Abstract
Cardiac myocyte-specific gene manipulation is facilitated by reagents permitting temporal control over transgene expression or gene ablation, and by physiological phenotyping platforms that complement post-mortem cellular and pathological analyses. The ease of creating cardiac-specific gene modified mice may have contributed to genetic mouse models lacking strong underlying mechanistic rationales; this was argued for genetic ablation of mitochondrial dynamics factors in cardiac myocytes that exhibit little evidence for mitochondrial dynamism. Here, I review recent published studies in which experimental in vivo manipulation of mitochondrial fusion and fission genes has revealed non-canonical functioning dynamics factors in mitochondrial quality and quantity control. Targeting mitochondrial dynamics proteins in the cardiac system, where mitochondrial dynamism is barely observed, was essential to uncovering novel functioning of these factors in other pathways.
Introduction: being special is not so special
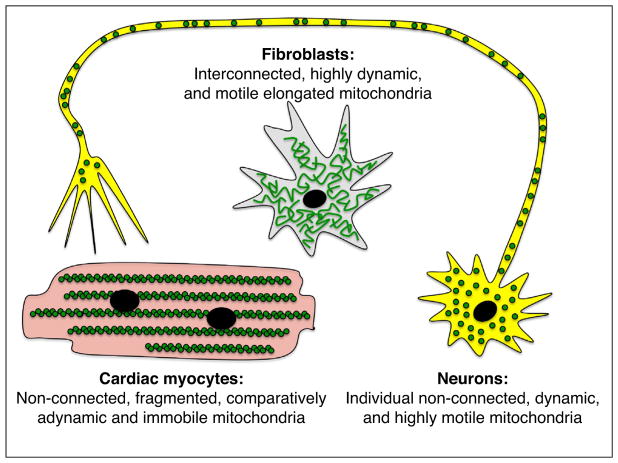
As cardiologists and cardiovascular researchers the concept that ‘the heart is a special case’ can become so deeply engrained in our scientific outlook that it becomes a major factor driving hypothesis development and experimental design, thereby limiting conclusions. Compared to a ‘generic’ cell, adult mammalian cardiac myocytes are atypical in that they are (for all practical purposes) non-replicative/non-regenerative, electrically excitable, and have among the highest basal metabolic activity and mitochondrial density of any cell type. Cardiac myocyte mitochondria do not conform to the classic description as being ‘highly dynamic and interconnected’ [1]. Mitochondria of adult cardiac myocytes are ovoid rather than elongated [2,3••], are static rather than dynamic [4–6], and exist as physically discreet organelles distributed along the long axis of the cell between sarcomeric elements rather than as members integrated into a greater mitochondrial continuum (although there is accumulating evidence that cardiac myocyte mitochondria can communicate between individuals [4] and function as a collective within discrete geographic zones [7]) (Figure 1). Thus, it has become common within the cardiovascular research community, and particularly among those who study mitochondria, to dismiss findings in non-cardiac myocytes as irrelevant to cardiac biology, even if the findings in different cell systems are fully concordant. Moreover, the non-cardiac community is fully aware of the distinguishing features of cardiac myocytes, and sometimes poses ‘the heart is a special case’ argument to negate the potential relevance to non-cardiac systems of primary data originating in cardiac myocyte models [8].
Figure 1.
Comparison of mitochondrial features in fibroblasts, cardiac myocytes, and neurons. Mitochondria (green) arrangement, morphology, and dynamism in three prototypical cell types wherein mitochondrial function is of particular interest.
Our experience over the past 5 years has taught us that investigating the unique qualities of cardiac myocyte mitochondria can provide a path toward understanding unanticipated non-canonical functioning of mitochondria in other (and perhaps most) cell types. For example, rather than blindly insisting that cardiac myocyte mitochondria are highly dynamic and undergo frequent cycles of fusion and fission in contrast to observational data, we asked whether highly abundant mitofusin (Mfn) proteins in mouse hearts might function in ways other than morphological mitochondrial remodeling. In exploring this question we uncovered a completely unexpected role for the mitochondrial fusion protein Mfn2 in PINK-Parkin mediated mitophagy signaling [9]. The particulars of this signaling pathway and the general crosstalk between mitochondrial dynamics and mitophagy have also been observed in fibroblasts and neurons [3••,10]. Because phenotypes observed after Mfn2 ablation in cells having ‘normally’ dynamic mitochondria were attributed to the default mechanism of interrupted mitochondrial dynamism, mitochondrial fusion proved to be a confounding variable obscuring a central role for Mfn2 in mitophagy signaling. It required studies of adynamic cardiac myocyte mitochondria to reveal non-fusogenic functioning of this mitochondrial fusion protein in the PINK-Parkin mitophagy pathway.
Hearts and brains; the mitochondrial connection
Notwithstanding that the human heart is the most mitochondria-rich and mitochondria-dependent organ, hereditary human diseases caused by loss-of-function mutations affecting key non-metabolic mitochondrial proteins are notable in that they do not typically affect the heart. Instead, there is an as-yet poorly understood predilection for mitochondrial mutations to provoke chronic neurodegenerative diseases. For example, damaging mutations affecting the mitophagy signaling factors Parkin or PINK1 can cause Parkinson’s disease [11–14], loss-of-function mutations affecting the outer mitochondrial membrane fusion protein Mfn2 can cause Charcot Marie Tooth disease type 2A [15,16], mutations affecting the inner mitochondrial membrane fusion/architecture protein Opa1 can cause dominant optic atrophy [17], and mutations affecting the mitochondrial fission protein Drp1 can cause encephalopathy [18–22]. It is perplexing that heart disease is not a typical feature of any of these syndromes since direct (Mfn1, Mfn2, Drp1) or indirect (Opa1) cardiac myocyte-specific genetic deletion of these factors in mice (excepting PINK1 which has yet to be conditionally ablated in hearts) provoked profound and frequently lethal cardiac phenotypes [3••,9,23–25,26•,27,28,29••].
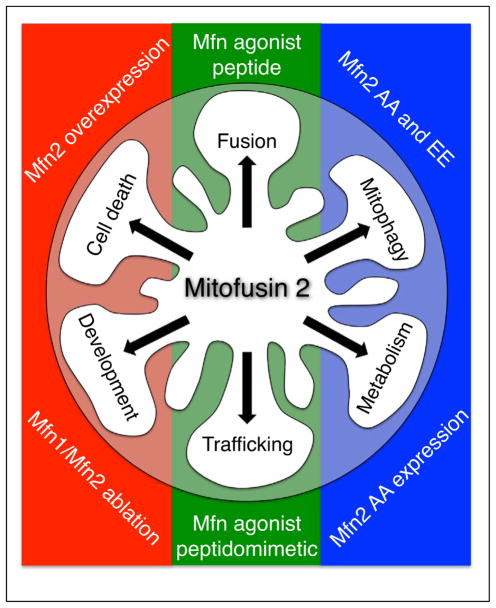
We asked why hearts are spared in clinical human neurodegenerative diseases caused by damaging mutations of mitochondrial proteins that are essential for normal cardiac functioning in experimental mouse models. Neurons and cardiac myocytes share many characteristics that we posit should make them jointly susceptible to mitochondrial injury: both are excitable cells with a high basal metabolic activity, neither cell type is capable of proliferation and therefore both are incapable of repairing through regeneration, and the mitochondria of both cell types tend to be arranged as individual organelles rather than in interconnected networks. The one obvious distinction, however, is cell morphology. An adult human cardiac myocyte is ~200–300 μm in length whereas a human sciatic nerve neuron originating in the lumbar spinal cord and terminating in the foot can be up to a meter in length (Figure 1). The orders of magnitude difference in long axis dimension suggests profound dissimilarities in the requirement for and mechanisms by which mitochondria are distributed throughout these two cell types. In this context the key feature underlying susceptibility of neurons to mitochondrial damage may be the requirement for neuronal mitochondrial trafficking over long physical distances (rather than any peculiarities in mitochondrial size, shape, or fission/fusion dynamics). Indeed, small molecule peptidomimetics of previously described minipeptide mitofusin agonists [30•] were recently proven to be remarkably effective at normalizing mitochondrial trafficking in both cultured neurons and mouse sciatic nerves expressing human Charcot Marie Tooth disease Mfn2 mutations [31••] (Figure 2). These results support an approach of using gene manipulation of mitochondrial dynamics proteins in the static mitochondria of in vivo adult mouse hearts not only to provide insights into the functioning of these factors in the ‘special cases’ of normal and diseased hearts, but to uncover previously unrecognized roles for these proteins in other tissues wherein their canonical effects on mitochondrial fusion/fission and trafficking obfuscate parallel functionality.
Figure 2.
Different roles played by Mfn2 in mitochondrial fusion, motility, and mitophagy and how they have been experimentally modulated. Mfn2 roles in cell death and cardiac development are not reviewed here and are included for completeness. Modulation of mitochondrial fusion and trafficking by Mfn agonist peptides and small molecule peptidomimetics is from Refs. [30•,31••]. Regulation of mitophagy and perinatal cardiac metabolic transitions by Mfn2 mutants with modified T111 and S442 PINK1 phosphorylation sites (Mfn2 AA and EE) is described in Refs. [3••,9].
Mitochondrial dynamism — a matter of balance
Functional interactions between mitochondrial fusion/ fission and quality control pathways occur at both the individual molecular and integrated organelle physiological levels. As an example of dual molecular functioning of a single protein in different pathways, PINK1-mediated phosphorylation of Mfn2 simultaneously converts it from a mitochondrial fusion protein that does not bind Parkin to a non-fusogenic protein that recruits Parkin to outer mitochondrial membranes [3••,9] (Figure 2). Indeed, this process likely contributes to functional sequestration of damaged mitochondria by abrogating their ability to fuse with other mitochondria while targeting them (via the downstream mitophagic effects of Parkin recruited to phospho-Mfn2) for elimination by autophagosomal engulfment and transfer to degradative lysosomes.
The processes of mitochondrial fusion and fission also play integral roles in mitophagic mitochondrial quality control, independent of their specific individual molecular mediators. The central role for mitochondrial fusion and fission in mitophagy was initially described by Shirihai and colleagues [32,33]. Briefly, mitochondria undergo asymmetric fission in a manner that segregates damaged from healthy mitochondrial components, producing two daughter organelles having opposing characteristics: The daughter that received healthy components is ‘rejuvenated’ and re-joins the functionally productive mitochondrial collective through a combination of fusion with other healthy mitochondria and incorporation of biogenically-derived new parts [34]. The other daughter mitochondrion, which received the damaged parental components, is typically smaller and depolarized, that is, the normal inner mitochondrial membrane electrochemical gradient that drives ATP production has partially or completely dissipated. As a consequence of its depolarization, the dysfunctional daughter organelle is targeted for mitophagic elimination via the standard PINK-Parkin pathway [35]. This paradigm, in which fission, fusion, and mitophagy are inextricably linked during mitochondrial quality control, has proven to be broadly applicable in in vitro systems wherein mitochondrial fusion, fission, and mitophagy are comparatively easy to identify and quantify. However, translating these findings to in vivo mammalian systems has proven to be challenging.
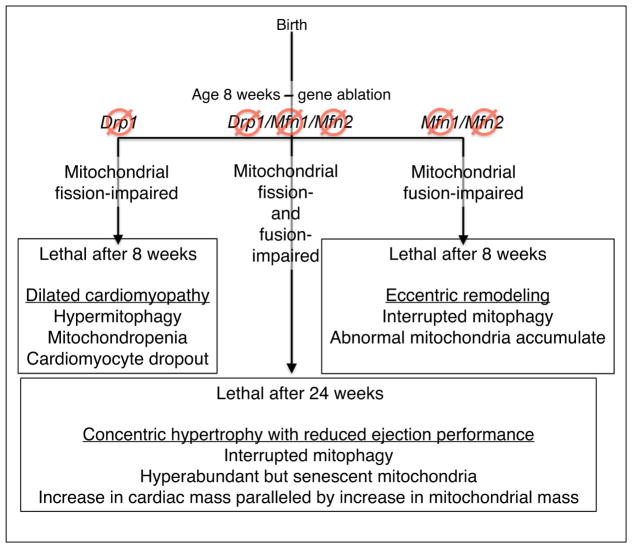
Because cardiac myocyte mitochondria do not utilize fission and fusion as a means to structurally modulate mitochondrial networks (because networks do not exist in adult cardiac myocytes), cardiac myocyte-targeted gene manipulation unexpectedly proved to be an effective means of evaluating the in vivo functional interactions between these mitochondrial dynamics and mitochondrial quality control independent of the confounding effects of mitochondrial interconnectivity. By employing available floxed allele mice [36,37] in combination with tamoxifen-activated cardiac myocyte-specific Cre mice [38], Moshi Song in our laboratory developed mouse lines in which the initiating factors for either mitochondrial fusion (the two outer membrane tethering and fusion proteins, Mfn1 and Mfn2) or mitochondrial fission (Drp1) could be conditionally ablated specifically in cardiac myocytes of adult (8 week old) mice [26•]. In parallel, Ms. Song developed primary cultured murine embryonic fibroblasts (MEFs) from each floxed mouse line (Mfn1/Mfn2 and Drp1) so that the targeted genes could be ablated in cultured cells using adenoviral Cre. In this manner the in vivo consequences on hearts and in vitro consequences on cultured MEFs of genetically interrupting mitochondrial fusion and fission could be directly compared. Interrupting mitochondrial fission (Drp1 ablation) evoked a delayed increase in unregulated mitophagy, ultimately producing mitochondropenia (loss of mitochondria). The converse experiment produced the reciprocal result: interrupting mitochondrial fusion (Mfn1/Mfn2 ablation) suppressed mitophagy, which resulted in progressive accumulation of damaged mitochondria [26•] (Figure 3). Abrogating mitochondrial fission and fusion in adult mouse hearts generated opposite, but equally lethal, cardiac phenotypes: Drp1 ablation caused a rapidly progressive dilated cardiomyopathy, whereas combined Mfn1/Mfn2 ablation caused eccentric cardiac hypertrophy; both conditions were uniformly lethal after ~8 weeks. These studies provided the first in vivo evidence supporting major pathophysiological importance of functional interactions between mitochondrial fission/fusion and mitochondrial quality control pathways as described in vitro in Shirihai’s studies [32].
Figure 3.
Interactions between mitochondrial dynamism and mitophagy in mitochondrial quality and quantity control pathways, as defined by studies of in vivo mouse hearts. This diagram summarizes the cardiac and mitochondrial phenotypes described in Refs. [26•,29••].
A question raised by the above results was whether it was absolute loss of mitochondrial fusion in Mfn1/Mfn2 null hearts, and fission in Drp1 null hearts, that provoked mitochondrial abnormalities and cardiac disease. The counter hypothesis posits that relative hyperactivity of Drp1-mediated fission in Mfn1/Mfn2 null hearts, and of Mfn1/Mfn2-mediated fusion in Drp1 null hearts, provoked mitochondrial and myocardial abnormalities. Ms. Song reasoned that simultaneous combined interruption of fission and fusion would exacerbate cardiac disease if the former hypothesis were correct, but would ameliorate cardiac disease if dynamic imbalance rather than absolute loss of fission or fusion was the key damaging feature. Accordingly, she crossed the fusion-defective Mfn1/Mfn2 and fission-defective Drp1 floxed allele mice to generate mice with hearts in which simultaneous defects in both mitochondrial fusion and fission (which she called ‘adynamic mitochondria’) could be genetically induced [29••]. Compared to the parent fusion-defective or fission-defective mice, mice with adynamic cardiac myocyte mitochondria lived 3-times longer and exhibited a third distinct phenotype: concentric hypertrophy that failed (i.e. developed severely reduced ejection performance) without transitioning through the typical stage of cardiac dilatation and wall thinning (Figure 3). As in the parent lines, the cardiac pathologies could be mechanistically linked to abnormalities in mitophagy. In adynamic mitochondria of Mfn1/Mfn2/Drp1 hearts, intrinsic mitophagy interruption prevented mitochondropenia seen when fission alone was interrupted, thus preempting the dilated cardiomyopathy provoked by hypermitophagic mitochondrial insufficiency. In the absence of the typical Shirihai mechanism for fission-mediated triage of healthy versus damaged mitochondrial components [32], the mitochondrial collective increased in both overall abundance and organellar senescence, that is, there was evidence of increased mitochondrial ‘wear and tear’ [29••]. The degree of adynamic mitochondrial dysfunction and proteotoxic stress in Mfn1/Mfn2/Drp1 null mouse hearts should have been sufficient to trigger normal mitophagic quality control mechanisms, but it could not because Mfn2 is a critical mediator of the PINK1-Parkin pathway. Nevertheless, compensatory mitochondrial quality control mechanisms were sufficient to prevent cytotoxicity. Indeed, cardiac hypertrophy and dysfunction paralleled the increase in mitochondrial mass rather than mitochondrial dysfunction. These results point to the mitochondrial dynamism/mitophagy interactome as a key regulator not only of mitochondrial quality, but also of mitochondrial quantity, in the in vivo mammalian heart. The importance of mitophagic mitochondrial quantity control in other tissues, and the nature of the regulatory mechanisms orchestrating biogenic and mitophagic regulation of mitochondrial mass, remains unclear at this time.
Summary
Just a few years ago mitochondrial dynamics (fission and fusion), mitochondrial biogenesis (a form of quantity control), and mitophagic mitochondrial quality control were considered to be largely separate and mechanistically distinct processes. This has been demonstrated to not be the case. Rather, the three processes function within a greater ‘interactome’ in which physiological, pathological, or genetic perturbations of one are reflected by co-regulation or counter-regulation of the other two. As reviewed above, the unique attributes of the heart have proven invaluable to discovering these functional interactions and to demonstrating their relevance to working organ systems in in vivo mammalian systems.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW., 2nd Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW., 2nd Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350:aad2459. doi: 10.1126/science.aad2459. In this manuscript cardiac-specific expression of an Mfn2 mutant incapable of being phosphorylated on the amino acids required for parkin binding was used to interrupt mitophagy in perinatal hearts and reveal the critical role of mitophagy in the normal postnatal metabolic transition from glycolytic to fatty acid metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, et al. Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci U S A. 2013;110:16669–16674. doi: 10.1073/pnas.1316622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorn GW., 2nd Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013;1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Dorn GW., 2nd Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glancy B, Hartnell LM, Combs CA, Fenmou A, Sun J, Murphy E, et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 2017;19:487–496. doi: 10.1016/j.celrep.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallanck L. Mitophagy: mitufusin recruits a mitochondrial killer. Curr Biol. 2013;23:R570–R572. doi: 10.1016/j.cub.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Sterky FH, Mourier A, Terzioglu M, Cullheim S, Olson L, et al. Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Hum Mol Genet. 2012;21:4827–4835. doi: 10.1093/hmg/dds352. [DOI] [PubMed] [Google Scholar]
- 11.Matsumine H, Saito M, Shimoda-Matsubayashi S, Tanaka H, Ishikawa A, Nakagawa-Hattori Y, et al. Localization of a gene for an autosomal recessive form of juvenile Parkinsonism to chromosome 6q25.2–27. Am J Hum Genet. 1997;60:588–596. [PMC free article] [PubMed] [Google Scholar]
- 12.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 13.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 14.Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H, et al. Novel PINK1 mutations in early-onset parkinsonism. Ann Neurol. 2004;56:424–427. doi: 10.1002/ana.20251. [DOI] [PubMed] [Google Scholar]
- 15.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 16.Zhu D, Kennerson ML, Walizada G, Zuchner S, Vance JM, Nicholson GA. Charcot-Marie-Tooth with pyramidal signs is genetically heterogeneous: families with and without MFN2 mutations. Neurology. 2005;65:496–497. doi: 10.1212/01.wnl.0000171345.62270.29. [DOI] [PubMed] [Google Scholar]
- 17.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 18.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 19.Yoon G, Malam Z, Paton T, Marshall CR, Hyatt E, Ivakine Z, et al. Lethal disorder of mitochondrial fission caused by mutations in DNM1L. J Pediatr. 2016;171:313–6. e1–2. doi: 10.1016/j.jpeds.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 20.Vanstone JR, Smith AM, McBride S, Naas T, Holcik M, Antoun G, et al. DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur J Hum Genet. 2016;24:1084–1088. doi: 10.1038/ejhg.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheffer R, Douiev L, Edvardson S, Shaag A, Tamimi K, Soiferman D, et al. Postnatal microcephaly and pain insensitivity due to a de novo heterozygous DNM1L mutation causing impaired mitochondrial fission and function. Am J Med Genet A. 2016;170:1603–1607. doi: 10.1002/ajmg.a.37624. [DOI] [PubMed] [Google Scholar]
- 22.Chao YH, Robak LA, Xia F, Koenig MK, Adesina A, Bacino CA, et al. Missense variants in the middle domain of DNM1L in cases of infantile encephalopathy alter peroxisomes and mitochondria when assayed in Drosophila. Hum Mol Genet. 2016;25:1846–1856. doi: 10.1093/hmg/ddw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara A, Cipolat S, Chen Y, Dorn GW, 2nd, Scorrano L. Mitochondrial fusion directs cardiac myocyte differentiation via calcineurin and Notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 25.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. This study directly compared in vitro and in vivo cardiac consequences of interrupting mitochondrial fusion and fission, revealing mechanistic involvement of mitochondrial dynamics in mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol. 2015;35:211–223. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:1221. doi: 10.1126/science.aad0116. [DOI] [PubMed] [Google Scholar]
- 29••.Song M, Franco A, Fleischer JA, Zhang L, Dorn GW., 2nd Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 2017;26:872–83. e5. doi: 10.1016/j.cmet.2017.09.023. This follow-up study to Ref. [26•] conditionally and simultaneously ablated both mitochondrial fission and fusion in vitro and in vivo mouse hearts, proving that balanced mitochodnrial dynamism is of greater import to mitochondrial health and heart function than the integrity of either pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, et al. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540:74–79. doi: 10.1038/nature20156. This study uncovered a novel allosteric mechanism for Mfn1 and Mfn2 activation and describes cell-permeant mini-peptides that act as mitofusin agonists or antagonists by modulating Mfn conformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Rocha AG, Franco A, Krezel AM, Runsey JM, Alberti JM, Knight WC, et al. Mfn2 agonists reverse mitochondrial defects in preclinical models of Charcot Marie Tooth disease type 2A. Science. 2018 doi: 10.1126/science.aao1785. in press (anticipated April 20). This follow-up study to Ref. [30•] revealed PINK1 phosphorylation of Mfn2 Ser378 to be the physiological trigger controling Mfn activation via conformational shifting, and describes peptidomimetic small molecule mitofusin agonists that correct mitochondrial motility defects in cultured Charcot Marie Tooth neurons and nerves. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirihai OS, Song M, Dorn GW., 2nd How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorn GW, 2nd, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–1991. doi: 10.1101/gad.269894.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 38.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]