Abstract
Temperature monitoring during critical care provides important data required to guide treatment delivery. Body temperature is an easily quantified clinical parameter that can yield much information concerning the health of an animal. In research settings, temperature has been adopted as a means to judge humane endpoints. Therefore, reliable, noninvasive, and inexpensive methods for temperature monitoring are becoming a necessity in research laboratories. This study aimed to determine the accuracy and agreement of using an infrared camera as an alternative method of temperature measurement in mice and to compare the accuracy of this noninvasive method with established subcutaneous, intraperitoneal, and rectal techniques. Measurement of body-surface temperature by using an infrared camera was compared with these 3 established methods in male NMRI nude mice (n = 10; age, 10 mo); data were obtained 3 times daily over 14 d. Subcutaneous temperatures were measured remotely by using a previously implanted subcutaneous temperature transponder, after which temperature was measured by using noncontact infrared thermometry and a rectal probe. Measurements from intraperitoneal data loggers were obtained retrospectively. The data show that using an infrared camera provides a simple, reliable method for measuring body temperature in male NMRI nu/nu mice that minimizes handling and is minimally invasive. Whether infrared thermometry is a useful method for measuring body temperature in furred mice warrants further investigation.
Abbreviations: BW, body weight; CCC, concordance correlation coefficient; DL, intraperitoneal data logger; IRC, infrared camera; ST, subcutaneous transponder
Monitoring the wellbeing of rodents has become routine after the formulation of the 3Rs principles.33 In addition, monitoring wellbeing has become a standard requirement for animal protection in science. Checking rodents daily represents a challenge in clinical studies because potential distress due to unnecessary manipulations should be avoided.
Body temperature measurement constitutes an important aspect of animal care and is a valuable clinical parameter for judging animal health both in general veterinary practice and in research settings,21,35 particularly for small rodents,9,37 such as rats and mice.1-3,6,8,10,28,30,36,45,46,48 In these species, numerous factors, including genetics,13 age,15 time of day,29,32,46 and husbandry conditions,12-15,29 influence body temperature overall and temperature distribution in the various regions of the body.27,31,42 In addition, the handling required for this assessment may increase body temperature and thus induce an experimental artifact.4,8,42,43 In experimental research, some models have adopted body temperature as an endpoint recommendation,1,8,16,41,43,45 whereas other studies have shown that measuring body temperature can help to evaluate an animal's chance of survival1 and thus to establish predetermined points that guide early termination of the research experiment. In this context, low body temperature is associated with a poor chance of survival,1,3,8,43 a finding that, in combination with other signs of morbidity,3,8 can be used to direct early euthanasia of affected animals to minimize pain and distress3,8,28,41,43,45,46 without loss of data.1
The number of studies using different methods to measure body temperature, each with its own advantages and disadvantages, is increasing. Commonly used methods include rectal thermometry,6,8,27 telemetry systems such as radiotransmitters29,32,43,46 and data loggers (DL),2,48 thermosensitive systems consisting of implanted transponders and external receivers,6,16,28,30,41,43,45 and infrared thermography.5,7,17,23 However, most of these methods require either frequent handling of the animals or anesthesia and surgery,2,4,6,8,28-32,35,41,46,48 and none has proven consistently sufficiently accurate for use in routine or critical care or is suitable for continuous monitoring of body temperature. Therefore, the ability to measure body temperature noninvasively is beneficial.11,12,31,36,40,42,44,45 The noncontact thermal imaging technique using an infrared camera (IRC) is well established in clinical veterinary medicine. Although IRC have been used to determine the body temperature of laboratory animals,5,7,17,23 surprisingly few comparisons between IRC and the more common methods of measuring body temperature are available.4,9,16,31,35,37,45
In the present study, we recorded the body temperature of mice by using an IRC. In addition, we compared these measurements with those obtained by using 3 common devices: rectal probes, subcutaneous transponders, and intraperitoneal data loggers.
The present study aimed to use an IRC to establish a direct and noninvasive method of body temperature measurement and to compare this new method with more common but invasive methods. We sought to demonstrate that an IRC be used as a tool to minimize the burden on mice during body temperature measurements and as a means for routinely evaluating the wellbeing of animals.
Materials and Methods
Animal husbandry and experimentation were conducted in compliance with the Home Office Guidance on the Operations of the Animals Act 1986.18 The experiments performed in this study were approved by the Animal Experiments Inspectorate under the State Office for Health and Social Affairs Berlin (license number B3001g0001). The experiments were performed in accordance with the European Union guidelines for keeping animals, as described in the Animal Welfare Act (EU 2010/63 Article 33, Appendix 3, Part A) and the Guide for the Care and Use of Laboratory Animals.19
Animals.
Male, retired breeder, Rj:NMRI nude mice (age, 10 mo) were purchased from Janvier Labs (albino-Tyrc/Tyrc; Saint-Berthevin Cedex, France). The mice were certified by the vendor to be free of specific rodent pathogens (in accordance with FELASA recommendations), including Bordetella bronchiseptica; cilia-associated respiratory bacillus; Citrobacter rodentium; Clostridium piliforme; Corynebacterium bovis; Corynebacterium kutscheri; Dermatophilus; Encephalitozoon cuniculi; Helicobacter spp.; Klebsiella oxytoca; Klebsiella pneumoniae; Mycoplasma pulmonis; Pasteurellacea, Actinobacillus spp.; Haemophilus spp.; Mannheimia haemolytica; Pasteurella multocida; Pasteurella pneumotropica; Pasteurella trehalosi; Pneumocystis spp.; Proteus mirabilis; Proteus vulgaris; Pseudomonas aeruginosa; Salmonella spp.; Staphylococcus aureus; Streptobacillus moniliformis; Streptococcus β-hemolytic groups A, B, C and G; Streptococcus pneumonia; ectoparasites including fleas, lice, and fur-, surface-, and follicle-dwelling mites; and endoparasites including protozoa (including Entamoeba spp. and flagellates), coccidia, helminths (including cestodes and nematodes). Furthermore, the mice were serologically negative for hantaviruses, lactate dehydrogenase elevating virus, lymphocytic choriomeningitis virus, minute virus of mice, mouse adenoviruses types 1 (FL) and 2 (K87), mouse cytomegalovirus, mouse hepatitis virus, mouse parvoviruses, mouse polyomavirus, mouse rotavirus, mouse thymic virus, ectromelia virus, mouse norovirus, pneumonia virus of mice, reovirus type 3, Sendai virus, and Theiler mouse encephalomyelitis virus.
The mice were individually housed in IVC (polysulfon type II long; model 1285L, Tecniplast, Buguggiate, Varese, Italy) under standard conditions at a room temperature of 22 °C ± 1 °C and relative humidity of 53% to 56%. The experimental animal room was regulated on a 12:12-h light:dark cycle (lights on, 0600 to 1800), with a minimum of 10 air changes hour. Personnel room-entry requirements included gloves, masks, hair caps, laboratory shoes, and clean overalls. The mice were free-choice fed X-irradiated (25 kGy) pellets (NM-1244-703, Ssniff, Soest, Germany) and autoclaved municipal water (Berlin, Germany). The mice were maintained on autoclaved poplar woodchip bedding (Poplar PAB 6, AsBe-wood, Buxtehude, Germany). Bite bricks consisting of aspen wood (Populus tremula gnawing sticks, size S, catalog no. NGS E-021, ABEDD, Vienna, Austria), nesting material composed of hemp (catalog no. H3279-10, Eco-hemps, Ssniff), and a red mouse tunnel (Plexx, Elst, Netherlands) were provided as environmental enrichment. A chrome running wheel (diameter, 12 cm; Heimtier-Land, Nürnberg, Germany) was placed free-standing in the IVC. The mice were acclimated to the animal facility for at least 1 wk before being used in experiments. The mice were habituated to cup handling for 1 wk before the temperature measurements started. For measurements, the experimenter transferred the mice by hand to a separate cage without a lid. Once in the cage, the mice were offered a small amount of oatmeal (Viva vital, Netto, Maxhütte-Haudhof, Germany) in the cage as positive reinforcement and a comforting distraction. Then the mice were transferred to a table top to allow for free movement, handling training, and restraint for rectal temperature measurement. Once weekly, each mouse was placed in a clean IVC and received a new water bottle after temperature readings were obtained.
Equipment.
Subcutaneous programmable temperature transponders (ST; diameter, 2.2 mm; length, 14 mm; mass, 0.12 g; IPTT-100, Bio Medic Data Systems, Seaford, DE) were inserted according to the manufacturer's instructions. The ST had a manufacturer-certified range of 32.0 to 42.0 °C, with a temperature resolution of 0.1 °C and an accuracy of 0.4 °C from 35 to 39 °C and 1.0 °C from 32 to 42 °C. A handheld portable pocket scanner (DAS-7007S, Bio Medic Data Systems) was used to scan the ST identification number and subcutaneous temperature.
In addition, intraperitoneally implanted data loggers (DL; diameter, 6 mm; length, 17 mm; mass, 1 g; DST Nano–T Temperature Recorder, Starr Oddi, Gardabaer, Iceland) were used. They had a temperature range of 5 to 45 °C, a resolution of 0.032 °C, and an accuracy of ±0.2 °C. Mercury software and a Wizard interface box (both from Starr Oddi) were used for programming in Windows. All time clocks (IRC, ST, DL) were synchronized throughout the entire process. Ten days before implantation, the real-time clock in the DL was synchronized with the computer time and programmed according to the user manual from Mercury Graphic Software. The manufacturer stated an accuracy of ±1 min per mo. The DL were set to perform one temperature measurement every 1 min.
An additional unimplanted ST and DL were placed in an empty IVC during the entire experiment, to assess their measurement accuracy. These additional data were compared with the readings from a room temperature monitoring system. Because the manufacturers reported that both the ST and DL were less accurate in the room-temperature range than in the body-temperature range, the data were not used for analysis.
A rectal probe thermometer (model 5885, Precision Digital Thermometer with PRT [platinum resistance thermometer], H Tinsley, New Addington, United Kingdom) provided a temperature accuracy of 0.01 °C. The rectal probe was calibrated prior to the start of the experiment, a comprehensive function control (calibration) was done on day 8, and the probe was routinely calibrated by measuring a fluid sample once every morning before the readings were taken in mice.
A noncontact IRC (model T660, FLIR Systems, Wilsonville, OR) with 640 × 480 pixel infrared resolution and a thermal sensitivity of less than 20 mK at 30 °C was set according to the manufacturer's instructions. The IRC has a spatial resolution of 0.68 milliradians and image frequency of 30 Hz and was used at a focal length of 25 mm. The accuracy stated by the manufacturers was ±1 °C for the target temperature range. The IRC was calibrated by the manufacturer; it also had a self-calibration function that could be applied during use. To produce measurements under standard conditions, infrared temperature was measured with settings as follows. An emissivity correction value of 0.98, specified for human skin in the manufacturer-provided emissivity table, was used for each measurement. Automatic image adjustment was used continuously. For the entire experiment, the IRC was mounted on a tripod (model 190X Aluminum 3-Section tripod with model 496RC2 Ball Head and model 322RC2 additional adapter, Manfrotto, Vicenza, Italy). It was positioned 50 cm above a clean and dry cage (Macrolon II, Tecniplast) without a lid. The temperature data were recorded on a memory card in radiometric CSQ format. The infrared videos were transferred and analyzed by using thermal camera software (FLIR Tools+ version 5.3.15320.1002, FLIR Systems).
The body weight (BW) of mice was measured automatically by using a calibrated balance (model PG2002-S, Mettler-Toledo, Giessen, Germany).
Implantation surgery.
All surgeries were performed by the same experienced veterinary surgeon on the same day. DL and ST were implanted in the mice 6 d prior to the start of the experiment. The mice were anesthetized by inhalation of 3% isoflurane (100% V/V Forene, AbbVie Deutschland, Ludwigshafen, Germany) with oxygen for surgery, in an anesthetic chamber with a sliding cover. To ascertain the depth of anesthesia, the pedal withdrawal and eyelid reflexes were monitored. The mice were transferred to a surgery tablet with a mouth inhalation adapter (nose cone) and then positioned on their backs. After the righting reflex had vanished, eye ointment (Artelac Splash MDO, Bausch and Lomb, Berlin, Germany) was administered to both eyes. The skin on the left flank was disinfected for injection by using 0.4 mL isopropyl alcohol prep pads (60 mm × 30 mm, catalog no. 999979, Paul Hartmann, Heideheim, Germany). All mice were injected subcutaneously with 0.4 mL Lactated Ringers Solution (Serag-Wiessner, Naila, Germany) containing 0.2 mg (5 mg/kg) caprofen (Rimadyl, Pfizer Animal Health, Louvain-la-Neuve, Belgium), a NSAID analgesic. The injection was administered subcutaneously in the flank region approximately 5 min prior to surgery. To disinfect the incision site on the abdomen, a solution containing 50% 2-propanol and 1% povidone–iodine (Braunoderm, B Braun Melsungen, Melsungen, Germany) was used. A sterilized scalpel (model 1001/A/a with model 1001/ST/0 surgical blades, Otto Rüttgers, Solingen, Germany) was used to make a 10-mm incision in the skin parallel to the region of the linea alba. The skin was separated from the peritoneum, and the peritoneal cavity was opened. A freely moving, sterilized DL was placed in the peritoneal cavity by using a pair of forceps. The peritoneal cavity was closed with 2 or 3 stitches of polyglycolic acid suture (4-0, with needles, Johnson and Johnson Medical, Norderstedt, Germany). The skin was closed by using 2 or 3 Michel suture clips (7.5 × 1.75 mm, Aesculap, Tuttlingen, Germany). The mouse was taken from the nose cone, and the neck region was disinfected by using 0.4 mL isopropyl alcohol prep pads.
The ST was implanted subcutaneously between the shoulder blades by using a needle applicator. The skin was closed by using tissue adhesive (blue Histoacryl, B Braun Surgical, Rubin, Spain). The mouse then was placed on a heating pad until it began to move. After the ST was inserted, the identification code and the measurement of subcutaneous temperature were tested. The first subcutaneous temperature measurements were taken immediately after anesthesia, but subcutaneous temperature data from the day of implantation were not used in the evaluation of the results. The mouse was transferred to a clean IVC home cage in a quiet, darkened recovery room. All mice were fully conscious and ambulatory not later than 40 min after surgery and were monitored closely by a veterinarian for 120 min in total.
Temperature measurement.
The temperature measurement procedure including weighing (measurement of subcutaneous temperature, infrared temperature, rectal temperature, and BW) was performed 3 times daily for 14 d. Thus, each of the 10 mice was handled 3 times daily (from 0700 to 0800, 1300 to 1400, and 1900 to 2000). On day 15, the mice were measured once in the morning between 0700 and 0800. The animals were handled and measured by the same veterinarian throughout the entire experiment.
First, the mouse was picked up by using the cup method and held within 2 cm of the ST scanner, and the subcutaneous temperature was obtained. Second, the mouse was placed in a separate lidless procedure cage for measurement of BW, after which the IRC recorded a 10-s video, with automatic focus on the mouse. Third, the mouse was transferred to a table and placed in such a way that it was unable to cling to anything, to prevent additional heat production due to muscle tension. The mouse stood relaxed on the table, lightly restrained by the veterinarian's grasping of the mouse's tail root and hips by using 4 fingers of one hand. The rectal temperature was taken by using a lubricated probe inserted 1 to 1.5 cm into the rectum, which was left in this position until a stable rectal temperature reading was obtained (approximately 15 s). The mouse then was returned to the home cage. The rectal probe was checked for blood traces by using a white handkerchief, cleaned with sterile water, and lubricated again for the next rectal measurement. After temperature readings were obtained from all 10 mice, the rectal probe was cleaned by using 70% alcohol.
After the measurement session on day 15, the mice were anesthetized by inhalation as described earlier and then euthanized by cervical dislocation for pathology. The DL was removed from the abdomen for data collection. The identification number of the DL was retrieved automatically. The subcutaneously implanted ST was removed also, with the integrity of the outer shell maintained.
Data collection.
For sample-size calculation, we used the concordance correlation coefficient (CCC) and calculated the number of samples necessary to yield a CCC of 0.3 with α = 0.05 and β = 0.9. This analysis revealed that we needed a sample size at least 344 measurements. To minimize the total number of animals, we decided to implant the temperature measurement systems (ST, DL) in 10 mice. This plan required repetition of measurements to generate sufficient data for comparison of the temperature measurement methods. Because we had to measure the same mice repeatedly and because we had only limited preliminary information on the expected CCC, we decided to increase the number of measurements to 430. Anticipating that 10% of the data would be lost if one of the 10 implanted systems (ST, DL) failed and that other smaller-scale failures of individual measurements might occur, the total number of measurements was set to 432. To limit the duration of the study, we decided to measure the temperature of the mice 3 times a day.
Data were collected from each of the 10 mice at each time point, 3 times daily over 14 d and once on day 15 morning and recorded. Data collection of ST temperatures (n = 430) with ST identification number and BW (n = 430) were inserted automatically and saved daily in an Excel spreadsheet for each day and for all 3 measurements. The rectal temperatures (n = 369) were recorded and saved to a Microsoft Excel spreadsheet. Infrared videos (n = 430) from the IRC were imported and analyzed by using FLIR Tool+ Software. For each measurement, the maximal temperature in the 10-s infrared video was extracted and transferred to the Excel spreadsheet.
The removed DL were connected to the interface box, and the intraperitoneal temperature data from the DL were exported by using Mercury software and transferred to an individual Microsoft Excel spreadsheet for each mouse. Although the DL produced temperature measurements every minute throughout the entire experimental period, only the intraperitoneal temperature values (n = 430) at the times of the other temperature measurements (subcutaneous, infrared, rectal) were used for statistical analysis. All of the data were collected into a single Excel spreadsheet for analysis.
Statistical analysis.
The results were analyzed by using SPSS (version 24, IBM, Armonk, NY). The statistical analysis included descriptive statistics for the temperature and BW data and a check for normality (Kolmogorow–Smirnow test for normality, histogram, QQ-plot, boxplot) for each mouse and each measurement model (ST, DL, IRC, rectal probe). Correlations among the different measurements were assessed by using the Pearson correlation coefficient as well as the CCC. Bland–Altman plots were used to compare the results of the rectal, subcutaneous, and intraperitoneal temperatures with the infrared temperature. ‘Yellow card zone’ outliers were those for which the distance between the outlier and interquartile range box was greater than 1.5 times the length of the interquartile range box; ‘red card zone’ outliers were those for which the distance between the outlier and interquartile range box exceeded 3 times the length of the interquartile range box.
To evaluate how well infrared thermography by IRC predicted the temperature obtained from the other measurements, mixed linear-regression models were implemented by using infrared temperature and BW as independent fixed factors, mouse as a random factor, and one of the measurements (rectal, subcutaneous, or intraperitoneal temperature) as the dependent factor. We decided to include BW as an influencing factor in each model, to control for it as a possible confounder. Interactions between infrared temperature and BW were included in the initial model but were removed when nonsignificant. Because several observations originated from each mouse, the data could not be regarded as completely independent. Including mouse as a random factor accounted for this effect and controlled its influence in the model. We considered P values less than 0.05 as statistically significant.
Although the same number of measurements was obtained for each of the subcutaneous, infrared, and intraperitoneal temperature methods, 62 rectal temperature measurements could not be taken as planned due to technical difficulties or animal welfare reasons and resulted in missing data. In particular, rectal temperature measurements were omitted due to technical issues (calibration) with the probe on day 8 and for animal welfare reasons (traces of blood on the probe) on other days.
Results
Table 1 gives descriptive statistics for BW and data from each temperature measurement technique for each NMRI nude male mouse. Figure 1 shows box-and-whisker plots of the temperature data for each mouse. The BW (n = 430) of the mice (n = 10) ranged from 34.1 to 47.9 g (mean ± 1 SD, 39.7 ± 3.3 g) over the course of the experiment.
Table 1.
The descriptive statistics of tests based on normal distribution of temperatures measured by using an infrared camera, rectal probe, subcutaneous transponder, or intraperitoneal data logger in each mouse (n= 10)
| Temperature (°C) |
||||||
| Mouse | BW (g) | Infrared | Rectal | Subcutaneous | Intraperitoneal | |
| 1 | n | 43 | 43 | 38 | 43 | 43 |
| Mean (1 SD) | 42.22 (1.04) | 37.31 (0.46) | 36.66 (0.82) | 36.33 (0.50) | 35.31 (0.84) | |
| Range | 39.95–43.52 | 36.02–38.13 | 34.81–38.20 | 35.30–37.50 | 34.00–37.21 | |
| 2 | n | 43 | 43 | 41 | 43 | 43 |
| Mean (1 SD) | 40.79 (0.51) | 37.44 (0.53) | 36.84 (0.79) | 36.07 (0.39) | 35.80 (0.55) | |
| Range | 39.82–41.92 | 36.00–38.47 | 34.88–38.88 | 35.20–36.90 | 34.43–37.00 | |
| 3 | n | 43 | 43 | 38 | 43 | 43 |
| Mean (1 SD) | 46.30 (1.27) | 36.57 (0.58) | 36.45 (0.70) | 36.30 (0.36) | 35.84 (0.54) | |
| Range | 43.38–47.90 | 35.46–37.93 | 34.43–37.60 | 35.70–37.00 | 34.07–37.06 | |
| 4 | n | 43 | 43 | 39 | 43 | 43 |
| Mean (1 SD) | 36.85 (0.63) | 37.40 (0.48) | 37.02 (0.75) | 36.81 (0.39) | 36.44 (0.50) | |
| Range | 35.58–38.43 | 36.41–38.42 | 35.29–38.12 | 36.20–37.80 | 35.20–37.42 | |
| 5 | n | 43 | 43 | 40 | 43 | 43 |
| Mean (1 SD) | 37.51 (0.70) | 37.51 (0.50) | 36.80 (0.83) | 36.75 (0.56) | 36.23 (0.58) | |
| Range | 36.07–38.68 | 36.60–38.47 | 34.34–38.60 | 35.40–37.70 | 34.90–37.31 | |
| 6 | n | 43 | 43 | 39 | 43 | 43 |
| Mean (1 SD) | 42.85 (1.16) | 37.00 (0.48) | 36.67 (0.62) | 39.33 (0.45) | 35.96 (0.48) | |
| Range | 38.91–44.36 | 35.98–38.21 | 35.18–37.62 | 38.50–40.30 | 34.87–37.09 | |
| 7 | n | 43 | 43 | 34 | 43 | 43 |
| Mean (1 SD) | 36.84 (0.67) | 37.53 (0.51) | 36.87 (0.64) | 36.62 (0.38) | 36.05 (0.56) | |
| Range | 35.61–38.31 | 36.40–38.64 | 34.35–38.10 | 35.50–37.50 | 34.42–37.14 | |
| 8 | n | 43 | 43 | 39 | 43 | 43 |
| Mean (1 SD) | 35.18 (0.52) | 38.05 (0.53) | 37.54 (0.50) | 40.82 (0.62) | 36.50 (0.57) | |
| Range | 34.14–36.18 | 36.94–39.28 | 36.26–28.45 | 39.70–42.10 | 35.32–37.76 | |
| 9 | n | 43 | 43 | 40 | 43 | 43 |
| Mean (1 SD) | 39.77 (0.58) | 37.15 (0.46) | 36.54 (0.80) | 37.26 (0.50) | 36.04 (0.50) | |
| Range | 38.65–41.00 | 36.03–38.36 | 34.01–37.92 | 36.20–38.60 | 34.54–36.75 | |
| 10 | n | 43 | 43 | 21 | 43 | 43 |
| Mean (1 SD) | 38.91 (1.00) | 37.63 (0.59) | 36.55 (0.71) | 36.47 (0.54) | 35.32 (0.67) | |
| Range | 37.11–41.32 | 36.23–38.69 | 35.41–38.05 | 35.00–37.30 | 33.21–36.31 | |
| Overall n | 430 | 430 | 369 | 430 | 430 | |
| Mean (1 SD) | 39.72 (3.32) | 37.36 (0.63) | 36.80 (0.78) | 37.27 (1.55) | 35.95 (0.70) | |
| Range | 34.14–47.90 | 35.46–39.28 | 34.01–38.87 | 35.00–42.10 | 33.21–37.76 | |
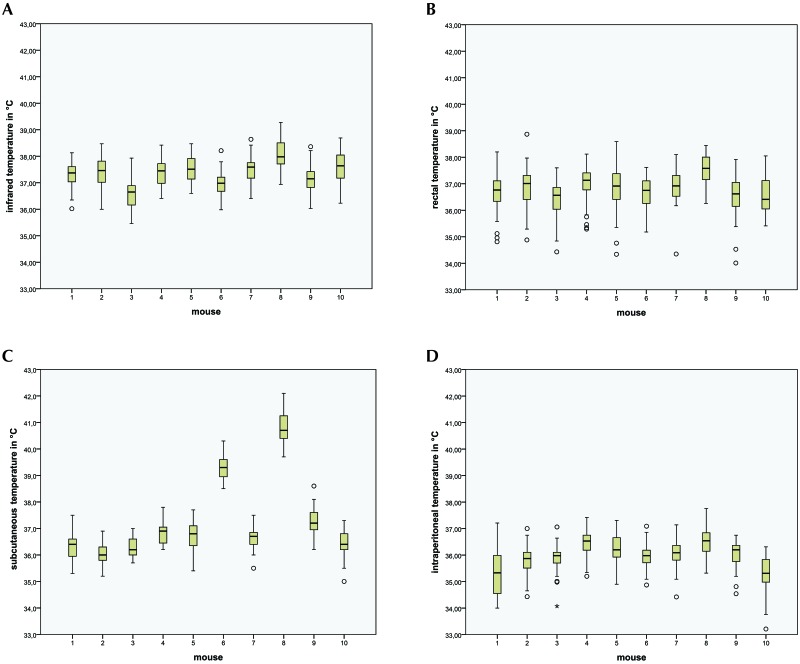
Figure 1.
Box-and-whisker plots of temperature data from 10 male NMRI mice. Box, 25% to 75% of values (quartile); whisker, range within 1.5 interquartiles; line, median value; °, `yellow card zone` outlier (distance between the outlier and box is greater than 1.5 times the interquartile range); *, `red card zone` outlier (distance between the outlier and box is greater than 3 times the interquartile range). Temperatures from (A) infrared thermography (n = 430), (B) rectal probe (n = 369), (C) subcutaneous transponder (n = 430), and (D) intraperitoneal data logger (n = 430).
The results show how mouse BW influences temperature readings, depending on the measurement method. For the rectal and infrared temperature methods, the distributions within mouse were quite similar, and the means differed only marginally. Intraperitoneal temperatures showed lower median values, whereas subcutaneous temperature showed substantially higher median values for 2 mice. The temperature values were normally distributed for each temperature measurement technique in each individual mouse.
More specifically, infrared temperature measurements (n = 430) within each mouse were similar and had small standard deviations (mean, 36.57 to 38.05 °C; 1 SD, 0.46 to 0.59 °C); the overall standard deviation was slightly higher (0.63 °C; Table 1). The boxplots for infrared temperature of the mice show 4 ‘yellow card zone’ outliers (Figure 1 A) the distance between the outlier and box is greater than 1.5 times the interquartile range.
The number of rectal temperature measurements (n = 369) per mouse differed from the numbers of infrared, subcutaneous, and intraperitoneal temperatures and, as noted in the Methods section, some rectal temperatures were unavailable for various reasons. The standard deviations for rectal temperature within each mouse differed (1 SD, 0.50 to 0.83 °C), and the overall standard deviation was quite large (0.78 °C; Table 1). The boxplots of rectal temperature show a total of 15 outliers in the yellow card zone (Figure 1 B).
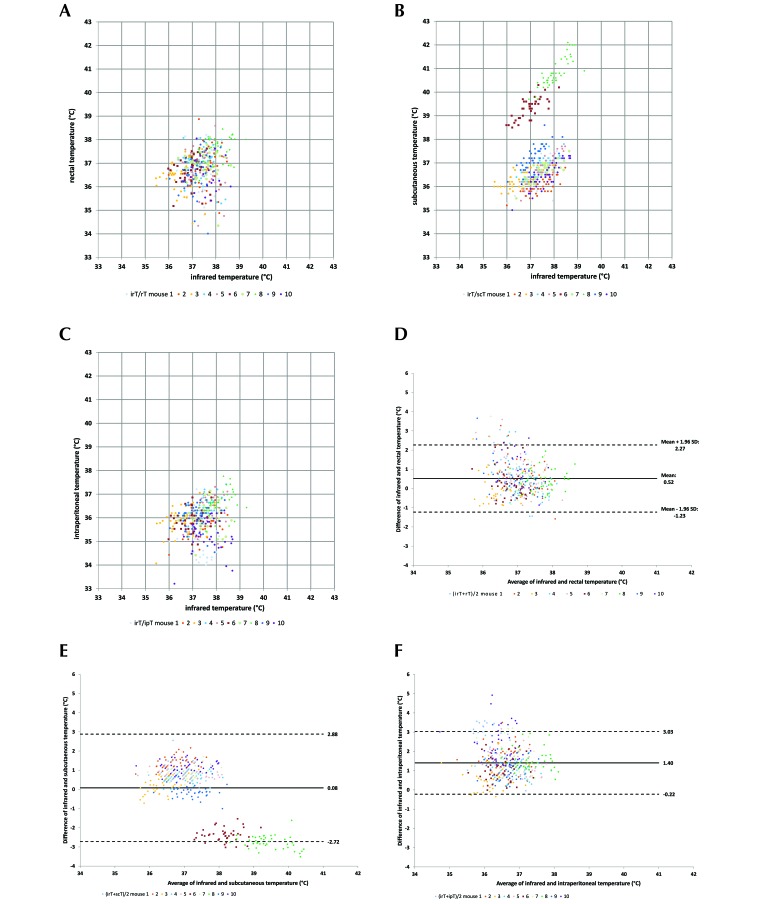
The comparison of infrared temperatures with rectal temperatures showed a CCC of 0.160 and a mean difference of 0.56 °C (1 SD, 0.89°C; Figure 2 A). Because these values do not account for repeated measurement of the same mice, a linear mixed-regression model was used. The analysis showed that BW had a significant influence on rectal temperature (P < 0.001), but the influence on infrared temperature was nonsignificant (P = 0.070). Estimation of the fixed effect with BW and infrared temperature as random effects and rectal temperature as the dependent variable found that an increase of infrared temperature by 1 °C caused an increase of rectal temperature of 0.13 °C, whereas a 1-g increase in BW increased rectal temperature by 0.21 °C. The random effect of mouse accounted for approximately 63.0% of the variance. These results show that rectal temperature varied markedly between mice and that this variation was independent of BW and infrared temperature.
Figure 2.
Bland–Altman plots comparing the temperature measured by using an infrared camera (IRC) and the compared with (A) the rectal temperature measured by using a rectal probe, (B) the subcutaneous temperature measured by using a transponder, or (C) the intraperitoneal temperature by using a data logger (n = 10 mice × 43 measures). Data points represent the values measured by using 2 methods; each mouse is represented by a different color. Structure of a Bland–Altman plot comparing temperatures obtained with the 2 compared methods. Scatterplots present the results of the differences between the values of the 2 methods on the y-axis and the average of the paired values of the 2 methods on the x-axis. The infrared temperature obtained by using the IRC is compared with the (D) rectal temperature by rectal probe, (E) subcutaneous temperature by using a transponder, and (F) intraperitoneal temperature by using a data logger.
The subcutaneous temperature (n = 430) measurements had the largest range, from 35.00 to 42.10 °C (mean, 37.27 °C). The standard deviations of subcutaneous temperature within each mouse were small (0.36 to 0.62 °C), but the overall standard deviation was large (1.55 °C; Table 1). The boxplots for subcutaneous temperature showed that mice 6 and 8 had higher mean values than the others (Figure 1 C). The descriptive statistics changed substantially when the ST of mice 6 and 8 were omitted (n = 344; mean, 36.58 °C; range, 35.0 to 38.20 °C). The subcutaneous temperature boxplots show a total of 3 yellow-zone outliers (Figure 1 C). The CCC of infrared temperature compared with subcutaneous temperature was 0.270 and thus slightly higher than the value for rectal temperature. The mean difference of both values was only 0.08 but had a large standard deviation of 1.43 due to the 2 mice with anomalously high values of subcutaneous temperature (Table 1). The mixed linear-regression model revealed that BW, infrared temperature, and their interaction all had significant effects on subcutaneous temperature (P < 0.001 for each). Estimation of the fixed effect with BW and infrared temperature as random effects and subcutaneous temperature as a dependent variable found that an increase of infrared temperature by 1 °C caused an increase of subcutaneous temperature of 2.21 °C, whereas a 1-g increase in BW increased subcutaneous temperature by 1.41 °C. The mouse factor accounted for 95.6% of the data variation and thus was an important random factor.
The intraperitoneal temperature measurement (n = 430) from the DL ranged from 33.21 to 37.76 °C, and the standard deviations differed markedly across mice (0.48 to 0.84 °C), so that the overall standard deviation was large (35.95 ± 0.70 °C, Table 1). The boxplots show that the average temperature levels were lower for intraperitoneal temperature than for the other methods, with a total of 11 outliers in the yellow zone and 1 in the red zone (Figure 1 D). The difference between the intraperitoneal and infrared temperatures averaged 1.4 °C (1 SD, 0.83 °C) with a CCC of 0.07 (Figure 2 F). In addition, BW (P = 0.050), infrared temperature (P = 0.017), and their interaction (P = 0.048) all showed significant influence on intraperitoneal temperature, although the influence of BW was at the boundary of significance. Estimation of the fixed effect with BW and infrared temperature as random effects and intraperitoneal temperature as a dependent variable found that an increase of infrared temperature by 1 °C caused an increase of intraperitoneal temperature of 1.41 °C, whereas a 1-g increase in BW increased intraperitoneal temperature by 1.08 °C. The mouse factor accounted for 28.4% of the variation, which was the lowest value among the 3 invasive measurement techniques.
Discussion
This study investigated whether noninvasive body temperature measurement in mice by using an IRC is valid method compared with other, more common methods of measuring temperature. The measurements for all techniques (subcutaneous, infrared, rectal, and intraperitoneal) were taken at almost exactly the same times.
In the past, rectal temperature has been used as the standard method for measuring body temperature.8 This was the reason for making a comparison between rectal temperature and other measurement methods in this study. The rectal temperature method has a number of serious disadvantages, including the necessity of restraining the animal and the use of a lubricated (oiled) rectal probe. Together these procedural necessities increase the risks of diarrhea and rectal prolapse, as well as the risk of infections due to perforation of the rectum by the probe.8,31 We checked the rectal probes for traces of blood after each measurement. When blood was present, the follow-up measurement was not performed due to animal welfare concerns. As a consequence, the rectal measuring method had fewer measurements (n = 369) than each of the other methods (ST, IRC, DL; Table 1). None of our mice showed clinical signs of rectal damage, such as diarrhea, infection, and loss of BW. The data generated by using rectal probes were unevenly distributed and covered a wide temperature range. The variation among individual mice was large, but intraindividual fluctuations were moderate (Table 1). This observation is an indicator of errors in rectal temperature measurement.10 Moreover, rectal measurement produced the highest number of outliers of any technique (Figure 1 B). We did not detect any increase in rectal temperature associated with handling of the mice, but the frequent and repeated measurements clearly were moderately stressful.
The comparison between rectal temperature and temperature measured by IRC did not show a significant correlation (Figure 2 A). The rectal and infrared temperatures increase at different rates. The rectal temperature does not correspond to the infrared temperature, and the influence of BW differed between infrared temperature as for rectal temperature. In this study, measuring temperature by using a rectal probe was a suboptimal standard temperature measurement procedure whose results failed to correspond to other studies.8,10,37
The implanted ST measurement system provides the advantage of technical ease in obtaining the temperature read-outs and the identification of the mouse.4,6,9,27,28 However, the frequency of handling16,42,43 must be regarded as a source of possible error during ST measurement. The signals are received through an antenna, and the device relies on energy supplied by the scanner; therefore, the mouse needs to be held within the range of the scanner. In addition, an animal's temperature might be affected by handling-associated stress and thus may not reflect the temperature measured at rest.10,31 The intraanimal standard deviations of the subcutaneous temperatures were very small, as might be expected for an implanted system. However, 2 of 10 ST produced measurements considerably above the temperature ranges of the others (Figures 1 C and 2 B). These ST deviations were systematic and thus not identified as outliers.4,6,9,31,41,42,45 The physiologic temperature of mice in the literature is reported to be 37.0 to 37.2 °C,20 and these temperatures should serve as a reference for healthy mice. The 2 anomalous ST (mice 6 and 8) appeared to show an incorrect temperature range (Figure 1 C). The discrepancy cannot be explained by the manufacturer's information on the temperature accuracy, which is given as 1.0 °C. Therefore, calibration of temperature-sensitive ST prior to an experiment is essential to exclude such erroneous effects.4,6,41
For measurement methods involving ST or DL, surgery generally is required. This requirement carries an increased risk for infections,4,6,31 and represents a burden due to the anesthesia.4,28,40 In some studies, ST have been implanted without anesthesia because the manufacturer did not consider it necessary.43 The adult male NMRI nude mice used in this study underwent surgery without complications, as in other studies.6 However, when an IRC is used, the risks of surgery do not exist.9,31,45
When interpreting temperature data collected by implanted systems, the implantation site should be considered.4,27,31,37 Temperature can vary widely between body regions.13,34,42 Others show a day–night rhythm in the analyzed temperature data,24,46 and several external factors such as handling may influence the measured temperature.8,10,16,43 To deal with these variations, temperature curves have been generated by using continuous measurement with DL.2 The DL system we used here measured temperature once every minute, and the DL software used oversampling and averaging to increase the resolution of the measured data. However, the calculated intraperitoneal temperature values seemed abnormally low (Figure 1 D), and apparently the DL processing is unsuitable for showing a stress-related influence on body temperature (Figure 2 F). The derived intraperitoneal temperature values were only partially comparable with the subcutaneous, infrared, and rectal temperature data, and the calculated intraperitoneal temperatures were not even in the physiologic range of mice.4,38,42,48 For most animals, the intraperitoneal temperature data showed a moderate standard deviation (0.70 °C), but the standard deviation for mouse 1 was so large that it substantially raised the overall standard deviation for the group (Table 1). In addition, the DL system produced the largest number of outliers, including one extreme outlier (Figure 1 D). A further disadvantage of the DL system is the need for intraperitoneal implantation, which requires invasive surgery.22 Especially in lines of small mice, there is the hazard of complications due to implanted foreign material.29 However, such complications did not occurred within the current study. Yet another disadvantage is that the DL could not be fixed in the peritoneum, raising the possibility that it could move, thereby compromising the measurements and potentially damaging tissues. Finally, DL could only be read after removal from the body, thus making it unusable for monitoring the ongoing status of an animal. Taking all these considerations into account, we do not recommend the use of DL for the measurement of body temperature in mice.42,48
The noninvasive infrared measurement method does not have the earlier mentioned disadvantages of the other methods. It is a noncontact procedure, needing no surgery or restraint of the animal. Our findings indicate that the published physiologic body temperatures of mice, ranging from 37.0 °C to 37.2 °C,20 can be reproduced through infrared thermography. Previous reports have established the dorsal skin temperature of nude mice at 37.2 ± 3 °C,13 and the IRC system produced temperature data similar to these in the current study (Table 1). In addition, the IRC showed favorably accurate temperature readings compared with other methods in rodents.3,34,45
Physiologically, body temperature is subject to fluctuations. Numerous variables affect thermoregulation and need to be considered in the analysis of infrared thermograms. Many animal housing situations create microclimates, which may influence on the mouse's surface temperature and consequently measurement results. Artifacts such as cold or hot spots in the thermography images can be caused, for example, by moisture from the environment, on the animals, or on their fur or skin.13,42 Some sources of error in the form of wet regions or radiation of enrichment materials have been circumvented by measuring infrared temperature in animals in a separate cage. An alternative procedure would involve removing all materials from the home cage, but doing so might stress animals and would prolong the measurement process. Therefore, other studies recommend allowing the animals to become accustomed to the measuring situation and to handling.4,42,43 The transfer of animals to a separate cage, which is used in rodents to measure BW, makes the infrared technique workable even when mice are group-housed. In the present study, to ensure that all infrared measurements were made in the same way, we placed mice individually in a separate dry and clean cage for infrared thermography. An additional factor that might affect infrared temperature measurements is the single housing of the mice. The adult male mice in our study were housed individually, because as retired breeders that could not be associated with other mice. In groups, mice can control their thermoregulation by means of social interaction such as cuddling,15 but they also can experience altered local skin temperature due to injuries from fighting.
The use of IRC as a valid temperature-measuring instrument requires some refinement regarding measurement technique. An IRC with sufficiently high resolution is necessary, especially when the system is used for mice. The smaller and faster the measured object moves, the higher the resolution of the IRC needs to be. Other authors working with rodents have used IRC with lower pixel resolution or number,5,23,35,36,40 but our present results indicate that a resolution of 640 × 480 is a prerequisite for measuring valid temperatures.7,11,16,27,36 Future projects will benefit from the rapid technical development of new IRC. According to the results of our current study, we conclude that the fluctuations of an IRC as a measuring device are not large enough to compromise its validity.
The set up for infrared measurement in this study was standardized. Standardization not only improves the quality of infrared temperature but also improves its reproducibility. A practical measurement setup allows daily use. To ensure reliable and reproducible temperature measurements from an IRC, the use of a tripod7,11,14,17 rather than a handheld device is helpful, because of the consistency it entails.12 Autofocusing is beneficial.44 Another way of increasing consistency and reducing stress is to offer animals food treats during infrared recording.
A further methodologic requirement for getting reproducible infrared temperatures is a correct emissivity setting in the IRC. Emissivity is a factor that corrects material-dependent differences between measured radiation and calculated infrared temperature. Some studies have used emissivity values between 0.957,12,44 and 1.0;5,44 the value for the skin of a nude mouse lies in a similar range. In an ideal scenario, the emissivity value would be determined for each mouse individually, but practical issues make this ideal infeasible, which is why an emissivity value of 0.98 for all measurements of this study was used as the standard.11,12,27,45,47
Other studies have performed infrared temperature measurement by using single infrared images.5,7,17,23,35 In our study, infrared temperature was calculated from a 10-s infrared video. The maximal temperature in the infrared videos was used for the analysis, irrespective of the body region of the mouse.44 A 10 s recording at 30 frames per second produces 300 infrared images per video. The 10 s video per infrared measurement of mouse should have given a high statistical chance of producing the correct infrared temperature value.
Other studies have used values averaged over regions of measured infrared images.5,7,17,23,35 To make our work reproducible and practical, we decided to avoid complicate banks of the thermogram analysis. The standardized settings allowed a field of view with a single mouse in a separate cage. Because temperature measurement needs to be simple, we used the maximum value from the infrared videos was used for analysis.23,44 The maximal value of infrared temperature mirrors the highest heat radiation from the animal, irrespective of the body region.
In comparison with the other temperature-measuring methods, the superiority of the contact-free infrared thermography measurement lies in its reduced invasiveness, without compromising validity. The radiation is influenced by the entire body. It is known that there is a connection between body size and body surface.25,26 The study showed a significant relation between BW and infrared temperature. This influence on the BW was not significant in the other methods (subcutaneous temperature, rectal temperature, intraperitoneal temperature). Furthermore, the thermal potential of the body is dependent on the body mass and its energy balance. The impaired health status or physical condition of an individual mouse may lead to hyperthermia or hypothermia.1,3,30,43
In addition to comparing the various temperature-measuring procedures, the current study examined the correlation between physiologic temperature increases in nude mice and the range of the measured temperatures. Comparison of the temperature data measured by using an IRC compared with a ST is the only evaluation that demonstrated temperature fluctuations within the physiologic range9 (Figure 2 B). The rectal temperature4 and intraperitoneal (that is, DL) temperatures48 proved to be less suitable for showing these physiologic fluctuations of body temperature (Figure 2 A and C). The current study only considered healthy adult NMRI nude mice, which is one of the most frequently used mouse lines in oncologic research. Differences associated with an animal's sex or genetics14,34 were not examined in the current study and should be considered in further studies.
To achieve the objective of introducing the infrared thermography as a standard method, additional investigations are required for validation in haired mice.13,42 In addition, the potential for sex-, genetics-, or age-associated differences should be investigated in follow-up studies.
The advantage of the measurement of temperatures by using the noninvasive IRC is the recording of direct temperatures under standard conditions during the husbandry of laboratory animals.39 The infrared method is a fast and stress-free measuring tool. Infrared temperature measurement requires neither surgical procedures nor implantation of foreign material into the mouse. IRC deliver temperature measurement results of comparable validity compared with those of the ST and rectal probes. The measurements from the IRC demonstrate a similar distribution to the temperature data. The temperatures measured by infrared thermography had a small standard deviation compared with the other methods tested (Table 1), not only for individual animals but across all mice. Compared with the other measuring systems, the IRC produced few outliers (Figure 1 A).
In summary, we conclude that infrared thermography is suitable for measuring the body temperature of NMRI nude mice and is as valid as the rectal and subcutaneous temperature-measurement methods. Compared with other methods, thermography by using IRC is much gentler for mice, thus representing an applied refinement in line with 3Rs concepts. In addition, we recommend using IRC thermography during routine checks of laboratory rodents. Further investigation to assess whether IRC thermography yields valid results from furred mice is warranted.
Acknowledgments
We wish to thank numerous people on the animal care team: Jens Sandau for animal ordering, Sven Kirstein and Maike Conerus (Bayer AG, Berlin) for all animal care, Natascha Manovski for assistance during surgery, and Oliver Schmücker (Bayer AG, Berlin) for providing essential equipment. We also thank the working group of Professor Dr Regitz-Zagrosek (CCR-Charité, Berlin) for the loan of the rodent running wheels and Monique Beyer (UNO Roestvastaal BV) for her help in programming the data loggers.
References
- 1.Adamson TW, Diaz-Arevalo D, Gonzalez TM, Liu X, Kalkum M. 2013. Hypothermic endpoint for an intranasal invasive pulmonary aspergillosis mouse model. Comp Med 63:477–481. [PMC free article] [PubMed] [Google Scholar]
- 2.Arata S, Watanabe J, Maeda M, Yamamoto M, Matsuhashi H, Mochizuki M, Kagami N, Honda K, Inagaki M. 2016. Continuous intake of the chaga mushroom (lnonotus obliquus) aqueous extract suppresses cancer progression and maintains body temperature in mice. Heliyon 2:1–16. 10.1016/j.heliyon.2016.e00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast DJ, Yue M, Chen X, Bell D, Dresser L, Saskin R, Mandell LA, Low DE, de Azavedo JC. 2004. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob Agents Chemother 48:3343–3348. 10.1128/AAC.48.9.3343-3348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunell MK. 2012. Comparison of noncontact infrared thermometry and 3 commercial subcutaneous temperature transponding microchips with rectal thermometry in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 51:479–484. [PMC free article] [PubMed] [Google Scholar]
- 5.Całkosiński I, Dobrzyński M, Rosińczuk J, Dudek K, Chrószcz A, Fita K, Dymarek R. 2015. The use of infrared thermography as a rapid, quantitative, and noninvasive method for evaluation of inflammation response in different anatomical regions of rats. BioMed Res Int 2015:1–9. 10.1155/2015/972535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 7.Carstens AM, Tambara EM, Colman D, Carstens MG, Matias JE. 2016. Infrared image monitoring of local anesthetic poisoning in rats. Braz J Anesthesiol 66:603–612. 10.1016/j.bjan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Cates CC, McCabe JG, Lawson GW, Couto MA. 2014. Core body temperature as adjunct to endpoint determination in murine median lethal dose testing of rattlesnake venom. Comp Med 64:440–447. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen PH, White CE. 2006. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 45:57–63. [PubMed] [Google Scholar]
- 10.Clement JG, Mills P, Brockway B. 1989. Use of telemetry to record body temperature and activity in mice. J Pharmacol Methods 21:129–140. 10.1016/0160-5402(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 11.Crane JD, Mottillo EP, Farncombe TH, Morrison KM, Steinberg GR. 2014. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Mol Metab 3:490–494. 10.1016/j.molmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David JM, Chatziioannou AF, Taschereau R, Wang H, Stout DB. 2013. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med 63:386–391. [PMC free article] [PubMed] [Google Scholar]
- 13.Funda DP, Houstĕk J, Holub M, Kazdová L, Michalský M, Burýsek L, Cervinková M, Síma P. 1998. Differences in thermoregulation between immunocompetent and immunodeficient hairless mice exposed to mild cold. Folia Microbiol (Praha) 43:487–489. 10.1007/BF02820799. [DOI] [PubMed] [Google Scholar]
- 14.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2013. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110-111:87–95. 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Gordon CJ, Becker P, Ali JS. 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262. 10.1016/S0031-9384(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 16.Hankenson FC, Ruskoski N, van Saun M, Ying GS, Oh J, Fraser NW. 2013. Weight loss and reduced body temperature determine humane endpoints in a mouse model of ocular herpesvirus infection. J Am Assoc Lab Anim Sci 52:277–285. [PMC free article] [PubMed] [Google Scholar]
- 17.Harshaw C, Culligan JJ, Alberts JR. 2014. Sex differences in thermogenesis structure behavior and contact within huddles of infant mice. PLoS One 9:1–15. 10.1371/journal.pone.0087405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollands C. 1986. The Animals (Scientific Procedures) Act. Lancet 2:32–33. PubMed [DOI] [PubMed] [Google Scholar]
- 19.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 20.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120. In: Fox JG, Anderson LC, Loew FM, Quimby FW, Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press. [Google Scholar]
- 21.Jennings M, Morton DB, Charton E, Cooper J, Hendriksen C, Martin S, Pearce MC, Price S, Redhead K, Reed N, Simmons H, Spencer S, Willingale H. 2010. Application of the 3 Rs to challenge assays used in vaccine testing: 10th report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Biologicals 38:684–695. 10.1016/j.biologicals.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Johnston NA, Bosgraaf C, Cox L, Reichensperger J, Verhulst S, Patten C, Jr, Toth LA. 2007. Strategies for refinement of abdominal device implantation in mice: strain, carboxymethylcellulose, thermal support, and atipamezole. J Am Assoc Lab Anim Sci 46:46–53. [PubMed] [Google Scholar]
- 23.Kambiz S, van Neck JW, Cosgun SG, van Velzen MH, Janssen JA, Avazverdi N, Hovius SE, Walbeehm ET. 2015. An early diagnostic tool for diabetic peripheral neuropathy in rats. PLoS One 10:1–15. 10.1371/journal.pone.0126892. Correction: an early diagnostic tool for diabetic peripheral neuropathy in rats. PLoS One 2015. 10: e0131144 . [DOI] [Google Scholar]
- 24.Keeney AJ, Hogg S, Marsden CA. 2001. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav 74:177–184. 10.1016/S0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- 25.Kleiber M. 1947. Body size and metabolic rate. Physiol Rev 27:511–541. 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 26.Kleiber M. 1961. The fire of life. An introduction to animal energetics. New York (NY): John Wiley and Sons. [Google Scholar]
- 27.Klir JJ, Heath JE, Bennani N. 1990. An infrared thermographic study of surface temperature in relation to external thermal stress in the Mongolian gerbil, Meriones unguiculatus. Comp Biochem Physiol A Comp Physiol 96:141–146. 10.1016/0300-9629(90)90055-W. PubMed [DOI] [PubMed] [Google Scholar]
- 28.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim 32:260–269. 10.1258/002367798780559329. [DOI] [PubMed] [Google Scholar]
- 29.Leon LR, Walker LD, DuBose DA, Stephenson LA. 2004. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am J Physiol Regul Integr Comp Physiol 286:R967–R974. 10.1152/ajpregu.00380.2003. [DOI] [PubMed] [Google Scholar]
- 30.Molins CR, Delorey MJ, Young JW, Yockey BM, Belisle JT, Schriefer ME, Petersen JM. 2012. Use of temperature for standardizing the progression of Francisella tularensis in mice. PLoS One 7:1–9. 10.1371/journal.pone.0045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newsom DM, Bolgos GL, Colby L, Nemzek JA. 2004. Comparison of body surface temperature measurement and conventional methods for measuring temperature in the mouse. Contemp Top Lab Anim Sci 43:13–18. [PubMed] [Google Scholar]
- 32.Ruf T, Heldmaier G. 1987. Computerized body-temperature telemetry in small animals: use of simple equipment and advanced noise suppression. Comput Biol Med 17:331–340. 10.1016/0010-4825(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 33.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (United Kingdom): Methuen. [Google Scholar]
- 34.Saegusa Y, Tabata H. 2003. Usefulness of infrared thermometry in determining body temperature in mice. J Vet Med Sci 65:1365–1367. 10.1292/jvms.65.1365. [DOI] [PubMed] [Google Scholar]
- 35.Shelton LJ, Jr, White CE, Felt SA. 2006. A comparison of noncontact, subcutaneous, and rectal temperatures in captive owl monkeys (Aotus spp.). J Med Primatol 35:346–351. 10.1111/j.1600-0684.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- 36.Song C, Appleyard V, Murray K, Frank T, Sibbett W, Cuschieri A, Thompson A. 2007. Thermographic assessment of tumor growth in mouse xenografts. Int J Cancer 121:1055–1058. 10.1002/ijc.22808. [DOI] [PubMed] [Google Scholar]
- 37.Stephens Devalle JM. 2005. Comparison of tympanic, transponder, and noncontact infrared laser thermometry with rectal thermometry in strain 13 guinea pigs (Cavia porcellus). Contemp Top Lab Anim Sci 44:35–38. [PubMed] [Google Scholar]
- 38.Su Y, Foppen E, Fliers E, Kalsbeek A. 2016. Effects of intracerebroventricular administration of neuropeptide y on metabolic gene expression and energy metabolism in male rats. Endocrinology 157:3070–3085. 10.1210/en.2016-1083. [DOI] [PubMed] [Google Scholar]
- 39.Tepper M, Gannot I. 2015. Monitoring tumor state from thermal images in animal and human models. Med Phys 42:1297–1306. 10.1118/1.4907967. [DOI] [PubMed] [Google Scholar]
- 40.Tepper M, Shoval A, Hoffer O, Confino H, Schmidt M, Kelson I, Keisari Y, Gannot I. 2013. Thermographic investigation of tumor size, and its correlation to tumor relative temperature, in mice with transplantable solid breast carcinoma. J Biomed Opt 18:111410 10.1117/1.JBO.18.11.111410. [DOI] [PubMed] [Google Scholar]
- 41.Trammell RA, Toth LA. 2011. Markers for predicting death as an outcome for mice used in infectious disease research. Comp Med 61:492–498. [PMC free article] [PubMed] [Google Scholar]
- 42.Vianna DM, Carrive P. 2005. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 21:2505–2512. 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- 43.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50:160–166. [PubMed] [Google Scholar]
- 44.Vogel B, Wagner H, Gmoser J, Worner A, Loschberger A, Peters L, Frey A, Hofmann U, Frantz S. 2016. Touch-free measurement of body temperature using close-up thermography of the ocular surface. MethodsX 3:407–416. 10.1016/j.mex.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 37:126–131. 10.1258/00236770360563769. [DOI] [PubMed] [Google Scholar]
- 46.Williamson ED, Savage VL, Lingard B, Russell P, Scott EA. 2007. A biocompatible microdevice for core body temperature monitoring in the early diagnosis of infectious disease. Biomed Microdevices 9:51–60. 10.1007/s10544-006-9007-5. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe WL, Zissis GJ. 1978. The infrared handbook. Arlington (VA): Office of Naval Research, Department of the Navy. [Google Scholar]
- 48.Zhang Z, Foppen E, Su Y, Bisschop PH, Kalsbeek A, Fliers E, Boelen A. 2016. Metabolic effects of chronic T3 administration in the hypothalamic paraventricular and ventromedial nucleus in male rats. Endocrinology 157:4076–4085. 10.1210/en.2016-1397. [DOI] [PubMed] [Google Scholar]