Abstract
Background: Integrin αvβ3 is a molecular marker for the estimation of tumor angiogenesis. 99mTc-IDA-D-[c(RGDfK)]2 (also known as BIK-505) is a recently developed radiotracer for single-photon emission computed tomography, with good affinity for integrin αvβ3. In this study, the authors investigated the whole-body distribution and internal radiation dosimetry of 99mTc-IDA-D-[c(RGDfK)]2 in elderly human participants.
Materials and Methods: Six healthy volunteers underwent whole-body simultaneous anterior and posterior scans, preceded by transmission scans using cobalt-57 flood source, with a dual head gamma camera system, at 0, 1, 2, 4, 8, and 24 h postinjection of 99mTc-IDA-D-[c(RGDfK)]2 (injected radioactivity [mean ± SD] = 388.7 ± 29.3 MBq). Anterior and posterior images were geometrically averaged and attenuation corrected to delineate the regions of interest in the liver, gallbladder, kidneys, urinary bladder, spleen, brain, and large intestine. Radiation dose for each organ and the effective doses (EDs) were estimated using OLINDA/EXM 1.1 software.
Results: High radiation doses of renal and biliary excretion tracks such as the urinary bladder wall, upper large intestine, kidneys, liver, and gallbladder wall (19.15 ± 6.84, 19.28 ± 4.78, 15.67 ± 0.90, 9.13 ± 1.71, and 9.09 ± 2.03 μGy/MBq, respectively) were observed. The ED and effective dose equivalent were 5.08 ± 0.53 and 7.11 ± 0.58 μSv/MBq, respectively.
Conclusions: Dosimetry results were comparable to other radiolabeled peptides and were considered safe and efficient for clinical usage.
Keywords: : 99mTc-IDA-D-[c(RGDfK)]2, angiogenesis, biodistribution, dosimetry, integrin αvβ3 expression
Introduction
Angiogenesis is the process of new blood vessel formation from the preexisting vasculature. It is well accepted as a biomarker for the growth, invasion, and metastasis of numerous solid tumors.1–3 Currently, there is a growing demand for novel noninvasive in vivo imaging techniques for response assessment and the pretherapeutic stratification of patients receiving antiangiogenic therapies. It has been suggested that angiogenesis-targeted imaging can provide an early diagnosis and aid in treatment planning and the monitoring of antiangiogenic cancer therapies.4–6
Integrin αvβ3 is a suitable target for both tumor angiogenesis imaging and antiangiogenic therapy because of its high expression on activated endothelial cells and new blood vessels found in tumors and surrounding tissues, while it is absent in most intact normal tissues.7,8 Therefore, integrin αvβ3 is considered an indicator of activated angiogenesis; imaging of integrin αvβ3 overexpression is a promising technique for the assessment of angiogenesis. Labeled synthetic ligands with demonstrated specificity for integrin αvβ3 have proven to be successful agents for in vivo imaging of tumor angiogenesis.4 In particular, agents based on the amino acid sequence Arg-Gly-Asp (RGD) have been identified as useful for tumor angiogenesis imaging.9–11 A significant correlation between the tracer uptake and the level of angiogenesis has been demonstrated in clinical in vivo studies of tumor imaging performed using radiolabeled RGD peptides.12,13
IDA-D-[c(RGDfK)]2 is a newly developed, cyclic synthetic ligand containing the RGD binding site, with a high affinity (IC50 = 50 nM) for integrin αvβ3 during angiogenesis.14 Preclinical imaging studies using 99mTc-IDA-D-[c(RGDfK)]2 (also known as BIK-505) and single-photon emission computed tomography (SPECT) have demonstrated substantial and specific uptake of the radiotracer at the sites of integrin αvβ3 overexpression in tumors14 and high-risk atherosclerotic plaques in discrimination with inflammation.15 Moreover, a recently published human study demonstrated the preliminary clinical efficacy of 99mTc-IDA-D-[c(RGDfK)]2 SPECT in the visualization and localization of activated angiogenesis in brain and lung tumors.16 In the study, angiogenesis imaging using 99mTc-IDA-D-[c(RGDfK)]2 SPECT facilitated the visualization of integrin αvβ3 overexpression in both lung and brain tumors, with no laboratory and clinical adverse events, whereas the relationship between the level of active angiogenesis and glucose metabolism (measured with 2-[18F]fluoro-2-deoxyglucose and positron emission tomography) was different between lung and brain tumors. Thus, 99mTc-IDA-D-[c(RGDfK)]2 SPECT is a potential tool for the in vivo assessment of angiogenesis through the visualization of integrin αvβ3 overexpression in solid tumors.
Human internal radiation dosimetry for a newly developed radiotracer is essential for the risk-benefit assessment of clinical application. In this study, the authors evaluated the whole-body distribution and radiation dosimetry of 99mTc-IDA-D-[c(RGDfK)]2 in healthy volunteers using serial emission data sets obtained using a dual head gamma camera system.
Materials and Methods
Healthy volunteers
The present study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB No.: B-1112-069-004). Informed consent was obtained from all of the individual participants. All the procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The participants were recruited through Internet advertisements and a hospital bulletin board. Six healthy participants (male:female = 2:4; mean age ± SD = 68.3 ± 3.2 years [range, 64–73 years]; mean body weight ± SD = 56.5 ± 10.7 kg [range, 47–77 kg]) voluntarily participated in the study. Participants were age matched with their previous study against brain tumor and lung cancer patients.16 They had no clinically significant medical or neurologic conditions and presented with no clinically significant abnormalities on physical, neurologic, and laboratory examinations.
Preparation of 99mTc-IDA-D-[c(RGDfK)]2
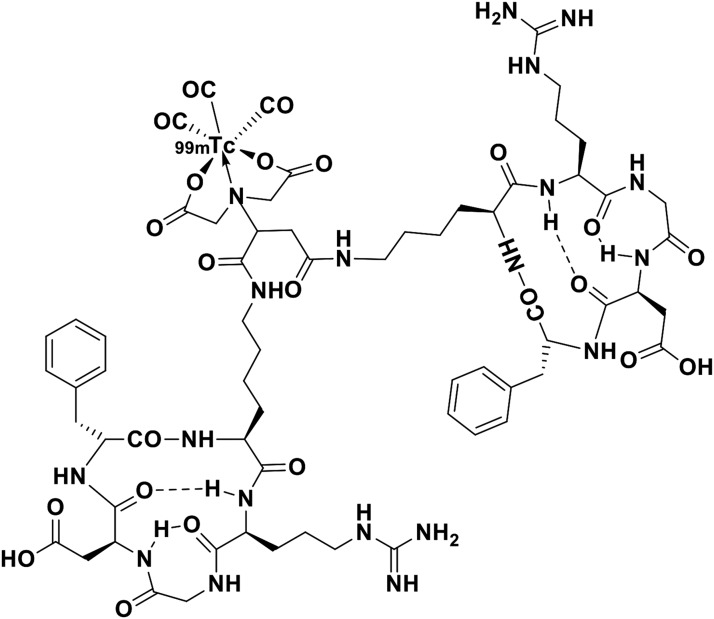
The precursor IDA-D-[c(RGDfK)]2 was generously provided by Bio Imaging Korea Co. Ltd. (Seoul, Republic of Korea), which owns the intellectual property rights. Sodium pertechnetate (99mTc) was eluted daily from 99Mo/99mTc-generator (Samyoung Unitech, Seoul, Republic of Korea). Structure of 99mTc-IDA-D-[c(RGDfK)]2 is provided in Figure 1. 99mTc-IDA-D-[c(RGDfK)]2 was synthesized following the method described in their previous work.14 A solution of [99mTc(H2O)3(CO)3]+ (370-740 MBq) in saline (200 μL) prepared according to the protocol described by Alberto et al.17 was added to the precursor in water (300 μL). After stirring the reaction mixture at 75°C for 30 min, the obtained 99mTc-IDA-D-[c(RGDfK)]2 was purified using semipreparative high-performance liquid chromatography (HPLC; Eclipse XDB-C18 column; 5 mm, 9.4 × 250 mm; Agilent Co., Palo Alto, CA). Their current study followed the same gradient conditions. All radiochemical processes, including 99mTc incorporation, HPLC purification, and tC18 Sep-Pak cartridge purification, were conducted within 60 ± 5 min. A quality control check, including pH, endotoxin testing, analytic HPLC, and residual solvents measurement by gas chromatography, was performed before human use; a radiochemical purity of 95% was mandatory. Molar radioactivity of 99mTc-IDA-D-[c(RGDfK)]2; 3.8 Ci/μmol (the injected chemical dose = 63 ng/kg) was obtained after HPLC purification.
FIG. 1.
Chemical structure of 99mTc-IDA-D-[c(RGDfK)]2.
Data acquisition
Participants were asked to urinate before the injection of 99mTc-IDA-D-[c(RGDfK)]2. Philips ADAC Forte dual-head gamma camera (Philips Medical Systems, Andover, MA) was used to acquire transmission and serial emission scan data sets. The photopeak was centered at 140 keV in a 20% width energy window using low-energy high-resolution collimators. Whole-body transmission scan was performed for attenuation correction using a Cobalt-57 flood source just before the injection of 99mTc-IDA-D-[c(RGDfK)]2. After the transmission scan, acquisition of simultaneous anterior and posterior whole-body emission scans was started at 0, 1, 2, 4, 8, and 24 h postinjection of 388.7 ± 29.3 MBq of 99mTc-IDA-D-[c(RGDfK)]2. The dose administrated was based on data from previous 99mTc-labeled RGD image studies.16,18–20 The scan speed was 20 cm/min for the whole body for both the transmission and emission scans. The duration of each whole-body scan was about 10.7 min.
The posterior image was mirrored, and the anterior-to-posterior pixel-by-pixel geometric average of the counts was determined. The calibration factor [(counts/pixel)/(Bq/pixel)] was obtained to convert counts/pixel into Bq/pixel using whole-body counts and injected doses (MBq). The attenuation was corrected using the transmission scan data and geometrically averaged first emission scan data under an assumption that the subject did not move during the transmission and first emission scans. Attenuation correction factors of the organs were obtained from attenuation corrected and nonattenuation corrected first scan data and were applied to all time-activity curves of the regions of interest (ROIs).
Quantitative analysis and radiation dose estimation
ROIs were manually drawn over the whole-body images at each time point, identifying high uptake organs such as the liver, kidneys, urinary bladder, gallbladder, spleen, brain, and large intestine to obtain the time-activity curves. The radioactivity of 99mTc-IDA-D-[c(RGDfK)]2 in each organ was expressed as the percentage injected dose (%ID) per organ calculated as the total activity in the organ ROI (Bq)/injected dose (Bq) × 100.
The number of disintegrations per unit activity administered (residence time) was obtained using the ratio of the cumulative activity and injected dose for each subject. The cumulative activity was obtained from the area under the time-activity curve. The area under the curve was calculated as the trapezoid sum of the observed data and the integral of physical decay for the curve tail after the last scan. The remainder of the body, an important input parameter for OLINDA/EXM 1.1 software (Organ Level Internal Dose Assessment Code, Vanderbilt University, Nashville, TN), was also assessed. If excretion of the radiotracer is prevented, the total residence time, which is the summation of the residence times in all of the organs in the body and the remainder of the body, can be calculated using the following simple equation:
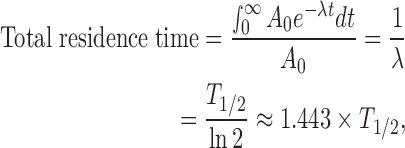 |
where A0 is the injected dose, λ is the decay constant, and T1/2 is the physical half-life of the radioisotope. In this study, the total residence time was about 8.67 h because the half-life of 99mTc was 6.01 h. The excretion of the radiotracer was assessed by the total radiation counts inside of the body contour of the geometrically averaged whole-body images. The residence time in the remainder of the body was obtained from the total residence time, summation of residence times in all of the organs, and excretion from the body.
Finally, the calculated residence times in each organ and the remainder of the body were used as input parameters for the OLINDA/EXM 1.1 software. The OLINDA/EXM 1.1 reports individual organ doses for 24 organs, effective dose (ED), and effective dose equivalent (EDE) using residence time data with reference to adult male and female models. The EDE and ED were calculated as the weighted sum of each individual organ dose. The weighting factors for the EDE and ED were defined from International Commission of Radiation Protection (ICRP) publications 26 (1979) and 60 (1990).
Results
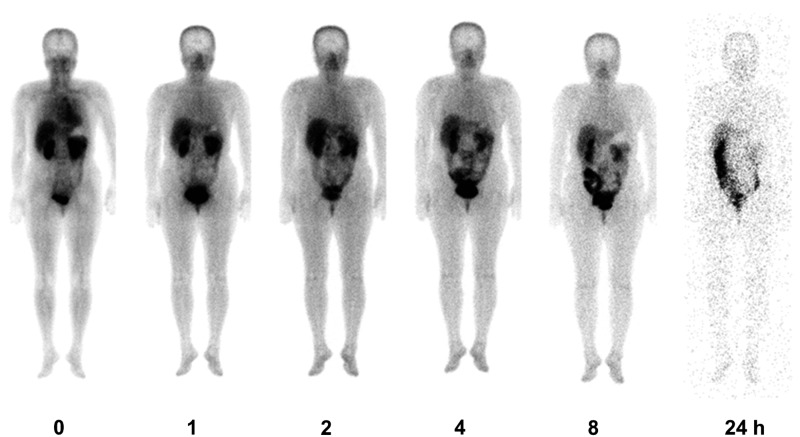
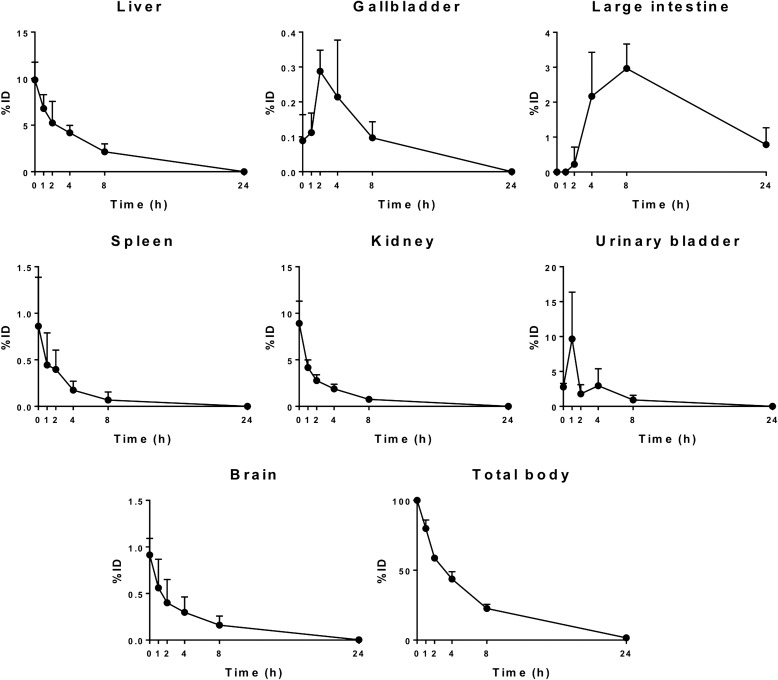
No adverse events were observed. Figure 2 shows a geometrically averaged whole-body image 0, 1, 2, 4, 8, and 24 h postinjection. After the intravenous injection, 99mTc-IDA-D-[c(RGDfK)]2 was rapidly taken up by various tissues and organs. High accumulations were observed in the heart, liver, kidneys, and urinary bladder at the initial phase and in the large intestine only at a very late phase. The residence times and cumulative activity percentage in each organ are shown in Table 1; high residence times were reported in the liver, large intestine, urinary bladder, and kidneys. 99mTc-IDA-D-[c(RGDfK)]2 was mainly eliminated through the hepatobiliary system and the renal system. Mean time-activity curves representing organ distribution details of %ID are shown in Figure 3. The radioactivity in the kidneys, liver, spleen, and brain decreased with time. Organs such as the urinary bladder, large intestine, and gallbladder showed initial accumulation and late washout according to the individuals' natural absorption and excretion cycles. At 24 h postinjection, the radioactivity of 99mTc-IDA-D-[c(RGDfK)]2 in almost all of organs had diminished completely, except in the large intestine.
FIG. 2.
Planar whole-body images showing biodistribution of 99mTc-IDA-D-[c(RGDfK)]2 at different times after i.v. injection (from left to right, 0, 1, 2, 4, 8, and 24 h) in a healthy volunteer.
Table 1.
Cumulative Activity Percentages (%ID) and Mean Residence Times (h)
| Organ | %ID | Residence time ( × 100, h) |
|---|---|---|
| Urinary Bladder | 3.78 ± 2.08 | 0.33 ± 0.18 |
| Brain | 0.48 ± 0.24 | 0.04 ± 0.02 |
| Gallbladder | 0.25 ± 0.12 | 0.02 ± 0.01 |
| Kidneys | 2.97 ± 0.29 | 0.26 ± 0.03 |
| Liver | 6.09 ± 1.83 | 0.53 ± 0.16 |
| Spleen | 0.30 ± 0.23 | 0.03 ± 0.02 |
| Large intestine | 5.40 ± 1.51 | 0.47 ± 0.13 |
| Total body | 48.24 ± 3.86 | 5.85 ± 0.41 |
| Remainder of the body | 67.51 ± 4.70 | 4.18 ± 0.33 |
| Outside of the body | 32.49 ± 4.70 | 2.82 ± 0.41 |
Values are mean ± SD.
FIG. 3.
Time-activity (%ID) curves for the organs. Data are mean ± SD.
The radiation dose to each organ and the effective doses (ED and EDE) obtained using OLINDA/EXM software are listed in Table 2. As expected, high radiation doses were reported in the upper large intestine (19.28 ± 4.78 μGy/MBq), urinary bladder wall (19.15 ± 6.84 μGy/MBq), kidneys (15.67 ± 0.90 μGy/MBq), liver (9.13 ± 1.71 μGy/MBq), and gallbladder wall (9.09 ± 2.03 μGy/MBq). Whole-body mean EDE and ED were 7.11 ± 0.58 μGy/MBq and 5.08 ± 0.53 μGy/MBq, respectively.
Table 2.
Organ Radiation Doses, Effective Doses, and Effective Dose Equivalents
| Organ | Radiation dose (μGy/MBq) |
|---|---|
| Adrenals | 4.81 ± 0.43 |
| Brain | 1.46 ± 0.33 |
| Breasts | 2.03 ± 0.26 |
| Gallbladder wall | 9.09 ± 2.03 |
| Lower large intestine wall | 4.44 ± 0.52 |
| Small intestine | 6.18 ± 0.78 |
| Stomach wall | 4.11 ± 0.48 |
| Upper large intestine wall | 19.28 ± 4.78 |
| Heart wall | 3.56 ± 0.40 |
| Kidneys | 15.67 ± 0.90 |
| Liver | 9.13 ± 1.71 |
| Lungs | 3.21 ± 0.42 |
| Muscle | 2.97 ± 0.29 |
| Ovaries | 5.56 ± 0.59 |
| Pancreas | 4.92 ± 0.49 |
| Red marrow | 3.07 ± 0.27 |
| Osteogenic cells | 7.77 ± 0.91 |
| Skin | 1.83 ± 0.19 |
| Spleen | 5.10 ± 1.72 |
| Testes | 2.72 ± 0.42 |
| Thymus | 2.80 ± 0.37 |
| Thyroid | 2.43 ± 0.21 |
| Urinary bladder wall | 19.15 ± 6.84 |
| Uterus | 5.70 ± 0.77 |
| Effective dose equivalent (μSv/MBq) | 7.11 ± 0.58 |
| Effective Dose (μSv/MBq) | 5.08 ± 0.53 |
Values are mean ± SD.
Discussion
Recently, angiogenesis imaging targeting integrin αvβ3 has gained widespread attention due to its value in tumor diagnostics and therapy. Studies, including ours, assessing the correlation of angiogenic activity in tumors with overexpression of integrin αvβ3 and tumor metabolism using 99mTc-IDA-D-[c(RGDfK)]2 SPECT and 18F-fluorodeoxyglucose (18F-FDG) PET, respectively, have reported on the clinical significance of angiogenesis imaging in solid tumor compared to metabolic imaging, compensating for the lack of biological characteristics in metabolic imaging.13,16,21 Although 99mTc-IDA-D-[c(RGDfK)]2 SPECT images demonstrated higher tumor-to-normal uptake ratios than 18F-FDG PET images in glioma patients and have shown its clinical potential, readily available and cost-effective radiotracers for monitoring angiogenesis are yet to be developed.
Radiotracers for angiogenesis imaging have a potentially broad field of application, spanning from cancer to cardiovascular theranostics. Several RGD peptides have been tested in the recent past, demonstrating that the number of RGD moieties, the isotope, and chelator profoundly affect the synthetic process, binding potential, and in vivo biodistribution.22 Other research groups have tried to synthesize 99mTc-labeled RGD analogs containing various 99mTc-chelating moieties such as 2-mercaptoacetyl-glycylglycyl (MAG2) and 6-hydrazinonicotinamide (HYNIC).23,24 However, these 99mTc-chelating moieties of the target compound have significant shortcomings, such as a bulky core size, low metabolic stability, and slow clearance from the normal tissue. Therefore, certain RGD analogs have been formulated to include polyethylene glycol (PEG4) or glycine (G3) linkers to increase the integrin αvβ3 binding affinity in a “bivalent” manner and to improve radiotracer excretion kinetics from normal organs.24 IDA-D-[c(RGDfK)]2 is a newly developed, cyclic synthetic ligand containing the RGD binding site with a high affinity for integrin αvβ3 during angiogenesis.14
SPECT/computed tomography (SPECT/CT) is a hybrid imaging modality that can longitudinally diagnose the target environment in the same subject across different time points noninvasively. RGD analogs that are labeled with radioisotopes suitable for SPECT detector systems, such as 99mTc, can be used as radiotracers for angiogenesis imaging with SPECT/CT. A clear advantage of the 99mTc-labeled SPECT radiotracer is its potential for clinical use as it is readily available and cost-effective compared to PET. 99mTc-labeled IDA-D-[c(RGDfK)]2 is a newly developed radiotracer for SPECT (or gamma camera) to visualize angiogenic lesions and is a promising agent for cancer imaging.
Human internal radiation dosimetry for newly developed radiotracers is essential for the risk-benefit assessment of clinical applications, which might influence the choice of the radiotracer. Therefore, in the present study, the authors evaluated the radiation dose exposure in humans who underwent a whole-body 99mTc-IDA-D-[c(RGDfK)]2 gamma camera scan. The results of the present study indicate that this tracer has favorable whole-body pharmacokinetics in terms of its high renal and hepatic extraction, rapid clearance through the kidneys, gallbladder, and GI tract and low doses in the other organs. The kidney exhibited high accumulation, suggesting the possibility to be the dose-limiting organ when targeting integrin αvβ3 for antiangiogenic therapy. Although the high kidney accumulation is probably caused by the increased arginine residues, the kidney uptake is comparable to other radiolabeled multimeric cyclic RGD peptides and other peptides.24–26 Activity in the liver and kidneys is substantially eliminated 8 h postinjection, with the excretion of the radiotracer through the large intestine and urinary bladder, with some residual activity in the large intestine. The ED and/or EDE values for other SPECT and/or gamma camera radiotracers commonly used clinically are listed in Table 3; the ED and EDE values for 99mTc-IDA-D-[c(RGDfK)]2 are comparable to those of other 99mTc-labeled radiotracers. To their knowledge, their study is the first reported evaluation of the human internal dosimetry of 99mTc-IDA-D-[c(RGDfK)]2 and represents an advancement in the quest for an ideal SPECT tracer for angiogenesis imaging.
Table 3.
The Effective Radiation Doses of 99mTc-Labeled Single Photon Emission Computed Tomography Radiotracers
The present study is limited by its small sample size. Because of the limited number of participants, the authors did not calculate the doses for subject-specific organ volumes. Rather, the authors computed the doses according to the OLINDA/EXM 1.1 reference phantoms. This approach is reasonable for low-dose range radiopharmaceutical compounds typically used in diagnostics, in which patient radioprotection is the main concern, because ED is a metric used for assessing stochastic risk in a population. On the contrary, the use of subject-specific organ masses would be crucial if a therapeutic approach was considered. Another limitation is the quantification of the dual-head gamma camera data. The geometric mean method using simultaneously acquired anterior and posterior images, which the authors adopted here, is certainly useful in activity quantification. However, its accuracy is limited to specific clinical situations. The most fundamental drawback of this method is the fact that it assumes constant attenuation of the photon intensity with the source depth; hence, it does not correct for the contribution of scatter to the counts obtained. This assumption is not valid for the relatively wide window settings used in nuclear medicine. It has been shown that the broad beam attenuation coefficient varies from 0.081 cm−1 for a source at 1-cm depth to 0.122 cm−1 for a source at 15-cm depth in tissue equivalent material using a 30% window. In addition, the method does not take into consideration the effects of the source thickness and inhomogeneity of the attenuating medium. The source thickness and inhomogeneity effects in vivo may be as high as 20% each.31
As mentioned earlier, integrin αvβ3 is a suitable target not only for tumor angiogenesis imaging but also for antiangiogenic therapy. In an aspect of radiotheranostics, the peptide IDA-D-[c(RGDfK)]2 is possibly transformed into a therapeutic radiopharmaceutical compound when it is labeled with beta-emitting radioisotopes such as rhenium-188 (188Re), as previously reported.14 Hence, target distribution and dosimetry studies of beta-emitting radioisotope labeled IDA-D-[c(RGDfK)]2 must be performed as previously mentioned, to utilize its strength as a radiotheranostic radiopharmaceutical. In specific, pretherapeutic scans with 99mTc-IDA-D[c[RGDfK]2 could aid the decision process of 188Re-IDA-D[c[RGDfK]2 radiation dosage prescription, predicting the possible radiation accumulation to critical nontarget organs such as the kidney.
To conclude, the radiation dosimetry results for 99mTc-IDA-D-[c(RGDfK)]2 are comparable to the data reported for other 99mTc-labeled radiotracers, and thus, possible diagnostic use of the new radiotracer does not escalate the radiation risk to the patient. This study demonstrates that 99mTc-IDA-D-[c(RGDfK)]2 is an efficacious and safe radiotracer for imaging integrin αvβ3 expression.
Acknowledgments
The authors thank Bio Imaging Korea Co., Ltd. for providing the precursor for radiolabeling and helping with radiolabeling procedures. This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1072), and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT, Republic of Korea (NRF-2013R1A1A2013175, NRF-2015R1D1A1A02061707, and NRF-2016R1D1A1A02937028).
Disclosure Statement
There are no existing financial conflicts.
References
- 1.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001;280:C1358. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9. [PubMed] [Google Scholar]
- 3.Veikkola T, Karkkainen M, Claesson-Welsh L, et al. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 2000;60:203. [PubMed] [Google Scholar]
- 4.Gaertner FC, Kessler H, Wester HJ, et al. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging 2012;39 Suppl 1:S126. [DOI] [PubMed] [Google Scholar]
- 5.Haubner R, Wester HJ. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of anti-angiogenic therapies. Curr Pharm Des 2004;10:1439. [DOI] [PubMed] [Google Scholar]
- 6.Laverman P, Sosabowski JK, Boerman OC, et al. Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging 2012;39 Suppl 1:S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks P, Clark R, Cheresh D. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569. [DOI] [PubMed] [Google Scholar]
- 8.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer 2002;2:83. [DOI] [PubMed] [Google Scholar]
- 9.Knetsch PA, Petrik M, Griessinger CM, et al. [68Ga] NODAGA-RGD for imaging αvβ3 integrin expression. Eur J Nucl Med Mol Imaging 2011;38:1303. [DOI] [PubMed] [Google Scholar]
- 10.Haubner R, Kuhnast B, Mang C, et al. [18F]Galacto-RGD: Synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem 2004;15:61. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Park R, Tohme M, et al. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem 2004;15:41. [DOI] [PubMed] [Google Scholar]
- 12.Mulder WJ, Griffioen AW. Imaging of angiogenesis. Angiogenesis 2010;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beer AJ, Lorenzen S, Metz S, et al. Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: A PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med 2008;49:22. [DOI] [PubMed] [Google Scholar]
- 14.Lee BC, Moon BS, Kim JS, et al. Synthesis and biological evaluation of RGD peptides with the 99mTc/188Re chelated iminodiacetate core: Highly enhanced uptake and excretion kinetics of theranostics against tumor angiogenesis. RSC Adv 2013;3:782 [Google Scholar]
- 15.Yoo JS, Lee J, Jung JH, et al. SPECT/CT Imaging of High-Risk Atherosclerotic Plaques using Integrin-Binding RGD Dimer Peptides. Sci Rep 2015;5:11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song YS, Park HS, Lee BC, et al. Imaging of Integrin alphavbeta3 Expression in Lung Cancers and Brain Tumors Using Single-Photon Emission Computed Tomography with a Novel Radiotracer (99m)Tc-IDA-D-[c(RGDfK)]2. Cancer Biother Radiopharm 2017;32:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberto R, Schibli R, Egli A, et al. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]- in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J Am Chem Soc 1998;120:7987 [Google Scholar]
- 18.Chen B, Zhao G, Ma Q, et al. (99m)Tc-3P-RGD2 SPECT to monitor early response to bevacizumab therapy in patients with advanced non-small cell lung cancer. Int J Clin Exp Pathol 2015;8:16064. [PMC free article] [PubMed] [Google Scholar]
- 19.Jin X, Liang N, Wang M, et al. Integrin Imaging with 99mTc-3PRGD2 SPECT/CT shows high specificity in the diagnosis of lymph node metastasis from non-small cell lung cancer. Radiology 2016;281:958. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Miao W, Li Q, et al. 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: A multicenter study. J Nucl Med 2012;53:716. [DOI] [PubMed] [Google Scholar]
- 21.Groves AM, Shastry M, Rodriguez-Justo M, et al. 18F-FDG PET and biomarkers for tumour angiogenesis in early breast cancer. Eur J Nucl Med Mol Imaging 2011;38:46. [DOI] [PubMed] [Google Scholar]
- 22.Liu S. Radiolabeled Cyclic RGD Peptide Bioconjugates as Radiotracers Targeting Multiple Integrins. Bioconjug Chem 2015;26:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decristoforo C, Santos I, Pietzsch HJ, et al. Comparison of in vitro and in vivo properties of [99mTc]cRGD peptides labeled using different novel Tc-cores. Q J Nucl Med Mol Imaging 2007;51:33. [PubMed] [Google Scholar]
- 24.Shi J, Kim YS, Zhai S, et al. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly(3) and PEG(4) linkers. Bioconjug Chem 2009;20:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akizawa H, Arano Y, Mifune M, et al. Effect of molecular charges on renal uptake of 111In-DTPA-conjugated peptides. Nucl Med Biol 2001;28:761. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Kim YS, Chakraborty S, et al. 2-Mercaptoacetylglycylglycyl (MAG2) as a bifunctional chelator for 99mTc-labeling of cyclic RGD dimers: Effect of technetium chelate on tumor uptake and pharmacokinetics. Bioconjug Chem 2009;20:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhiri A, Slim I, Ghezaiel M, et al. Estimation of Radiation Dosimetry for some Common SPECT-CT Exams. International Journal of Biotechnology for Wellness Industries 2012;1:266 [Google Scholar]
- 28.Stabin M, Taylor A, Jr., Eshima D, et al. Radiation dosimetry for technetium-99m-MAG3, technetium-99m-DTPA, and iodine-131-OIH based on human biodistribution studies. J Nucl Med Jan 1992;33:33. [PubMed] [Google Scholar]
- 29.Mozley PD, Stubbs JB, Plossl K, et al. Biodistribution and dosimetry of TRODAT-1: a technetium-99m tropane for imaging dopamine transporters. J Nucl Med 1998;39:2069. [PubMed] [Google Scholar]
- 30.Leide S, Diemer H, Ahlgren L, Mattsson S. In: Schlafke-Stelson AT. (ed.), In vivo distribution and dosimetry of Tc-99m MIBI in man (CONF-910529–). United States: 1992 [Google Scholar]
- 31.Hammond ND, Moldofsky PJ, Beardsley MR, et al. External imaging techniques for quantitation of distribution of I-131 F(ab')2 fragments of monoclonal antibody in humans. Med Phys 1984;11:778. [DOI] [PubMed] [Google Scholar]