Abstract
Hypothesis:
Magnetic vestibular stimulation (MVS) elicits nystagmus in C57BL/6J mice but not head tilt mice lacking Nox3, which is required for normal otoconial development.
Background:
Humans have vertigo and nystagmus in strong magnetic fields within MRI machines. The hypothesized mechanism is a Lorentz force driven by electrical current entering the utricular neuroepithelium, acting indirectly on crista hair cells via endolymph movement deflecting cupulae. We tested an alternate hypothesized mechanism: Lorentz action directly on crista hair cell stereocilia, driven by their currents independent of the utricle.
Methods:
Before MVS, vestibulo-ocular reflex (VOR) responses of 8 C57BL/6J mice and 6 head tilt mice were measured during whole-body sinusoidal rotations and tilts using video-oculography. Mice were then placed within a 4.7 Tesla magnetic field with the horizontal semicircular canals approximately Earth-horizontal for ≥1 minute in several head orientations, while eye movements were recorded via infrared video in darkness.
Results:
Outside the magnet, both C57BL/6J and head tilt mice had intact horizontal VOR, but only C57BL/6J mice exhibited static counter-roll responses to tilt (normal utiruclo-ocular reflex). When placed in the magnet nose-first, C57BL/6J mice had left-beating nystagmus, lasting a median of 32.8s. When tail-first, nystagmus was right-beating and similar duration (median 28.0s, p>0.05). In contrast, head tilt mice lacked magnetic field-induced nystagmus (p<0.001).
Conclusions:
C57BL/6J mice generate nystagmus in response to MVS, while mice deficient in Nox3 do not. This suggests (1) a normal utricle is necessary, and (2) functioning semicircular canals are insufficient, to generate MVS-induced nystagmus in mice.
INTRODUCTION
Static magnetic fields like those in magnetic resonance imaging (MRI) machines have been shown to induce nystagmus in humans and the mechanism is thought to be peripheral vestibular stimulation via a magneto-hydrodynamic force1. With elimination of visual fixation: (1) all humans with intact peripheral vestibular function have a mixed horizontal and torsional nystagmus while lying in static magnetic fields of at least 1.5 Tesla (T); (2) the direction of nystagmus changes with the pitch of the head and with direction of entry into the magnet bore; (3) the nystagmus persists throughout the duration of time in the magnetic field (up to 90 minutes thus far)2; (4) the nystagmus does not depend on motion into or out of the field; and (5) the effect on eye movement scales with the intensity of the magnetic field1. Despite the nystagmus persisting, the perception of rotation adapts quickly 3.
A Lorentz force is exerted by a magnetic field on a charged particle or a conductor carrying electrical current. When the charged particles are ions moving through a fluid, the force tends to cause the fluid to flow in a direction perpendicular both to the magnetic field and the direction of the ionic current. This phenomenon is the basis of magnetohydrodynamics. In the inner ear a Lorentz force, engendered by both a strong static magnetic field and current entering utricular hair cells, is believed to create a force that acts on the endolymph above the utricle, causing semicircular canal cupular deflection and consequently, an observable nystagmus. This phenomenon has subsequently been corroborated in humans by other groups, and models have predicted that in strong MRI machines the forces are sufficient to deflect the cupulae 3–5. Built upon both assessments of nystagmus direction in humans with functioning and diseased labyrinths and models of hair cell currents, the present theory of magnetic vestibular stimulation (MVS) is that the stimulated labyrinthine structures are the lateral and superior semicircular canals 6–8, and that the utricle is the primary current source 1,4. Experimental evidence, however, regarding the source of current that generates this force, as well as the affected inner ear structures is needed.
Earlier behavioral studies in rats and mice also indicate the labyrinth is involved in producing magnetic field-induced vertigo in strong static magnetic fields 9–11. Rats avoid static magnetic fields of at least 2T, and these reactions can be extinguished following labyrinthectomy 9. Rats exposed to a 14T magnetic field also show c-fos induction in the brainstem (indicating neural activation), a finding that is then prevented by labyrinthectomy 12. Furthermore, mice placed in different pitch positions in an MRI have varying amounts of post-exposure circling depending on the pitch angle, consistent with a Lorentz force hypothesis as explained above 13. These findings indicate that strong magnetic fields influence the mouse labyrinth in ways that may be consistent with the nystagmus observed in humans.
A mouse model of strong magnetic field-induced nystagmus would allow exploration of this phenomenon using mutant mice with targeted inner ear lesions. An unanswered question in the mechanism of MVS is the relative contributions of the semicircular canal cupulae and otolith maculae to the current that generates a Lorentz force, in particular whether the current entering the semicircular canal cristae is capable of generating a Lorentz force sufficient to displace its cupula. The utricular macula, with both its close proximity to the superior and lateral canal cupulae and its greater hair cell count—and presumed greater current density—is thought to be primarily responsible for generating the force that displaces the cupulae 1,4. Mice deficient in Nox3 (the gene that encodes NADPH oxidase) lack otoconia in those mice homozygous for the mutation 14, but have anatomically intact hair cells and synapses 15. While these mice have expected impairment of peripheral otolith organ function 16,17, they are capable of generating a vestibulo-ocular reflex (VOR) 17, indicating functioning semicircular canals. We hypothesize that MVS elicits nystagmus in C57BL/6J mice with normal utricular and semicircular canals, but not in mice lacking Nox3, which is required for normal otoconial development.
MATERIALS AND METHODS
We measured eye movements in darkness in C57BL/6J mice and in head tilt mice deficient in otoconia, while in different orientations inside a 4.7 T magnetic field. Eye movement responses of C57BL/6J mice (n=8) to the static magnetic field were compared to those of head tilt mice (n=6). Prior to placement in the MRI, vestibular function was characterized in each mouse by transient horizontal rotations to assess semicircular canal function and by static tilts to assess ocular counter-roll and otolith function. All experiments were approved by the Johns Hopkins Animal Care and Use Committee, in compliance with all applicable laws and guidelines.
Animal Preparation
To minimize head and body movements during experimentation, the mouse’s head was restrained to pitch the animal’s head 30° ‘nose-down’ from the Earth horizontal position (Figure 1A)18. With the animal in this position, the axes of the animal’s horizontal semicircular canals (SCC) were aligned to within 10° of the Earth-horizontal when the holding container was in the Earth-horizontal position19.
Figure 1.
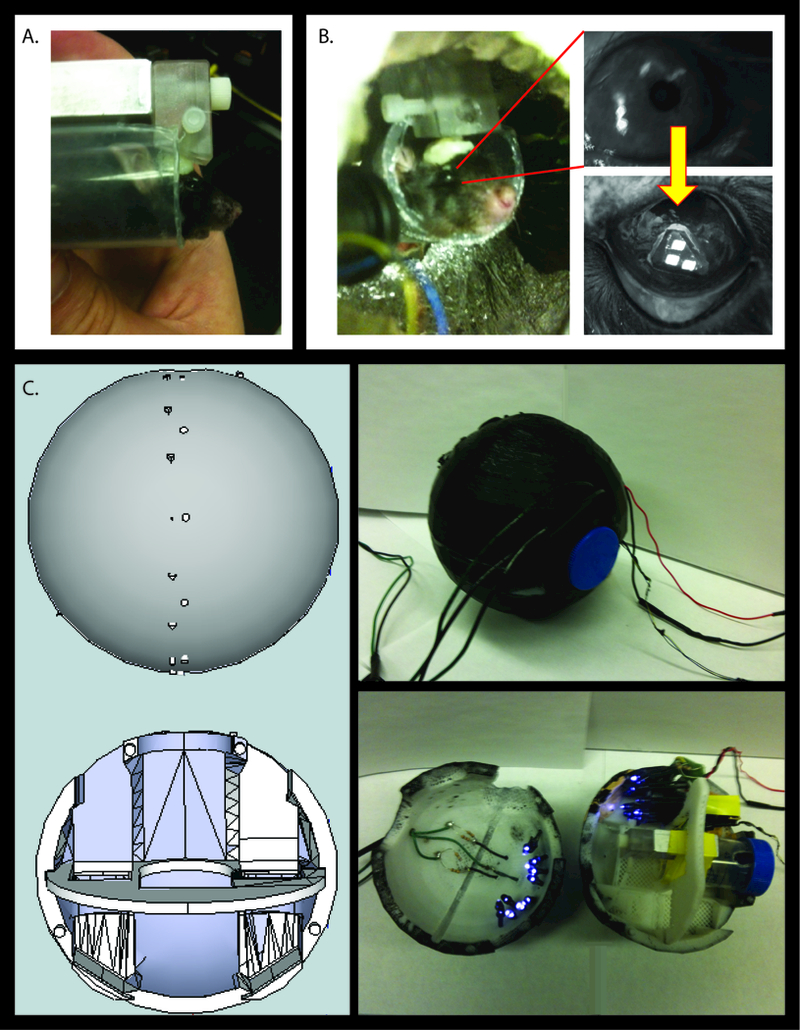
Methods for recording eye movements in mice in an MRI. A) The mouse is positioned such that its horizontal semicircular canals are roughly parallel to the Earth. B) The camera is focused on the mouse’s right eye, where a marker is placed to allow tracking of the pupil during all testing. C) The mouse container is a sphere that was printed using a 3D printer. Infrared LEDs are used to illuminate the fluorescent marker such that the camera records movement of the three squares on the marker.
Video-Oculography
A MRI-compatible video-oculography (VOG) system was used to measure eye movements in horizontal and vertical directions. Eye movement recordings were obtained using marker arrays that were secured to the mouse’s pupil after administering general and topical anesthesia (Figure 1B). For all experiments, the mouse was positioned so that a video camera focused on the pupil and markers of the right eye. The mouse was placed in darkness to preclude visual fixation suppression of nystagmus. When entering the bore nose first, the magnetic field lines were directed from the animal’s tail towards its head. Horizontal and vertical eye movement data were computed and stored using custom-designed software to track the position of the eye marker windows and eye velocity was then obtained using software and techniques as described previously18,20,21,22.
Head tilt mice
B6.129S1-Nox3het-3J/GrsrJ mice (head tilt mice) with mutations in the Nox3 gene were used to assess influence of otoconia on MVS. Mice heterozygous for the Nox3het–3J mutation were obtained from the Jackson Laboratory (Bar Harbor, ME). The Nox3het–3J allele has been made congenic on a background of C57BL/6J strain. All physiologic comparisons were therefore made to C57BL/6J mice. The mice were bred for two generations prior to testing. Forced swim testing was performed on all head tilt mice at 3 months of age to screen for likelihood of the desired phenotype23. Later, to confirm that the screened mice had two copies of the mutation, Sanger sequencing was also performed24. Two copies of this mutation were identified in all 6 presumed head tilt mice used in the study, and no copies in either of two control wild type C57BL/6J mice.
Vestibulo-ocular Reflex testing
Prior to placing the mice in the magnetic field, a quantitative VOR assessment was performed in all mice in response to static tilt position, sinusoidal horizontal rotations and horizontal transient accelerations. Head tilt mice should lack compensatory eye movements in response to head tilt due to absent otoconia, but continue to have residual VOR function in response to horizontal rotations17. Experimental protocols were completed as described previously18.
Magnet Experimental Protocol
The mouse was secured in a 3D printed sphere of acrylonitrile butadiene styrene plastic (Figure 1C). All mice were placed inside the magnetic field with heads pitched so that the horizontal semicircular canals were approximately aligned with the magnetic force vector. Eye movements were recorded throughout each placement into the magnet bore. Mice were placed into the magnet bore for at least 30 seconds each in the nose first and tail first positions. Note that no scans were performed; only the static field of the MRI machine was used. For additional methods details on animal preparation, design of the testing apparatus, forced swim testing, and Sanger sequencing, please see methods Supplementary Digital Content 1.
Data Analysis
For each head orientation position in the bore, VOG eye position data were converted to head-fixed rotation vectors from which canal-plane referenced eye rotation velocities were computed. Eye and head data are reported in a right-hand-rule head coordinate system centered on the skull stereotactic origin as described previously by our laboratory18. The number of beats of nystagmus over the duration in the MRI, nystagmus frequency, and mean slow-phase eye velocity was calculated for each static head position. Nonparametric between groups tests were used to compare results of C57BL/6J mice and head tilt mice. All data was analyzed with Stata 10 (StataCorp, Inc., College Station, TX, USA) and p<0.05 were considered significant.
RESULTS
Head tilt mice had no or minimal eye movement response to static tilt across the axes assessed, when compared to C57BL/6J mice (Figure 2). However, head tilt mice demonstrated intact VOR in response to transient horizontal rotations and sinusoidal rotation (Figure 3), but with slightly lower gain in response to transient accerletaions (Figure 3, right panel).
Figure 2.
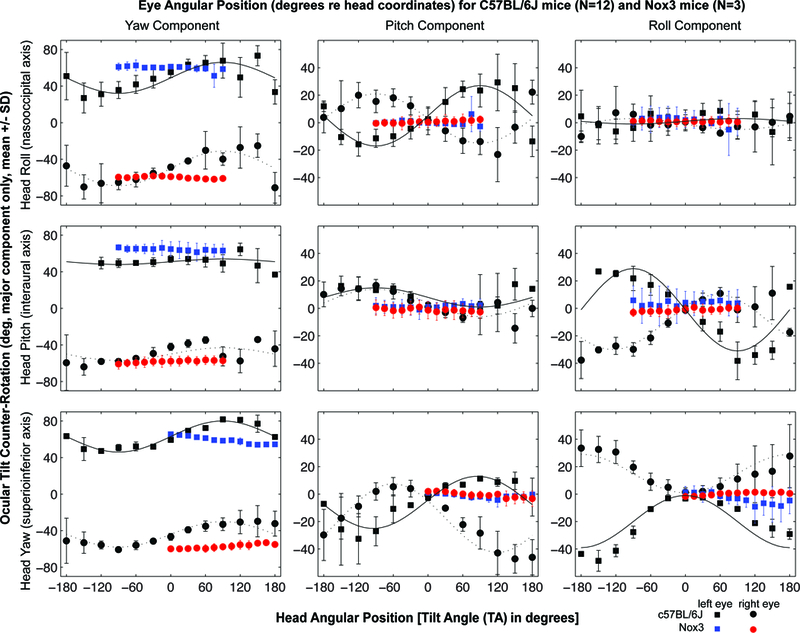
Head tilt (Nox3) mice have minimal eye movements in response to static, passive whole-body tilts about an Earth-horizontal axis in a gravitational field (red and blue marks), as compared to C57BL/6J mice (black marks). SD, standard deviation; deg, degrees; het, head tilt.
Figure 3.
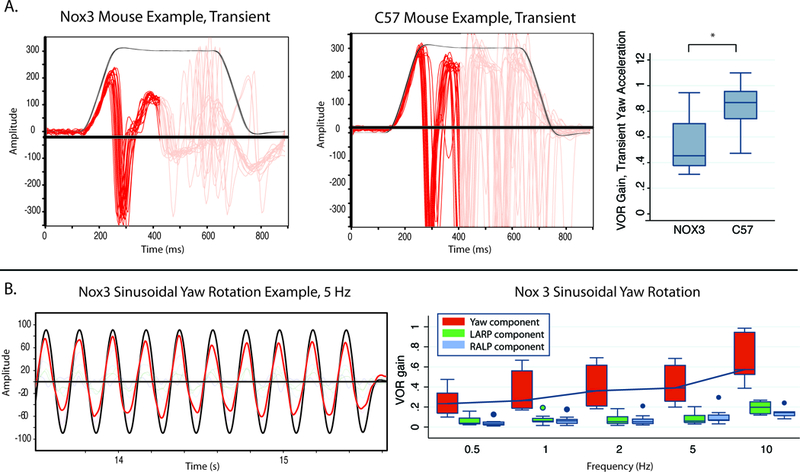
VOR response in head tilt (Nox3) mice A) Example of eye movement traces in response to transient, rightward,horizontal whole-body rotation in a head-tilt mouse and an exceptionally good example of a C57 mouse, with acceleration 3000 deg/s2 for 100 msec to a 300 deg/s constant-velocity plateau. Black trace represents the horizontal head velocity stimulus and the red trace the horizontal component of the eye velocity response, which comprises an initial, VOR-driven slow phase nystagmus during the constant-acceleration portion of the stimulus and then an alternating series of rightward nystagmus quick phases (toward negative values in this plot) and slow phases (positive value segments). Right panel shows head tilt mice have a robust and well aligned 3D angular VOR in response to quick, passive horizontal head rotations, with the horizontal component gain ranging from 0.3–0.7, nevertheless this is lower than C57 litter mates, p<0.05. C) Responses to horizontal 100 deg/s peak sinusoidal rotations demonstrate median gain increasing from 0.3 to 0.7 across frequencies from 0.5 Hz to 10 Hz, with other components of the 3D VOR small. VOR, vestibulo-ocular reflex; LARP, left anterior-right posterior plane; RALP, right anterior-left posterior canal plane
Magnetic field-induced nystagmus
C57BL/6J mice demonstrated a nystagmus that varies with position relative to the magnetic field. After nose-first entry into the magnetic field, C57BL/6J mice developed a left-beating nystagmus lasting a median of 32.8 seconds (Figure 4). On tail-first entry into the magnet bore, the direction reversed and was of similar duration (median 28.0s, p>0.05). C57BL/6J mice additionally had null positions at 90 degrees relative to the magnetic field vector, where minimal nystagmus was observed. SPV could be reliably calculated for 5 C57BL/6J mice on nose-first entry and had a mean velocity of 118.0 (SD 81.5) degrees per second when entering the magnet nose first (see figure 4 for example). Despite the conservation of VOR in response to sinusoidal and transient rotations, head tilt mice had no nystagmus inside the magnetic field in any of the tested orientations (Figures 4 and 5). To ensure the head tilt mice were not oriented in a ‘null’ position in the head first or tail first orientations, they were additionally positioned with their nose 30 degrees up and 30 degrees nose down from horizontal. Again, nystagmus was not observed in these additional orientations.
Figure 4.
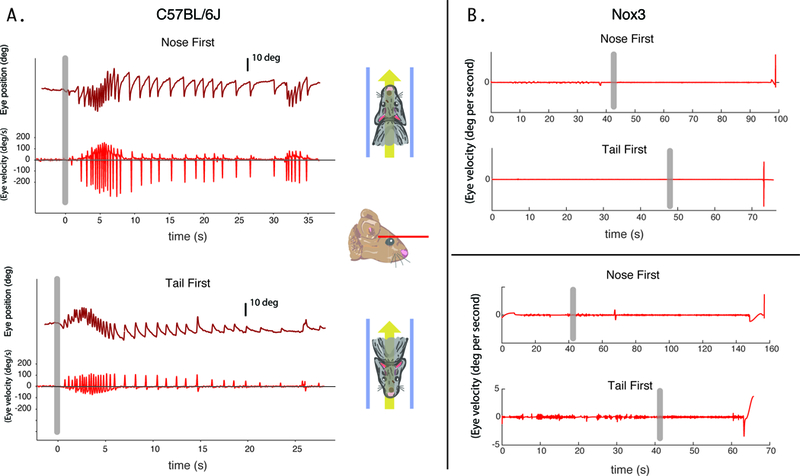
Eye movement recordings of a mouse entering in the nose-first Earth-horizontal position (legend). Grey vertical bar signifies time of entry into the magnetic field. For C57BL/6J mice, a horizontal nystagmus develops after nose-first entry into the magnetic field (A, left panels). The velocity increases to a peak after entry, before decreasing over time while the mouse remains in the magnetic field. No nystagmus was seen for head tilt mice (n=2 shown) in the tested positions in the MRI machine (B, right panels).
Figure 5.
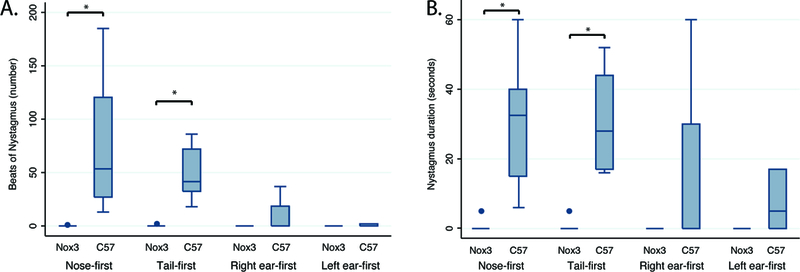
Group data for C57BL6/J mice (n=8) and Nox3 mice (n=6) entering the magnetic field in each of 4 positions. C57 mice develop nystagmus in head-first and tail-first positions that is not present in Nox3-deficient mice. *, p<0.01
DISCUSSION
Mice with intact vestibular function have nystagmus in strong static magnetic fields, and this response is dependent on the presence of Nox3 or functioning otolith end organs. C57BL/6J mice had a primarily horizontal nystagmus that changed direction with body position relative to the magnetic field, reversing from left-beating on nose-first entry to right-beating on tail-first entry into the magnetic field. As in humans, the direction of the magnetic field vector with respect to the head influences the direction of nystagmus, suggesting that the mechanism generating this nystagmus response depends upon the polarity of the magnetic field.
Several mechanisms have been proposed for the interactions of magnetic fields with the inner ear (See recent reviews 25,26), many of which have been ruled out as causing nystagmus. Notably, there has never been ferromagnetic particles detected in the mammalian inner ear. Static magneto-hydrodynamics is thought to be the origin of the nystagmus humans experience inside strong magnetic fields like MRI machines. Static magneto-hydrodynamics (MHD) requires no gross head movement—only a static magnetic field and an ionic current—to produce a Lorentz force on a conductive fluid (See figure, Supplementary Digital Content 2). In the presence of a strong magnetic field, normal ionic currents present in neural/vestibular tissue produce sufficient force within vestibular organs to account for the observed nystagmus, depending on the alignment of the head with the magnetic field 1,4.
Similarities between mouse and human MVS
The nystagmus velocity in C56BL/6J mice peaked shortly after magnetic field entry, and persisted for tens of seconds after the mouse was stationary in the center of the strong static magnetic field. These findings argue against a mechanism that relies upon motion through the magnetic field like electromagnetic induction, as motion-induced effects would peak during movement into the magnetic field and would be absent inside the center of the magnetic field. Furthermore, the directions of nystagmus responses observed are those that would be predicted by a static magnetohydrodynamic mechanism (i.e. a Lorentz force, figure, Supplementary Digital Content 2) 1,7.
The velocity of the eye movements in mice demonstrated an exponential decay from a peak SPV, that occurs after entry into the magnetic field. This is a finding seen with a constant deflection of the cupula as occurs in a constant acceleration input, in which a static deflection of the cupula is induced, such as rotation at constant acceleration 27. We have hypothesized that the stimulus for MVS is similar to a constant acceleration input in that it induces a constant pressure on the semicircular canal cupula.
In prior reports, all humans tested had a null position where no nystagmus was seen, and this null position was hypothesized to occur when the head was pitched such that the plane of the horizontal semicircular canals and utricles was orthogonal to the magnetic field vector 1. Based on the static MHD hypothesis, a null should occur when aligning net utricular current with the magnetic field vector. When these vectors are aligned, there is no cross product and no Lorentz force generated. It was also predicted that a null position could occur when, despite a Lorentz force being generated sufficient to displace the cupula, the direction of the force vector is not aligned with its shear sensor (the canal cupula). This situation occurred when the C57BL/6J mice demonstrated little or no eye movements (except in one mouse) when entering the magnetic field left-ear first or right-ear first (Figure 6). In current MRI machines, it is not possible to orient the human labyrinth in this position with respect to the magnetic field.
Figure 6.
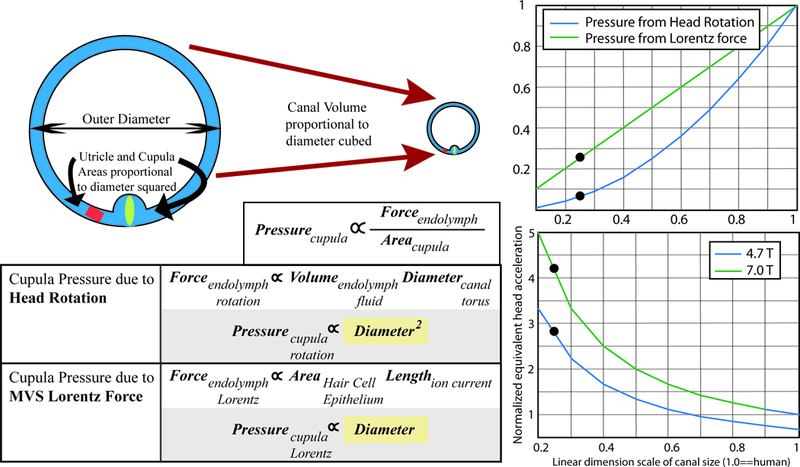
Hypothesis for increased velocity of mouse eye movements in the magnetic field relative to prior reports in humans. Laboratory mice have much greater slow-phase nystagmus velocities than humans, despite a weaker magnetic field. This may be explained by differential scaling of the cupula pressures with labyrinth size due to a Lorentz force relative to a head rotation. For head rotations, the torque force due to the moment of inertia of the endolymph is related to the endolymph volume and the canal diameter. Therefore, cupula pressure due to head rotation is proportional to the diameter of the canal squared. For magnetic vestibular stimulation (MVS), the Lorentz force in the endolymph is related to the ionic current flowing into the area of hair cells, and to the length over which the current flows. Therefore, cupula pressure due to MVS is directly proportional to canal diameter. The right panels are graphical representations of the relationships between pressure on the cupula as a function of labyrinthine dimension, normalized for human labyrinthine size (1.0). The right upper panel shows that as canal dimensions scale down linearly, the cupula pressure for a Lorentz force (green line) scales slower than for a head acceleration (blue line), such that a mouse should experience a relatively greater force (blue dots depict approximate relative labyrinthine size for a mouse). The right lower panel adjusts for the relative MRI strengths of prior human experiments in 7T MRI compared to those in mice. In a 4.7T MRI, mice should experience an approximately 3 times greater cupula pressure than a human, and in a 7T scanner over a 4 times greater pressure, akin to a much greater head acceleration.
Differences between mouse and human MVS
Although the directions of nystagmus and the presence of an initial peak in eye velocity are similar between mice and humans, there are differences in both the amplitude of the peak eye velocity as well as in the longer-term dynamics of the eye movement responses in mice. A vestibular nystagmus has slow and fast phases; and the slow-phase component of a vestibular nystagmus is driven by the vestibular system. In humans, there is a characteristic peak in the velocity of the slow-phase component shortly after entering the magnet, followed by a slowly-declining plateau 1. The maximum recorded peak slow-phase velocity (SPV) in humans in a 7T magnetic field is 40 degrees per second, and the mean SPV of the plateau is approximately 10 degrees per second 27. The peak SPV of the C57BL/6J mice in a weaker 4.7T magnetic field was substantially greater than that of the humans exposed to a 7T magnetic field.
An explanation for this difference in SPV may be differences in dimensions of the mouse semicircular canals relative to the human’s and the ways in which a Lorentz force scales with decreasing labyrinth size relative to how the mechanics of a head acceleration scales with decreasing labyrinth size. The pressure on the cupula due to head rotations decreases much more quickly with labyrinthine dimensions than the pressure on the cupula due to a Lorentz force. Using a simple first-order steady-state approximation equation from Oman and Young 28, the pressure on the cupula during a head rotation is proportional to the diameter of the canal torus squared. The pressure on the cupula generated by a Lorentz force, however, is proportional to a linear dimension. Figure 6 shows that although the magnitude of the Lorentz force decreases linearly with labyrinth size, the sensitivity of the crista to a rotational force decreases more (i.e. by a factor of three). Thus a mouse with a smaller labyrinth would experience a relatively larger force on the cupula than a human. This could account for approximately 3 times the difference in pressure on the cupula. Of course this needs to be confirmed in animals with various sized labyrinths, but it may explain some of the differences we observe in SPV.
There are also differences in the longer-term dynamics of the eye movement responses in mice compared to humans. In humans, nystagmus persists during the entire exposure in the MRI (up to 90 minutes) and can be modeled as a cascade of adaptation parameters with increasing time constants 27. This slowly-declining plateau in eye movement velocity is absent in mice. In C57BL/6J mice, the nystagmus decayed in most instances within approximately 30 seconds. Furthermore, in humans, an aftereffect occurs in which nystagmus beats in the opposite direction, reflecting adaptation inside the MRI 1,27. This was observed in C57BL/6J mice in less than half of trials, with a few beats of nystagmus in the opposite direction upon withdrawal from the magnet. These differences in adaptation dynamics may be explained by interspecies differences in processing of vestibular input between mice and humans. The time constant of the mouse VOR as compared to the human is significantly smaller 29.
Effect of Nox3 on MVS
We found that MVS nystagmus was absent in Nox3-deficient or head tilt mice that lack functional otolith organs, despite the presence of a VOR in response to horizontal head rotations. To ensure that the nose-first or tail-first positions were not by chance in the mouse’s null position, we reoriented these mice in different pitch positions. No nystagmus was seen in any position for Nox3-deficient mice. This implies that a functioning otolith end organ is critical for the development of magnetic field-induced nystagmus.
Some features of head tilt mice are important to keep in mind when interpreting these results. Despite having an intact VOR in response to head rotations, head tilt mice, however, have reduced VOR gain 17. The inner ear morphology of these mice previously have been well characterized and have shown intact hair cells with ribbon synapses in the utricle 15. They additionally appear to have spontaneous discharge from the superior vestibular nerves 30. As shown in this study, however, the otolith-ocular reflexes are absent, and other studies have also shown an absence of otolith-mediated potentials 16,17.
Based on the current hypothesized mechanism of MVS, the utricle is the primary source of the current that generates the Lorentz force, as the utricle has the largest density of hair cells coupled with its close proximity to the lateral and superior semicircular canal cupula. This force is then sensed by the semicircular canals as a constant acceleration. Theoretically, any hair cells in the inner ear can generate a Lorentz force, but the current must be great enough and in the proper direction to generate a force that must then be strong enough to deflect the cupula. While head tilt mice have been found to have a spontaneous discharge from superior vestibular nerve afferents 15, the afferent response differs from that of control mice 15 and it is unknown whether there is current flowing through the endolymph and into the apical portion of the utricular hair cells. We hypothesize that without the mass of otoconia on utricular hair cells that these hair cells carry less current. This hair cell current is required based on the MHD hypothesis of MVS to generate nystagmus. The results of this study imply that functioning semicircular canal cristae are insufficient to generate nystagmus in a strong magnetic field.
Magnetoreception
The physiology underlying animal migration remains controversial. Among vertebrates, many species have been reported to be sensitive to magnetic fields 31. Orientation with respect to magnetic fields in mammals has additionally been reported for species of subterranean rodents 32,33, and more recently for laboratory mice 34. Furthermore, rodents have been shown to use this magnetic field cue for path integration 33. Several theories for transduction of magnetic field cues have been proposed, including light-dependent radical pairs and ferromagnetic particles like magnetite. Subterranean rodents that live entirely in darkness, are unlikely to benefit from a light dependent radical pair mechanism of magnetoreception. Furthermore, magnetite has not yet been detected in mammals. Although the strength of the magnetic field used in these experiments far exceeds that of the Earth’s magnetic field, a labyrinthine signal generated by a Lorentz force could be a polarity-sensitive mechanism by which mice could orient with respect to a North/South magnetic pole.
Limitations
This study has several limitations in part due to the technical challenges of obtaining video oculography in mice in an MRI. We adapted a previously developed system in our laboratory for use in an MRI, and for simplicity, we limited the eye movement analysis to the horizontal component, as this appeared to be the principal component. This technique could be further validated for obtaining accurate 3-dimensional eye movements. There was variability in the eye velocity in those C57BL/6J mice from which we could obtain clean velocity data, similar to the variability seen in humans depending on the location of the ‘null’ position1. Additional investigations could assess variability in null position among mice, as well as the influence of varying magnetic field intensities on the slow-phase eye velocity.
Future directions and Clinical Implications
MRI has revolutionized the diagnosis and treatment of neurological disease and the scientific understanding of the function of the brain, yet we know surprisingly little about the effects of magnetic fields on brain function. People near MRI scanners occasionally report dizziness and this is most prominent with 7T magnets. The potential health implications of these symptoms are unknown; however, we reported a case of a patient who experienced a medical complication after entering an MRI scanner, due to the dizziness caused by the MRI machine 35. Although human studies provided evidence that magnet induced nystagmus is evoked by magneto-hydrodynamic forces, they are insufficient to fully characterize this effect and to work out why it occurs. By clarifying the characteristics and mechanisms of MVS, a mouse model can allow for further exploration of the health and safety of people exposed to MRI scanners with high-strength magnets. As a first step towards this goal, this study has found that functioning otoconial organs are required for MVS to occur and that intact crista hair cells are insufficient to produce the nystagmus observed in strong MRI machines. The observations here and in other work in humans with vestibular disease5,6 clarify the pathophysiology underlying vertigo in MRI scanners and may translate to new diagnostic tests for patients with dizziness and vertigo. Furthermore, we anticipate MVS may become a useful technique for vestibular rehabilitation.
Supplementary Material
Additional methods details on animal preparation, design of the testing apparatus, forced swim testing, and Sanger sequencing.
Hypothesized static magnetohydrodynamic force model with force vectors. F, Lorentz force; L, distance over which the current flows; j, net current; B, magnetic field. Shown are mouse orientation and presence/direction of observed magnetic field induced nystagmus; arrows indicate suspected vectors contributing to the Lorentz force. Legend (left) shows right-hand rule relationship among hair cell ionic currents (green), magnetic field (yellow), resulting force (red) and flow of endolymph (orange). Based on the results of the study, utricular current is believed to be the principal ionic current. Two-dimensional lateral canal cartoons are drawn for nose-first and tail-first mouse orientation with larger utricular current directed into the image. When the mouse is tail-first, the Lorentz force vectors do not change direction, but the horizontal canal stimulation is like a head acceleration in the opposite direction. SCC, semicircular canal.
Acknowledgments:
The authors would like to acknowledge Lani Swarthout for assistance with animal care and data acquisition, and Catie Copley for assistance genetic testing and Vadappuram Chacko for use of the MRI magnet. This work was in part funded by T32 DC000027–24 and a resident research fellowship grant from the American Otological Society. We appreciate the support of the Johns Hopkins University Center for Hearing and Balance.
Footnotes
Data from this project was presented as a poster at the Association for Research in Otolaryngology in San Diego, CA in February, 2016 and has not been submitted elsewhere for publication.
The authors have no financial disclosures to report pertinent to this project.
REFERENCES:
- 1.Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI magnetic field stimulates rotational sensors of the brain. Curr Biol. 2011;21(19):1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward B, Zee D. Dizziness and Vertigo during MRI. N Engl J Med. 2016;375(21):e44. [DOI] [PubMed] [Google Scholar]
- 3.Mian OS, Li Y, Antunes A, Glover PM, Day BL. On the vertigo due to static magnetic fields. PLoS One. 2013;8(10):e78748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes A, Glover PM, Li Y, Mian OS, Day BL. Magnetic field effects on the vestibular system: calculation of the pressure on the cupula due to ionic current-induced Lorentz force. Phys Med Biol. 2012;57(14):4477–4487. [DOI] [PubMed] [Google Scholar]
- 5.Ward BK, Otero-Millan J, Jareonsettasin P, Schubert MC, Roberts DC, Zee DS. Magnetic Vestibular Stimulation (MVS) As a Technique for Understanding the Normal and Diseased Labyrinth. Front Neurol. 2017;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Magnetic vestibular stimulation in subjects with unilateral labyrinthine disorders. Front Neurol. 2014;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mian OS, Glover PM, Day BL. Reconciling Magnetically Induced Vertigo and Nystagmus. Front Neurol. 2015;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otero-Millan J, Zee DS, Schubert MC, Roberts DC, Ward BK. Three-dimensional eye movement recordings during magnetic vestibular stimulation. J Neurol. March 2017. doi:10.1007/s00415-017-8420-4. [DOI] [PubMed] [Google Scholar]
- 9.Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC. Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav. 2007;92(4):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houpt TA, Houpt CE. Circular swimming in mice after exposure to a high magnetic field. Physiol Behav. 2010;100(4):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder DJ, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brain stem by a 9.4 T magnetic field. Neuroreport. 2000;11(12):2681–2685. [DOI] [PubMed] [Google Scholar]
- 12.Cason AM, Kwon B, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav. 2009;97(1):36–43. [DOI] [PubMed] [Google Scholar]
- 13.Houpt TA, Kwon B, Houpt CE, Neth B, Smith JC. Orientation within a high magnetic field determines swimming direction and laterality of c-Fos induction in mice. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R793–R803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergstrom RA, You Y, Erway LC, Lyon MF, Schimenti JC. Deletion mapping of the head tilt (het) gene in mice: a vestibular mutation causing specific absence of otoliths. Genetics. 1998;150(2):815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman LF, Ross MD, Varelas J, Jones SM, Jones TA. Afferent synapses are present in utricular hair cells from otoconia-deficient mice. Hear Res. 2006;222(1–2):35–42. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res. 1999;135(1–2):56–60. [DOI] [PubMed] [Google Scholar]
- 17.Harrod CG, Baker JF. The vestibulo ocular reflex (VOR) in otoconia deficient head tilt (het) mutant mice versus wild type C57BL/6 mice. Brain Res. 2003;972(1–2):75–83. [DOI] [PubMed] [Google Scholar]
- 18.Migliaccio AA, Meierhofer R, Della Santina CC. Characterization of the 3D angular vestibulo-ocular reflex in C57BL6 mice. Exp Brain Res. 2011;210(3–4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese DR, Hullar TE. Planar relationships of the semicircular canals in two strains of mice. J Assoc Res Otolaryngol. 2006;7(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migliaccio AA, Macdougall HG, Minor LB, Della Santina CC. Inexpensive system for real-time 3-dimensional video-oculography using a fluorescent marker array. J Neurosci Methods. 2005;143(2):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliaccio AA, Minor LB, Della Santina CC. Adaptation of the vestibulo-ocular reflex for forward-eyed foveate vision. J Physiol. 2010;588(Pt 20):3855–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashima Y, Géléoc GSG, Kurima K, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest. 2011;121(12):4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paffenholz R, Bergstrom RA, Pasutto F, et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18(5):486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty JP, Fairfield HE, Spruce CA, McCarty CM, Bergstrom DE. Molecular characterization of an allelic series of mutations in the mouse Nox3 gene. Mamm Genome. 2011;22(3–4):156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover P Magnetic Field-Induced Vertigo in the MRI Environment. Curr Radiol Rep. 2015;3(8):29. [Google Scholar]
- 26.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Vestibular stimulation by magnetic fields. Ann N Y Acad Sci. 2015;1343:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jareonsettasin P, Otero-Millan J, Ward BK, Roberts DC, Schubert MC, Zee DS. Multiple Time Courses of Vestibular Set-Point Adaptation Revealed by Sustained Magnetic Field Stimulation of the Labyrinth. Curr Biol. 2016;26(10):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oman CM, Young LR. The physiological range of pressure difference and cupula deflections in the human semicircular canal. Theoretical considerations. Acta Otolaryngol. 1972;74(5):324–331. [DOI] [PubMed] [Google Scholar]
- 29.van Alphen AM, Stahl JS, De Zeeuw CI. The dynamic characteristics of the mouse horizontal vestibulo-ocular and optokinetic response. Brain Res. 2001;890(2):296–305. [DOI] [PubMed] [Google Scholar]
- 30.Jones TA, Jones SM, Hoffman LF. Resting discharge patterns of macular primary afferents in otoconia-deficient mice. J Assoc Res Otolaryngol. 2008;9(4):490–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiltschko R, Wiltschko W. Magnetoreception. Adv Exp Med Biol. 2012;739:126–141. [DOI] [PubMed] [Google Scholar]
- 32.Burda H, Marhold S, Westenberger T, Wiltschko R, Wiltschko W. Magnetic compass orientation in the subterranean rodent Cryptomys hottentotus (Bathyergidae). Experientia. 1990;46(5):528–530. [DOI] [PubMed] [Google Scholar]
- 33.Kimchi T, Etienne AS, Terkel J. A subterranean mammal uses the magnetic compass for path integration. Proc Natl Acad Sci U S A. 2004;101(4):1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips JB, Youmans PW, Muheim R, et al. Rapid learning of magnetic compass direction by C57BL/6 mice in a 4-armed “plus” water maze. PLoS One. 2013;8(8):e73112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward BK, Zee DS, Solomon D, Gallia GL, Reh DD. CSF leak: A complication from vomiting after magnetic vestibular stimulation. Neurology. 2015;85(6):551–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional methods details on animal preparation, design of the testing apparatus, forced swim testing, and Sanger sequencing.
Hypothesized static magnetohydrodynamic force model with force vectors. F, Lorentz force; L, distance over which the current flows; j, net current; B, magnetic field. Shown are mouse orientation and presence/direction of observed magnetic field induced nystagmus; arrows indicate suspected vectors contributing to the Lorentz force. Legend (left) shows right-hand rule relationship among hair cell ionic currents (green), magnetic field (yellow), resulting force (red) and flow of endolymph (orange). Based on the results of the study, utricular current is believed to be the principal ionic current. Two-dimensional lateral canal cartoons are drawn for nose-first and tail-first mouse orientation with larger utricular current directed into the image. When the mouse is tail-first, the Lorentz force vectors do not change direction, but the horizontal canal stimulation is like a head acceleration in the opposite direction. SCC, semicircular canal.


