector to create the SRS5:GUS fusion.
GUS Staining
GUS staining was performed according to methods described previously (Li et al., 2015). Briefly, seedlings were incubated at 37°C in staining solution (100 mM sodium phosphate buffer, pH 7.5, containing 10.0 mM EDTA, pH 8.0, 0.5 mM K3[Fe(CN)6], 0.5 mM K4[Fe(CN)6], 0.1% Triton X-100, and 1.0 mM 5-bromo-chloro-3-indolyl-β-d-glucuronide). Images of seedlings were acquired using a Nikon camera (DXM1200F) coupled to a stereomicroscope (Olympus SZX12). Biological triplicates, involving three sets of plant grown at separate times, were included for each treatment.
RNA Extraction and RT-qPCR Analysis
Total RNA was isolated from whole seedlings using TRIzol reagent (Invitrogen) as previously described (Zhang et al., 2013). Following treatment with RQ1 RNase-free DNase I (Promega), first-strand cDNA synthesis was performed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. The RT-qPCR analysis was performed using a Bio-Rad CFX96 (Bio-Rad) and SYBR Green I (Invitrogen). PCR was performed in 96-well plates according the following protocol: 3 min at 95°C followed by 40 cycles of 15 s denaturation at 95°C, 15 s annealing at 58°C, and 20 s extension at 72°C. ACTIN2 (AT3G18780) and EIF4A (AT3G13920) were used as internal controls. All experiments were performed with three independent biological replicates using RNA samples extracted from three independent plant materials grown under identical conditions and three technical repetitions. The primers used for RT-qPCR analysis are listed in Supplemental Table 1.
Immunoblot Analysis
Protein immunoblot analyses were conducted as described previously (Gendrel et al., 2005). Briefly, total protein was extracted from Arabidopsis seedlings, separated by SDS-PAGE, and then electroblotted onto a polyvinylidene difluoride membrane. Membranes were incubated with indicated primary antibodies separately and then incubated with goat anti-rabbit or goat anti-mouse IgG peroxidase conjugate (Sigma-Aldrich) as the secondary antibody. Coomassie Brilliant Blue staining indicates equal total protein loading. When the SRS5 protein level was detected in estradiol-treated SRS5ox seedlings after dark treatment, the antibody anti-actin (M20009; Abmart) was used as a loading control. The photographs shown are representative results of three independent assays. The intensity of each band was measured with an image processing and analysis software package (ImageJ). The ratio was calculated by normalizing the intensity of each band to the intensity of the input.
For the detection of HY5 and SRS5 proteins, the antibody anti-HY5 (R1245; Abiocode) and antibody anti-SRS5 (diluted in 0.02 M PBS, pH 7.4, and 0.01% NaN3) were used, respectively. To prepare antibody anti-SRS5, full-length recombinant Arabidopsis SRS5 was expressed and purified using Escherichia coli and then the purified protein was used to generate a polyclonal anti-SRS5 antibody in the rabbit as previously described (Gendrel et al., 2005). The specificity of anti-SRS5 was tested in Arabidopsis by immunoblot analysis. SRS5 protein was detected in the wild type but not in srs5-2 mutant seedlings. Besides, the significant increase in SRS5 abundance was observed in estradiol-treated SRS5ox seedlings (Supplemental Figure 3).
ChIP
According to a previously described method (Gendrel et al., 2005), 7-d-old estradiol-treated and nontreated SRS5ox seedlings grown under white light were fixed at room temperature in 1% formaldehyde under vacuum for 15 min. Fixed tissues were homogenized, and the chromatin was isolated and sonicated. The anti-SRS5 antibody was used for immunoprecipitation. About 5% of nonimmunoprecipitated sonicated chromatin was reverse cross-linked and used as an input DNA control. The immunoprecipitated DNA was recovered and analyzed by RT-qPCR in triplicate replicates with ACTIN7 (AT5G09810) as negative control. qPCR data were analyzed according to the percentage of input method (Haring et al., 2007; Binkert et al., 2014). All experiments were performed with three independent biological replicates using chromatin samples from three independent plant materials grown under identical conditions. The experiments were repeated three times with similar results. Data shown are representative of three independent experiments. All primers used in the ChIP assays are listed in Supplemental Table 1.
EMSA
The full-length SRS5 cDNA sequence was cloned in the pET28a vector and introduced into the E. coli strain BL21 (Invitrogen) to produce SRS5. Purified SRS5 was used for EMSA. Oligonucleotide probes were synthesized and labeled with biotin at their 3′-ends (Invitrogen). EMSA was performed using a Light Shift Chemiluminescent EMSA kit (Thermo Scientific). Briefly, biotin-labeled probes were incubated in 1× binding buffer, 2.5% glycerol, 50 mM KCl, 5 mM MgCl2, and 10 mM EDTA with or without proteins at room temperature for 20 min. For competition experiments, nonlabeled probes were added to the binding reactions. The probe sequences are listed in Supplemental Table 2.
Yeast Two-Hybrid Assays
To verify the interaction between SRS5 and COP1 in yeast, the coding sequence (CDS) of SRS5 was fused to the DNA binding domain (BD) in pGBKT7, and the CDSs of COP1 were individually cloned into the pGADT7. The interactions between proteins were assayed by the mating method as described (Clontech Laboratories; Matchmaker GAL4 Two-Hybrid System and Libraries User Manual) (Yuan et al., 2017).
BiFC Assays
For the BiFC assays, the CDS of Arabidopsis SRS5 was cloned into pUC-YNE (containing the YFP N terminus), and the CDS of COP1 was cloned into pUC-YCE (containing the YFP C terminus) (Walter et al., 2004). The sequences of the primers used to generate these constructs are listed in Supplemental Table 1. Constructs for the expression of SRS5-nYFP and COP1-cYFP were introduced into Nicotiana benthamiana leaves via agroinfiltration. After 2 d of incubation, YFP fluorescence was observed in transformed leaf epidermal cells using a laser confocal microscope (Olympus FluoView 1000-Confocal laser scanning microscope).
Co-IP Assays
For co-IP assays, total proteins were extracted from 7-d-old Pro35S:SRS5-GFP seedlings and immunoprecipitated with anti-GFP (Sigma-Aldrich). The immunoprecipitated proteins were separated by SDS-PAGE and subject to immunoblot analysis using anti-COP1 antibody (Abiocode) and anti-GFP (Sigma-Aldrich).
In Vitro Ubiquitination Assays
The full-length COP1 cDNA sequence was fused with N-terminal MBP coding region, and MBP-COP1 fusion was cloned into the pET28a vector and introduced into the E. coli strain BL21 (Invitrogen) to produce MBP-COP1. According to a previously described method (Saijo et al., 2003), ubiquitination reaction mixtures (30 μL) contained 50 ng of UBE1 (E1; Boston Biochem), 200 ng of UbcH5b (E2; Boston Biochem), 5 μg of myc-tagged ubiquitin (myc-Ub; Boston Biochem), 500 ng of SRS5, and 200 ng of MBP-COP1 in a reaction buffer containing 50 mM Tris, pH 7.5, 5 mM MgCl2, 2 mM ATP, and 2 mM DTT. After incubation at 30°C for 2 h, the reactions were stopped with sample loading buffer by boiling at 100°C, separated by SDS-PAGE, and analyzed by immunoblots using antibody monoclonal anti-myc (M4439; Sigma-Aldrich) and antibody anti-SRS5, respectively.
RNA Sequencing
RNA samples were collected from 5-d-old SRS5ox seedlings grown under white light treated with or without estradiol for 2 h, respectively. Library construction and sequencing were performed by the Beijing Genomic Institution (Shenzhen, China). Clean tags were mapped to the reference genome and genes available at the ABRC (http://www.arabidopsis.org/abrc). The expression ratios were calculated using the formula: ratio = SRS5ox +E/SRS5ox −E.
Accession Numbers
The TAIR accession numbers for the sequences used in this study are as follows: SRS5 (AT1G75520), HY5 (AT5G11260), COP1 (AT2G32950), BBX21 (AT1G75540), BBX22 (AT1G78600), SHI (AT5G66350), SRS7 (AT1G19790), LRP1 (AT5G12330), CHS (AT5G13930), CHI (AT3G55120), F3H (AT3G51240), EXT3 (AT1G21310), IAA19 (AT3G11540), and XTR6 (AT4G25810). RNA-seq data are available at the National Center for Biotechnology Information Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra) under accession number SRP158356.
Supplemental Data
Supplemental Figure 1. Phenotypes of SRS5ox and srs5-2 seedlings under red or far-red light.
Supplemental Figure 2. Expression patterns of SRS5 in response to red or far-red light.
Supplemental Figure 3. Immunodetection of SRS5 in the wild type, estradiol-treated SRS5ox, and srs5-2.
Supplemental Figure 4. Light-induced accumulation of SRS5 proteins.
Supplemental Figure 5. Immunodetection of SRS5 in wild-type and cop1-4 seedling following a transition from the light to the dark.
Supplemental Figure 6. Immunodetection of SRS5 in cop1-4, estradiol-treated cop1-4 SRS5ox, and wild-type seedlings grown in darkness.
Supplemental Figure 7. SRS5 directly binds to the promoters of CHS and CHI.
Supplemental Figure 8. Phenotypes of single or double mutants of SHI family genes under dark, blue, red, or far-red light.
Supplemental Table 1. List of the primers used in this study.
Supplemental Table 2. List of oligonucleotides used for EMSA.
Supplemental Data Set 1. Raw RNA-seq data for differential expression genes in estradiol-treated SRS5ox plants.
Supplemental File 1. Statistical analysis.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Jianru Zuo at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, for generously providing seeds for the XVE T-DNA-tagged mutant library. This work was supported by the National Natural Science Foundation of China (31470378).
AUTHOR CONTRIBUTIONS
T.-T.Y. and Y.-T.L. designed the experiments. T.-T.Y., H.-H.X., Q.Z., and L.-Y.Z. performed the experiments. T.-T.Y. and Y.-T.L. wrote the article.
References
- Abbas N., Maurya J.P., Senapati D., Gangappa S.N., Chattopadhyay S. (2014). Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 26: 1036–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.H., Chattopadhyay S., Wei N., Oyama T., Okada K., Batschauer A., Deng X.W. (1998). Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1: 213–222. [DOI] [PubMed] [Google Scholar]
- Baylis T., Cierlik I., Sundberg E., Mattsson J. (2013). SHORT INTERNODES/STYLISH genes, regulators of auxin biosynthesis, are involved in leaf vein development in Arabidopsis thaliana. New Phytol. 197: 737–750. [DOI] [PubMed] [Google Scholar]
- Binkert M., Kozma-Bognár L., Terecskei K., De Veylder L., Nagy F., Ulm R. (2014). UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26: 4200–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.S., Maloof J.N., Wu S.H. (2011). COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 156: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Chory J., Fankhauser C. (2004). Light signal transduction in higher plants. Annu. Rev. Genet. 38: 87–117. [DOI] [PubMed] [Google Scholar]
- Datta S., Johansson H., Hettiarachchi C., Irigoyen M.L., Desai M., Rubio V., Holm M. (2008). LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 20: 2324–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund D.M., Ståldal V., Valsecchi I., Cierlik I., Eriksson C., Hiratsu K., Ohme-Takagi M., Sundström J.F., Thelander M., Ezcurra I., Sundberg E. (2010). The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. Plant Cell 22: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridborg I., Kuusk S., Moritz T., Sundberg E. (1999). The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein. Plant Cell 11: 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridborg I., Kuusk S., Robertson M., Sundberg E. (2001). The Arabidopsis protein SHI represses gibberellin responses in Arabidopsis and barley. Plant Physiol. 127: 937–948. [PMC free article] [PubMed] [Google Scholar]
- Gangappa S.N., Botto J.F. (2014). The BBX family of plant transcription factors. Trends Plant Sci. 19: 460–470. [DOI] [PubMed] [Google Scholar]
- Gendrel A.V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218. [DOI] [PubMed] [Google Scholar]
- Haring M., Offermann S., Danker T., Horst I., Peterhansel C., Stam M. (2007). Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U., Quail P.H. (2001). The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signaling in Arabidopsis. J. Biol. Chem. 276: 38173–38178. [DOI] [PubMed] [Google Scholar]
- Holm M., Ma L.G., Qu L.J., Deng X.W. (2002). Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 16: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Deng X.W. (2014). Beyond repression of photomorphogenesis: role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 21: 96–103. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230. [DOI] [PubMed] [Google Scholar]
- Jing Y., Zhang D., Wang X., Tang W., Wang W., Huai J., Xu G., Chen D., Li Y., Lin R. (2013). Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C., Lorrain S., Hornitschek P., Fankhauser C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91: 29–66. [DOI] [PubMed] [Google Scholar]
- Kuusk S., Sohlberg J.J., Long J.A., Fridborg I., Sundberg E. (2002). STY1 and STY2 promote the formation of apical tissues during Arabidopsis gynoecium development. Development 129: 4707–4717. [DOI] [PubMed] [Google Scholar]
- Kuusk S., Sohlberg J.J., Magnus Eklund D., Sundberg E. (2006). Functionally redundant SHI family genes regulate Arabidopsis gynoecium development in a dose-dependent manner. Plant J. 47: 99–111. [DOI] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13: 571–577. [DOI] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17: 584–593. [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu H.H., Liu W.C., Zhang X.W., Lu Y.T. (2015). Ethylene Inhibits Root Elongation during Alkaline Stress through AUXIN1 and Associated Changes in Auxin Accumulation. Plant Physiol. 168: 1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.G., Mitsukawa N., Oosumi T., Whittier R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8: 457–463. [DOI] [PubMed] [Google Scholar]
- Ma L., Li J., Qu L., Hager J., Chen Z., Zhao H., Deng X.W. (2001). Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Zhang Y.C., Sang Y., Li Q.H., Yang H.Q. (2005). From The Cover: A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 102: 12270–12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch-Martínez N., Pereira A. (2011). Activation tagging with En/Spm-I /dSpm transposons in Arabidopsis. Methods Mol. Biol. 678: 91–105. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000b). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Wei N., Deng X.W. (2000a). The roles of photoreceptor systems and the COP1-targeted destabilization of HY5 in light control of Arabidopsis seedling development. Plant Physiol. 124: 1520–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Sullivan J.A., Wang H., Yang J., Shen Y., Rubio V., Ma L., Hoecker U., Deng X.W. (2003). The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17: 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y., Li Q.H., Rubio V., Zhang Y.C., Mao J., Deng X.W., Yang H.Q. (2005). N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis CRYPTOCHROME 1. Plant Cell 17: 1569–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Park E., Choi G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49: 981–994. [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.W. (1994). Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79: 1035–1045. [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Osterlund M.T., Kwok S.F., Deng X.W. (1997). Genetic and developmental control of nuclear accumulation of COP1, a repressor of photomorphogenesis in Arabidopsis. Plant Physiol. 114: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schutze K., Batistic O., Weckermann K., Nake C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438. [DOI] [PubMed] [Google Scholar]
- Wang H., Ma L.G., Li J.M., Zhao H.Y., Deng X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154–158. [DOI] [PubMed] [Google Scholar]
- Wang J., Yan D.W., Yuan T.T., Gao X., Lu Y.T. (2013). A gain-of-function mutation in IAA8 alters Arabidopsis floral organ development by change of jasmonic acid level. Plant Mol. Biol. 82: 71–83. [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang Y., Li J., Lin F., Holm M., Deng X.W. (2016). BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 113: 7655–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.Q., Wu Y.J., Tang R.H., Liu D., Liu Y., Cashmore A.R. (2000). The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103: 815–827. [DOI] [PubMed] [Google Scholar]
- Yang H.Q., Tang R.H., Cashmore A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13: 2573–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.M., Liu W.C., Lu Y.T. (2017). CATALASE2 coordinates SA-mediated repression of both auxin accumulation and JA biosynthesis in plant defenses. Cell Host Microbe 21: 143–155. [DOI] [PubMed] [Google Scholar]
- Yuan T.T., Xu H.H., Zhang K.X., Guo T.T., Lu Y.T. (2014). Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ. 37: 1338–1350. [DOI] [PubMed] [Google Scholar]
- Zhang K.X., Xu H.H., Yuan T.T., Zhang L., Lu Y.T. (2013). Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 76: 308–321. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng S., Liu Z., Wang L., Bi Y. (2011). Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J. Plant Physiol. 168: 367–374. [DOI] [PubMed] [Google Scholar]
- Zuo J., Niu Q.W., Chua N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273. [DOI] [PubMed] [Google Scholar]