An unusual approach was used to identify a transcription factor that regulates γ-zein gene expression during maize endosperm development together with other transcription factors.
Abstract
Zeins are the most abundant storage proteins in maize (Zea mays) kernels, thereby affecting the nutritional quality and texture of this crop. 27-kD γ-zein is highly expressed and plays a crucial role in protein body formation. Several transcription factors (TFs) (O2, PBF1, OHP1, and OHP2) regulate the expression of the 27-kD γ-zein gene, but the complexity of its transcriptional regulation is not fully understood. Here, using probe affinity purification and mass spectrometry analysis, we identified ZmbZIP22, a TF that binds to the 27-kD γ-zein promoter. ZmbZIP22 is a bZIP-type TF that is specifically expressed in endosperm. ZmbZIP22 bound directly to the ACAGCTCA box in the 27-kD γ-zein promoter and activated its expression in wild tobacco (Nicotiana benthamiana) cells. 27-kD γ-zein gene expression was significantly reduced in CRISPR/Cas9-generated zmbzip22 mutants. ChIP-seq (chromatin immunoprecipitation coupled to high-throughput sequencing) confirmed that ZmbZIP22 binds to the 27-kD γ-zein promoter in vivo and identified additional direct targets of ZmbZIP22. ZmbZIP22 can interact with PBF1, OHP1, and OHP2, but not O2. Transactivation assays using various combinations of these TFs revealed multiple interaction modes for the transcriptional activity of the 27-kD γ-zein promoter. Therefore, ZmbZIP22 regulates 27-kD γ-zein gene expression together with other known TFs.
INTRODUCTION
Zeins are the largest group of storage proteins in the maize (Zea mays) kernel, comprising 50 to 70% of total proteins. Zeins are encoded by a large supergene family. The complexity of the zein gene family has been well described in several reviews (Thompson and Larkins, 1994; Holding and Larkins, 2009; Holding and Messing, 2013). Zeins are identified as α-, β-, γ-, and δ-types based on their solubility and ability to form disulfide bonds (Esen, 1987; Coleman and Larkins, 1999). The α-zeins are the zein-1 (z1) fraction of zeins, as they can be extracted by alcohol without a reducing agent. The zeins of the z1 fraction can be divided into two subclasses: 19- and 22-kD zeins. The 19-kD zeins are further divided into three multimember classes based on their cDNA sequences, z1A, z1B, and z1D, while the 22-kD zein group consists of a single multimember class, z1C (Wienand et al., 1981; Pedersen et al., 1982; Song et al., 2001; Song and Messing, 2002). Unlike α-zeins, the other types of zeins belong to the zein-2 fraction, and an additional reducing reagent is required to extract these proteins. The β-, γ-, and δ-types of zeins are usually encoded by a single gene. An exception to this rule is the duplication of the 27-kD γ-zein gene in quality protein maize lines, which have even higher levels of this protein (Liu et al., 2016). The 15-kD zein is a β-type zein (Pedersen et al., 1986), while the 10- and 18-kD zeins are δ-type (Kirihara et al., 1988; Chui and Falco, 1995). The three other zein genes, including the 16-, 27-, and 50-kD zein genes, belong to the γ-zein gene subfamily (Prat et al., 1987; Woo et al., 2001).
Zeins, the largest group of endosperm storage proteins, lack two essential amino acids, lysine and tryptophan, resulting in poor kernel protein quality (Osborne et al., 1914). Thus, maize, one of the most productive crops worldwide, does not provide balanced nutrition to poultry and nonruminant livestock without additional lysine and tryptophan (Bhan et al., 2003). Efforts have focused on improving kernel protein quality by reducing zein contents. Suppression of the expression of a particular type of zein by RNA interference (RNAi) resulted in a reduction in zein content but caused the endosperm to appear opaque (Wu and Messing, 2010b; Guo et al., 2013). Increased lysine and tryptophan contents have been observed in many maize kernel mutants, such as opaque2 (o2), o7, and o11 (Mertz et al., 1964; Wang et al., 2011; Feng et al., 2018). However, these mutants also exhibit pleiotropic effects, including chalky endosperm and low insect and pathogen resistance, which are adverse agronomic traits.
Zeins accumulate in protein bodies (PBs) within the lumen of the rough endoplasmic reticulum in endosperm cells beginning at ∼10 d after pollination (DAP) (Larkins and Hurkman, 1978). PBs begin as small accretions consisting mainly of γ-zein, which is consistent with the slightly earlier initiation of γ-zein gene expression compared with the others (Woo et al., 2001). As PBs expand, α- and δ-zeins enter the PB core, becoming encapsulated in a shell of γ-zeins (Lending and Larkins, 1989).
Zein sequences accounted for nearly 50% of the cDNAs in a non-normalized endosperm cDNA library, with α-zeins accounting for ∼30% and γ-zeins accounting for ∼15%. Unlike α-zeins, which are encoded by multiple genes, the 27-kD γ-zein gene alone provided the most abundant transcripts in a non-normalized endosperm cDNA library (Woo et al., 2001). Thus, the transcriptional regulatory mechanism of the exceptional magnitude of expression of this gene is important. RNAi modification of zein gene expression led to a reduced number of PBs specifically in response to the downregulation of the 27-kD γ-zein gene and not the downregulation of other zein family members, indicating that the 27-kD γ-zein plays a central role in PB initiation (Guo et al., 2013).
Transcriptional regulation of zein genes is crucial for zein protein accumulation. To date, only four types of transcription factors (TFs) (O2, PBF1 [prolamin box binding factor 1], OHP1/2, and MADS box TF protein MADS47) have been identified as functional TFs that directly regulate the transcription of zein genes. o2, a well-known endosperm-specific TF for zein genes, was cloned by transposon tagging (Schmidt et al., 1987). O2 directly regulates almost all zein genes, except for genes encoding 16-kD γ- and 18-kD δ-zein, but O2 exhibits different levels of activity for different zein gene promoters (Schmidt et al., 1990; Cord Neto et al., 1995; Li et al., 2015). Although 27-kD γ-zein gene expression was only slightly reduced in an o2 mutant, ChIP-seq (chromatin immunoprecipitation coupled to high-throughput sequencing) analysis of O2 suggested that the 27-kD γ-zein gene is a direct target of O2 for transactivation (Li et al., 2015). The endosperm-specific Dof (DNA binding one zinc finger) TF, PBF1, specifically recognizes the prolamin boxes of most zein gene promoters (Vicente-Carbajosa et al., 1997). Silencing of PBF1 using RNAi caused a severe reduction in 27-kD γ-zein levels (Wu and Messing, 2012), suggesting that PBF1 is a regulator of 27-kD γ-zein gene transcription. Two other bZIP TFs, OHP1 and OHP2, are O2 heterodimerizing proteins that bind to the O2-like box in the 27-kD γ-zein promoter (Pysh et al., 1993; Pysh and Schmidt, 1996). Silencing of OHPs leads to a significant reduction in 27-kD γ-zein levels (Zhang et al., 2015). ZmMADS47 is a MADS box-containing TF that controls the activation of 16-kD and 50-kD zein genes by interacting with O2 (Qiao et al., 2016). O11, a bHLH-type TF, cannot directly bind to zein gene promoters, but it regulates zein gene transcription by directly affecting O2 and PBF1 expression (Feng et al., 2018).
There is evidence that other TFs for 27-kD γ-zein gene remain to be discovered. O2, PBF1, OHP1, and OHP2 are known TFs that regulate the expression of the 27-kD γ-zein gene. These proteins interact with each other (Pysh et al., 1993; Vicente-Carbajosa et al., 1997; Zhang et al., 2015), suggesting that the regulatory mechanism of the 27-kD γ-zein gene is complex. Triple mutations of O2, PBF1, and OHPs resulted in a severe reduction in zein accumulation, but some 27-kD γ-zein was still produced (Zhang et al., 2015), indicating that additional TFs regulate this gene.
In this study, we identified a protein that binds to the 27-kD γ-zein gene promoter using an electrophoretic mobility shift assay (EMSA). ZmbZIP22 can activate the 27-kD γ-zein promoter in vivo. RNA sequencing (RNA-seq) analysis of zmbzip22 mutant endosperm versus the wild type identified 1756 differentially expressed genes (DEGs), including the 27-kD γ-zein gene. ChIP-seq identified the 27-kD γ-zein and other functional genes as direct targets of ZmbZIP22. ZmbZIP22 physically interacts with PBF1 and the two OHPs, but not with O2. The combination of ZmbZIP22 and these TFs indicates that the expression of the 27-kD γ-zein gene is regulated by a complex mechanism.
RESULTS
Identification of a Nuclear Protein That Binds to 27-kD Zein Promoter
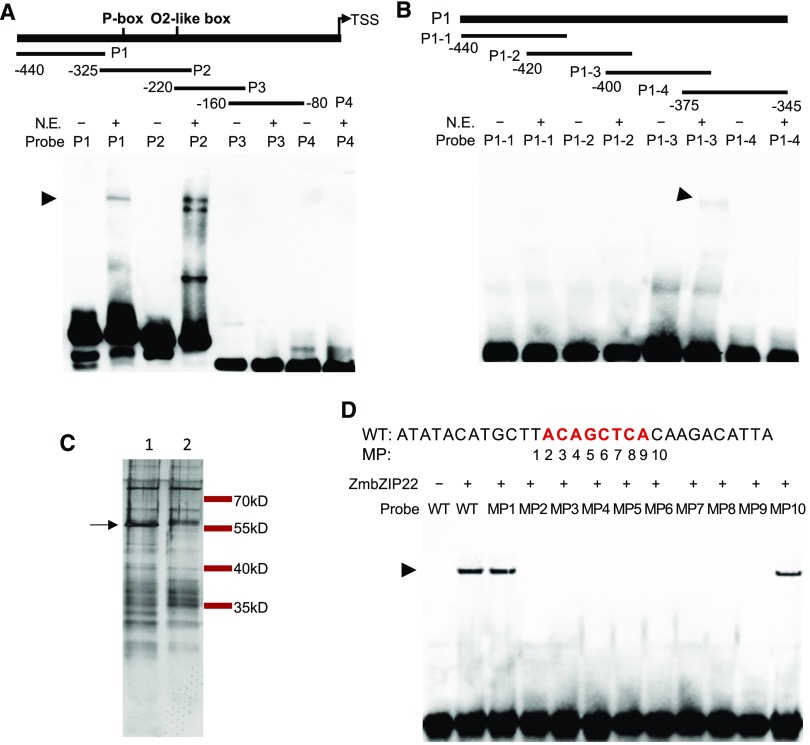
To identify transcriptional regulators of the 27-kD γ-zein gene, the −80 to −440 bp region upstream from the transcription start site (TSS) was divided into four segments (Probe1–Probe4), each 80 to 125 bp in length (Figure 1A, upper panel; Supplemental Table 1). The TSS was determined according to previous S1 nuclease mapping results (Ueda and Messing, 1991). We subjected these segments to biotin labeling and used them as probes for EMSA with nuclear proteins extracted from maize endosperm 15 DAP. Probe2 (−205 to −325 bp) contained the prolamin box and O2-like box. It was not surprising to detect strong band shifts when this probe was incubated with nuclear extract (Figure 1A). These band shifts are believed to be caused by the binding of the probe to PBF1, O2, or the OHPs (Ueda et al., 1992; Vicente-Carbajosa et al., 1997; Zhang et al., 2015). No significant band shift was detected when Probe3 (−141 to −220 bp) and Probe4 (−80 to −160 bp) were incubated with nuclear extract. A previous report using DNase I footprinting indicated that the cis-elements Pb3 and GZM motifs lie in Probe1 (−316 to −440 bp), whereas the factors binding to Probe1 are unknown (Marzábal et al., 1998). A strong band shift was detected when Probe1 was incubated with nuclear extract (Figure 1A).
Figure 1.
Identification of a Nuclear Protein That Interacts with the 27-kD γ-Zein Gene Promoter.
(A) EMSA of four probes (P1–P4) from the 27-kD γ-zein promoter with nuclear extract from 15 DAP endosperm. Upper panel shows a schematic representation of the 27-kD γ-zein promoter fragments. Arrowhead points to the nuclear extract-induced shifted bands. P1–P4, Probe1 to Probe 4; N.E., nuclear extract.
(B) EMSA of four probes truncated from P1. Upper panel shows a schematic representation of the P1 fragments. Arrowhead points to the nuclear extract-induced shifted band.
(C) Nuclear proteins from 15-DAP endosperm purified by affinity chromatography using P1-3 (lane 1) and P1-m3 (lane 2) as baits. Proteins were separated by SDS-PAGE and visualized by silver staining. The arrow points to the protein band specifically purified by P1-3. Numbers on the right indicate the positions of the molecular mass standards.
(D) EMSA of ZmbZIP22 with probes containing the second mutation site of P1-m3. DNA probe containing the second mutation site (WT) and a series of probes each with 1-bp point mutations across the 10-bp segment centered around the second mutation site (MP1–MP10) were used. Arrowhead points to the ZmbZIP22-induced shifted bands.
To determine if the band shift associated with Probe1 and a protein in the endosperm nuclear extract was due to the presence of the Pb3 or GZM motif, we further divided Probe1 into four segments, namely, P1-1 to P1-4, for EMSA (Figure 1B, upper panel; Supplemental Table 1). P1-1 (−405 to −440) contained the Pb3 and GZM motifs. After incubation with nuclear extract, only P1-3 (−350 to −387 bp) showed a band shift (Figure 1B), indicating that P1-3 contains the binding sequence.
To identify the protein binding P1-3, we performed a DNA affinity chromatography pull-down assay. A negative control probe of P1-3 was designed with point mutations every 7 bp (P1-m3; Supplemental Figure 1, upper panel), which abolished the binding of the unknown protein (Supplemental Figure 1). The DNA affinity pull-down assay was performed using three tandem repeats of P1-3, with three tandem repeats of P1-m3 as the negative control (Supplemental Table 1). The proteins that were recovered from the affinity pull-down assay were separated by SDS-PAGE and visualized by silver staining. The specific band that formed with P1-3 but not P1-m3 was subjected to liquid chromatography-mass spectrometry (LC-MS) analysis (Figure 1C). Among the proteins identified, only one was unique to those pulled down by P1-3 (Table 1), which was annotated as the TF ZmbZIP22.
Table 1. Proteins Identified by LC-MS (P < 0.01).
| Accession | Score in Sample P1-3 | Score in Sample P1-m3 | Annotation |
|---|---|---|---|
| GRMZM2G043600 | 376 | – | ZmbZIP22 transcription factor |
| GRMZM2G044128 | 42 | 19 | H/ACA ribonucleoprotein complex subunit |
| GRMZM2G082365 | – | 47 | tRNA pseudouridylate synthase B |
| GRMZM2G017847 | – | 35 | Nucleotide-binding domain of the sugar kinase |
To confirm that ZmbZIP22 can bind to the P1-3 probe, we performed an EMSA using bacterially expressed ZmbZIP22. A band shift was observed when ZmbZIP22 was incubated with P1-3 but not with P1-m3 (Supplemental Figure 2A). To locate the exact binding site of ZmbZIP22, we designed a series of recovery mutation probes based on P1-m3 and designated them as m3-1 to m3-4 (Supplemental Figure 2B, upper panel). The probe m3-2 recovered the protein/DNA complex with ZmbZIP22, indicating that the binding site flanks the second mutation site of P1-3 (Supplemental Figure 2B).
To test the core sequence of the ZmbZIP22 binding motif, we subjected a 30-bp probe across the second mutation site to point mutation analysis. A series of 1-bp point mutations were designed across the 10-bp segment centered by the second mutation site. The EMSA was performed by incubating these probes with bacterially expressed ZmbZIP22. As shown in Figure 1D, point mutations at MP1 and MP10 did not affect the interaction with ZmbZIP22. However, point mutations at MP2 to MP9 abolished the interaction with ZmbZIP22. These results indicate that the binding motif for ZmbZIP22 in the 27-kD γ-zein gene promoter is ACAGCTCA (Figure 1D).
ZmbZIP22 Is an Endosperm-Specific Transcription Factor
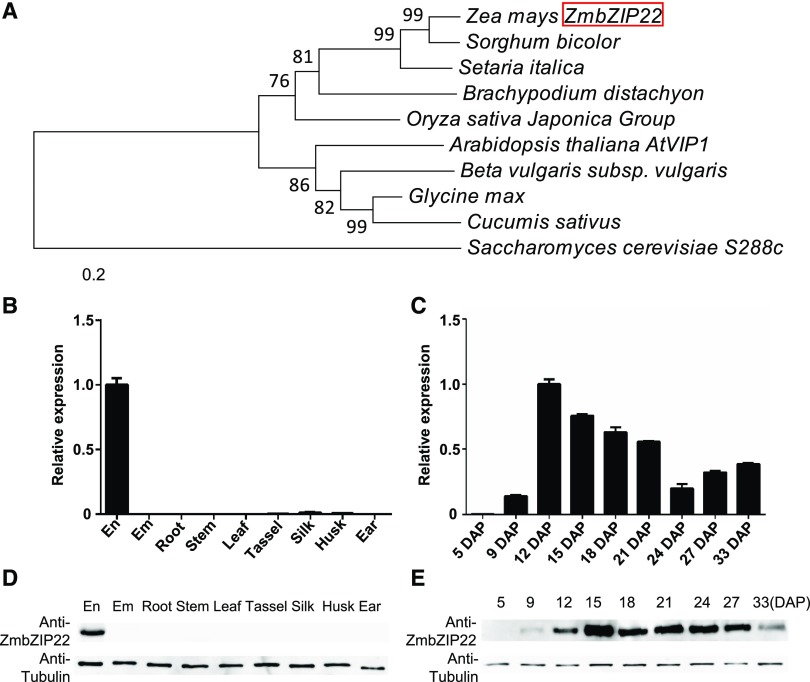
We constructed a phylogenetic tree based on the full-length protein sequence of ZmbZIP22 and its homologous proteins in sorghum (Sorghum bicolor), Brachypodium distachyon, rice (Oryza sativa), foxtail millet (Setaria italica), Arabidopsis thaliana, soybean (Glycine max), beet (Beta vulgaris), cucumber (Cucumis sativus), the yeast Saccharomyces cerevisiae (as the outgroup), and so on (Supplemental Data Set 1). The results indicated that ZmbZIP22 is highly conserved in other plants (Figure 2A). Sequence alignment analysis indicated that ZmbZIP22 shares high similarity with the I-type bZIP TF, VIRE2-INTERACTING PROTEIN1 (AtVIP1), in Arabidopsis (Tzfira et al., 2001; Pitzschke et al., 2009).
Figure 2.
Phylogenetic Relationships and Expression Pattern of ZmbZIP22.
(A) Phylogenetic relationships of ZmbZIP22 and its homologs in plants. Distances were estimated using the neighbor-joining algorithm. The numbers at the nodes (100) represent the percentage of 1000 bootstraps. The scale bar indicates the average number of amino acid substitutions per site. The S. cerevisiae homologous protein was used as an outgroup.
(B) and (C) Expression analysis of ZmbZIP22 by RT-qPCR. RT-qPCR analysis of ZmbZIP22 expression in various tissues (B) and developing kernels (C). The endosperm (En) and embryo (Em) samples in (B) were harvested at 15 DAP. Other tissues (root, stem, third leaf, tassel, silk, husk, and ear) were collected from field-cultivated W22 plants at the V12 stage. The kernel samples in (C) were collected at different developmental stages, as indicated by days after pollination. The Ubiquitin gene was used as internal control. Three biological replicates were made with tissues from three plants. Error bars indicate ±sd (n = 3).
(D) and (E) Immunoblot analysis of ZmbZIP22 expression in various tissues (D) and developing kernels (E). The embryo and endosperm samples in (D) were harvested at 15 DAP. Other tissues (root, stem, third leaf, tassel, silk, husk, and ear) were collected from field-cultivated W22 plants at the V12 stage. The kernel samples were collected at different developmental stages, as indicated by days after pollination. Antitubulin was used as a loading control.
The ZmbZIP22 antibody was synthesized in rabbit using 6×His tagged ZmbZIP22 recombinant protein purified from bacteria (see Methods). We investigated the temporal and spatial expression patterns of ZmbZIP22 by qPCR (Figures 2B and 2C) and protein gel blot analysis with ZmbZIP22-specific antibody (Figures 2D and 2E). The results indicate that ZmbZIP22 is expressed specifically in endosperm, begins to accumulate at 9 DAP and maintains a high level of expression from 15 to 33 DAP.
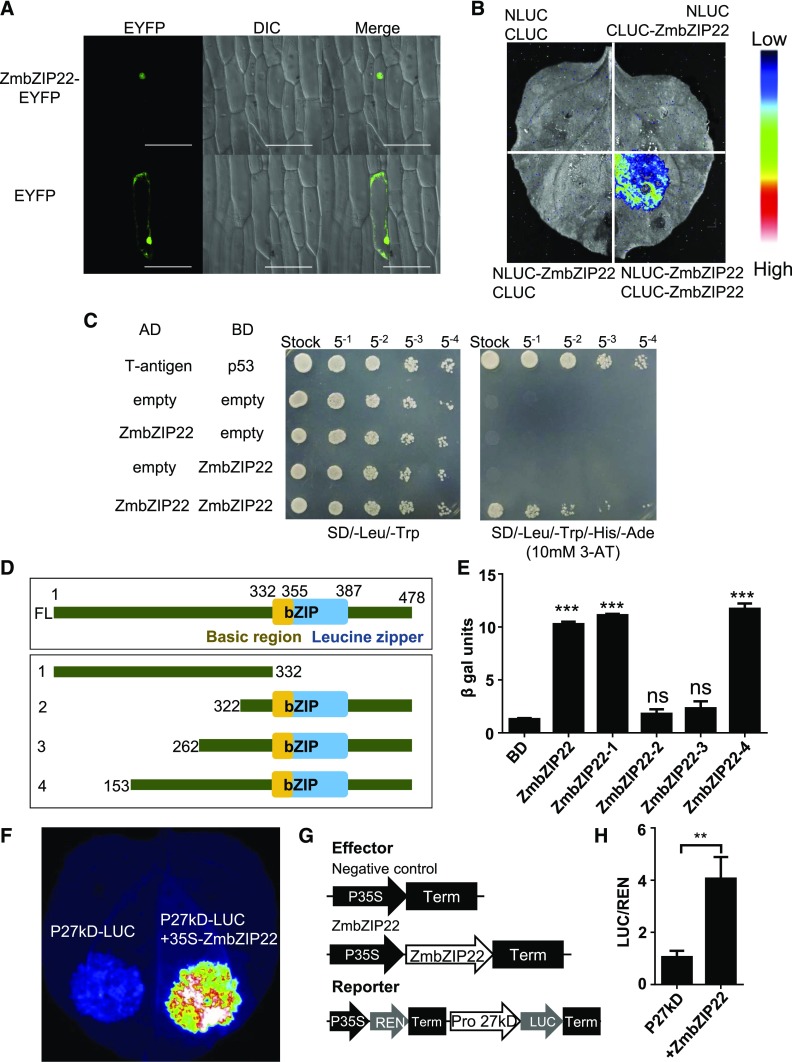
We examined the subcellular localization of ZmbZIP22 using an enhanced YFP (eYFP) fusion at the C terminus and transiently expressing the recombinant protein in onion (Allium cepa) epidermal cells. We visualized the onion nuclei by differential interference contrast microscopy. Compared with the eYFP control, ZmbZIP22-eYFP signals were only concentrated in nuclei, indicating that ZmbZIP22 functions in the nucleus (Figure 3A).
Figure 3.
Transcription Factor Features and Homodimerization of ZmbZIP22.
(A) Fluorescent signals resulting from the expression of ZmbZIP22-eYFP and eYFP alone in onion epidermal cells. Signals from eYFP, DIC (differential interference contrast microscopy), and merging of the two signals are shown in the panels. Similar results were observed in at least 30 cells from three independent experiments. Bar = 50 μm.
(B) LCI assay showing homodimerization of ZmbZIP22 in N. benthamiana. The lower-right section shows the self-interaction signal of ZmbZIP22; the three other sections show the signals of the negative controls. The specific combinations are labeled next to each section. The fluorescent signal intensity represents their interaction activities.
(C) Yeast two-hybrid analysis of the self-interaction of ZmbZIP22. AD, GAL4 activation domain; BD, GAL4 DNA binding domain; 10 mM 3-AT was added to the SD/-Leu/-Trp/-His/-Ade plate to repress the self-activation.
(D) Schematic representation of the structures of ZmbZIP22 and multiple truncated ZmbZIP22 constructions for the transactivation assay in yeast cells.
(E) β-Galactosidase activity resulting from TF transactivation. The pGBK-T7 vector alone was used as a negative control. The significance was calculated by comparing with the BD empty control. Error bars indicate ±sd (n = 3). ns, not significant, ***P < 0.001; Student’s t test.
(F) Transactivation of the 27-kD γ-zein promoter by ZmbZIP22. A representative image of an N. benthamiana leaf 48 h after infiltration is shown.
(G) The 35S:REN-Pro 27-kD γ-zein:LUC reporter constructs, which were transiently expressed in wild tobacco leaf cells together with control vector or 35S:ZmbZIP22 effector.
(H) The expression level of Renilla (REN) was used as an internal control. The LUC/REN ratio represents the relative activity of 27-kD γ-zein promoters. Data are values of three independent experiments. Error bars indicate ±sd (n = 3). **P < 0.01; Student’s t test.
Since ZmbZIP22 is a bZIP protein, it was predicted to form homodimers (Deppmann et al., 2006). This was confirmed by luciferase complementation image (LCI) and yeast two-hybrid assays (Figures 3B and 3C). Consequently, when expressed alone, ZmbZIP22 may be able to bind DNA and activate downstream genes by forming homodimers.
We subjected the ZmbZIP22 protein sequence to domain annotation by CDD/SPARCLE (Marchler-Bauer et al., 2017). The basic leucine zipper (bZIP) domain is found at 332 to 387 amino acids, the basic region of the bZIP domain spans 332 to 355 amino acids, and the leucine zipper region is located at 354 to 387 amino acids, as shown in Figure 3D, upper panel. The basic region is responsible for the DNA binding activity, while the leucine zipper region is responsible for the dimerization (Jakoby et al., 2002). To investigate if ZmbZIP22 has transactivation activity, we performed a yeast transactivation assay (Ye et al., 2004; Li et al., 2006). The full-length ZmbZIP22 open reading frame (ORF) was cloned into the pGBK-T7 vector. Meanwhile, to further locate the activation domain of ZmbZIP22, the protein was truncated, as shown in Figure 3D. The truncated coding sequences were also cloned into the pGBK-T7. The pGBK-T7 vector contains the DNA binding domain of GAL4 TF (GAL4-BD), which was fused to the C terminus of the inserted protein. The vectors were separately cotransformed into the EGY48 yeast reporter strain with the pG221 vector. pG221 contains a β-galactosidase reporter gene with a minimum promoter that can be bound by the DNA binding domain of GAL4 (GAL4-BD) expressed from the pGBK-T7 vector. The β-galactosidase assay showed that ZmbZIP22 has very strong transactivation activity compared with the negative control. Among the truncated fragments, ZmbZIP22-1 (1–332 amino acids) and ZmbZIP22-4 (153–478 amino acids) showed strong activation activity, while ZmbZIP22-2 (322–478 amino acids) and ZmbZIP22-3 (262–478 amino acids) had no activation activity (Figure 3E). These results indicate that ZmbZIP22 is an effective transcriptional activator. Moreover, the activation domain lies between amino acids 153 and 262.
ZmbZIP22 Transactivates the 27-kD Zein Gene Promoter in Wild Tobacco Cells
To determine if ZmbZIP22 has transactivation activity on the 27-kD γ-zein gene promoter in vivo, we fused the 500-bp sequence including the ACAGCTCA box upstream from the TSS with the luciferase coding sequence, yielding the reporter vector, P27kD-LUC. The coding region of ZmbZIP22 was driven by the CaMV 35S promoter, yielding the effector plasmid 35S-ZmbZIP22. Infiltration of Agrobacterium tumefaciens harboring P27-LUC into wild tobacco (Nicotiana benthamiana) leaves produced only basal luciferase activity. When P27-LUC was cotransfected with 35S-ZmbZIP22, significant luciferase activity was detected (Figure 3F), indicating activation of the 27-kD zein gene promoter by ZmbZIP22.
We also performed dual-luciferase transient transcriptional activity assays in wild tobacco leaf cells (Hellens et al., 2005). Expression of ZmbZIP22 and the REN internal control was driven by the CaMV 35S promoter and that of the LUC reporter gene was driven by the 500-bp sequence upstream from the 27-kD zein gene TSS (Figure 3G). Consistent with the qualitative test shown in Figure 3F, significant transactivation was detected when ZmbZIP22 was coexpressed with the reporter construct (Figure 3H).
Loss of Function of ZmbZIP22 Leads to Reduced 27-kD Zein Protein Levels and a Thinner PB Periphery
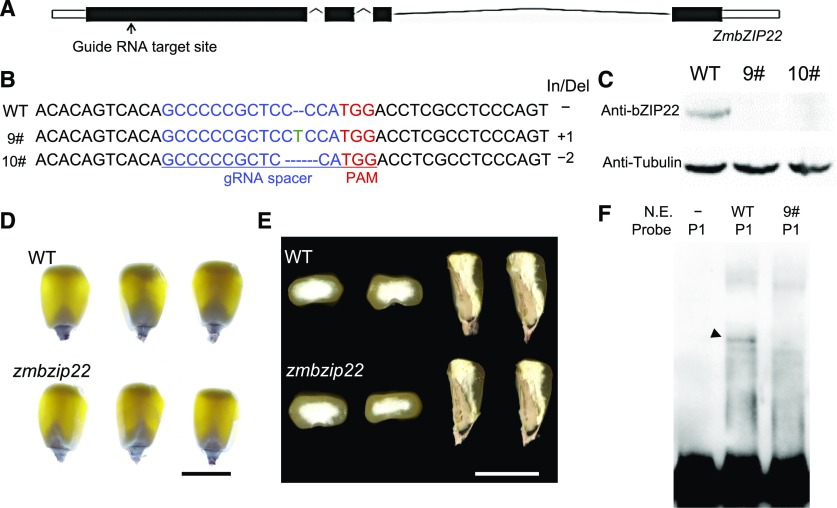
To investigate the effects of the loss of function of ZmbZIP22, we generated knockout mutants using CRISPR-Cas9 (Qi et al., 2016). The gRNA target site was designed based on the first exon of ZmbZIP22 (Figure 4A). A total of 10 CRISPR-Cas9 edited events were identified. zmbzip22-mu9 has a single-nucleotide “T” insertion after the 100th base pair of the ZmbZIP22 ORF, and zmbzip22-mu10 has a two-nucleotide “CC” deletion at 100 to 101 bp of the ZmbZIP22 ORF (Figure 4B). Both events caused loss of function of ZmbZIP22 due to a frame shift and premature termination. We crossed the mutants into the W22 genetic background. We examined the expression of ZmbZIP22 in immature kernels of the zmbzip22-mu9 and zmbzip22-mu10 mutants at 15 DAP in F2 segregating ears by protein gel analysis blot using anti-ZmbZIP22 antibody, finding that ZmbZIP22 was absent from both CRISPR-Cas9 edited mutants (Figure 4C; Supplemental Figure 3).
Figure 4.
CRISPR-Cas9-Based Mutation of ZmbZIP22 and Phenotype Analysis of zmbzip22.
(A) Schematic representation of the gene model of ZmbZIP22. The arrow indicates the gRNA target site.
(B) The DNA sequences of the Cas9-edited ZmbZIP22 gene. The 20-bp guide RNA (gRNA) spacer sequence for the Cas9/gRNA complex is shown in blue, and the protospacer adjacent motif (PAM) site is shown in red. Deleted nucleotides are depicted as dashes, and inserted nucleotides are shown in green. The lengths of the insertions and/or deletions (In/Del) are shown. 9#, zmbzip22-mu9; 10#, zmbzip22-mu10.
(C) Protein gel blot analysis of ZmbZIP22 showing the absence of ZmbZIP22 in zmbzip22-mu9 and zmbzip22-mu10. Antitubulin antibody was used as the internal control. Total proteins were extracted from whole kernels at 15 DAP.
(D) Light transmission analysis of wild-type (WT) and zmbzip22 mature kernels. The homozygous mutant kernels and homozygous wild-type kernels were randomly selected from a segregating F2 population, genotyped and viewed on a light box. Bar = 1 cm.
(E) Transverse and sagittal sections of wild-type and zmbzip22 mature kernels. The homozygous mutant kernels and homozygous wild-type kernels were randomly selected from a segregating F2 population. Bar = 1 cm.
(F) EMSA of wild-type and zmbzip22-mu9 nuclear extract with Probe 1. Arrowhead points to the nuclear extract-induced shifted band. P1, Probe1; N.E., nuclear extract.
We backcrossed zmbzip22-mu9 to plants in the W22 genetic background for at least five generations and collected mature wild-type and zmbzip22-mu9 kernels from F2 ears for phenotype analysis. The zmbzip22 mature kernels showed vitreous endosperm and could not be distinguished from the wild-type kernels by visual inspection (Figures 4D and 4E).
We incubated nuclear extracts from 15-DAP endosperm of zmbzip22-mu9 and the wild type with Probe1 for EMSA. An obvious band shift was observed only with wild-type nuclear extract, but not with zmbzip22-mu9 nuclear extract (Figure 4F). This result confirms the notion that the shifted band identified in Figure 1A resulted from the binding of ZmbZIP22.
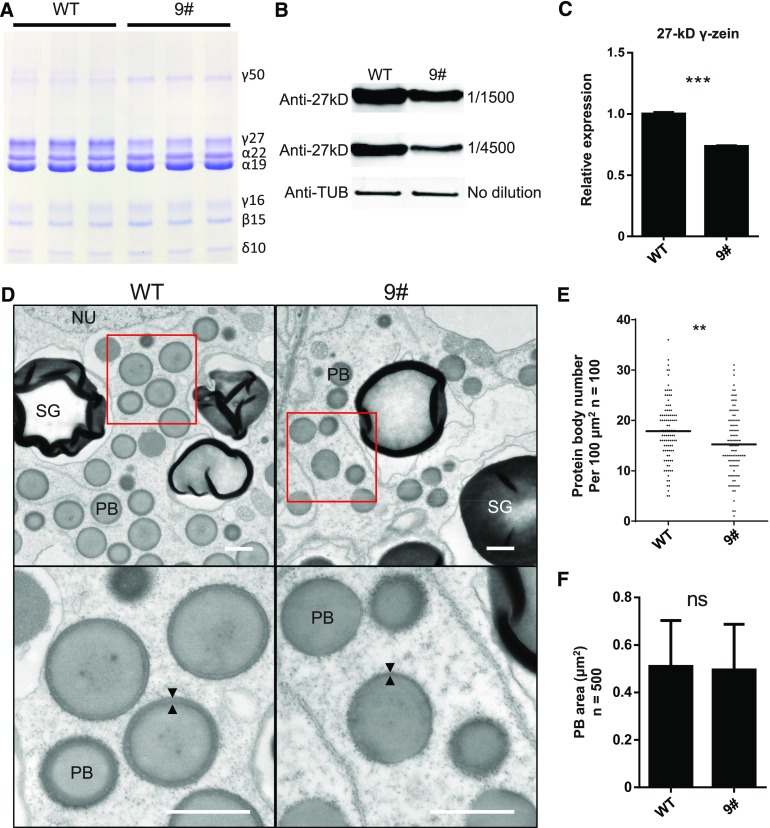
We extracted zein proteins from mature kernels of the wild type and zmbzip22-mu9 and analyzed them by SDS-PAGE. The accumulation of 27-kD γ-zein was significantly reduced in mutant kernels. 50-kD γ-zein protein levels showed a slight increase in the mutant, whereas the levels of the other zeins showed no noticeable changes (Figure 5A). A similar pattern of zein accumulation was observed in zmbzip22-mu10 (Supplemental Figure 4). HPLC profiling of zein proteins confirmed that the level of 27-kD γ-zein was considerably reduced in mature zmbzip22-mu9 kernels compared with the wild type (Supplemental Figure 5). The reduction in 27-kD γ-zein levels was also confirmed by protein gel blot analysis using an anti-27-kD γ-zein antibody (Figure 5B). To determine if the decrease in 27-kD γ-zein gene transcription caused the reduction in 27-kD γ-zein accumulation, we quantified 27-kD γ-zein gene transcript levels in 15-DAP wild-type and zmbzip22-mu9/10 endosperm by qPCR. 27-kD γ-zein transcript levels were reduced to ∼70% in the mutants, indicating that the reduction in 27-kD γ-zein accumulation was caused by the reduced transcription of the 27-kD γ-zein gene (Figure 5C; Supplemental Figure 6).
Figure 5.
Zein and TEM Analysis of zmbzip22 Endosperm.
(A) SDS-PAGE detection of zein accumulation in mature seeds of zmbzip22-mu9. 9#, zmbzip22-mu9.
(B) Protein gel blot detection of 27-kD γ-zein protein in zmbzip22-mu9 with a specific 27-kD γ-zein antibody. Anti-27kD, anti-27-kD γ-zein; Anti-TUB, antitubulin.
(C) Quantitative RT-PCR showing the reduction of 27-kD γ-zein transcription in 15-DAP zmbzip22-mu9 endosperm. Data are values of three independent experiments. Error bars indicate ±sd (n = 3). ***P < 0.001; Student’s t test.
(D) Observation of PBs in wild-type and zmbzip22-mu9 developing kernels at 15 DAP by TEM. The PBs in the fourth endosperm cell layer from the aleurone layer were examined. The lower panels are the magnified sections (indicated by red line boxes) from the upper panels. The arrowheads indicate the peripheral layer of the PB. SG, starch granules; NU, nucleus. Bars = 1 μm.
(E) PB number per 100 μm2 of the fourth endosperm cell layer from the aleurone layer (100 × 100 μm2 from three independent kernels each for zmbzip22 and the wild type). Black lines indicate mean (n = 100). **P < 0.01; Student’s t test.
(F) PB area analysis of the fourth endosperm cell layer from the aleurone layer (500 PBs from three independent kernels each of zmbzip22 and the wild type). Error bars indicate ±sd (n = 500). ns, not significant; Student’s t test.
To examine the structures of PBs in developing zmbzip22-mu9 kernels, we prepared 15-DAP wild-type and zmbzip22-mu9 kernels from the same segregating ear for transmission electron microscopy (TEM) and examined the morphology and sizes of PBs in the fourth endosperm cell layer from the aleurone layer. The periphery of the PBs was uneven and thinner in zmbzip22-mu9 kernels at 15 DAP compared with the wild type (Figure 5D). We calculated PB number and size in the endosperm cells of the fourth layer from aleurone layer. The PB number showed a noticeable decrease in zmbzip22-mu9, while the PB size showed no difference compared with the wild type (Figures 5E and 5F; Supplemental Figure 7). This result is consistent with earlier observations of PBs in which 27-kD zein expression was suppressed by RNAi (Guo et al., 2013).
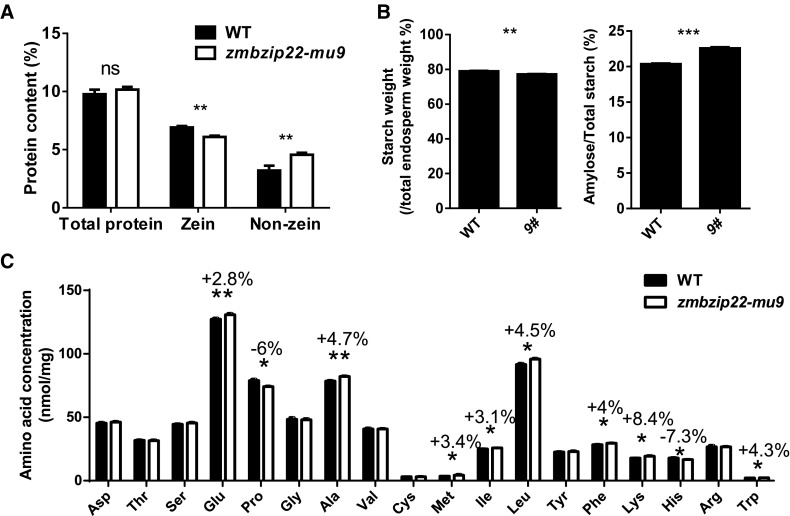
We quantified protein content in mature zmbzip22-mu9 and wild-type endosperm from the same segregating ears to determine if the reduced PB number caused quantitative changes in the levels of zein proteins and nonzein proteins. Zein protein levels in zmbzip22-mu9 endosperm decreased significantly, while nonzein protein levels increased compared with the wild type, resulting in an unchanged total protein content (Figure 6A). We also quantified starch and amylose content in mature zmbzip22-mu9 endosperm. The total starch level in the mutant endosperm was essentially unchanged, whereas the amylose ratio in total starch showed a slight increase in zmbzip22-mu9 (Figure 6B; Supplemental Table 2).
Figure 6.
Quantification of Nutritional Quality of Mature zmbzip22 Endosperm.
(A) Comparison of total proteins, zein, and nonzein from wild-type and zmbzip22-mu9 endosperm. The measurements were done on w/w % of dried endosperm. Error bars indicate ±se (n = 3). **P < 0.01; ns, not significant; Student’s t test.
(B) Comparison of total starch content and amylose content in wild-type and zmbzip22-mu9 mature endosperm. Endosperm from 20 mature kernels each of the wild type and zmbzip22 from the same segregating ear was pooled as one replicate. Three biological replicates were performed using three different segregating ears. The measurements were done on w/w % of dried endosperm. 9#, zmbzip22-mu9. Error bars indicate ±se (n = 3). **P < 0.01 and ***P < 0.001; Student’s t test.
(C) Quantification of TAA content of mature endosperm in the wild type and zmbzip22-mu9. Endosperm from 20 mature kernels each of the wild type and zmbzip22 from the same segregating ear was pooled as one replicate. Three biological replicates were performed using three different segregating ears. The amino acid without an asterisk showed no significant difference. The error bar indicates ±se (n = 3). *P < 0.05 and **P < 0.01; Student’s t test.
Zein proteins lack lysine, tryptophan, and methionine (Mertz et al., 1964). The altered proportion of zein and nonzein proteins in zmbzip22-mu9 may affect the amino acid content and quality of the endosperm. We therefore measured total amino acid (TAA) contents in mature zmbzip22-mu9 endosperm. Compared with the wild type, the lysine content increased ∼8%, tryptophan content increased ∼4% and methionine content increased ∼3% in the mutant (Figure 6C).
Loss of Function of ZmbZIP22 Affects the Transcription of Genes Involved in Multiple Biological Processes
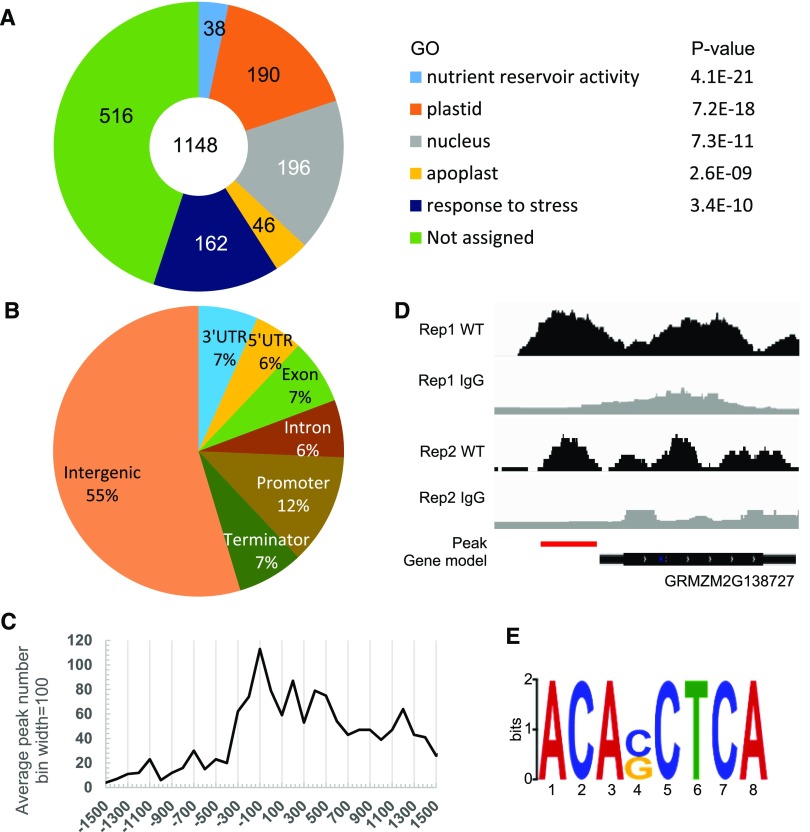
To further explore the potential regulatory function of ZmbZIP22 during maize endosperm development, we performed RNA-seq analysis of 15-DAP developing endosperm from zmbzip22-mu9 and the wild type using segregating F2 ears. DEGs were identified as those with Q value < 0.001 (Supplemental Data Set 2). A total of 1756 DEGs were identified and characterized using the Gene Ontology (GO) database (http://bioinfo.cau.edu.cn/agriGO/). A total of 1118 DEGs annotated by the GO database corresponded to five major GO terms: nutrient reservoir activity (GO: 0045735, P value = 4.1E-21), plastid (GO: 0009507, P value = 7.2e-18), nucleus (GO: 0005634, P value = 7.30E-11), apoplast (GO: 0048046, P value = 2.6E-9), and response to stress (GO: 0006950, P value = 3.4E-10) (Figure 7A; Supplemental Data Set 3).
Figure 7.
GO Classification of DEGs Based on RNA-Seq Analysis and Genome-Wide Binding Profiles from ChIP-Seq Analysis.
(A) GO classification of DEGs based on RNA-seq analysis of zmbzip22-mu9. The number of genes classified within each GO term as well as its P value are shown. In total, 1148 genes were functionally annotated and classified using the GO database. E indicates 10 raised to a power in scientific notation.
(B) Distribution of ZmbZIP22 binding regions in the maize genome. Promoter region, −1 to +100 bp of the TSS; terminator, −100 bp to +1 kb of the TTS; intergenic region, 1 kb upstream of the TSS or 1 kb downstream of the TTS.
(C) Distribution of ZmbZIP22 binding sites per 100 bp bin corresponding to the −1500- to +1500-bp region flanking the TSS. The ZmbZIP22 binding sites are significantly concentrated in the 100 bp immediately upstream of the TSS.
(D) Distribution of the ZmbZIP22 binding site for 27-kD γ-zein gene loci, as shown using the Integrated Genome Browser. Red line indicates significant peaks calculated by MACS2. The binding sites were obtained from two independent biological replicates (rep1 and rep2).
(E) ZmbZIP22 binding motif identified by MEME-ChIP in the 1-kb flanking sequences around the genic peak summits and the density plot of this motif around the summits of the peaks. The ACASCTCA motif was identified as the most prominent ZmbZIP22 binding motif.
Most of the DEGs in the nutrient reservoir term were zein genes. Among the differentially expressed zein genes, the transcript level of the 27-kD γ-zein gene was reduced nearly 20%. This gene showed the most significant reduction in the zmbzip22 mutant, which is consistent with the results of protein analysis (Figures 5A to 5C). The transcript level of the 15-kD β-zein gene significantly increased in zmbzip22 compared with the wild type, even though 15-kD β-zein protein accumulation showed no obvious change in this mutant. Conversely, although the 50-kD γ-zein showed a noticeable increase at the protein level in zmbzip22 (Figure 5A), its transcript level did not differ from that of the wild type. Other DEGs included the 19- and 22-kD α-zein genes. However, although the transcript levels of individual 19-kD α-zein genes or 22-kD α-zein genes varied, the total transcript levels of all genes in these multigene families did not vary between the mutant and the wild type (Supplemental Figure 8).
Starch synthesis in maize endosperm occurs in the amyloplast, a specialized plastid (Buttrose, 1960). Several enzymes are critical for this process, including sucrose synthase, ADP-glucose pyrophosphorylase (AGPase), granule-bound starch synthase, and starch branching and debranching enzymes (Hannah, 1997). AGPase is mainly localized to the cytosol in endosperm, while its catalysis product, ADP-glucose, is transported from the cytosol to the amyloplast via the adenylate translocator BT1 and is used as substrate for starch synthesis (Shannon et al., 1998). Intriguingly, of the DEGs related to starch synthesis, the transcript level of both subunits of AGPase genes, AGPS1a (Bt2, GRMZM2G068506) and AGPL1 (Sh2, GRMZM2G429899), were significantly reduced, whereas the transcript levels of granule-bound starch synthase gene GBSSI (Wx, GRMZM2G024993) and starch branching enzyme gene SBEIIa (Sbe3, GRMZM2G073054) showed an ∼10% increase in the zmbzip22 mutant compared with the wild type (Supplemental Data Set 3).
Of the 1756 DEGs, 162 are closely related to stress responses. Of these, five are defensin-encoding genes and four are proteinase inhibitor genes. Notably, ZmGRP1, a homolog of AtGRP7, showed a significant reduction at the transcript level (Heintzen et al., 1997; Cao et al., 2006). Forty-six of the DEGs are related to the apoplast. Half of these encode ion binding proteins. ZmGRP1 is one of the DEGs in this term. Finally, 196 DEGs are related to nuclear function, including 22 encoding ribosomal proteins.
ChIP-Seq Identifies Genomic Sites That Are Directly Bound by ZmbZIP22
To further investigate the target genes of ZmbZIP22, we performed a ChIP-seq assay using a ZmbZIP22-specific antibody and chromatin extracted from 15-DAP wild-type endosperm (Li et al., 2015). The IgG antibody was used as a negative control. We confirmed the specificity and immunoprecipitation efficiency of the ZmbZIP22 antibodies used in the ChIP experiments by protein gel blot analysis (Supplemental Figure 9). For quality control, ChIP-qPCR for 27-kD zein gene promoter was performed using DNA that had been precipitated by ChIP. The chromatin fragment around the ACAGCTCA box in the 27-kD γ-zein promoter was highly enriched in this DNA bound by ZmbZIP22 antibody (Supplemental Figure 10).
We performed ChIP-seq with two biological replicates (repeat experiments) and predicted the ZmbZIP22 binding sites by Model Based Analysis of ChIP-Seq (MACS) 2 (Zhang et al., 2008; Q value < 0.05, based on a Poisson distribution comparing the ZmbZIP22 and IgG ChIP-seq samples). The 1550 peaks detected in both replicates were used in further analyses (Supplemental Data Set 4).
We analyzed the distribution of binding peaks in the ZmbZIP22 ChIP-seq experiment. The genic region was defined as the DNA sequences containing the coding sequences as well as 1 kb upstream from the TSS to 1 kb downstream of the transcription termination site (TTS). Approximately 45% of the 1550 ZmbZIP22 binding sequences are located in the genic regions of 514 genes. Of these, 12% are located in the promoter regions (−1 kb to +100 bp of the TSS), 6% are located in the 5′-UTRs (untranslated regions), 6% are located in intron regions, 7% are located in exon regions, 7% are located in 3′-UTRs, and 7% are located in terminator regions (−100 bp to +1 kb of the TTS) (Figure 7B). To investigate the binding of ZmbZIP22 to the promoter regions, we calculated the distance between each peak summit and its nearest gene’s TSS. The ZmbZIP22 peaks were highly concentrated in the 200 bp immediately upstream of the TSSs in the core promoter regions (Figure 7C). The 27-kD γ-zein gene was among the 514 genes with ZmbZIP22 binding sites, as determined by ChIP-seq (Figure 7D).
To explore conserved ZmbZIP22 binding motifs across the genome, we subjected the 1-kb flanking sequences around all of the genic peak summits to the motif discovery tool, MEME-ChIP (http://meme-suite.org/tools/meme-chip; Machanick and Bailey, 2011). ACASCTCA was identified as a statistically defined motif (E-value = 1.1E-174; Figure 7E). ZmbZIP22’s binding motif is enriched at the center of the 1-kb sequences examined (Supplemental Figure 11). These results indicate that ACASCTCA is the most highly conserved binding motif for ZmbZIP22 and shows high similarity to the motif identified by EMSA (Figure 1).
Genes That Are Bound and Activated by ZmbZIP22
RNA-seq revealed 1756 DEGs between wild-type and zmbzip22 endosperm, and ChIP-seq identified 33 of these as putative targets bound at their genic region by ZmbZIP22 (Table 2). Of the 33 candidate target genes, 10 genes were bound by ZmbZIP22 at promoter or 5′-UTR. Five of these genes showed reduced transcript levels in zmbzip22, and the five other genes showed increased transcript levels in the mutant. In addition to the 27-kD γ-zein gene, the four other genes were bound by ZmbZIP22 at the promoter or 5′-UTR and showed reduced transcript levels, ZmGRP1, a remorin-encoding gene, a C2H2 RING domain-containing protein gene, and a RRM domain-containing protein gene were used for further validation.
Table 2. The 33 High-Confidence Potential Targets of ZmbZIP22.
| Gene | Function | Fold Change | P Value | Binding region |
|---|---|---|---|---|
| GRMZM2G438538 | – | −1.33 | 0 | 3′-UTR |
| GRMZM2G138727 | 27-kD γ-zein | −1.28 | 0 | Promoter |
| GRMZM2G080603 | ZmGRP1 | −1.09 | 9.28E-166 | 5′-UTR |
| GRMZM5G836166 | – | −1.12 | 6.79E-92 | Terminator |
| GRMZM2G013801 | Zinc finger (MYND type) family protein | −2.30 | 1.33E-54 | 3′-UTR |
| GRMZM2G360097 | Nucleotide binding domain | −4.63 | 5.78E-45 | Terminator |
| GRMZM2G147459 | Uncharacterized protein | 1.60 | 7.55E-33 | Exon |
| GRMZM2G001645 | Uncharacterized protein | 1.28 | 2.33E-26 | Promoter |
| GRMZM2G089631 | Cation transport regulator-like protein 1 | 1.13 | 1.17E-19 | 5′-UTR |
| GRMZM2G137352 | Remorin | −3.24 | 2.27E-15 | Promoter |
| GRMZM2G154156 | Transducin/WD40 repeat-like protein | −1.56 | 5.50E-13 | 3′-UTR |
| GRMZM2G005939 | HLH DNA binding domain superfamily protein | 1.26 | 1.75E-12 | 5′-UTR |
| GRMZM2G432796 | Protein kinase protein | 1.34 | 4.29E-12 | 3′-UTR |
| GRMZM2G585923 | Protein of unknown function (DUF674) | 1.54 | 1.17E-11 | Exon |
| GRMZM2G153292 | Tubulin α-1 chain | −1.04 | 1.51E-09 | Exon |
| GRMZM2G116292 | Ubiquitin fusion protein | 1.10 | 2.62E-09 | Intron |
| GRMZM2G109252 | Lipid binding START domain-containing protein | 1.41 | 2.77E-09 | Promoter |
| GRMZM2G180044 | Protein of unknown function (DUF607) | 2.46 | 3.91E-09 | Promoter |
| GRMZM2G075828 | Transparent testa 12 protein | 3.61 | 5.42E-09 | Exon |
| GRMZM2G127232 | Gibberellin 20 oxidase | 1.53 | 9.25E-09 | Terminator |
| GRMZM2G439784 | Disease resistance family protein/LRR family protein | −1.52 | 1.56E-08 | Exon |
| GRMZM2G143210 | RING/U-box superfamily protein | 1.55 | 1.64E-08 | 5′-UTR |
| GRMZM2G144645 | C2H2 RING | −1.25 | 1.87E-08 | 5′-UTR |
| GRMZM2G099317 | Arg/Ser-rich zinc knuckle-containing protein | 1.11 | 2.13E-08 | 3′-UTR |
| GRMZM2G417455 | β-Galactosidase | 1.17 | 3.75E-08 | Terminator |
| GRMZM2G137174 | Phospholipase A 2A | 1.14 | 1.09E-07 | Terminator |
| GRMZM2G305685 | Nuclear RNA polymerase C2 | 1.25 | 3.18E-07 | Intron |
| GRMZM5G811373 | Mps one binder kinase activator-like 1A | −1.52 | 4.86E-07 | Intron |
| GRMZM2G080176 | Nuclear RNA polymerase C1 | 1.17 | 4.89E-07 | Intron |
| GRMZM2G179090 | RAB GTPase homolog E1E | 1.39 | 1.89E-06 | Promoter |
| GRMZM2G167591 | Uncharacterized protein | 2.53 | 4.10E-06 | 5′-UTR |
| GRMZM2G094497 | Putative ATPase, V1 complex, subunit B protein | −1.07 | 1.59E-05 | Intron |
| GRMZM2G141386 | RNA binding (RRM motifs) family protein | −1.17 | 2.97E-05 | Promoter |
E indicates 10 raised to a power in scientific notation.
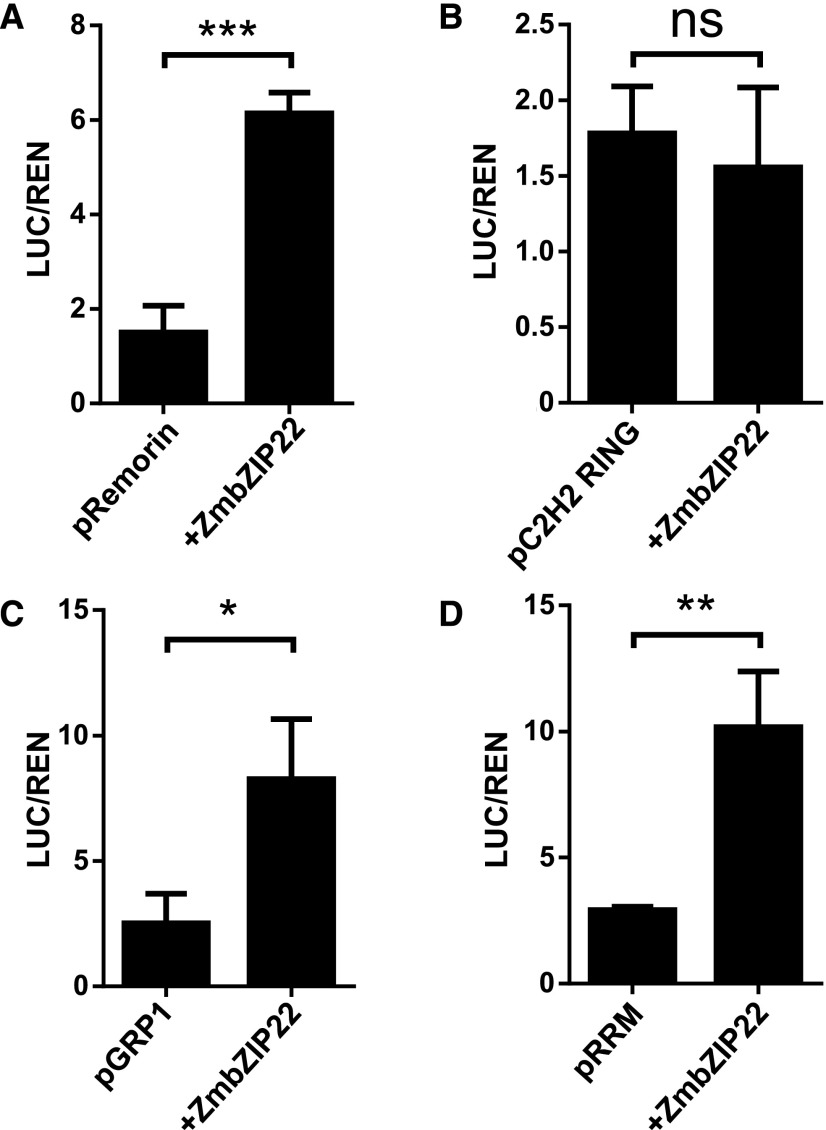
To determine if ZmbZIP22 is a transcriptional activator for these four genes, we performed a dual-luciferase transient transcriptional activity assay. ZmbZIP22 driven by the CaMV 35S promoter was used as the effecter, and LUC driven by the −1000 to −1 bp from the translation start codon of each of the four putative target genes was used as the reporter. ZmbZIP22 specifically induced the expression of LUC driven by the ZmGRP1, remorin coding gene, and RRM domain-containing protein gene promoters (Figure 8). Along with genes previously shown to be direct targets of 27-kD γ-zein, all four genes were shown to be directly activated by ZmbZIP22.
Figure 8.
Dual-Luciferase Activation Assay with Potential Targets of ZmbZIP22.
Relative reporter activities (LUC/REN) of ZmbZIP22 to its potential target promoters (1 kb upstream sequence of the start codon) in N. benthamiana plants. Relative LUC activities (normalized to the REN activity) are shown. The tested promoters are indicated on the x axis. Overexpressed ZmbZIP22 protein was used as the effector. Error bars indicate ±sd (n = 3). ns, not significant; *P < 0.05, **P < 0.01, and ***P < 0.001; Student’s t test.
ZmbZIP22 Interacts with PBF1, OHP1, and OHP2
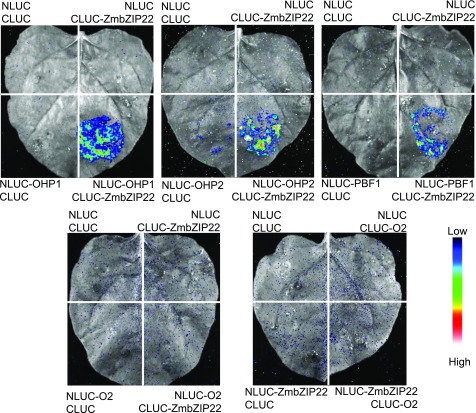
PBF1, OHP1, OHP2, and O2 are known TFs for the 27-kD zein gene (Vicente-Carbajosa et al., 1997; Li et al., 2015; Zhang et al., 2015). The ACAGCTCA box lies 86 bp upstream of the prolamin box and 142 bp upstream of the O2-like box, suggesting that ZmbZIP22 could interact with these TFs. To examine this hypothesis in vivo, we performed an LCI assay. We fused ZmbZIP22 and the four known 27-kD zein gene TFs to the C- and N-terminal domains of LUCIFERASE (CLUC and NLUC, respectively). Cotransfection of ZmbZIP22-NLUC with either OHP1-CLUC, OHP2-CLUC, or PBF1-CLUC produced strong luciferase activity, while individual infiltration of the four vectors with the corresponding empty construct failed to produce a visible signal. Cotransfection of ZmbZIP22-NLUC with O2-CLUC or O2-NLUC with ZmbZIP22-CLUC did not produce visible signals (Figure 9), indicating that ZmbZIP22 interacts with PBF1 and OHPs, but not with O2. These interactions were further examined by yeast two-hybrid assays, yielding similar results (Supplemental Figure 12).
Figure 9.
Interaction Analysis of ZmbZIP22 with PBF1, OHPs, or O2, as Tested by the LCI Assay in N. benthamiana.
For each panel, the lower-right section shows the interaction signal between ZmbZIP22 and the protein of interest; the three other sections show the signals of the negative controls. The specific combinations used for each interaction are labeled next to each panel. The fluorescent signal intensity represents their interaction activities.
ZmbZIP22 Coregulates the Transcription of the 27-kD γ-Zein Promoter with PBF1, O2, and OHPs
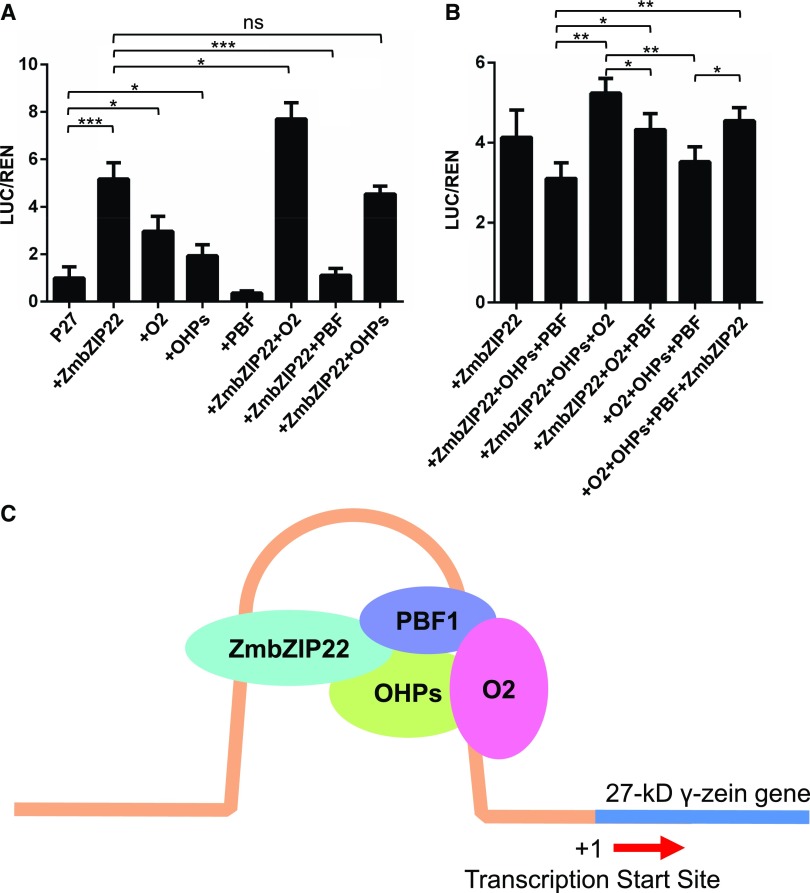
To elucidate the regulatory relationships of PBF1, OHPs, O2, and ZmbZIP22 in the transactivation of the 27-kD γ-zein promoter, we performed a dual-luciferase transient transcriptional activity assay using the firefly luciferase gene driven by the 500-bp 27-kD γ-zein promoter as a reporter. ZmbZIP22, PBF1, and OHPs driven by the CaMV 35S promoter served as effectors.
These effectors were individually transiently expressed with the reporter in wild tobacco leaves (Figure 10A). The two OHPs were treated as one TF in this assay because they share high similarity in amino acid sequence and are paralogs that descended from the two maize allotetraploid progenitors (Xu and Messing, 2008). O2 alone transactivated the 27-kD γ-zein promoter, which is consistent with previous findings (Li et al., 2015). OHPs showed low but significant activation on the 27-kD γ-zein promoter, which is also consistent an earlier report (Zhang et al., 2015). However, PBF1 showed nondetectable activation on 27-kD γ-zein promoter in this assay. Meanwhile, ZmbZIP22 showed an even higher activation level on the 27-kD γ-zein promoter than O2 and OHPs.
Figure 10.
The Cooperation of ZmbZIP22 with Other TFs Known to Regulate 27-kD γ-Zein Gene Expression.
(A) Relative reporter activity (LUC/REN) of different effectors (ZmbZIP22, O2, OHPs, PBF1, and ZmbZIP22 combined with each of the remaining TFs) with the 27-kD γ-zein promoter (500 bp upstream sequence of the TSS). Relative LUC activities (normalized to the REN activity) are shown. Error bars indicate ±sd (n = 3). *P < 0.05 and ***P < 0.001; Student’s t test.
(B) Relative reporter activity (LUC/REN) of different effectors (ZmbZIP22, combination of every three of the four TFs and all four TFs) with the 27-kD γ-zein promoter (500 bp upstream sequence of the TSS). Relative LUC activities (normalized to the REN activity) are shown. The unmarked comparisons are not significant. Error bars indicate ±sd (n = 3). *P < 0.05 and ***P < 0.001; Student’s t test.
(C) Schematic diagram of the cooperation of the 27-kD γ-zein gene transcriptional regulators in maize endosperm.
We then combined each of the three previously described TFs of the 27-kD γ-zein gene with ZmbZIP22 in a dual-luciferase transient transcriptional activity assay (Figure 10A). PBF1 completely repressed ZmbZIP22’s transactivation activity, OHPs did not enhance ZmbZIP22’s activation of the 27-kD γ-zein promoter, and O2 acted additively with ZmbZIP22 in activating the 27-kD γ-zein promoter.
To determine the mechanism underlying how the four TFs cooperate in the transcriptional regulation of the 27-kD γ-zein gene, we coexpressed three of the four TFs in a transactivation assay (Figure 10B). When the four TFs were coexpressed, the activation of the 27-kD γ-zein promoter showed no difference compared with the expression of ZmbZIP22 alone. The absence of either O2 or ZmbZIP22 from the four TFs caused similarly low levels of activation of the 27-kD γ-zein promoter. The absence of PBF1 caused the highest activation of 27-kD γ-zein promoter. The absence of the OHPs did not alter the activation of the promoter. Finally, the absence of any one of the four TFs only caused a minor reduction or increase in the activation of the reporter.
DISCUSSION
ZmbZIP22 Is a TF That Regulates the 27-kD γ-Zein Gene
Identifying proteins that bind to a given gene promoter has always been a challenge due to the shortage of reliable and convenient methods for analysis. Although yeast one-hybrid assays has been broadly used for this type of analysis, this method has many limitations, such as the high background and heavy workload (Reece-Hoyes and Marian Walhout, 2012). Much effort has focused on studying the regulation of zein gene expression since this protein was first characterized in 1821 (Gorham, 1821). It took a long time for O2, the first TF for zein genes, to be characterized (Schmidt et al., 1990). PBF1 was found to bind the prolamin boxes in most zein gene promoters by DNase I footprinting, and a study in Arabidopsis suggested that the Dof TF OBP1 interacts with the AAAG motif (Vicente-Carbajosa et al., 1997). ZmMADS47 was previously identified as an interacting protein with O2 (Qiao et al., 2016). In this study, we used a different approach based on nuclear protein EMSA and DNA pull-down to identify ZmbZIP22, a TF that interacts with the 27-kD γ-zein gene promoter. The interaction between ZmbZIP22 and the 27-kD γ-zein promoter was validated in vitro and in vivo by EMSA and ChIP assays. This approach opens the possibility to identify other DNA binding regulators for a given gene promoter.
Four TFs were previously found to regulate the 27-kD γ-zein gene. PBF1 binds to the prolamin-box, which lies 282 bp upstream of the TSS (Vicente-Carbajosa et al., 1997). O2, OHP1, and OHP2 bind to the O2-like box 49 bp downstream of the prolamin-box (Li et al., 2015; Zhang et al., 2015). Of the four TFs, only PBF1 and O2 are specifically expressed in the endosperm. In this study, we identified ZmbZIP22, a bZIP TF that interacts with the ACAGCTCA motif in the 27-kD γ-zein promoter. Subcellular localization analysis, yeast activation assays, and expression pattern analysis indicated that, like PBF1 and O2, ZmbZIP22 is a nucleus-localized transcriptional activator that functions in developing endosperm (Figures 2B to 2E and 3A). An activation assay indicated that ZmbZIP22 activates the 27-kD γ-zein promoter. A ChIP assay confirmed that ZmbZIP22 directly binds to the ACAGCTCA box of the 27-kD γ-zein gene promoter in vivo. These results indicate that, acting along with PBF1, O2, and the two OHPs, ZmbZIP22 is a component of the transcriptional activation machinery for the 27-kD γ-zein gene.
γ-Zeins, which localize to the PB periphery, function at the early stages of PB formation. Of the γ-zein genes, the 27-kD γ-zein gene has the most abundant transcripts and plays a key role in PB initiation (Guo et al., 2013). In maize kernels with knocked down 27-kD γ-zein expression via RNAi, PBs usually show reduced number, shape distortions, and a thinner periphery compared with the wild type. In zmbzip22 endosperm, the 27-kD γ-zein had ∼20% lower transcript levels and a corresponding reduction in protein accumulation compared with the wild type (Figures 5A to 5C). Protein bodies in 15 DAP zmbzip22 endosperm had an uneven and thinner γ-zein-rich periphery, and the number of PBs was also reduced (Figures 5D to 5F). These results indicate that ZmbZIP22 influences PB initiation.
ChIP-seq analysis revealed that the 27-kD γ-zein is the only zein-encoding gene directly regulated by ZmbZIP22 (Figure 7D). By contrast, the other known zein gene regulators, O2, PBF1, OHPs, and ZmMADS47, affect the expression of multiple zein genes (Li et al., 2015; Zhang et al., 2015; Qiao et al., 2016). ZmbZIP22 starts to accumulate as early as 9 DAP (Figure 2C), and the slightly earlier expression of the 27-kD γ-zein compared with other types of zeins could be due to its transcriptional activation by ZmbZIP22. Some zein genes showed different expression patterns in zmbzip22 compared with the wild type but are not directly regulated by ZmbZIP22. For example, the 50-kD γ-zein showed increased expression at the protein level in the zmbzip22 mutant but is not directly regulated by ZmbZIP22. The 50-kD γ-zein is believed to function in PB expansion (Guo et al., 2013). The increased synthesis of the 50-kD γ-zein is likely due to the feedback response of the reduced PB number caused by the reduction in 27-kD γ-zein expression. Meanwhile, the obvious increase in 15-kD β-zein at the transcriptional level did not cause a noticeable increase in this zein at the protein level. This finding is consistent with the results of a previous RNAi study (Guo et al., 2013), i.e., the reduced transcription of the 15-kD β-zein gene did not result in noticeably lower-than-wild-type levels of 15-kD β-zein protein. Our results confirm the notion that the accumulation of this protein is not primarily limited by its transcript level.
Taken together, our results indicate that the regulation of 27-kD γ-zein gene expression by ZmbZIP22 contributes to the accumulation of 27-kD γ-zein protein and affects PB initiation.
Mutation of ZmbZIP22 Improves the Protein Quality of the Endosperm
Zein, accounting for 70% of the storage protein in maize endosperm, lacks lysine and tryptophan. Reducing the zein content in the endosperm can increase lysine and tryptophan levels, but it usually leads to irregular PB formation and opaque endosperm (Wu and Messing, 2010b; Guo et al., 2013). In zmbzip22 mutant kernels, zein levels decreased while nonzein protein levels increased, resulting in unchanged total protein content (Figure 6A). The reduction in zein content was mainly due to the decreased accumulation of 27-kD γ-zein (Figure 5A). The amino acids making up the 27-kD γ-zein protein include no lysine or tryptophan, and methionine accounts for only 0.9% in frequency. The mutation of zmbzip22 elevated the ratio of nonzeins in storage protein, thus increasing the total content of lysine (∼8%), tryptophan (∼4%), and methionine (∼3%) (Figure 6C). Meanwhile, in zmbzip22, the specific reduction in 27-kD γ-zein levels did not lead to the formation of opaque endosperm. The vitreous phenotype of zmbzip22 is consistent with the observation that the loss of 27-kD γ-zein alone is insufficient to cause opacity (Wu and Messing, 2010a, 2010b; Zhang et al., 2015). The rise in lysine and tryptophan content in zmbzip22 was limited and was not sufficient to reach the protein quality goal via a single gene mutation. However, in combination with other mutations with similar effects (slightly increased protein quality without causing opaque endosperm), this could still be a promising route toward optimizing the amino acid balance in maize endosperm storage proteins. Possible candidates for such combinations could be OHPs and ZmMADS47, whose mutation decreased zein content but without visible changes to kernel texture (Zhang et al., 2015; Qiao et al., 2016).
The Complex Transcriptional Regulation of 27-kD γ-Zein Gene Expression
In eukaryotes, TFs do not usually act alone, instead working in combination: Two or more TFs act synergistically to control target gene transcription (Arnone and Davidson, 1997). The interaction of TFs that regulate the same target genes may be a beneficial for their binding activity to the promoter and for their regulatory efficiency (Zaret and Carroll, 2011). For example, in Arabidopsis, ABI5 (ABA INSENSITIVE5), a bZIP TF, interacts with the AP2/B3-type TF, ABI3, to enhance its activation of downstream targets in the regulation of ABA response genes (Nakamura et al., 2001). Previous research in maize showed that O2, PBF1, and OHPs can interact and cooperate to regulate their target genes (Pysh et al., 1993; Vicente-Carbajosa et al., 1997; Zhang et al., 2015). Our interaction study showed that ZmbZIP22 can interact with the three major 27-kD γ-zein regulators, PBF1, and the two OHPs, but not with O2 (Figure 9; Supplemental Figure 12). A recent study on 27-kD γ-zein gene regulation showed that OHPs and PBF1 play critical rules in this process, whereas O2 plays only a minor role (Yang et al., 2016). ZmbZIP22 specifically regulates the 27-kD γ-zein gene. We speculate that ZmbZIP22 established the ability to interact with PBF1 and the OHPs, but not with O2, during its early evolution to maximize its regulatory efficiency.
Dof-type TFs can act as transcriptional activators or repressors. In maize, ZmDof1 functions as a transcriptional activator. ZmDof2 inhibits ZmDof1 activity in stems and roots through their competitive binding and interaction (Yanagisawa and Sheen, 1998). In banana (Musa acuminata), MaDof23 acts as a repressor and inhibits the activation activity of MaERF9 in regulating ripening-related genes (Feng et al., 2016). In this study, when PBF1 was tested alone with the 27-kD γ-zein promoter, it showed nondetectable activation activity. Coexpression of PBF1 with ZmbZIP22 created much less activation activity than ZmbZIP22 alone (Figure 10A). These findings suggest that PBF1 might act as a suppressor of ZmbZIP22. Perhaps PBF1 can block ZmbZIP22’s activation ability through their interaction.
There is evidence indicating that zeins are subjected to fine-tuned regulation and that the subtle differences in zein content are important for maize endosperm development. In the endosperm, zeins are efficiently packaged as PBs through protein-protein interactions (Hurkman et al., 1981). Moreover, PBs play a central role in the formation of vitreous endosperm (Holding, 2014). Suppressing the expression of a particular type of zein by RNAi results in abnormal PBs and opaque endosperm, indicating that the proper level of each type of zeins is critical for normal PB development and the formation of vitreous endosperm. During endosperm development, the transcription of α-zein shows an “up-and-down” oscillating expression patterns, indicating that α-zein gene expression is precisely regulated during endosperm development (Feng et al., 2009). O2 is a master TF of most zein genes; its activity is regulated by diurnal phosphorylation modification, indicating that zein transcription is regulated diurnally (Ciceri et al., 1997). ZmbZIP22, O2, and the OHPs are all transcriptional activators, and they act together to maintain a high expression level of the 27-kD γ-zein gene. A negative regulator might be needed to modulate the activity of these activators to balance 27-kD γ-zein gene expression. PBF1’s repressive property may be important to fine-tune zein transcription to an optimal level.
The absence of any one of the 27-kD γ-zein TFs (ZmbZIP22, O2, PBF1, or OHPs) did not cause a large reduction or increase in 27-kD γ-zein activation (Figure 10B). This result indicates that these TFs function redundantly to regulate the 27-kD γ-zein gene. The 27-kD γ-zein is crucial for zein PB formation, making it a functional protein as well as a storage protein. The regulatory activity of the four TFs could optimize 27-kD γ-zein gene transcription in the face of diverse physiological or environmental challenges. Stable 27-kD γ-zein gene transcription ensures the successful formation of PBs and the efficient accumulation of other zein storage proteins (Figure 10C).
In addition to ZmbZIP22, other unknown nuclear factors could also participate in 27-kD γ-zein gene regulation. Previous studies suggested that the Pb3 and GZM motifs, lying 430 and 417 bp upstream of the TSS, interact with unknown nuclear factors (Marzábal et al., 1998). However, these two putative cis-elements were not detected in this study. Possible reasons include the following: (1) The radioactive labeling assay is more sensitive than biotin-labeled EMSA; and (2) Probe1 may alter the in vivo DNA structure flanking the Pb3 and GZM region, thus disturbing the interaction of the two motifs with nuclear proteins. Further studies should focus on identifying the nuclear proteins that interact with the Pb3 and GZM motifs.
Multiple Effects of ZmbZIP22 on Endosperm Development
By performing genome-wide gene expression profiling of zmbzip22 using RNA-seq, we identified 1765 DEGs in 15-DAP endosperm of zmbzip22 compared with the wild type, which can be classified into five GO terms. In addition to the expected GO term nutrient reservoir activity, the four other significant GO terms suggest that ZmbZIP22 directly or indirectly participates in the biological processes in the plastid, nucleus, and apoplast and in the response to stress.
Amyloplasts, the specialized plastids in endosperm, are dedicated to starch synthesis. Starch levels showed no major changes in zmbzip22, while the amylose/total starch ratio increased slightly (Figure 6B). Granule-bound starch synthase (GBSS) mediates the synthesis of amylose from ADP-glucose, and GBSSI is the dominate enzyme in amylose synthesis in endosperm (Hannah and James, 2008). The transcript level of the GBSSI gene increased 10% in zmbzip22 compared with the wild type. The slightly elevated amylose ratio might be due to the increased expression of GBSSI. The ChIP-seq assay indicated that ZmbZIP22 does not directly regulate any starch synthesis genes. PBF1 directly influences starch synthesis (Zhang et al., 2016). ZmbZIP22 interacts with PBF1 in vivo, and their interaction might explain the potential indirect regulatory effect of ZmbZIP22 on starch synthesis genes (Figure 9; Supplemental Figure 12).
The ChIP-seq assay revealed 514 ZmbZIP22 binding genes. Overlay of the RNA-seq and ChIP-seq results revealed 33 ZmbZIP22-modulated target genes. Of the 33 genes, the 27-kD γ-zein gene, ZmGRP1, remorin coding gene, and RRM domain-containing protein-coding gene were shown to be directly activated by ZmbZIP22. Meanwhile, ZmbZIP22 interacted with PBF1, OHP1, and OHP2. The altered biological processes in the plastid, nucleus, and apoplast and altered response to stress in zmbzip22 may be modulated directly or indirectly by the targets of ZmbZIP22 or proteins that interact with ZmbZIP22.
METHODS
Plant Materials and Growth Conditions
Maize (Zea mays) inbred line W22 and HiII maize transformation parental lines PA and PB for the maize transgene were obtained from the Maize Genetics Cooperation Stock Center and maintained in our laboratory.
Maize plants were cultivated in the field, greenhouse, or growth chamber at the campus of Shanghai University. Wild-type Nicotiana benthamiana plants were grown in a growth chamber (Ningbo Jiangnan; RXZ-500) at 22°C and 70% relative humidity under a 16-h-light (LED bulb, 52,000 LUX)/8-h-dark photoperiod for ∼4 to 5 weeks before infiltration. After infiltration, the plants were kept under the same growth conditions.
Maize kernels were harvested between 5 and 33 DAP, immediately frozen in liquid nitrogen, and stored at −80°C for RNA and protein extraction (Feng et al., 2009). Root, stem, the third leaf, tassel, husk, silk, and ear tissues were harvested at the V12 stage as previously described (Wang et al., 2012). Tissue samples were collected from at least three individual plants for each developmental stage.
ZmbZIP22 CRISPR-Cas9 transgenic lines were generated in the laboratory according a previous method using the simplex strategy (Qi et al., 2016). The 20-bp target editing sequence was in the first exon of ZmbZIP22 (the 20-bp target sequence was GTCACAGCCCCCGCTCCCCA). The Agrobacterium tumefaciens-mediated maize transformation procedure was performed as described for the simplex strategy. Ten independent Cas9 edited knockout alleles (named zmbzip22-mu1 to zmbzip22-mu10) were recovered and separated from the Cas9 transgene. Zmbzip22-mu9 and zmbzip22-mu10 were backcrossed to the W22 genetic background for at least five generations. The segregating F2 ears were used in this study.
Genotyping of zmbzip22 Mutant Kernels Generated by CRISPR-Cas9
To genotype kernels from a segregating F2 ear, a small piece of the endosperm was cut from a mature kernel and placed into a 2-mL tube containing a stainless steel ball. The small piece of endosperm was ground using a Geno/Grinder (Qiagen; TissueLyser II) in 500 μL TPS buffer (0.1 M Tris-Cl, pH 8.0; , 0.01 M EDTA, pH 8.0, and 1 M KCl). After centrifugation, the DNA was precipitated by adding an equal amount of isopropanol to the supernatant, washed with 70% ethanol, and resuspended in 50 μL distilled water. The CRISPR-Cas9 targeted site was amplified from the genomic DNA using specific primers (Supplemental Table 3), and the PCR product was analyzed by Sanger sequencing for genotyping.
Protein Extraction
Nuclear extracts were prepared from 15-DAP endosperm (W22) for EMSA as described (Schmidt et al., 1992). The extracts were stored in aliquots at −80°C. Total proteins for protein gel blot analysis were prepared from developing kernels and other tissues as described previously (Bernard et al., 1994). Preparation of zein and nonzein from mature maize kernels was described previously (Wallace et al., 1990).
Measurement of Protein, Starch, and TAA Content
Quantification of the proteins was performed as previously described (Smith et al., 1985). Total starch content was measured using an amyloglucosidase/α-amylase starch assay kit (Megazyme) according to the manufacturer’s protocol and adapted as previously described (Feng et al., 2018). Amylose content was measured with an amylose/amylopectin assay kit (Megazyme) according to the manufacturer’s instructions. TAA content was quantified as described previously (Wang et al., 2011). Total tryptophan content was determined as described previously (Hernández, 1969).
To measure zein, nonzein, total starch, and amylose/amylopectin content, endosperm from 20 mature kernels each from the wild type and zmbzip22 from the same segregating ear was pooled as one replicate. Three biological replicates were performed using pooled tissues from three different segregating ears.
Polyclonal Antibody Preparation and Protein Gel Blot Analysis
To generate His-tagged ZmbZIP22 protein, the full-length ZmbZIP22 ORF was amplified and cloned into the EcoRI/SalI sites of pET-32a (Novagen) and the construct was transformed into Escherichia coli Rosetta (DE3) cells. The cells were grown at 37°C and induced by the addition of isopropylthio-β-galactoside to a final concentration of 0.5 mM when the optical density at 600 nm was 0.6. The 6xHis fusion recombinant ZmbZIP22 protein was purified with the ÄKTA purification system using a 1-mL HisTrap FF crude column (GE Healthcare). The antibody was prepared in rabbits by Abclonal according to their standard protocol.
Protein gel blot analysis was performed according to a previously described method (Wang et al., 2014). The ZmbZIP22 antibody was used at dilution of 1:1000. The α-tubulin (Sigma-Aldrich) 27-kD γ-zein antibodies were used at dilution of 1:5000 (Woo et al., 2001).
EMSA
Oligonucleotide probes were synthesized and labeled by Suzhou Synbio Technology. The standard reaction mixture for EMSA contained 8 μg of nuclear extract or 40 ng of purified His-ZmbZIP22 fusion protein, 6 ng of biotin-labeled annealed oligonucleotides, 2 μL of 10× binding buffer (100 mM Tris, 500 mM KCl, and 10 mM DTT, pH 7.5), 1 mL of 50% (v/v) glycerol, 1 mL of 100 mM MgCl2, 1 mL of 1 mg/mL poly(dI-dC), 1 mL of 1% (v/v) Nonidet P-40, and double-distilled water to a final volume of 20 mL. The reactions were incubated at 25°C for 20 min, electrophoresed on 6% (w/v) polyacrylamide gels, and transferred to N+ nylon membranes (Millipore). Biotin-labeled DNA was detected using a LightShift Chemiluminescent EMSA kit (Thermo Scientific). Signals were visualized using the Tanon-5200 image system.
DNA Affinity Pull-Down Assay
The DNA pull-down method used in this assay was modified based on a previous report (Jutras et al., 2012). Maize endosperm nuclear proteins were used as input proteins instead of total cellular proteins to increase the relative abundance of TFs. The probes with three tandem repeats of P1-3 and P1-m3 were synthesized by Suzhou Synbio Technology. The nuclear extract proteins were dialyzed for 4 h against BS-THES buffer before they were used as input proteins in the DNA affinity pull-down assay. The pull-down proteins of the control probe were distinguished from specific proteins pulled down by the nonmutated probe using silver staining. Gel staining was performed using a Protein Silver Stain Kit (CWBio). The specific gel bands were excised and subjected to digestion and LC-MS analysis.
LC/-MS
LC-MS was performed by Mass Spectrum Laboratory of China Agricultural University. Protein digestion was performed using the FASP method with some modifications (Wiśniewski et al., 2009). Briefly, the specific gel bands were destained, dehydrated, reduced with DTT at 56°C for 45 min, and alkylated with IAM (iodoacetamide) at room temperature for 30 min in the dark. The solution was transferred into a 10K ultrafiltration tube (Vivacon 500; Satrorius) and centrifuged at 14,000g for 20 min. The protein was washed three times with 50 mM ABC solution. Two micrograms of trypsin in 50 μL 50 mM ABC was added to the sample and incubated at 37°C overnight. The ultrafiltration tube was centrifuged at 14,000g for 20 min with a new collection tube to collect digested peptides. ABC solution was added to the ultrafiltration tube to wash the digested peptide into the collection tube. The collected solution was diluted with 0.1% formic acid(FA) for nanoLC-MS analysis.
NanoLC separation was achieved with a Waters nanoAcquity nanoHPLC. The trap column was Thermo Acclaim PepMap 100 (75 μm × 2 mm, C18, 3 μm). The analytical column was constructed in the laboratory using a 100 μm i.d. fused silica capillary (Polymicro) filled with 20 cm of C18 stationary phase (Phenomenex; Aqua 3 μm C18 125A). A gradient elution program was used with mobile phase increases linearly from 1% B to 35% B in 65 min: mobile phase A, 0.1% FA in water; B, 0.1% FA in acetonitrile.
Nanospray ESI-MS was performed on a Thermo Q-Exactive high-resolution mass spectrometer (Thermo Fisher Scientific) with 70,000 MS scan resolution and 17,500 MS/MS scan resolution and top-10 MS/MS selection. Raw data from the mass spectrometer were preprocessed with Mascot Distiller 2.4 for peak picking. The resulting peak lists were searched against the UniProt maize database using the Mascot 2.5 search engine. The search parameters were as follows: fixed modifications, carbamidomethyl (C); variable modifications, oxidation (M); enzyme, trypsin; maximum missed cleavages, 2; MS mass tolerance, 10 ppm; MS/MS mass tolerance, 0.02 D.
Domain Annotation and Phylogenetic Analysis
For domain annotation, the ZmbZIP22 protein sequence was subjected to the CDD/SPARCLE tool with default parameters (Marchler-Bauer et al., 2017). ZmbZIP22 homologous protein sequences in other species were identified in the NCBI database by performing a BLASTp (nr) search using the ZmbZIP22 protein sequence as a query. Amino acid sequences were aligned with MUSCLE in MEGA5.2 software. The evolutionary distances were computed using Poisson correction analysis. The bootstrap method was performed using 1000 replicates for the phylogeny test.
RNA Extraction and Quantitative PCR
Total RNA was extracted from developing kernels using an RNAprep Pure Plant Kit (polysaccharides and polyphenolics-rich) (Tiangen; code DP441). Total RNA was extracted from other tissues using an RNAprep Pure Plant Kit (Tiangen; code DP432). cDNA was synthesized using ReverTra Ace qPCR RT Master Mix (Toyobo; code FSQ-201). Primer pairs for quantitative PCR were designed using Quantiprime software (http://www.quantprime.de), and the Ubiquitin gene (GenBank accession number BT018032) was used as an internal control.
For quantitative PCR, the reaction mixture was composed of cDNA first-strand template, primer mix, and SYBR Green Mix (Toyobo; code QPK-201) to a final volume of 20 μL. The reactions were performed using a Mastercycler ep realplex 2 (Eppendorf). Three biological replicates were made with kernels from three F2 ears. The data were analyzed by the ∆∆Ct method as previously described (Zhang et al., 2012).
Subcellular Localization
The full-length ZmbZIP22 ORF was cloned into the transit expression vector pSAT6-EYFP-N1 at the EcoRI/SalI site. Living onion (Allium cepa) epidermal cells were peeled and cultured on Murashige and Skoog medium in the dark for 8 h. One microgram of plasmid per construct was used to coat 0.3 mg of 1.0-μm-diameter tungsten particles and bombarded into the cells using the Biolistic PDS-1000/He system (Bio-Rad). The bombarded samples were cultured for 12 h in the dark. The samples were then observed under a confocal laser scanning microscope (LSM710; Zeiss).
Yeast Two-Hybrid Assay
Yeast two-hybrid assays were performed as previously described (Zhang et al., 2012). The full-length ZmbZIP22, OHP1, OHP2, PBF1, and O2 ORFs were cloned into the EcoRI and XhoI sites of the pGAD-T7 vector (Clontech) using a ClonExpress II one-step cloning kit (Vazyme). The full-length ZmbZIP22-3 was cloned into EcoRI and SalI sites of the pGBK-T7 vector (Clontech) using a ClonExpress II one-step cloning kit. The pGAD-T7-ZmbZIP22, pGAD-T7-OHP1, pGAD-T7-OHP2, pGAD-T7-PBF1, and pGAD-T7-O2 plasmids were cotransformed with pGBK-T7-ZmbZIP22-3 separately as prey and bait vectors into yeast reporter strain AH109 and cultured until OD600 reached 0.5. AD+BD, AD+ZmbZIP22-3, and ZmbZIP22 (OHP1, OHP2, PBF1, or O2) + BD were used as negative controls. Different yeast strains were grown on DDO (SD/-Leu/-Trp) plate and QDO (SD/-Leu/-Trp/-Ade/-His) plates.
Yeast Transactivation Assay
The yeast transactivation assay was performed as previously described (Qiao et al., 2016). The full-length ZmbZIP22 ORF, and ZmbZIP22-1, -2, -3, and -4 were cloned into yeast expression binary vector pGBK-T7 at the EcoRI and SalI sites and then separately cotransformed into yeast strain EGY48 with pG221. The empty vector pGBK-T7 was used as a negative control.
LCI Assay and Transactivation of the 27-kD γ-Zein Promoter
The ORFs of ZmbZIP22, PBF1, O2, OHP1, and OHP2 were cloned into JW771 (NLUC) and JW772 (CLUC), yielding the ZmbZIP22-CLUC and ZmbZIP22/PBF1/O2/OHP1/OHP2-NLUC constructs for the LCI assay as described elsewhere (Gou et al., 2011).
For the qualitative transactivation assay, the reporter P27-LUC was generated by inserting the 27-kD γ-zein promoter (500 bp from TSS) into the HindIII and BamHI sites of the pLL00R vector. The 35S-ZmbZIP22 effector was created by cloning its coding sequences into the pHB vector (Yao et al., 2016). The empty vector was used as a negative control.
Agrobacterium GV3101 harboring the above constructs was infiltrated into 5-week-old N. benthamiana leaves for LCI and transactivation analyses. After growing for 48 h under a 16-h-light/8-h-dark cycle, the leaves were injected with 1 mM luciferin and the resulting luciferase signals were captured using the Tanon-5200 image system. The experiments were repeated at least three times with similar results.
Dual-Luciferase Transient Transcriptional Activity Assay
To generate the p27-kD:LUC, pRemorin:LUC, pC2H2:LUC, pGRP1:LUC, and pRRM:LUC reporters for the dual-luciferase assays, the 500-bp sequence upstream from the TSS of the 27-kD γ-zein gene and −1000 bp from the translation start codons of the GRMZM2G080603, GRMZM2G137352, GRMZM2G144645, and GRMZM2G141386 promoters were inserted into the HindIII and BamHI sites of pGreenII-0800 using a ClonExpress II one-step cloning kit. The 35S-ZmbZIP22, PBF1, O2, OHP1, and OHP2 effectors were created by cloning their coding sequences into the HindIII and XbaI sites of the pHB vector using a ClonExpress II one-step cloning kit. The empty vector was used as a negative control. Transient dual-luciferase assays in 5-week-old N. benthamiana leaves were performed and checked using dual-luciferase assay reagents (Promega). For this analysis, the ratio between LUC and REN activity was measured with three biological replicates from three leaves.
HPLC Analysis of Zeins
HPLC analysis of zeins was performed as previously described (Yao et al., 2016). Briefly, 10 wild-type and 10 zmbzip22-mu9 kernels from the same F2 ear were ground into a powder in liquid N2, and the dry powder was dissolved in extraction buffer (70% ethanol, 5% β-mercaptoethanol, and 0.5 sodium acetate [w/v]). The samples were shaken at room temperature for at least 4 h, and the supernatants were diluted 5-fold and measured by HPLC. The relative concentrations of the zeins were computed using their peak areas. All measurements were performed via analysis of three F2 ears.
TEM
For TEM, immature kernels (15 DAP) of zmbzip22-mu9 and the wild type were prepared as previously described (Wang et al., 2011). The samples were observed under a Hitachi H7600 transmission electron microscope at Shanghai Normal University.
ChIP-qPCR
ChIP with ZmbZIP22 antibody was performed as previously described (Li et al., 2015). Antibodies against ZmbZIP22 and the IgG (Sigma-Aldrich) control were used for immunoprecipitation. The precipitated DNA was recovered using a QIAquick PCR purification kit (Qiagen) and analyzed by RT-qPCR using the appropriate DNA primers and SYBR Green Real-Time PCR Master Mix (Toyobo). Equal amounts of starchy endosperm tissue and ChIP products were used for each reaction. ChIP values were normalized to their respective DNA input values, and the fold changes in concentration were calculated based on the relative enrichment in anti-ZmbZIP22 compared with anti-IgG immunoprecipitates. Replicates were made using kernels from three wild-type ears. The means and standard deviations were calculated from three biological replicates.
RNA-Seq Analysis
For RNA-seq, immature endosperm tissues from individual kernels (15 DAP) were harvested from the same F2 ear. An embryo from the same kernel was subjected to DNA extraction and genotyping to determine whether the kernel was wild type or zmbzip22. The endosperm from 20 wild-type or zmbzip22 kernels was pooled for RNA isolation. Replicates were made using endosperm from three F2 ears. RNA-seq libraries were prepared according to the Illumina standard instructions (TruSeq Standard RNA LT Guide), followed by a quality check with the Agilent 2100 bioanalyzer, and sequenced on the Illumina HiSeq 2500 platform according to the manufacturer’s instructions (HiSeq 2500 User Guide) by Shanghai Hanyu-Bio. Effective reads were aligned to Ensembl plants release-30 zea_mays genome build Zea_mays.AGPv3.30 using TopHat software (Trapnell et al., 2009). The unique reads were normalized as reads per kilobase of exon model per million mapped reads using Samtools v0.1.19 (Li et al., 2009). DEGs were defined as those with Q value < 0.001.
ChIP-Seq
ChIP-seq was performed according to Li et al. (2015). ChIP DNA samples with two biological replicates (wild-type 15-DAP kernels from two ears) were subjected to end repair and A-base addition, followed by ligation to adapters. The barcode sequences of adapters correspond to the following: CTCAGA, ChIP of ZmbZIP22; GCGCTA, ChIP of IgG. Illumina Genome Analyzer (GA) sequencing was performed by the OSUCCC Nucleic Acid Shared Resource-Illumina GA II Core by Hanyu-Bio. Target genes were defined as those containing ChIP-seq peaks located within transcribed regions of genes, in introns or 1 kb upstream of the TSS, or 1 kb downstream of the TTS. ChIP-seq reads were aligned to the maize genome (Schnable et al., 2009) using Bowtie2 (Langmead et al., 2009), which supports gapped and paired-end alignment modes. Bowtie version 2.2.3 with default parameters was used, and only unique alignments were reported. ChIP-seq peaks were detected by MACS2 (Zhang et al., 2008). MACS version 2.0.10 with default parameters was used, as duplicates were allowed and P value < 1e-5. Peaks between replicates were defined as mutual peaks only when the distance of peak summits between replicates was <500 bp.
Statistical Analysis
Tables for all Student’s t tests are shown in Supplemental Table 4.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: ZmbZIP22, XP_008652952.1, GRMZM2G043600; 27-kD γ-zein gene, NP_001337252.1, GRMZM2G138727; O2, XP_008651859.1, GRMZM2G015534; PBF1, NP_001105400.2, GRMZM2G146283; OHP1, NP_001105687.1, GRMZM2G016150; OHP2, AQK96350.1, GRMZM2G143469; ZmGRP1, NP_001105707.1, GRMZM2G080603; Remorin, XP_023158194.1, GRMZM2G137352; C2H2 RING, NP_001145789.1, GRMZM2G144645; RRM domain, NP_001336748.1, GRMZM2G141386; and AtGRP7, NP_179760.1. Sequences used for phylogenetic analysis are as follows: Sorghum bicolor, XP_002460540.1; Setaria italica, KQL25366.1; Brachypodium distachyon, XP_003576738.1; Oryza sativa japonica group, XP_015651132.1; AtVIP1, NP_564486.1; Beta vulgaris subsp vulgaris, XP_010670890.1; Cucumis sativus, XP_011658569.1 Glycine max, ABI34695.1; and Saccharomyces cerevisiae S288c, NP_116622.1. RNA-seq and ChIP-seq data are available from the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under the series entries GSE113048 and GSE112917.
Supplemental Data
Supplemental Figure 1. EMSA showing that the mutation in P1-3 abolishes its interaction with the nuclear protein.
Supplemental Figure 2. Identification of the core binding sites using EMSA with bacterially expressed ZmbZIP22.
Supplemental Figure 3. Whole scan image of Figure 4C showing the specificity of the ZmbZIP22 antibody.
Supplemental Figure 4. SDS-PAGE detection of zein accumulation in mature zmbzip22-mu10 seeds.
Supplemental Figure 5. Diagram of HPLC peaks based on the determination of the zein content in mature kernels of the wild type and zmbzip22-mu9.
Supplemental Figure 6. Quantitative RT-PCR showing the reduction of 27-kD γ-zein transcription in 15-DAP endosperm of zmbzip22-mu10.
Supplemental Figure 7. Distribution of PB number per 100 μm2 of the fourth endosperm cell layer from the aleurone layer in zmbzip22 and the wild type.
Supplemental Figure 8. RPKM summation of 19-kD α-zein and 22-kD α-zein transcripts in wild-type and zmbzip22-mu9 endosperm based on 15-DAP RNA-seq data.
Supplemental Figure 9. Determination of immunoprecipitation efficiency of the ZmbZIP22 antibody by protein gel blot analysis.
Supplemental Figure 10. ChIP-qPCR assays examining the in vivo binding activity of ZmbZIP22 to the 27-kD γ-zein promoter.
Supplemental Figure 11. Density plot of the ACASCTCA motif around the summits of the peaks.
Supplemental Figure 12. Yeast two-hybrid analysis of the interaction of ZmbZIP22 with OHP1, OHP2, PBF1, or O2.
Supplemental Table 1. List of probes used in EMSA and DNA affinity pull-down assay.
Supplemental Table 2. Raw data use to quantify total starch and amylose in zmbzip22-mu9 mature endosperm.
Supplemental Table 3. List of primers.
Supplemental Table 4. Student’s t test tables.
Supplemental Data Set 1. Text file of the alignment used for the phylogenetic analysis shown in Figure 2A.
Supplemental Data Set 2. Significantly differentially expressed genes identified from RNA-seq data of zmbzip22-mu9 at 15 DAP.
Supplemental Data Set 3. Gene Ontology classifications of zmbzip22-mu9 DEGs with functional annotations.
Supplemental Data Set 4. ZmbZIP22 binding sites identified by ChIP-seq.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Tongdan Zhu, Ying Jin, Yingying Xing, and Huiling Ling (Shanghai University) for their technical support. We thank Brian A. Larkins for critical reading of the article. This work is supported by the National Natural Science Foundation of China (31730065, 91635303, and 31425019 to R.S.), by the National Key Research and Development Program of China (2016YFD0100503 to W.Q. and 2016YFD0101003 to R.S.), and by the National Postdoctoral Program for Innovative Talents (BX201700283 to C.L.).
Acknowledgments
R.S. and C.L. designed research. C.L., Y.Y., and H.C. performed the research. C.L., R.S., W.Q., and Y.Y. analyzed data. C.L. and R.S. wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Arnone M.I., Davidson E.H. (1997). The hardwiring of development: organization and function of genomic regulatory systems. Development 124: 1851–1864. [DOI] [PubMed] [Google Scholar]
- Bernard L., Ciceri P., Viotti A. (1994). Molecular analysis of wild-type and mutant alleles at the Opaque-2 regulatory locus of maize reveals different mutations and types of O2 products. Plant Mol. Biol. 24: 949–959. [DOI] [PubMed] [Google Scholar]
- Bhan M.K., Bhandari N., Bahl R. (2003). Management of the severely malnourished child: perspective from developing countries. BMJ 326: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttrose M.S. (1960). Submicroscopic development and structure of starch granules in cereal endosperms. J. Ultrastruct. Res. 4: 231–257. [DOI] [PubMed] [Google Scholar]
- Cao S., Jiang L., Song S., Jing R., Xu G. (2006). AtGRP7 is involved in the regulation of abscisic acid and stress responses in Arabidopsis. Cell. Mol. Biol. Lett. 11: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui C.F., Falco S.C. (1995). A new methionine-rich seed storage protein from maize. Plant Physiol. 107: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri P., Gianazza E., Lazzari B., Lippoli G., Genga A., Hoscheck G., Schmidt R.J., Viotti A. (1997). Phosphorylation of Opaque2 changes diurnally and impacts its DNA binding activity. Plant Cell 9: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C.E., Larkins B.A. (1999). The prolamins of maize. In Seed Proteins, Shewry P.R., Casey R., eds (Dordrecht, The Netherlands: Springer; ), pp. 109–139. [Google Scholar]
- Cord Neto G., Yunes J.A., da Silva M.J., Vettore A.L., Arruda P., Leite A. (1995). The involvement of Opaque 2 on beta-prolamin gene regulation in maize and Coix suggests a more general role for this transcriptional activator. Plant Mol. Biol. 27: 1015–1029. [DOI] [PubMed] [Google Scholar]
- Deppmann C.D., Alvania R.S., Taparowsky E.J. (2006). Cross-species annotation of basic leucine zipper factor interactions: Insight into the evolution of closed interaction networks. Mol. Biol. Evol. 23: 1480–1492. [DOI] [PubMed] [Google Scholar]
- Esen A. (1987). A proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.). J. Cereal Sci. 5: 117–128. [Google Scholar]
- Feng B.H., Han Y.C., Xiao Y.Y., Kuang J.F., Fan Z.Q., Chen J.Y., Lu W.J. (2016). The banana fruit Dof transcription factor MaDof23 acts as a repressor and interacts with MaERF9 in regulating ripening-related genes. J. Exp. Bot. 67: 2263–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F., Qi W., Lv Y., Yan S., Xu L., Yang W., Yuan Y., Chen Y., Zhao H., Song R. (2018). OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell 30: 375–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Zhu J., Wang G., Tang Y., Chen H., Jin W., Wang F., Mei B., Xu Z., Song R. (2009). Expressional profiling study revealed unique expressional patterns and dramatic expressional divergence of maize alpha-zein super gene family. Plant Mol. Biol. 69: 649–659. [DOI] [PubMed] [Google Scholar]
- Gorham J. (1821). Analysis of Indian corn. Quart. J. Sci. Lit. Art. 2: 206–208. [Google Scholar]
- Gou J.Y., Felippes F.F., Liu C.J., Weigel D., Wang J.W. (2011). Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23: 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Yuan L., Chen H., Sato S.J., Clemente T.E., Holding D.R. (2013). Nonredundant function of zeins and their correct stoichiometric ratio drive protein body formation in maize endosperm. Plant Physiol. 162: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L.C. (1997). Starch synthesis in the maize seed. In Cellular and Molecular Biology of Plant Seed Development, Larkins B.A., Vasil I.K., eds (Dordrecht, The Netherlands: Springer; ), pp. 375–405. [Google Scholar]
- Hannah L.C., James M. (2008). The complexities of starch biosynthesis in cereal endosperms. Curr. Opin. Biotechnol. 19: 160–165. [DOI] [PubMed] [Google Scholar]
- Heintzen C., Nater M., Apel K., Staiger D. (1997). AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94: 8515–8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández H.H. (1969). A Modified Method for Rapid Tryptophan Analysis of Maize. (Mexico: International Maize and Wheat Improvement Center; ). [Google Scholar]
- Holding D.R. (2014). Recent advances in the study of prolamin storage protein organization and function. Front. Plant Sci. 5: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding D.R., Larkins B.A. (2009). Zein storage proteins. In Molecular Genetic Approaches to Maize Improvement, Kriz A.L., Larkins B.A., eds (Berlin, Heidelberg, Germany: Springer; ), pp. 269–286. [Google Scholar]
- Holding D.R., Messing J. (2013). Evolution, structure, and function of prolamin storage proteins. In Seed Genomics, Becraft P., ed (New York: John Wiley & Sons; ), pp. 139–158. [Google Scholar]
- Hurkman W.J., Smith L.D., Richter J., Larkins B.A. (1981). Subcellular compartmentalization of maize storage proteins in Xenopus oocytes injected with zein messenger RNAs. J. Cell Biol. 89: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby M., Weisshaar B., Dröge-Laser W., Vicente-Carbajosa J., Tiedemann J., Kroj T., Parcy F.; bZIP Research Group (2002). bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7: 106–111. [DOI] [PubMed] [Google Scholar]
- Jutras B.L., Verma A., Stevenson B. (2012). Identification of novel DNA binding proteins using DNA affinity chromatography-pulldown. Curr. Protoc. Microbiol. 24: 1F.1.1–1F.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirihara J.A., Petri J.B., Messing J. (1988). Isolation and sequence of a gene encoding a methionine-rich 10-kDa zein protein from maize. Gene 71: 359–370. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B.A., Hurkman W.J. (1978). Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 62: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending C.R., Larkins B.A. (1989). Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Qiao Z., Qi W., Wang Q., Yuan Y., Yang X., Tang Y., Mei B., Lv Y., Zhao H., Xiao H., Song R. (2015). Genome-wide characterization of cis-acting DNA targets reveals the transcriptional regulatory framework of opaque2 in maize. Plant Cell 27: 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R.; 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang X., Wang Y., Li X., Gao Z., Pei M., Chen Z., Qu L.-J., Gu H. (2006). Two groups of MYB transcription factors share a motif which enhances trans-activation activity. Biochem. Biophys. Res. Commun. 341: 1155–1163. [DOI] [PubMed] [Google Scholar]
- Liu H., et al. (2016). Gene duplication confers enhanced expression of 27-kDa γ-zein for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. USA 113: 4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P., Bailey T.L. (2011). MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27: 1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., et al. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45: D200–D203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzábal P., Busk P.K., Ludevid M.D., Torrent M. (1998). The bifactorial endosperm box of γ-zein gene: characterisation and function of the Pb3 and GZM cis-acting elements. Plant J. 16: 41–52. [DOI] [PubMed] [Google Scholar]
- Mertz E.T., Bates L.S., Nelson O.E. (1964). Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145: 279–280. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Lynch T.J., Finkelstein R.R. (2001). Physical interactions between ABA response loci of Arabidopsis. Plant J. 26: 627–635. [DOI] [PubMed] [Google Scholar]
- Osborne T.B., Mendel L.B., Ferry E.L., Wakeman J.A. (1914). Nutritive properties of proteins of the maize kernel. J. Biol. Chem. 18: 1–16. [Google Scholar]
- Pedersen K., Devereux J., Wilson D.R., Sheldon E., Larkins B.A. (1982). Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell 29: 1015–1026. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Argos P., Naravana S.V., Larkins B.A. (1986). Sequence analysis and characterization of a maize gene encoding a high-sulfur zein protein of Mr 15,000. J. Biol. Chem. 261: 6279–6284. [PubMed] [Google Scholar]
- Pitzschke A., Djamei A., Teige M., Hirt H. (2009). VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc. Natl. Acad. Sci. USA 106: 18414–18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat S., Pérez-Grau L., Puigdomènech P. (1987). Multiple variability in the sequence of a family of maize endosperm proteins. Gene 52: 41–49. [DOI] [PubMed] [Google Scholar]
- Pysh L.D., Schmidt R.J. (1996). Characterization of the maize OHP1 gene: evidence of gene copy variability among inbreds. Gene 177: 203–208. [DOI] [PubMed] [Google Scholar]
- Pysh L.D., Aukerman M.J., Schmidt R.J. (1993). OHP1: a maize basic domain/leucine zipper protein that interacts with opaque2. Plant Cell 5: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Zhu T., Tian Z., Li C., Zhang W., Song R. (2016). High-efficiency CRISPR/Cas9 multiplex gene editing using the glycine tRNA-processing system-based strategy in maize. BMC Biotechnol. 16: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z., Qi W., Wang Q., Feng Y., Yang Q., Zhang N., Wang S., Tang Y., Song R. (2016). ZmMADS47 regulates zein gene transcription through interaction with Opaque2. PLoS Genet. 12: e1005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece-Hoyes J.S., Marian Walhout A.J. (2012). Yeast one-hybrid assays: a historical and technical perspective. Methods 57: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.J., Burr F.A., Burr B. (1987). Transposon tagging and molecular analysis of the maize regulatory locus opaque-2. Science 238: 960–963. [DOI] [PubMed] [Google Scholar]
- Schmidt R.J., Burr F.A., Aukerman M.J., Burr B. (1990). Maize regulatory gene opaque-2 encodes a protein with a “leucine-zipper” motif that binds to zein DNA. Proc. Natl. Acad. Sci. USA 87: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.J., Ketudat M., Aukerman M.J., Hoschek G. (1992). Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell 4: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable P.S., et al. (2009). The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Shannon J.C., Pien F.M., Cao H., Liu K.C. (1998). Brittle-1, an adenylate translocator, facilitates transfer of extraplastidial synthesized ADP--glucose into amyloplasts of maize endosperms. Plant Physiol. 117: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- Song R., Messing J. (2002). Contiguous genomic DNA sequence comprising the 19-kD zein gene family from maize. Plant Physiol. 130: 1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Llaca V., Linton E., Messing J. (2001). Sequence, regulation, and evolution of the maize 22-kD α zein gene family. Genome Res. 11: 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G.A., Larkins B.A. (1994). Characterization of Zein Genes and Their Regulation in Maize Endosperm. In The Maize Handbook, Freeling M., Walbot V., eds (New York: Springer; ), pp. 639–647. [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. (2009). TopHat: discovering splice junctions with RNA-seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T., Vaidya M., Citovsky V. (2001). VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 20: 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Messing J. (1991). A homologous expression system for cloned zein genes. Theor. Appl. Genet. 82: 93–100. [DOI] [PubMed] [Google Scholar]
- Ueda T., Waverczak W., Ward K., Sher N., Ketudat M., Schmidt R.J., Messing J. (1992). Mutations of the 22- and 27-kD zein promoters affect transactivation by the Opaque-2 protein. Plant Cell 4: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Carbajosa J., Moose S.P., Parsons R.L., Schmidt R.J. (1997). A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc. Natl. Acad. Sci. USA 94: 7685–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J.C., Lopes M.A., Paiva E., Larkins B.A. (1990). New methods for extraction and quantitation of zeins reveal a high content of gamma-zein in modified opaque-2 maize. Plant Physiol. 92: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Sun X., Wang G., Wang F., Gao Q., Sun X., Tang Y., Chang C., Lai J., Zhu L., Xu Z., Song R. (2011). Opaque7 encodes an acyl-activating enzyme-like protein that affects storage protein synthesis in maize endosperm. Genetics 189: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang G., Wang F., Wang G., Wang F., Zhang X., Zhong M., Zhang J., Lin D., Tang Y., Xu Z., Song R. (2012). Opaque1 encodes a myosin XI motor protein that is required for endoplasmic reticulum motility and protein body formation in maize endosperm. Plant Cell 24: 3447–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang J., Wang G., Fan X., Sun X., Qin H., Xu N., Zhong M., Qiao Z., Tang Y., Song R. (2014). Proline responding1 plays a critical role in regulating general protein synthesis and the cell cycle in maize. Plant Cell 26: 2582–2600. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wienand U., Langridge P., Feix G. (1981). Isolation and characterization of a genomic sequence of maize coding for a zein gene. Mol. Gen. Genet. 182: 440–444. [Google Scholar]
- Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. (2009). Universal sample preparation method for proteome analysis. Nat. Methods 6: 359–362. [DOI] [PubMed] [Google Scholar]
- Woo Y.M., Hu D.W., Larkins B.A., Jung R. (2001). Genomics analysis of genes expressed in maize endosperm identifies novel seed proteins and clarifies patterns of zein gene expression. Plant Cell 13: 2297–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Messing J. (2010a). Rescue of a dominant mutant with RNA interference. Genetics 186: 1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Messing J. (2010b). RNA interference-mediated change in protein body morphology and seed opacity through loss of different zein proteins. Plant Physiol. 153: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Messing J. (2012). Rapid divergence of prolamin gene promoters of maize after gene amplification and dispersal. Genetics 192: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.H., Messing J. (2008). Diverged copies of the seed regulatory Opaque-2 gene by a segmental duplication in the progenitor genome of rice, sorghum, and maize. Mol. Plant 1: 760–769. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S., Sheen J. (1998). Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell 10: 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ji C., Wu Y. (2016). Divergent transactivation of maize storage protein zein genes by the transcription factors Opaque2 and OHPs. Genetics 204: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D., Qi W., Li X., Yang Q., Yan S., Ling H., Wang G., Wang G., Song R. (2016). Maize opaque10 encodes a cereal-specific protein that is essential for the proper distribution of zeins in endosperm protein bodies. PLoS Genet. 12: e1006270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ye R., Yao Q.-H., Xu Z.-H., Xue H.-W. (2004). Development of an efficient method for the isolation of factors involved in gene transcription during rice embryo development. Plant J. 38: 348–357. [DOI] [PubMed] [Google Scholar]
- Zaret K.S., Carroll J.S. (2011). Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25: 2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Qiao Z., Liang Z., Mei B., Xu Z., Song R. (2012). Zea mays Taxilin protein negatively regulates opaque-2 transcriptional activity by causing a change in its sub-cellular distribution. PLoS One 7: e43822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yang J., Wu Y. (2015). Transcriptional regulation of zein gene expression in maize through the additive and synergistic action of opaque2, Prolamine-Box Binding Factor, and O2 heterodimerizing proteins. Plant Cell 27: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zheng X., Yang J., Messing J., Wu Y. (2016). Maize endosperm-specific transcription factors O2 and PBF network the regulation of protein and starch synthesis. Proc. Natl. Acad. Sci. USA 113: 10842–10847. [DOI] [PMC free article] [PubMed] [Google Scholar]